Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development
Abstract
1. Introduction
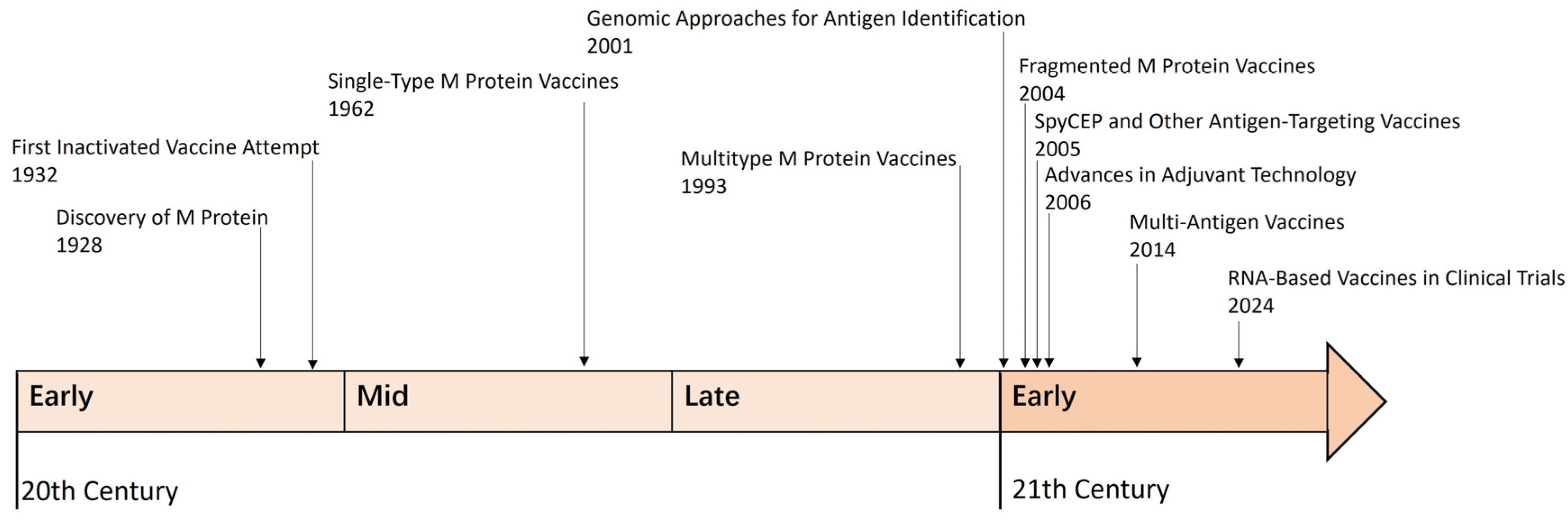
2. The Development of GAS Vaccines
3. Clinical Trials
3.1. M Protein Vaccines
3.1.1. Multivalent Vaccine
3.1.2. J8-DT Vaccine
3.1.3. Others
3.2. Non-M Protein Vaccines
4. Health Economics Evaluation and Vaccination Strategy Optimization for GAS Vaccines
4.1. Health Economics Evaluation
4.2. Vaccination Strategy Optimization
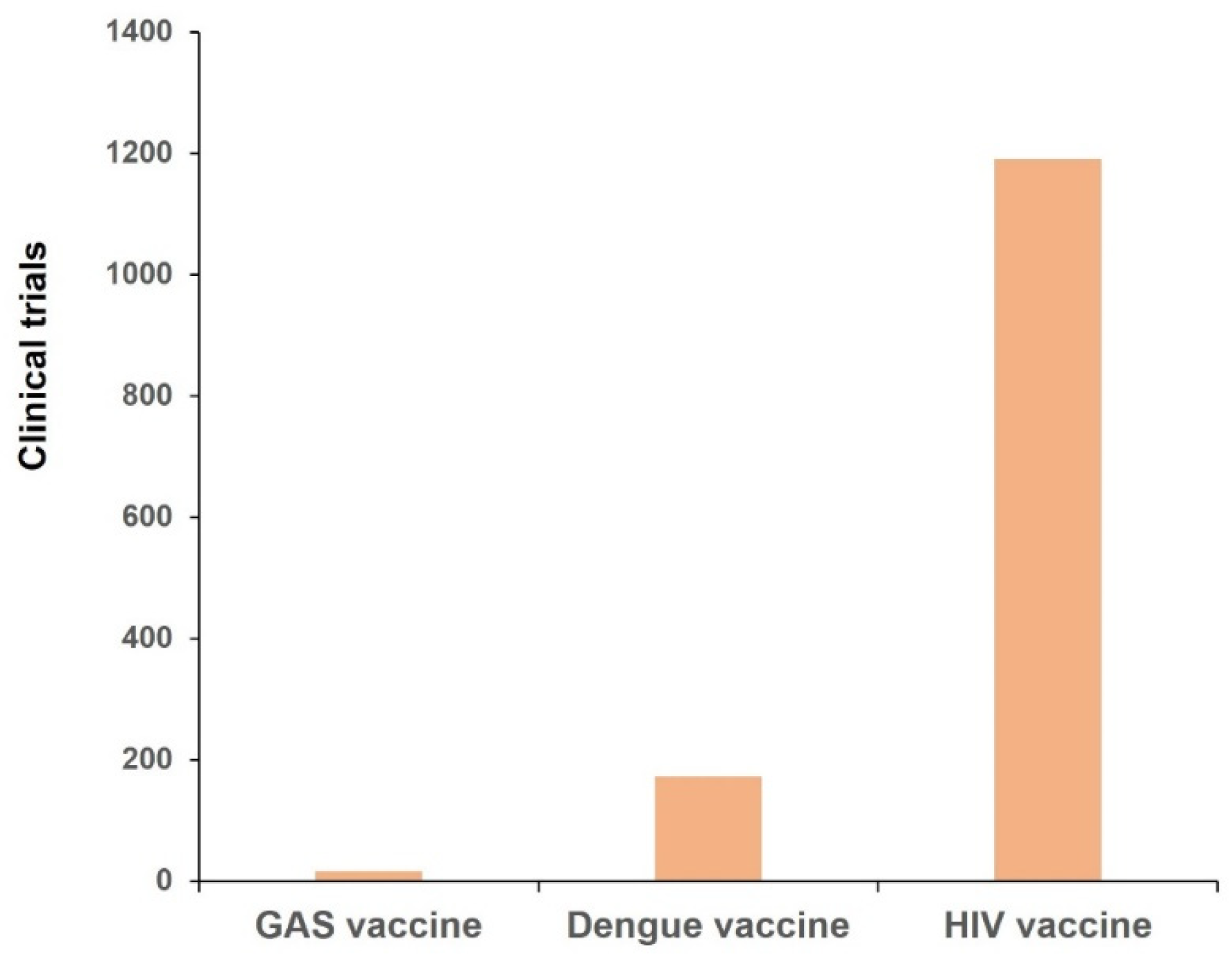
5. Accelerating the Development and Approval of GAS Vaccines
5.1. Enhanced Funding and Global Collaboration
5.2. Leverage Advanced Vaccine Technologies
5.3. Streamline Clinical Trials
5.4. Increase Advocacy and Public Awareness
6. New Avenues for Future Research
6.1. Novel Insights into the Infection Spectrum, Disease Spectrum, and Pathogen Profile
6.2. Exploration of Novel Antigens, Innovative Technologies, and Next-Generation Vaccine Platforms
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Chan, T.C.; Yap, L.W.; Luo, Y.; Xu, W.; Qin, S.; Zhao, N.; Yu, Z.; Geng, X.; Liu, S.L.; et al. Resurgence of scarlet fever in China: A 13-year population-based surveillance study. Lancet Infect. Dis. 2018, 18, 903–912. [Google Scholar] [CrossRef]
- Lamagni, T.; Guy, R.; Chand, M.; Henderson, K.L.; Chalker, V.; Lewis, J.; Saliba, V.; Elliot, A.J.; Smith, G.E.; Rushton, S.; et al. Resurgence of scarlet fever in England, 2014–2016: A population-based surveillance study. Lancet Infect. Dis. 2018, 18, 180–187. [Google Scholar] [CrossRef]
- Keuleyan, E.; Todorov, T.; Donchev, D.; Kevorkyan, A.; Vazharova, R.; Kukov, A.; Todorov, G.; Georgieva, B.; Altankova, I.; Uzunova, Y.; et al. Characterization of Streptococcus pyogenes Strains from Tonsillopharyngitis and Scarlet Fever Resurgence, 2023-FIRST Detection of M1UK in Bulgaria. Microorganisms 2025, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, O.P.; Rana, R.; Priyanka null Ali, A.B.; Sharma, V. Emergence of STSS in Japan: An assessment of the threat and containment strategies. New Microbes New Infect. 2024, 60–61, 101449. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M. Emerging Trends in Streptococcal Toxic Shock Syndrome, Japan. Emerg. Infect. Dis. 2025, 31, 847–849. [Google Scholar] [CrossRef]
- Gregory, C.J.; Okaro, J.O.; Reingold, A.; Chai, S.; Herlihy, R.; Petit, S.; Farley, M.M.; Harrison, L.H.; Como-Sabetti, K.; Lynfield, R.; et al. Invasive Group A Streptococcal Infections in 10 US States. JAMA 2025, 333, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Lond. Engl. 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Mehta, S.; McGeer, A.; Low, D.E.; Hallett, D.; Bowman, D.J.; Grossman, S.L.; Stewart, T.E. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006, 130, 1679–1686. [Google Scholar] [CrossRef]
- Andrejko, K.; Whittles, L.K.; Lewnard, J.A. Health-Economic Value of Vaccination Against Group A Streptococcus in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.F.; LaRock, C.N. Antibiotic Treatment, Mechanisms for Failure, and Adjunctive Therapies for Infections by Group A Streptococcus. Front. Microbiol. 2021, 12, 760255. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.; Sistla, S. Trends in Antimicrobial Resistance Patterns of Group A Streptococci, Molecular Basis and Implications. Indian J. Med. Microbiol. 2018, 36, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.; Sistla, S. Molecular epidemiology of macrolide resistant Group A streptococci from Puducherry, India. J. Infect. Dev. Ctries 2017, 11, 679–683. [Google Scholar] [CrossRef]
- Dicuonzo, G.; Fiscarelli, E.; Gherardi, G.; Lorino, G.; Battistoni, F.; Landi, S.; De Cesaris, M.; Petitti, T.; Beall, B. Erythromycin-resistant pharyngeal isolates of Streptococcus pyogenes recovered in Italy. Antimicrob. Agents Chemother. 2002, 46, 3987–3990. [Google Scholar] [CrossRef]
- Bahnan, W.; Hashwa, F.; Araj, G.; Tokajian, S. emm typing, antibiotic resistance and PFGE analysis of Streptococcus pyogenes in Lebanon. J. Med. Microbiol. 2011, 60, 98–101. [Google Scholar] [CrossRef]
- Tamayo, J.; Pérez-Trallero, E.; Gómez-Garcés, J.L.; Alós, J.I.; Spanish Group for the Study of Infection in the Primary Health Care Setting. Resistance to macrolides, clindamycin and telithromycin in Streptococcus pyogenes isolated in Spain during 2004. J. Antimicrob. Chemother. 2005, 56, 780–782. [Google Scholar] [CrossRef][Green Version]
- Lapthorne, S.; McWade, R.; Scanlon, N.; Ní Bhaoill, S.; Page, A.; O’Donnell, C.; Dornikova, G.; Hannan, M.; Lynch, B.; Lynch, M.; et al. Rising clindamycin resistance in group A Streptococcus in an Irish healthcare institution. Access. Microbiol. 2024, 6, 000772.v4. [Google Scholar] [CrossRef]
- Silva-Costa, C.; Ramirez, M.; Melo-Cristino, J. Rapid Inversion of the Prevalences of Macrolide Resistance Phenotypes Paralleled by a Diversification of T and emm Types among Streptococcus pyogenes in Portugal. Antimicrob. Agents Chemother. 2005, 49, 2109–2111. [Google Scholar] [CrossRef]
- Wilson, M.G. The biologic products of streptococcus cardioarthritidis: Therapeutic and prophylactic value in rheumatic disease in children. J. Am. Med. Assoc. 1930, 94, 842–844. [Google Scholar] [CrossRef]
- Collis, W.R.F.; Sheldon, W. Intravenous vaccines of hæmolytic streptococci in acute rheumatism in childhood. Lancet 1932, 220, 1261–1264. [Google Scholar] [CrossRef]
- Todd, E.W.; Lancefield, R.C. Variants of hemolytic streptococci; their relation to type-specific substance, virulence, and toxin. J. Exp. Med. 1928, 48, 751–767. [Google Scholar] [CrossRef]
- Lancefield, R.C. Current Knowledge of Type-Specific M Antigens of Group A Streptococci1. J. Immunol. 1962, 89, 307–313. [Google Scholar] [CrossRef]
- Fischetti, V.A. Streptococcal M protein: Molecular design and biological behavior. Clin. Microbiol. Rev. 1989, 2, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Simmons, M.; Chiang, E.C.; Chiang, E.Y. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 1996, 14, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Chiang, E.Y.; Lederer, J.W. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. Baltim. Md. 1950 1993, 151, 2188–2194. [Google Scholar] [CrossRef]
- Dale, J.B.; Penfound, T.; Chiang, E.Y.; Long, V.; Shulman, S.T.; Beall, B. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin. Diagn. Lab. Immunol. 2005, 12, 833–836. [Google Scholar] [CrossRef]
- Pastural, É.; McNeil, S.A.; MacKinnon-Cameron, D.; Ye, L.; Langley, J.M.; Stewart, R.; Martin, L.H.; Hurley, G.J.; Salehi, S.; Penfound, T.A.; et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: A randomized, controlled phase I study. Vaccine. 2020, 38, 1384–1392. [Google Scholar] [CrossRef]
- Ferretti, J.J.; McShan, W.M.; Ajdic, D.; Savic, D.J.; Savic, G.; Lyon, K.; Primeaux, C.; Sezate, S.; Suvorov, A.N.; Kenton, S.; et al. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 2001, 98, 4658–4663. [Google Scholar] [CrossRef]
- Dale, J.B. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 1999, 17, 193–200. [Google Scholar] [CrossRef]
- Georgousakis, M.M.; McMillan, D.J.; Batzloff, M.R.; Sriprakash, K.S. Moving forward: A mucosal vaccine against group A streptococcus. Expert Rev. Vaccines 2009, 8, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Sumby, P.; Barbian, K.D.; Gardner, D.J.; Whitney, A.R.; Welty, D.M.; Long, R.D.; Bailey, J.R.; Parnell, M.J.; Hoe, N.P.; Adams, G.G.; et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. USA 2005, 102, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.B.; Penfound, T.A.; Salehi, S.; Ogega, C.O.; Dold, C.; Plante, O.; Dale, J.B. Immunogenicity of a 30-valent M protein mRNA group A Streptococcus vaccine. Vaccine 2024, 42, 126205. [Google Scholar] [CrossRef]
- McNeil, S.A.; Halperin, S.A.; Langley, J.M.; Smith, B.; Baxendale, D.M.; Warren, A.; Sharratt, G.P.; Reddish, M.A.; Fries, L.F.; Vink, P.E.; et al. A double-blind, randomized phase II trial of the safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adults. Int. Congr. Ser. 2006, 1289, 303–306. [Google Scholar] [CrossRef]
- Batzloff, M.R.; Hayman, W.A.; Davies, M.R.; Zeng, M.; Pruksakorn, S.; Brandt, E.R.; Good, M.F. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: Contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 2003, 187, 1598–1608. [Google Scholar] [CrossRef]
- Sanduja, P.; Gupta, M.; Somani, V.K.; Yadav, V.; Dua, M.; Hanski, E.; Sharma, A.; Bhatnagar, R.; Johri, A.K. Cross-serotype protection against group A Streptococcal infections induced by immunization with SPy_2191. Nat. Commun. 2020, 11, 3545. [Google Scholar] [CrossRef]
- Reglinski, M.; Lynskey, N.N.; Choi, Y.J.; Edwards, R.J.; Sriskandan, S. Development of a multicomponent vaccine for Streptococcus pyogenes based on the antigenic targets of IVIG. J. Infect. 2016, 72, 450–459. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Corretti, M.; Palmer, K.; Campbell, J.D.; Reddish, M.A.; Hu, M.C.; Wasserman, S.S.; Dale, J.B. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: Phase 1 trial. JAMA 2004, 292, 709–715. [Google Scholar] [CrossRef]
- McNeil, S.A.; Halperin, S.A.; Langley, J.M.; Smith, B.; Warren, A.; Sharratt, G.P.; Baxendale, D.M.; Reddish, M.A.; Hu, M.C.; Stroop, S.D.; et al. Safety and Immunogenicity of 26-Valent Group A Streptococcus Vaccine in Healthy Adult Volunteers. Clin. Infect. Dis. 2005, 41, 1114–1122. [Google Scholar] [CrossRef]
- Hayman, W.A.; Brandt, E.R.; Relf, W.A.; Cooper, J.; Saul, A.; Good, M.F. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int. Immunol. 1997, 9, 1723–1733. [Google Scholar] [CrossRef]
- Rivera-Hernandez, T.; Hartas, J.; Wu, Y.; Chuan, Y.P.; Lua, L.H.; Good, M.; Batzloff, M.R.; Middelberg, A.P. Self-adjuvanting modular virus-like particles for mucosal vaccination against group A streptococcus (GAS). Vaccine 2013, 31, 1950–1955. [Google Scholar] [CrossRef]
- Pandey, M.; Wykes, M.N.; Hartas, J.; Good, M.F.; Batzloff, M.R. Long-term antibody memory induced by synthetic peptide vaccination is protective against Streptococcus pyogenes infection and is independent of memory T cell help. J. Immunol. Baltim. Md. 1950 2013, 190, 2692–2701. [Google Scholar] [CrossRef]
- Sekuloski, S.; Batzloff, M.R.; Griffin, P.; Parsonage, W.; Elliott, S.; Hartas, J.; O’Rourke, P.; Marquart, L.; Pandey, M.; Rubin, F.A.; et al. Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS ONE 2018, 13, e0198658. [Google Scholar] [CrossRef]
- Zaman, M.; Abdel-Aal, A.B.; Fujita, Y.; Phillipps, K.S.; Batzloff, M.R.; Good, M.F.; Toth, I. Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: Structure-activity relationship. PLoS ONE 2012, 7, e30146. [Google Scholar] [CrossRef]
- Batzloff, M.R.; Hartas, J.; Zeng, W.; Jackson, D.C.; Good, M.F. Intranasal vaccination with a lipopeptide containing a conformationally constrained conserved minimal peptide, a universal T cell epitope, and a self-adjuvanting lipid protects mice from group A streptococcus challenge and reduces throat colonization. J. Infect. Dis. 2006, 194, 325–330. [Google Scholar] [CrossRef]
- Shaila, M.S.; Nayak, R.; Prakash, S.S.; Georgousakis, M.; Brandt, E.; McMillan, D.J.; Batzloff, M.R.; Pruksakorn, S.; Good, M.F.; Sriprakash, K.S. Comparative in silico analysis of two vaccine candidates for group A streptococcus predicts that they both may have similar safety profiles. Vaccine 2007, 25, 3567–3573. [Google Scholar] [CrossRef] [PubMed]
- University of Alberta. A Randomized Double Blinded Within Dose, Controlled, Safety and Immunogenicity Study of GAS Vaccine Candidate in Healthy Individuals. clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT04882514 (accessed on 29 April 2025).
- Meier-Stephenson, V.; Hawkes, M.T.; Burton, C.; Calcutt, A.; Davis, C.; Dooley, J.; Good, M.; Houghton, M.; Keeffe, E.; Kim, K.; et al. A phase 1 randomized controlled trial of a peptide-based group A streptococcal vaccine in healthy volunteers. Trials 2024, 25, 781. [Google Scholar] [CrossRef]
- Brandt, E.R.; Hayman, W.A.; Currie, B.; Carapetis, J.; Wood, Y.; Jackson, D.C.; Cooper, J.; Melrose, W.D.; Saul, A.J.; Good, M.F. Opsonic human antibodies from an endemic population specific for a conserved epitope on the M protein of group A streptococci. Immunology 1996, 89, 331–337. [Google Scholar] [CrossRef]
- Brandt, E.R.; Hayman, W.A.; Currie, B.; Pruksakorn, S.; Good, M.F. Human antibodies to the conserved region of the M protein: Opsonization of heterologous strains of group A streptococci. Vaccine 1997, 15, 1805–1812. [Google Scholar] [CrossRef]
- Brandt, E.R.; Hayman, W.A.; Currie, B.; Carapetis, J.; Jackson, D.C.; Do, K.A.; Good, M.F. Functional analysis of IgA antibodies specific for a conserved epitope within the M protein of group A streptococci from Australian Aboriginal endemic communities. Int. Immunol. 1999, 11, 569–576. [Google Scholar] [CrossRef][Green Version]
- Skwarczynski, M.; Kamaruzaman, K.A.; Srinivasan, S.; Zaman, M.; Lin, I.C.; Batzloff, M.R.; Good, M.F.; Toth, I. M-protein-derived conformational peptide epitope vaccine candidate against Group A Streptococcus. Curr. Drug Deliv. 2013, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.J.; Davies, M.R.; Good, M.F.; Sriprakash, K.S. Immune response to superoxide dismutase in group A streptococcal infection. FEMS Immunol. Med. Microbiol. 2004, 40, 249–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McArthur, J.; Medina, E.; Mueller, A.; Chin, J.; Currie, B.J.; Sriprakash, K.S.; Talay, S.R.; Chhatwal, G.S.; Walker, M.J. Intranasal vaccination with streptococcal fibronectin binding protein Sfb1 fails to prevent growth and dissemination of Streptococcus pyogenes in a murine skin infection model. Infect. Immun. 2004, 72, 7342–7345. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.; Schulze, K.; Chin, J.; Currie, B.J.; Sriprakash, K.S.; Talay, S.R.; Chhatwal, G.S.; Guzmán, C.A.; Walker, M.J. Immune responses of a liposome/ISCOM vaccine adjuvant against streptococcal fibronectin binding protein 1 (Sfb1) in mice. Indian J. Med. Res 2024, 119, 115–120. [Google Scholar]
- Wizemann, T.M.; Adamou, J.E.; Langermann, S. Adhesins as targets for vaccine development. Emerg. Infect. Dis. 1999, 5, 395–403. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. (Eds.) Handbook of Bacterial Adhesion: Principles, Methods, and Applications; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Loh, J.M.S.; Rivera-Hernandez, T.; McGregor, R.; Khemlani, A.H.J.; Tay, M.L.; Cork, A.J.; MRaynes, J.; Moreland, N.J.; Walker, M.J.; Proft, T.; et al. A multivalent T-antigen-based vaccine for Group A Streptococcus. Sci. Rep. 2021, 11, 4353. [Google Scholar] [CrossRef]
- Loh, J.M.S.; Lorenz, N.; Tsai, C.J.; Khemlani, A.H.J.; Proft, T. Mucosal vaccination with pili from Group A Streptococcus expressed on Lactococcus lactis generates protective immune responses. Sci. Rep. 2017, 7, 7174. [Google Scholar] [CrossRef]
- J-Khemlani, A.H.; Pilapitiya, D.; Tsai, C.J.Y.; Proft, T.; Loh, J.M.S. Expanding strain coverage of a group A Streptococcus pilus-expressing Lactococcus lactis mucosal vaccine. Immunol. Cell Biol. 2023, 101, 545–555. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Harabuchi, Y. Intranasal immunization with lipoteichoic acid and cholera toxin evokes specific pharyngeal IgA and systemic IgG responses and inhibits streptococcal adherence to pharyngeal epithelial cells in mice. Int. J. Pediatr. Otorhinolaryngol. 2002, 63, 235–241. [Google Scholar] [CrossRef]
- van Sorge, N.M.; Cole, J.N.; Kuipers, K.; Henningham, A.; Aziz, R.K.; Kasirer-Friede, A.; Lin, L.; Berends, E.T.M.; Davies, M.R.; Dougan, G.; et al. The classical lancefield antigen of group a Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 2014, 15, 729–740. [Google Scholar] [CrossRef]
- Sabharwal, H.; Michon, F.; Nelson, D.; Dong, W.; Fuchs, K.; Manjarrez, R.C.; Sarkar, A.; Uitz, C.; Viteri-Jackson, A.; Suarez, R.S.; et al. Group A streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis. 2006, 193, 129–135. [Google Scholar] [CrossRef]
- Khatun, F.; Dai, C.C.; Rivera-Hernandez, T.; Hussein, W.M.; Khalil, Z.G.; Capon, R.J.; Toth, I.; Stephenson, R.J. Immunogenicity Assessment of Cell Wall Carbohydrates of Group A Streptococcus via Self-Adjuvanted Glyco-lipopeptides. ACS Infect. Dis. 2021, 7, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, X.; Li, N.; Cui, H.; Hou, B.; Gao, B.; Cleary, P.P.; Wang, B. Sortase A induces Th17-mediated and antibody-independent immunity to heterologous serotypes of group A streptococci. PLoS ONE 2014, 9, e107638. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Zhao, Y.; Wang, S.; Feng, S.; Gu, G. Immunogenicity Assessment of Different Segments and Domains of Group a Streptococcal C5a Peptidase and Their Application Potential as Carrier Protein for Glycoconjugate Vaccine Development. Vaccines 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.; Kaplan, E.L.; Johnson, D.R.; Cleary, P.P. Immune response to group A streptococcal C5a peptidase in children: Implications for vaccine development. J. Infect. Dis. 2003, 188, 809–817. [Google Scholar] [CrossRef]
- Rivera-Hernandez, T.; Carnathan, D.G.; Jones, S.; Cork, A.J.; Davies, M.R.; Moyle, P.M.; Toth, I.; Batzloff, M.R.; McCarthy, J.; Nizet, V.; et al. An Experimental Group A Streptococcus Vaccine That Reduces Pharyngitis and Tonsillitis in a Nonhuman Primate Model. mBio. 2019, 10, e00693-19. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Cleary, P.P. Active and passive intranasal immunizations with streptococcal surface protein C5a peptidase prevent infection of murine nasal mucosa-associated lymphoid tissue, a functional homologue of human tonsils. Infect. Immun. 2005, 73, 7878–7886. [Google Scholar] [CrossRef]
- Rivera-Hernandez, T.; Pandey, M.; Henningham, A.; Cole, J.; Choudhury, B.; Cork, A.J.; Gillen, C.M.; Ghaffar, K.A.; West, N.P.; Silvestri, G.; et al. Differing Efficacies of Lead Group A Streptococcal Vaccine Candidates and Full-Length M Protein in Cutaneous and Invasive Disease Models. mBio 2016, 7, e00618-16. [Google Scholar] [CrossRef]
- Herrera, A.L.; Van Hove, C.; Hanson, M.; Dale, J.B.; Tweten, R.K.; Huber, V.C.; Diel, D.; Chaussee, M.S. Immunotherapy targeting the Streptococcus pyogenes M protein or streptolysin O to treat or prevent influenza A superinfection. PLoS ONE 2020, 15, e0235139. [Google Scholar] [CrossRef]
- Kapoor, N.; Uchiyama, S.; Pill, L.; Bautista, L.; Sedra, A.; Yin, L.; Regan, M.; Chu, E.; Rabara, T.; Wong, M.; et al. Non-Native Amino Acid Click Chemistry-Based Technology for Site-Specific Polysaccharide Conjugation to a Bacterial Protein Serving as Both Carrier and Vaccine Antigen. ACS Omega 2022, 7, 24111–24120. [Google Scholar] [CrossRef]
- Troese, M.J.; Burlet, E.; Cunningham, M.W.; Alvarez, K.; Bentley, R.; Thomas, N.; Carwell, S.; Morefield, G.L. Group A Streptococcus Vaccine Targeting the Erythrogenic Toxins SpeA and SpeB Is Safe and Immunogenic in Rabbits and Does Not Induce Antibodies Associated with Autoimmunity. Vaccines 2023, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Burlet, E.; HogenEsch, H.; Dunham, A.; Morefield, G. Evaluation of the Potency, Neutralizing Antibody Response, and Stability of a Recombinant Fusion Protein Vaccine for Streptococcus pyogenes. AAPS J. 2017, 19, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Morefield, G.; Touhey, G.; Lu, F.; Dunham, A.; HogenEsch, H. Development of a recombinant fusion protein vaccine formulation to protect against Streptococcus pyogenes. Vaccine 2014, 32, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Ericsson, D.J.; Langer, K.; Casey, L.W.; Jovcevski, B.; Chhatwal, G.S.; Aquilina, J.A.; Batzloff, M.R.; Kobe, B.; Walker, M.J. Structure-informed design of an enzymatically inactive vaccine component for group A Streptococcus. mBio 2013, 4, e00509-13. [Google Scholar] [CrossRef]
- Xu, Z.; Rivera-Hernandez, T.; Moyle, P.M. Development of an Enzyme-Mediated, Site-Specific Method to Conjugate Toll-Like Receptor 2 Agonists onto Protein Antigens: Toward a Broadly Protective, Four Component, Group A Streptococcal Self-Adjuvanting Lipoprotein-Fusion Combination Vaccine. ACS Infect. Dis. 2020, 6, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Abate, F.; Malito, E.; Falugi, F.; Margarit YRos, I.; Bottomley, M.J. Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of SpyCEP, a candidate antigen for a vaccine against Streptococcus pyogenes. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1103–1106. [Google Scholar] [CrossRef]
- Henningham, A.; Chiarot, E.; Gillen, C.M.; Cole, J.N.; Rohde, M.; Fulde, M.; Ramachandran, V.; Cork, A.J.; Hartas, J.; Magor, G.; et al. Conserved anchorless surface proteins as group A streptococcal vaccine candidates. J. Mol. Med. Berl. Ger. 2012, 90, 1197–1207. [Google Scholar] [CrossRef]
- Cannon, J.W.; Zhung, J.; Bennett, J.; Moreland, N.J.; Baker, M.G.; Geelhoed, E.; Fraser, J.; Carapetis, J.R.; Jack, S. The economic and health burdens of diseases caused by group A Streptococcus in New Zealand. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 103, 176–181. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.; Excler, J.L.; Kim, J.H.; Mogasale, V. Global economic burden per episode for multiple diseases caused by group A Streptococcus. npj Vaccines 2023, 8, 69. [Google Scholar] [CrossRef]
- Cannon, J.W.; Jack, S.; Wu, Y.; Zhang, J.; Baker, M.G.; Geelhoed, E.; Fraser, J.; Carapetis, J.R. An economic case for a vaccine to prevent group A streptococcus skin infections. Vaccine. 2018, 36, 6968–6978. [Google Scholar] [CrossRef]
- WHO Preferred Product Characteristics for Group A Streptococcus Vaccines. Available online: https://www.who.int/publications/i/item/who-preferred-product-characteristics-for-group-a-streptococcus-vaccines (accessed on 20 May 2025).
- Search for: Other Terms: “Group A Streptococcus” AND “Vaccine”|List Expert Search|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/expert-search?term=%22Group%20A%20Streptococcus%22%20AND%20%22vaccine%22 (accessed on 27 April 2025).
- Search for: Other Terms: Dengue Vaccine|List Expert Search|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/expert-search?term=Dengue%20Vaccine (accessed on 27 April 2025).
- Search for: Other Terms: HIV Vaccine|List Expert Search|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/expert-search?term=HIV%20vaccine (accessed on 27 April 2025).
- Tortorice, D.; Ferranna, M.; Bloom, D.E. Optimal global spending for group A Streptococcus vaccine research and development. npj Vaccines 2023, 8, 62. [Google Scholar] [CrossRef]
- SAVAC Web. Available online: https://savac.ivi.int/ (accessed on 27 April 2025).
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef]
- Kaizer, A.M.; Belli, H.M.; Ma, Z.; Nicklawsky, A.G.; Roberts, S.C.; Wild, J.; Wogu, A.F.; Xiao, M.; Sabo, R.T. Recent innovations in adaptive trial designs: A review of design opportunities in translational research. J. Clin. Transl. Sci. 2023, 7, e125. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Lin, J.; Lin, Y.; Hoffman, E. Innovative trial designs and analyses for vaccine clinical development. Contemp. Clin. Trials. 2021, 100, 106225. [Google Scholar] [CrossRef] [PubMed]
- Damle, N.; Shah, S.; Nagraj, P.; Tabrizi, P.; Rodriguez, G.E.; Bhambri, R. FDA’s Expedited Programs and Their Impact on the Availability of New Therapies. Ther. Innov. Regul. Sci. 2017, 51, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.; Lao, C.T.; Yousif, M.M.; Luga, J.M. Fast Tracking-Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review. Vaccines 2022, 10, 1256. [Google Scholar] [CrossRef]
- Dada, S.; McKay, G.; Mateus, A.; Lees, S. Lessons learned from engaging communities for Ebola vaccine trials in Sierra Leone: Reciprocity, relatability, relationships and respect (the four R’s). BMC Public Health 2019, 19, 1665. [Google Scholar] [CrossRef]
- Slaoui, M.; Hepburn, M. Developing Safe and Effective COVID Vaccines—Operation Warp Speed’s Strategy and Approach. N. Engl. J. Med. 2020, 383, 1701–1703. [Google Scholar] [CrossRef]
- Schwartz, J.L. Evaluating and Deploying COVID-19 Vaccines—The Importance of Transparency, Scientific Integrity, and Public Trust. N. Engl. J. Med. 2020, 383, 1703–1705. [Google Scholar] [CrossRef]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- CDC. How Influenza (Flu) Vaccines Are Made. Influenza (Flu). 30 September 2024. Available online: https://www.cdc.gov/flu/vaccine-process/index.html (accessed on 28 April 2025).
- Xu, M.A.; Choi, J.; Capasso, A.; DiClemente, R.J. Improving HPV Vaccination Uptake Among Adolescents in Low Resource Settings: Sociocultural and Socioeconomic Barriers and Facilitators. Adolesc. Health Med. Ther. 2024, 15, 73–82. [Google Scholar] [CrossRef]
- Kyei, G.K.; Kyei, E.F.; Ansong, R. HPV Vaccine Hesitancy and Uptake: A Conceptual Analysis Using Rodgers’ Evolutionary Approach. J. Adv. Nurs. 2025, 81, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, O.E.; Provenzano, S.; Di Martino, G.; Ferrara, P. COVID-19 Vaccination and Public Health: Addressing Global, Regional, and Within-Country Inequalities. Vaccines 2024, 12, 885. [Google Scholar] [CrossRef]
- Vaccine Equity. Available online: https://www.who.int/campaigns/vaccine-equity (accessed on 28 April 2025).
- Vaccine Inequity Undermining Global Economic Recovery. Available online: https://www.who.int/news/item/22-07-2021-vaccine-inequity-undermining-global-economic-recovery (accessed on 28 April 2025).
- Macrì, S.; Spinello, C.; Widomska, J.; Magliozzi, R.; Poelmans, G.; Invernizzi, R.W.; Creti, R.; Roessner, V.; Bartolini, E.; Margarit, I.; et al. Neonatal corticosterone mitigates autoimmune neuropsychiatric disorders associated with streptococcus in mice. Sci. Rep. 2018, 8, 10188. [Google Scholar] [CrossRef]
- Menendez, C.M.; Zuccolo, J.; Swedo, S.E.; Reim, S.; Richmand, B.; Ben-Pazi, H.; Kovoor, A.; Cunningham, M.W. Dopamine receptor autoantibody signaling in infectious sequelae differentiates movement versus neuropsychiatric disorders. JCI Insight 2024, 9, e164762. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Steer, A.C.; Smeesters, P.R.; Keeble, J.; Inouye, M.; Carapetis, J.; Wicks, I.P. Post-infectious group A streptococcal autoimmune syndromes and the heart. Autoimmun. Rev. 2015, 14, 710–725. [Google Scholar] [CrossRef]
- Snider, L.A.; Swedo, S.E. PANDAS: Current status and directions for research. Mol. Psychiatry 2004, 9, 900–907. [Google Scholar] [CrossRef]
- Putra, T.M.H.; Rodriguez-Fernandez, R.; Widodo, W.A.; Elfiana, M.; Laksono, S.; Nguyen, Q.N.; Tan, J.W.C.; Narula, J. Myocardial fibrosis in rheumatic heart disease: Emerging concepts and clinical implications. Front. Cardiovasc. Med. 2023, 10, 1230894. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ng, J.S.W.; Ottakandathil Babu, R.; Woo, J.; Nahler, J.; Hardman, C.S.; Kurupati, P.; Nussbaum, L.; Gao, F.; Dong, T.; et al. Group A Streptococcus induces CD1a-autoreactive T cells and promotes psoriatic inflammation. Sci. Immunol. 2023, 8, eadd9232. [Google Scholar] [CrossRef]
- Li, Y.; Rivers, J.; Mathis, S.; Li, Z.; Chochua, S.; Metcalf, B.J.; Beall, B.; Onukwube, J.; Gregory, C.J.; McGee, L. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019–2021. Emerg. Infect. Dis. 2023, 29, 2116–2120. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, J.P.; Lin, Q.; Lammens, C.; Smeesters, P.R.; van Kleef-van Koeveringe, S.; Matheeussen, V.; Malhotra-Kumar, S. Increase in bloodstream infections caused by emm1 group A Streptococcus correlates with emergence of toxigenic M1UK, Belgium, May 2022 to August 2023. Euro Surveill. Bull. Eur. Sur. Mal. Transm. Eur. Commun. Dis. Bull. 2023, 28, 2300422. [Google Scholar] [CrossRef]
- Vieira, A.; Wan, Y.; Ryan, Y.; Li, H.K.; Guy, R.L.; Papangeli, M.; Huse, K.K.; Reeves, L.C.; Soo, V.W.C.; Daniel, R.; et al. Rapid expansion and international spread of M1UK in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat. Commun. 2024, 15, 3916. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Li, H.K.; Li, H.; Loboda, Z.; Charles, S.; Vieira, A.; Huse, K.; Jauneikaite, E.; Reeves, L.; Mok, K.Y.; et al. Emerging Invasive Group A Streptococcus M1UK Lineage Detected by Allele-Specific PCR, England, 20201. Emerg. Infect. Dis. 2023, 29, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar] [CrossRef]
- Bowman, B.N.; McAdam, P.R.; Vivona, S.; Zhang, J.X.; Luong, T.; Belew, R.K.; Sahota, H.; Guiney, D.; Valafar, F.; Fierer, J.; et al. Improving reverse vaccinology with a machine learning approach. Vaccine 2011, 29, 8156–8164. [Google Scholar] [CrossRef] [PubMed]
- Teahan, B.; Ong, E.; Yang, Z. Identification of Mycobacterium tuberculosis Antigens with Vaccine Potential Using a Machine Learning-Based Reverse Vaccinology Approach. Vaccines 2021, 9, 1098. [Google Scholar] [CrossRef]
- Ong, E.; Cooke, M.F.; Huffman, A.; Xiang, Z.; Wong, M.U.; Wang, H.; Seetharaman, M.; Valdez, N.; He, Y. Vaxign2: The second generation of the first Web-based vaccine design program using reverse vaccinology and machine learning. Nucleic Acids Res. 2021, 49, W671–W678. [Google Scholar] [CrossRef]
- Derking, R.; Sanders, R.W. Structure-guided envelope trimer design in HIV-1 vaccine development: A narrative review. J. Int. AIDS Soc. 2021, 24 (Suppl. S7), e25797. [Google Scholar] [CrossRef]
- Huang, Y.; Song, F.; Zeng, Y.; Sun, H.; Sheng, R.; Wang, X.; Liu, L.; Luo, G.; Jiang, Y.; Chen, Y.; et al. A single residue switch mediates the broad neutralization of Rotaviruses. Nat. Commun. 2025, 16, 838. [Google Scholar] [CrossRef]
- Kaushal, N.; Jain, S.; Baranwal, M. Computational design of immunogenic peptide constructs comprising multiple human leukocyte antigen restricted dengue virus envelope epitopes. J. Mol. Recognit. JMR 2022, 35, e2961. [Google Scholar] [CrossRef]
- Forouharmehr, A. Engineering an efficient poly-epitope vaccine against Toxoplasma gondii infection: A computational vaccinology study. Microb. Pathog. 2021, 152, 104646. [Google Scholar] [CrossRef]
- He, L.; Zhu, J. Computational tools for epitope vaccine design and evaluation. Curr. Opin. Virol. 2015, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Sessevmez, M.; Koray Gök, M.; Özgümüş, S.; Okyar, A.; Oya Alpar, H.; Cevher, E. Nasal vaccination with poly(β-amino ester)-poly(d,l-lactide-co-glycolide) hybrid nanoparticles. Int. J. Pharm. 2017, 529, 1–14. [Google Scholar] [CrossRef]
- Pavot, V.; Climent, N.; Rochereau, N.; Garcia, F.; Genin, C.; Tiraby, G.; Vernejoul, F.; Perouzel, E.; Lioux, T.; Verrier, B.; et al. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials 2016, 75, 327–339. [Google Scholar] [CrossRef]
- Longet, S.; Lundahl, M.L.E.; Lavelle, E.C. Targeted Strategies for Mucosal Vaccination. Bioconjug. Chem. 2018, 29, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yan, Q.; Yu, Y.; Wu, M.X. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J. Control Release Off. J. Control Release Soc. 2017, 255, 36–44. [Google Scholar] [CrossRef]
- An, M.; Liu, H. Dissolving Microneedle Arrays for Transdermal Delivery of Amphiphilic Vaccines. Small Weinh. Bergstr. Ger. 2017, 13, 1700164. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Ahlén, G.; Appelberg, K.S.; Meade-White, K.; Hanley, P.W.; Scott, D.; Monteil, V.; Devignot, S.; Okumura, A.; Weber, F.; et al. A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a Cynomolgus macaque model. Nat. Microbiol. 2021, 6, 187–195. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, D.; Wang, X.; Li, C.; Yang, T.; Du, L.; Wei, Z.; Cheng, Q.; Cao, H.; Liang, Z.; et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics 2018, 8, 2361–2376. [Google Scholar] [CrossRef]
- Alameh, M.G.; Semon, A.; Bayard, N.U.; Pan, Y.G.; Dwivedi, G.; Knox, J.; Glover, R.C.; Rangel, P.C.; Tanes, C.; Bittinger, K.; et al. A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection. Science 2024, 386, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.Z.; Qin, Z. The mRNA-LNP vaccines—The good, the bad and the ugly? Front. Immunol. 2024, 15, 1336906. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Lottenbach, K.R.; Hill, H.; Blevins, T.P.; Yu, Y.; Zhang, Y.; Brenneman, K.E.; Kelly-Aehle, S.M.; McDonald, C.; Jansen, A.; et al. A Phase I, dose-escalation trial in adults of three recombinant attenuated Salmonella Typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine 2013, 31, 4874–4880. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jeza, V.T.; Pan, Q. Salmonella typhi: From a human pathogen to a vaccine vector. Cell. Mol. Immunol. 2008, 5, 91–97. [Google Scholar] [CrossRef]
- Kurup, D.; Wirblich, C.; Feldmann, H.; Marzi, A.; Schnell, M.J. Rhabdovirus-based vaccine platforms against henipaviruses. J. Virol. 2015, 89, 144–154. [Google Scholar] [CrossRef]
- Scher, G.; Schnell, M.J. Rhabdoviruses as vectors for vaccines and therapeutics. Curr. Opin. Virol. 2020, 44, 169–182. [Google Scholar] [CrossRef]
| Type | Vaccine Target Region of M Protein | Clinical Trial Phase | Developers |
|---|---|---|---|
| 6-valent vaccine | the N-terminal | Phase I | James B Dale’s team |
| 26-valent vaccine | the N-terminal | Phase II | James B Dale’s team |
| 30-valent vaccine | the N-terminal | Phase I | James B Dale’s team |
| Novel 30-valent mRNA vaccine | the N-terminal | Phase I | James B Dale’s team |
| J8-DT Vaccine | the C-repeat | Phase I | Michael F. Good’s team |
| P*17 vaccine | the C-repeat | Phase I | Vanessa Meier-Stephenson’s team |
| Cellular Localization | Biological Function | Antigens | Protective Mechanism/Evidence |
|---|---|---|---|
| Surface-Exposed Proteins | Adhesion Factors | Fibronectin-binding protein (Sfb1) | Protection against lethal GAS challenge, bacterial attachment prevention, and colonization inhibition [54,55,56,57] |
| Streptococcal pili/T-antigen | Anti-adhesion neutralizing activity [58,59,60] | ||
| Lipoteichoic acid (LTA) | Anti-adherence activity to pharyngeal epithelium by GAS [61] | ||
| Cell Wall Components | GAS carbohydrate/lacking GlcNAc side chain | Experimentally induced passive protection in mice [62,63] | |
| Trirhamnosyl-lipopeptide | 75–97% opsonic activity against GAS clinical isolates comparable to J8-lipopeptide subunit vaccine [64] | ||
| Enzymes and Anchors | Sortase A | Nasal-associated lymphoid tissue colonization suppression [65] | |
| C5a peptidase (ScpA) | Immune evasion protease neutralization [66,67,68,69,70] | ||
| Secreted Virulence Factors | Toxins | Streptolysin O (SLO) | Neutralization of SLO-mediated hemolysis [71,72] |
| SpeAB fusion protein | Superantigen-neutralizing antibody response induction [73,74,75] | ||
| Metabolic Enzymes | Arginine deiminase (ADI) | Multi-serotype GAS immunity induction [76,77] | |
| SpyCEP (IL-8 protease) | Multi-serotype GAS protection in animal models [68,78] | ||
| Immune Modulators | Trigger factor (TF)-TLR2 | Protective immunity elicitation [77,79] |
| Technology | Advantages | limitations |
|---|---|---|
| Respiratory Mucosal Vaccines | Induces mucosal immunity | Potential immune tolerance issues |
| Needle-free administration improves compliance | Precise delivery requirements | |
| Rapid pathogen blockade at entry site | Stability challenges | |
| Novel Adjuvant Platforms | Enhances immunogenicity | Risk of excessive immune activation |
| Enables antigen dose sparing | May cause respiratory irritation | |
| mRNA-LNP | Rapid development | Low delivery efficiency in certain tissues |
| Non-integrating and safe | Requires booster doses | |
| Encodes multiple antigens | Cold chain dependency | |
| Self-Amplifying RNA | Ultra-low dose efficacy | Large molecular size requires optimized delivery |
| Prolonged antigen expression | Potential innate immune suppression of translation | |
| Built-in adjuvant effect via dsRNA intermediates | Viral-derived components may raise safety concerns | |
| Live Vector Systems | Mimics natural infection | Replication-competent vectors risk virulence reversion |
| Multivalent antigen capacity | Complex manufacturing increases costs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Pan, H.; Wu, H.; Chen, J. Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development. Vaccines 2025, 13, 734. https://doi.org/10.3390/vaccines13070734
Kong D, Pan H, Wu H, Chen J. Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development. Vaccines. 2025; 13(7):734. https://doi.org/10.3390/vaccines13070734
Chicago/Turabian StyleKong, Dechuan, Hao Pan, Huanyu Wu, and Jian Chen. 2025. "Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development" Vaccines 13, no. 7: 734. https://doi.org/10.3390/vaccines13070734
APA StyleKong, D., Pan, H., Wu, H., & Chen, J. (2025). Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development. Vaccines, 13(7), 734. https://doi.org/10.3390/vaccines13070734






