An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii
Abstract
1. Introduction
2. Materials and Methods
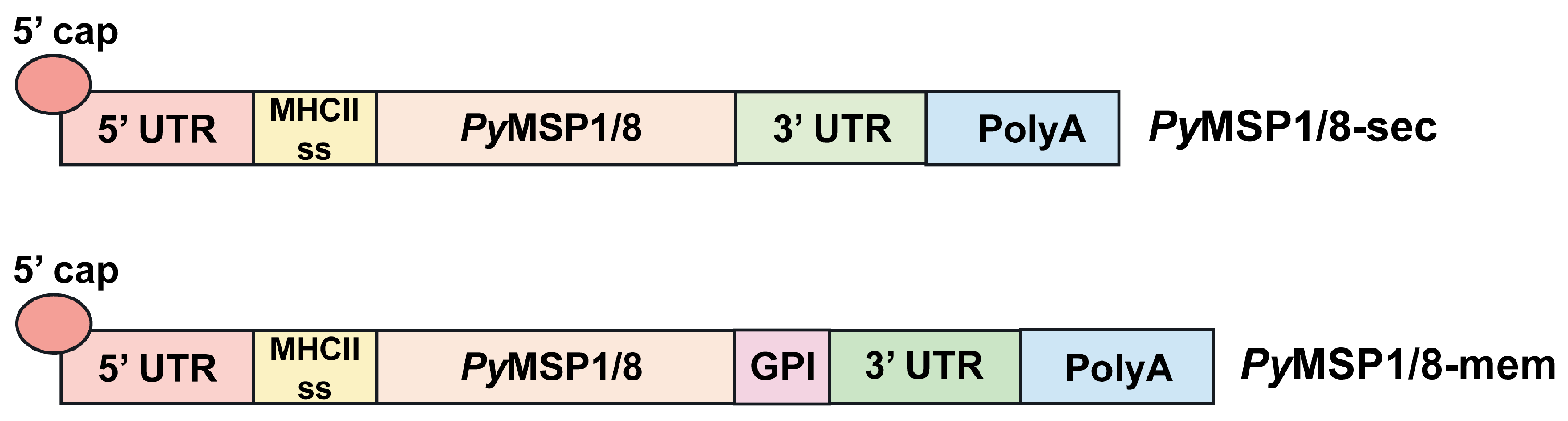
2.1. mRNA Vaccine Construct Design
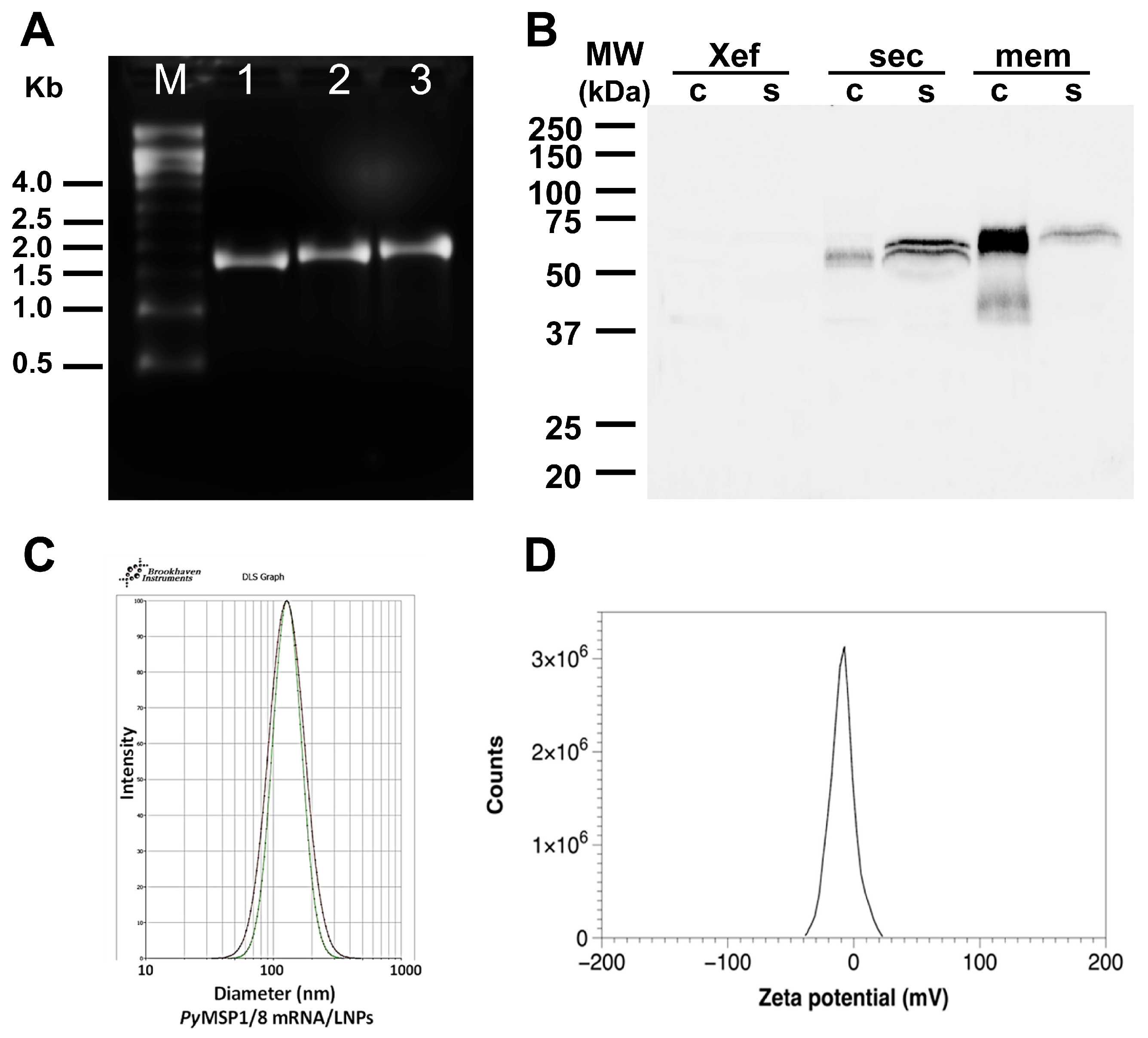
2.2. Production of Capped, Purified mRNA, and CHO Cell Transfection
2.3. Immunoblot Assays
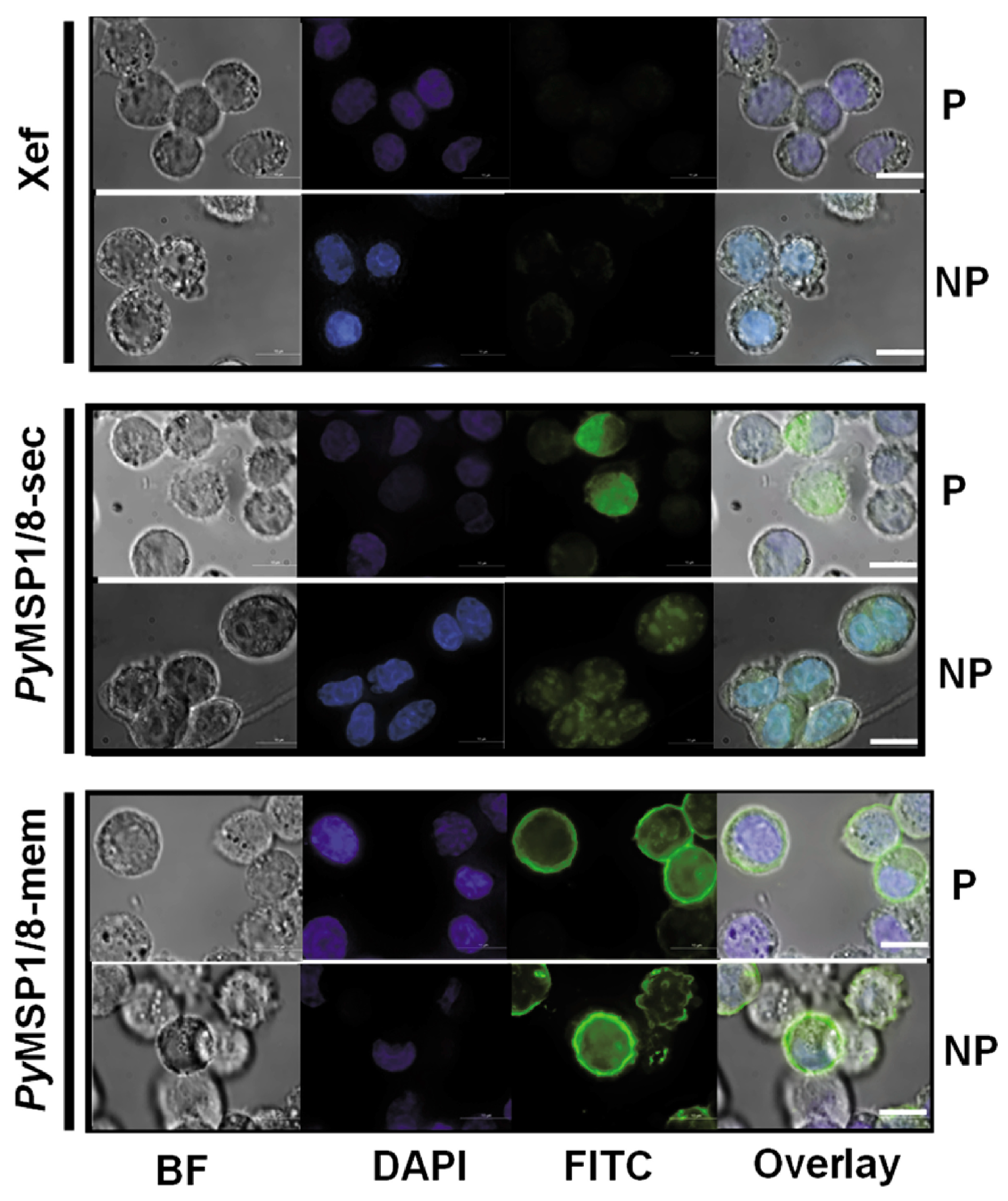
2.4. Immunofluorescence Assay
2.5. Production of mRNA-LNP Vaccines
2.6. Immunization and Challenge
2.7. ELISA
2.8. Statistical Analysis
2.9. Generative Artificial Intelligence
3. Results
3.1. PyMSP1/8-Sec and PyMSP1/8-Mem mRNAs Are Expressed in Mammalian Cells
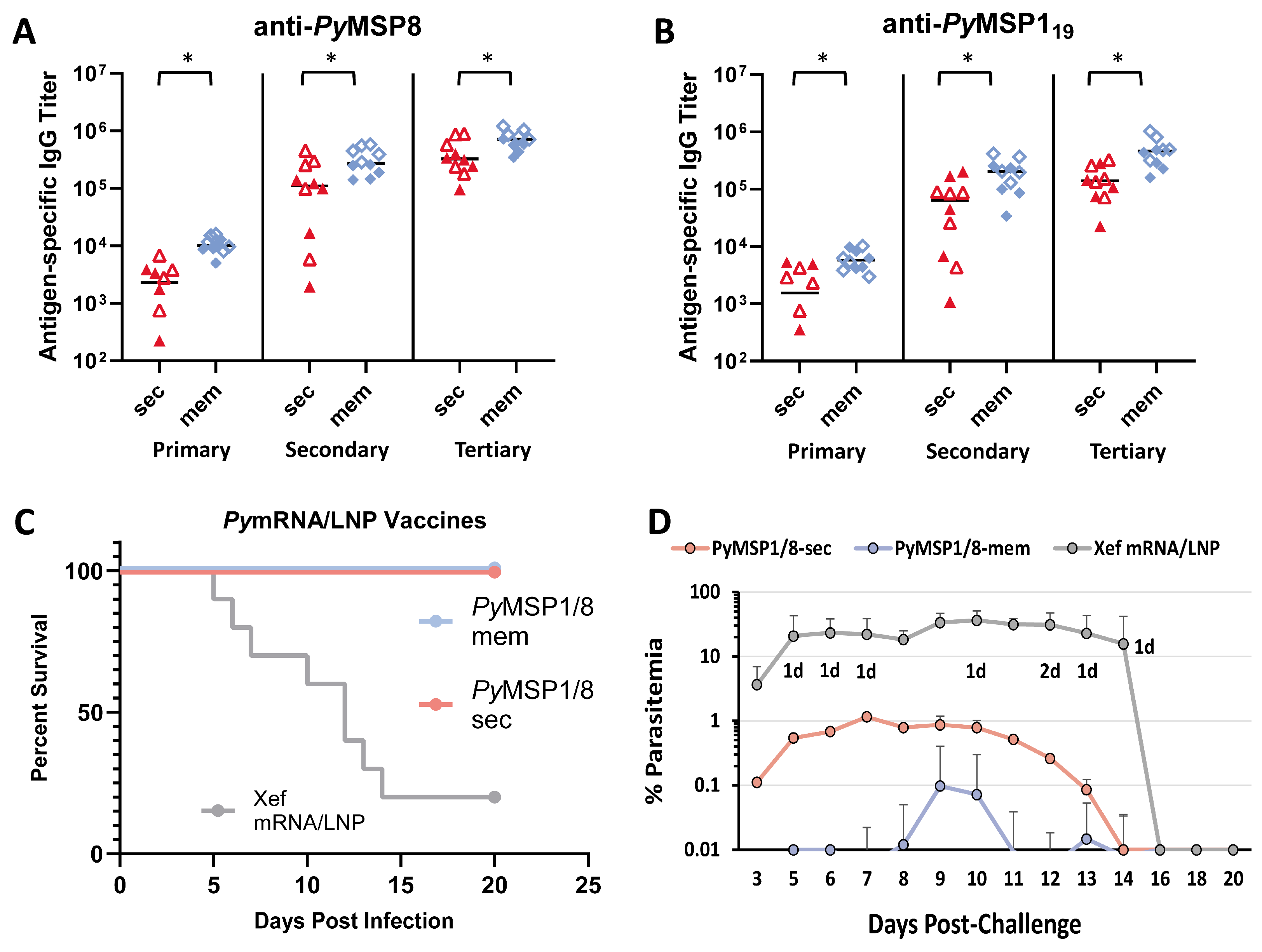
3.2. The PyMSP1/8-Sec mRNA Vaccine Is Potently Immunogenic and Protective Against Lethal Blood-Stage Malaria, Superior to a Recombinant Antigen/Adjuvant Formulation
3.3. The Immunogenicity and Efficacy of the PyMSP1/8-Mem mRNA Vaccine Are Superior to the PyMSP1/8-Sec mRNA Vaccine When Administered at Low Dose
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghehreyesus, T. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Daily, J.P.; Parikh, S. Malaria. N. Engl. J. Med. 2025, 392, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E.; Gorres, J.P.; Healy, S.A.; Fried, M. Malaria vaccines: A new era of prevention and control. Nat. Rev. Microbiol. 2024, 22, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Zavala, F. RTS,S: The first malaria vaccine. J. Clin. Investig. 2022, 132, e156588. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, C.; Peissig, K.; Kurup, S.P. Pre-Erythrocytic Vaccines against Malaria. Vaccines 2020, 8, 400. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouedraogo, J.B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Takashima, E.; Otsuki, H.; Morita, M.; Ito, D.; Nagaoka, H.; Yuguchi, T.; Hassan, I.; Tsuboi, T. The Need for Novel Asexual Blood-Stage Malaria Vaccine Candidates for Plasmodium falciparum. Biomolecules 2024, 14, 100. [Google Scholar] [CrossRef]
- Long, C.A.; Zavala, F. Malaria vaccines and human immune responses. Curr. Opin. Microbiol. 2016, 32, 96–102. [Google Scholar] [CrossRef]
- Ly, A.; Hansen, D.S. Development of B Cell Memory in Malaria. Front. Immunol. 2019, 10, 559. [Google Scholar] [CrossRef]
- El-Moamly, A.A.; El-Sweify, M.A. Malaria vaccines: The 60-year journey of hope and final success—Lessons learned and future prospects. Trop. Med. Health 2023, 51, 29. [Google Scholar] [CrossRef]
- Cohen, S.; McGregor, I.A.; Carrington, S. Gamma-Globulin and Acquired Immunity to Human Malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef]
- Silk, S.E.; Kalinga, W.F.; Salkeld, J.; Mtaka, I.M.; Ahmed, S.; Milando, F.; Diouf, A.; Bundi, C.K.; Balige, N.; Hassan, O.; et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet Infect. Dis. 2024, 24, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Tsoumani, M.E.; Voyiatzaki, C.; Efstathiou, A. Malaria Vaccines: From the Past towards the mRNA Vaccine Era. Vaccines 2023, 11, 1452. [Google Scholar] [CrossRef]
- Wu, Q.; Tong, J.; Zhang, B.; Zhang, D.; Chen, J.; Lei, Y.; Lu, Y.; Wang, Y.; Li, L.; Shen, Y.; et al. Real-World Effectiveness of BNT162b2 Against Infection and Severe Diseases in Children and Adolescents. Ann. Intern. Med. 2024, 177, 165–176. [Google Scholar] [CrossRef]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef]
- Beladiya, J.; Kumar, A.; Vasava, Y.; Parmar, K.; Patel, D.; Patel, S.; Dholakia, S.; Sheth, D.; Boddu, S.H.S.; Patel, C. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of controlled and randomized clinical trials. Rev. Med. Virol. 2024, 34, e2507. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Matarazzo, L.; Bettencourt, P.J.G. mRNA vaccines: A new opportunity for malaria, tuberculosis and HIV. Front. Immunol. 2023, 14, 1172691. [Google Scholar] [CrossRef]
- Pardi, N.; Krammer, F. mRNA vaccines for infectious diseases—advances, challenges and opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef]
- Mallory, K.L.; Taylor, J.A.; Zou, X.; Waghela, I.N.; Schneider, C.G.; Sibilo, M.Q.; Punde, N.M.; Perazzo, L.C.; Savransky, T.; Sedegah, M.; et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines 2021, 6, 84. [Google Scholar] [CrossRef]
- Hayashi, C.T.H.; Cao, Y.; Clark, L.C.; Tripathi, A.K.; Zavala, F.; Dwivedi, G.; Knox, J.; Alameh, M.G.; Lin, P.J.C.; Tam, Y.K.; et al. mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines 2022, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Waghela, I.N.; Mallory, K.L.; Taylor, J.A.; Schneider, C.G.; Savransky, T.; Janse, C.J.; Lin, P.J.C.; Tam, Y.K.; Weissman, D.; Angov, E. Exploring in vitro expression and immune potency in mice using mRNA encoding the Plasmodium falciparum malaria antigen, CelTOS. Front. Immunol. 2022, 13, 1026052. [Google Scholar] [CrossRef] [PubMed]
- Yanik, S.; Venkatesh, V.; Gordy, J.T.; Alameh, M.G.; Meza, J.; Li, Y.; Glass, E.; Flores-Garcia, Y.; Tam, Y.; Chaiyawong, N.; et al. iDC-targeting PfCSP mRNA vaccine confers superior protection against Plasmodium compared to conventional mRNA. NPJ Vaccines 2025, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.K.; Das Mohapatra, A.; Jnawali, A.; Zuromski, J.; Jha, A.; Cham-Kpu, G.; Sherman, B.; Rudlaff, R.M.; Nixon, C.E.; Hilton, N.; et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature 2020, 582, 104–108. [Google Scholar] [CrossRef]
- Fotoran, W.L.; Silva, J.R.D.; Glitz, C.; Ferreira, L.C.S.; Wunderlich, G. Establishment of an Antiplasmodial Vaccine Based on PfRH5-Encoding RNA Replicons Stabilized by Cationic Liposomes. Pharmaceutics 2023, 15, 1223. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Freyn, A.W.; Pine, M.; Rosado, V.C.; Benz, M.; Muramatsu, H.; Beattie, M.; Tam, Y.K.; Krammer, F.; Palese, P.; Nachbagauer, R.; et al. Antigen modifications improve nucleoside-modified mRNA-based influenza virus vaccines in mice. Mol. Ther. Methods Clin. Dev. 2021, 22, 84–95. [Google Scholar] [CrossRef]
- Melzi, E.; Willis, J.R.; Ma, K.M.; Lin, Y.C.; Kratochvil, S.; Berndsen, Z.T.; Landais, E.A.; Kalyuzhniy, O.; Nair, U.; Warner, J.; et al. Membrane-bound mRNA immunogens lower the threshold to activate HIV Env V2 apex-directed broadly neutralizing B cell precursors in humanized mice. Immunity 2022, 55, 2168–2186.e2166. [Google Scholar] [CrossRef]
- Scaria, P.V.; Roth, N.; Schwendt, K.; Muratova, O.V.; Alani, N.; Lambert, L.E.; Barnafo, E.K.; Rowe, C.G.; Zaidi, I.U.; Rausch, K.M.; et al. mRNA vaccines expressing malaria transmission-blocking antigens Pfs25 and Pfs230D1 induce a functional immune response. NPJ Vaccines 2024, 9, 9. [Google Scholar] [CrossRef]
- Holder, A.A.; Freeman, R.R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J. Exp. Med. 1984, 160, 624–629. [Google Scholar] [CrossRef]
- Lyon, J.A.; Geller, R.H.; Haynes, J.D.; Chulay, J.D.; Weber, J.L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc. Natl. Acad. Sci. USA 1986, 83, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.S.; Heidrich, H.G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 1987, 23, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Daly, T.M.; Long, C.A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 1995, 155, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hirunpetcharat, C.; Tian, J.H.; Kaslow, D.C.; van Rooijen, N.; Kumar, S.; Berzofsky, J.A.; Miller, L.H.; Good, M.F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: Correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 1997, 159, 3400–3411. [Google Scholar]
- O’Donnell, R.A.; de Koning-Ward, T.F.; Burt, R.A.; Bockarie, M.; Reeder, J.C.; Cowman, A.F.; Crabb, B.S. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 2001, 193, 1403–1412. [Google Scholar] [CrossRef]
- Egan, A.; Waterfall, M.; Pinder, M.; Holder, A.; Riley, E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: Evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 1997, 65, 3024–3031. [Google Scholar] [CrossRef]
- Hensmann, M.; Li, C.; Moss, C.; Lindo, V.; Greer, F.; Watts, C.; Ogun, S.A.; Holder, A.A.; Langhorne, J. Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur. J. Immunol. 2004, 34, 639–648. [Google Scholar] [CrossRef]
- Burns, J.M., Jr.; Belk, C.C.; Dunn, P.D. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect. Immun. 2000, 68, 6189–6195. [Google Scholar] [CrossRef]
- Shi, Q.; Cernetich, A.; Daly, T.M.; Galvan, G.; Vaidya, A.B.; Bergman, L.W.; Burns, J.M., Jr. Alteration in host cell tropism limits the efficacy of immunization with a surface protein of malaria merozoites. Infect. Immun. 2005, 73, 6363–6371. [Google Scholar] [CrossRef][Green Version]
- Petritus, P.M.; Burns, J.M., Jr. Suppression of lethal Plasmodium yoelii malaria following protective immunization requires antibody-, IL-4-, and IFN-gamma-dependent responses induced by vaccination and/or challenge infection. J. Immunol. 2008, 180, 444–453. [Google Scholar] [CrossRef]
- Alaro, J.R.; Partridge, A.; Miura, K.; Diouf, A.; Lopez, A.M.; Angov, E.; Long, C.A.; Burns, J.M., Jr. A chimeric Plasmodium falciparum merozoite surface protein vaccine induces high titers of parasite growth inhibitory antibodies. Infect. Immun. 2013, 81, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Lynch, M.M.; Romero, M.; Burns, J.M., Jr. Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect. Immun. 2007, 75, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Angov, E.; Hillier, C.J.; Kincaid, R.L.; Lyon, J.A. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS ONE 2008, 3, e2189. [Google Scholar] [CrossRef]
- Stowers, A.W.; Chen Lh, L.H.; Zhang, Y.; Kennedy, M.C.; Zou, L.; Lambert, L.; Rice, T.J.; Kaslow, D.C.; Saul, A.; Long, C.A.; et al. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2002, 99, 339–344. [Google Scholar] [CrossRef]
- Hailman, E.; Lichenstein, H.S.; Wurfel, M.M.; Miller, D.S.; Johnson, D.A.; Kelley, M.; Busse, L.A.; Zukowski, M.M.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994, 179, 269–277. [Google Scholar] [CrossRef]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Alaro, J.R.; Lynch, M.M.; Burns, J.M., Jr. Protective immune responses elicited by immunization with a chimeric blood-stage malaria vaccine persist but are not boosted by Plasmodium yoelii challenge infection. Vaccine 2010, 28, 6876–6884. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Guler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Sher, L.D.; Sabharwal, C.; Gurtman, A.; Xu, X.; Kitchin, N.; Lockhart, S.; Riesenberg, R.; Sexter, J.M.; Czajka, H.; et al. Evaluation of BNT162b2 COVID-19 Vaccine in Children Younger than 5 Years of Age. N. Engl. J. Med. 2023, 388, 621–634. [Google Scholar] [CrossRef]
- Eacret, J.S.; Parzych, E.M.; Gonzales, D.M.; Burns, J.M., Jr. Inclusion of an Optimized Plasmodium falciparum Merozoite Surface Protein 2-Based Antigen in a Trivalent, Multistage Malaria Vaccine. J. Immunol. 2021, 206, 1817–1831. [Google Scholar] [CrossRef]
- Parzych, E.M.; Miura, K.; Long, C.A.; Burns, J.M. Maintaining immunogenicity of blood stage and sexual stage subunit malaria vaccines when formulated in combination. PLoS ONE 2020, 15, e0232355. [Google Scholar] [CrossRef]
- Stump, W.H.; Klingenberg, H.J.; Ott, A.C.; Gonzales, D.M.; Burns, J.M., Jr. Design and Evaluation of Chimeric Plasmodium falciparum Circumsporozoite Protein-Based Malaria Vaccines. Vaccines 2024, 12, 351. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Meng, L.; Li, F.; Yu, C. Comparison of Immune Responses Elicited by SARS-CoV-2 mRNA and Recombinant Protein Vaccine Candidates. Front. Immunol. 2022, 13, 906457. [Google Scholar] [CrossRef]
- Guo, C.; Chai, X.; Baerlike, M.; Liu, Y.; Wang, Y.; Shao, F.; Huang, Q.; Zhang, W.; Cen, S.; Dong, Y.; et al. Comparison of antigen-specific B cell responses reveals disparity in immunogenicity and memory B cell formation across COVID-19 vaccine platforms. hLife 2024, 2, 625–640. [Google Scholar] [CrossRef]
- Johnson, Y.; Shakri, A.R.; Pond-Tor, S.; Jnawali, A.; Najrana, T.; Wu, H.; Badhai, J.; Alameh, M.G.; Weissman, D.; Kabyemela, E.; et al. Immunization with PfGBP130 generates antibodies that inhibit RBC invasion by P. falciparum parasites. Front. Immunol. 2024, 15, 1350560. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Ver, A.T.; Velasco, J.V.; Pastrana, A.; Catahay, J.A.; Salvagno, G.L.; Yap, E.P.H.; Martinez-Sobrido, L.; Torrelles, J.B.; Lippi, G.; et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: A systematic review. Crit. Rev. Clin. Lab. Sci. 2022, 59, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, E.; Mateo-Urdiales, A.; Bietta, C.; Cesaroni, G.; Anticoli, S.; Di Maggio, E.; Ancona, A.; Petrone, D.; Cannone, A.; Sacco, C.; et al. Sex differences in response to COVID-19 mRNA vaccines in Italian population. Epidemiol. Infect. 2024, 152, e139. [Google Scholar] [CrossRef] [PubMed]
- Anticoli, S.; Dorrucci, M.; Iessi, E.; Chiarotti, F.; Di Prinzio, R.R.; Vinci, M.R.; Zaffina, S.; Puro, V.; Colavita, F.; Mizzoni, K.; et al. Association between sex hormones and anti-S/RBD antibody responses to COVID-19 vaccines in healthcare workers. Hum. Vaccines Immunother. 2023, 19, 2273697. [Google Scholar] [CrossRef]
- Kunkeaw, N.; Nguitragool, W.; Takashima, E.; Kangwanrangsan, N.; Muramatsu, H.; Tachibana, M.; Ishino, T.; Lin, P.J.C.; Tam, Y.K.; Pichyangkul, S.; et al. A Pvs25 mRNA vaccine induces complete and durable transmission-blocking immunity to Plasmodium vivax. NPJ Vaccines 2023, 8, 187. [Google Scholar] [CrossRef]
- De Oliveira, A.S.; Versteeg, L.; Briggs, N.; Adhikari, R.; Villar, M.J.; Redd, J.R.; Hotez, P.; Bottazzi, M.E.; Pollet, J. Altering the intracellular trafficking of Necator americanus GST-1 antigen yields novel hookworm mRNA vaccine candidates. PLoS Negl. Trop. Dis. 2025, 19, e0012809. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ott, A.C.; Loll, P.J.; Burns, J.M., Jr. An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii. Vaccines 2025, 13, 702. https://doi.org/10.3390/vaccines13070702
Ott AC, Loll PJ, Burns JM Jr. An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii. Vaccines. 2025; 13(7):702. https://doi.org/10.3390/vaccines13070702
Chicago/Turabian StyleOtt, Amy C., Patrick J. Loll, and James M. Burns, Jr. 2025. "An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii" Vaccines 13, no. 7: 702. https://doi.org/10.3390/vaccines13070702
APA StyleOtt, A. C., Loll, P. J., & Burns, J. M., Jr. (2025). An mRNA Vaccine Expressing Blood-Stage Malaria Antigens Induces Complete Protection Against Lethal Plasmodium yoelii. Vaccines, 13(7), 702. https://doi.org/10.3390/vaccines13070702





