Innovation in mRNA Vaccines and RNAi via Protein Nanocages
Abstract
1. Introduction
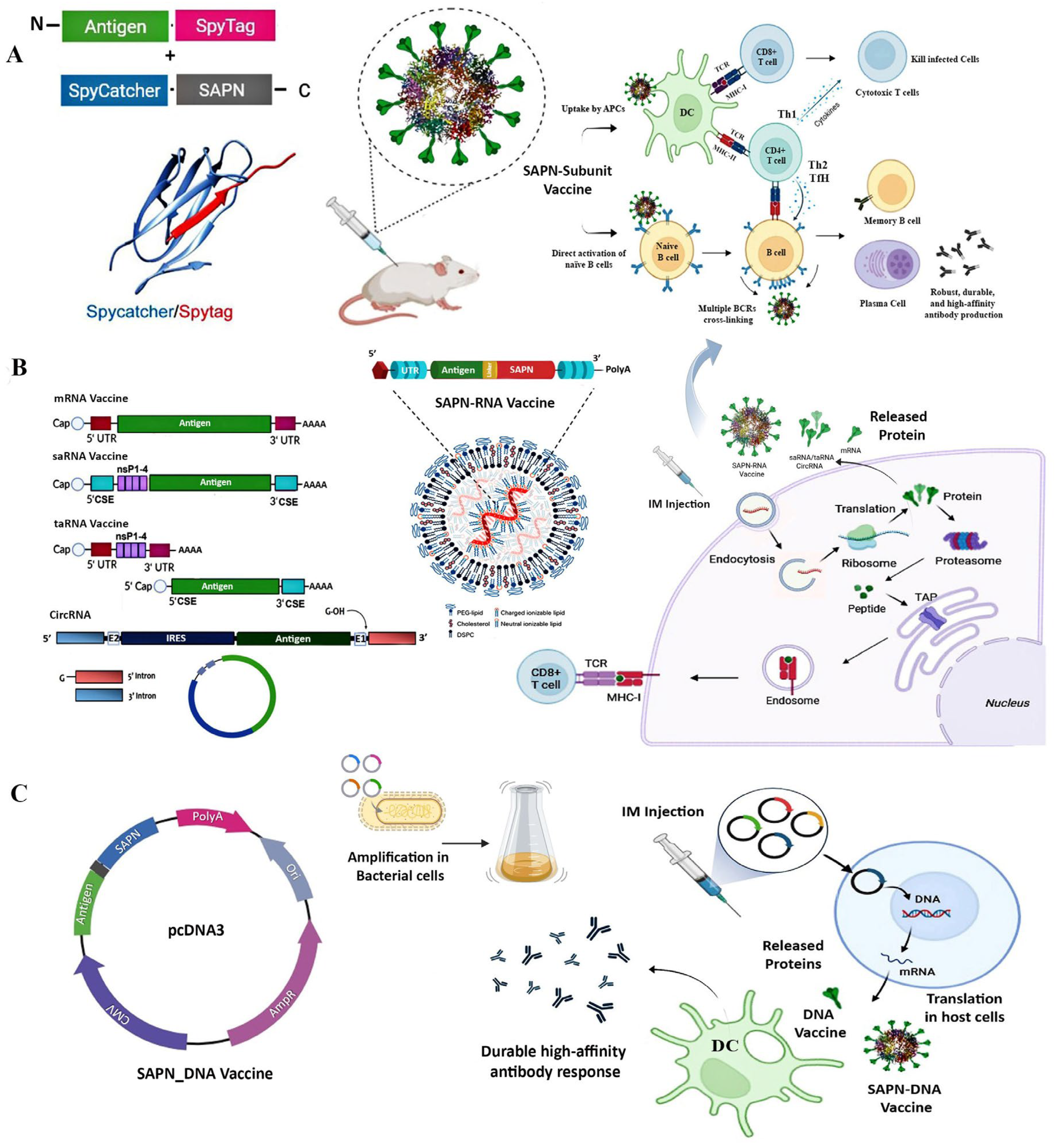
2. Self-Assembling Protein Nanocages (SAPNs)
2.1. Characteristics
2.2. Production
3. Self-Assembling Protein Nanocages (SAPNs) as Vaccine Platform
3.1. SAPN-Subunit Vaccines
3.2. SAPN-RNA Vaccines: Synergizing mRNA Vaccines with Protein Nanocages
3.2.1. mRNA Vaccines and Mechanisms
3.2.2. Self-Assembling Protein Nanocages for mRNA Vaccines Development
3.3. SAPN-DNA Vaccines: Integration with DNA Plasmids for Multivalent Antigen Display
3.4. SAPN-Adjuvant: Multivalent Display of Adjuvant and Co-Delivery with Antigen
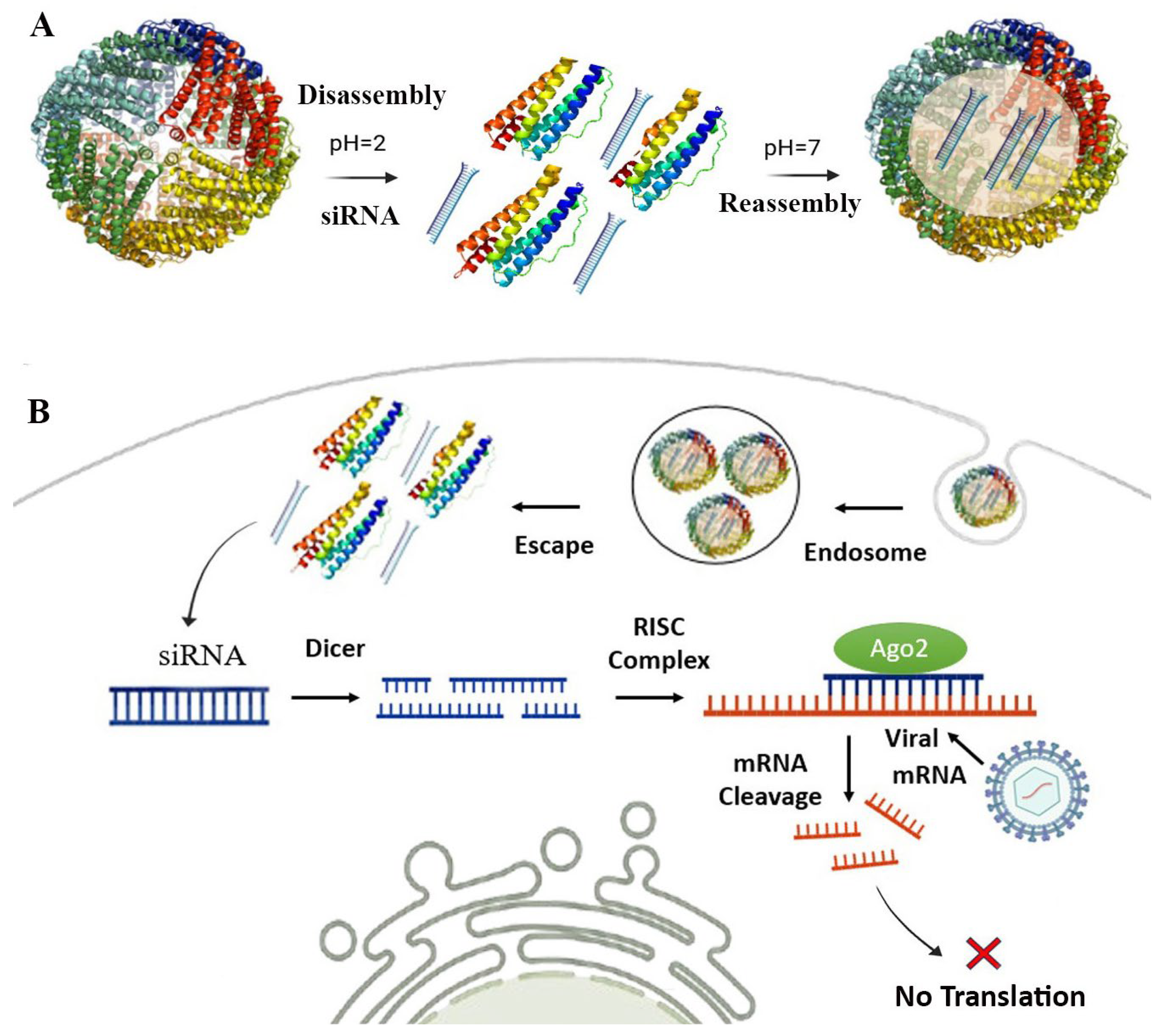
4. SAPN-RNAi: Self-Assembling Protein Nanocages for RNAi Delivery
4.1. RNA Interference (RNAi) and Mechanisms
4.2. Self-Assembling Protein Nanocages for RNAi Delivery Against Viral Infection
5. Challenges and Future Direction
Funding
Conflicts of Interest
References
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA vaccine development for emerging animal and zoonotic diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Krammer, F. mRNA vaccines for infectious diseases—Advances, challenges and opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Yin, J. RNAi, a new therapeutic strategy against viral infection. Cell Res. 2004, 14, 460–466. [Google Scholar] [CrossRef]
- Kelleher, A.D.; Cortez-Jugo, C.; Cavalieri, F.; Qu, Y.; Glanville, A.R.; Caruso, F.; Symonds, G.; Ahlenstiel, C.L. RNAi therapeutics: An antiviral strategy for human infections. Curr. Opin. Pharmacol. 2020, 54, 121–129. [Google Scholar] [CrossRef]
- Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. pH-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules 2011, 12, 1629–1640. [Google Scholar] [CrossRef]
- Boyton, I.; Goodchild, S.C.; Diaz, D.; Elbourne, A.; Collins-Praino, L.E.; Care, A. Characterizing the Dynamic Disassembly/Reassembly Mechanisms of Encapsulin Protein Nanocages. ACS Omega 2022, 7, 823–836. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, H.; Zhang, Y.; Liu, G.; Niu, G.; Chen, X. Functional Ferritin Nanoparticles for Biomedical Applications. Front. Chem. Sci. Eng. 2017, 11, 633–646. [Google Scholar] [CrossRef]
- Mohanty, A.; Parida, A.; Raut, R.K.; Behera, R.K. Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology. ACS Bio Med. Chem. Au 2022, 2, 258–281. [Google Scholar] [CrossRef]
- Kwon, S.; Giessen, T.W. Engineered Protein Nanocages for Concurrent RNA and Protein Packaging In Vivo. ACS Synth Biol. 2022, 11, 3504–3515. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Archambault, D. Vaccination strategies based on bacterial self-assembling proteins as antigen delivery nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-M.; Feng, M.-F.; Sun, B.; Yu, X.; Wu, Y. Targeted Delivery of BCL-2 siRNA Using Modified Ferritin Nanocarriers for Antitumor Therapy. ACS Appl. Nano Mater. 2024, 7, 22487–22496. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Krpetic, Z.; Martínez, M.M.; García-Ordoñez, M.; Roher, N.; Palić, D. Self-Assembling Ferritin Nanoplatform for the Development of Infectious Hematopoietic Necrosis Virus Vaccine. Front. Immunol. 2024, 15, 1346512. [Google Scholar] [CrossRef]
- Hauser, H.; Lopez-Sagaseta, J.; Malito, E. Self-Assembling Protein Nanoparticles in the Design of Vaccines: 2022 Update. Vaccines 2022, 10, 1447. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palić, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef]
- Jiang, R.; Yuan, S.; Zhou, Y.; Wei, Y.; Li, F.; Wang, M.; Chen, B.; Yu, H. Strategies to overcome the challenges of low or no expression of heterologous proteins in Escherichia coli . Biotechnol. Adv. 2024, 75, 108417. [Google Scholar] [CrossRef]
- Powell, A.E.; Caruso, H.; Park, S.; Chen, J.-L.; O’Rear, J.; Ferrer, B.J.; Walker, A.; Bruening, A.; Hartwig, A.; Ahyong, V.; et al. A stabilized MERS-CoV spike ferritin nanoparticle vaccine elicits robust and protective neutralizing antibody responses. bioRxiv 2024. 2024.07.01.601243. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palić, D. Role of T Follicular Helper Cells in Viral Infections and Vaccine Design. Cells 2025, 14, 508. [Google Scholar] [CrossRef]
- Guo, Y.; He, W.; Mou, H.; Zhang, L.; Chang, J.; Peng, S.; Ojha, A.; Tavora, R.; Parcells, M.S.; Luo, G.; et al. An engineered receptor-binding domain improves the immunogenicity of multivalent SARS-CoV-2 vaccines. mBio 2021, 12, e00930-21. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Prabhakaran, M.; Muthui, M.; Naidoo, A.; Sincomb, T.; Wu, W.; Cottrell, C.A.; Landais, E.; deCamp, A.C.; Keshavarzi, N.R. Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science 2025, 0, eadr8382. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liguori, A.; Skog, P.; Landais, E.; Menis, S.; et al. Immunogenicity of RNA replicons encoding HIV Env immunogens designed for self-assembly into nanoparticles. Mol. Ther. 2019, 27, 2080–2090. [Google Scholar] [CrossRef]
- Li, J.; Helal, Z.H.; Karch, C.P.; Mishra, N.; Girshick, T.; Garmendia, A.; Burkhard, P.; Khan, M.I. A Self-Adjuvanted Nanoparticle-Based Vaccine Against Infectious Bronchitis Virus. PLoS ONE 2018, 13, e0203771. [Google Scholar] [CrossRef]
- Lee, E.B.; Jeon, H.M.; Kim, C.U.; Park, S.M.; Cho, G.; Kim, H.J.; Kim, Y.; Kim, D.J.; Kim, Y.S.; Lee, H.; et al. Attachment of flagellin enhances the immunostimulatory activity of a hemagglutinin-ferritin nano-cage. Nanomedicine 2019, 17, 223–235. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, X.; Liao, Y.; Zeng, S.; Li, Y.; Zhang, Y.; Gao, C.; Zhang, Y.; Wan, J.; Gu, J.; et al. Recombinant Pseudomonas aeruginosa flagellin delivered using ferritin nanoparticles provides enhanced cross-protection against lung infection in mice. Mol. Immunol. 2023, 163, 235–242. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Li, L.; Muñoz-Culla, M.; Carmona, U.; Lopez, M.P.; Yang, F.; Trigueros, C.; Otaegui, D.; Zhang, L.; Knez, M. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials. 2016, 98, 143–151. [Google Scholar] [CrossRef]
- Rajabinejad, M.; Valadan, R.; Tehrani, M.; Najafi, A.; Negarandeh, R.; Saeedi, M.; Asgarian-Omran, H. Effective delivery of anti-PD-L1 siRNA with human heavy chain ferritin (HFn) in acute myeloid leukemia cell lines. Med Oncol. 2024, 41, 149. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, B.; Li, J.; Guo, Z.; Zhang, C.; Chen, X.; Ma, L.; Wang, Z.; Yang, H.; Li, Y.; et al. Bioengineered protein nanocarrier facilitating siRNA escape from lysosomes for targeted RNAi therapy in glioblastoma. Sci Adv. 2025, 11, eadr9266. [Google Scholar] [CrossRef]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Q.; Alves, P.M.; Roldão, A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics 2021, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.R.; Pearl, V.M.; Denotti, A.R.; Lee, J.B.; Swaminathan, S.; Scindia, Y.M.; Charlton, N.P.; Baldelomar, E.J.; Beeman, S.C.; Bennett, K.M. Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomedicine: Nanotechnology. Biol. Med. 2016, 12, 1735–1745. [Google Scholar]
- Zhang, Y.; Orner, B.P. Self-Assembly in the Ferritin Nano-Cage Protein Superfamily. Int. J. Mol. Sci. 2011, 12, 5406–5421. [Google Scholar] [CrossRef]
- Ladenstein, R.; Morgunova, E. Second career of a biosynthetic enzyme: Lumazine synthase as a virus-like nanoparticle in vaccine development. Biotechnol. Rep. 2020, 27, e00494. [Google Scholar] [CrossRef]
- Ladenstein, R.; Fischer, M.; Bacher, A. The lumazine synthase/riboflavin synthase complex: Shapes and functions of a highly variable enzyme system. FEBS J. 2013, 280, 2537–2563. [Google Scholar] [CrossRef]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.-A.; Liu, A.; Traulsen, C.H.-H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R.; et al. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for in Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796–12804. [Google Scholar] [CrossRef]
- Lagoutte, P.; Mignon, C.; Stadthagen, G.; Potisopon, S.; Donnat, S.; Mast, J.; Lugari, A.; Werle, B. Simultaneous Surface Display and Cargo Loading of Encapsulin Nanocompartments and Their Use for Rational Vaccine Design. Vaccine 2018, 36, 3622–3628. [Google Scholar] [CrossRef]
- Wiryaman, T.; Toor, N. Recent Advances in the Structural Biology of Encapsulin Bacterial Nanocompartments. J. Struct. Biol. X 2022, 6, 100062. [Google Scholar] [CrossRef] [PubMed]
- Chmelyuk, N.S.; Oda, V.V.; Gabashvili, A.N.; Abakumov, M.A. Encapsulins: Structure, Properties, and Biotechnological Applications. Biochemistry 2023, 88, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.; Khaleeq, S.; Garg, P.; Bhat, M.; Reddy, P.; Vignesh, V.S.; Upadhyaya, A.; Das, M.; Chakshusmathi, G.; Pandey, S.; et al. Comparative Immunogenicity of Bacterially Expressed Soluble Trimers and Nanoparticle Displayed Influenza Hemagglutinin Stem Immunogens. Front. Immunol. 2022, 13, 890622. [Google Scholar] [CrossRef] [PubMed]
- Khaleeq, S.; Sengupta, N.; Kumar, S.; Patel, U.R.; Rajmani, R.S.; Reddy, P.; Pandey, S.; Singh, R.; Dutta, S.; Ringe, R.P.; et al. Neutralizing Efficacy of Encapsulin Nanoparticles against SARS-CoV2 Variants of Concern. Viruses 2023, 15, 346. [Google Scholar] [CrossRef]
- Jung, H.G.; Jeong, S.; Kang, M.J.; Hong, I.; Park, Y.S.; Ko, E.; Kim, J.O.; Choi, D.Y. Molecular Design of Encapsulin Protein Nanoparticles to Display Rotavirus Antigens for Enhancing Immunogenicity. Vaccines 2024, 12, 1020. [Google Scholar] [CrossRef]
- Allen, M.D.; Perham, R.N. The Catalytic Domain of Dihydrolipoyl Acetyltransferase from the Pyruvate Dehydrogenase Multienzyme Complex of Bacillus stearothermophilus: Expression, Purification and Reversible Denaturation. FEBS Lett. 1997, 413, 339–343. [Google Scholar] [CrossRef]
- Izard, T.; Aevarsson, A.; Allen, M.D.; Westphal, A.H.; Perham, R.N.; de Kok, A.; Hol, W.G. Principles of Quasi-Equivalence and Euclidean Geometry Govern the Assembly of Cubic and Dodecahedral Cores of Pyruvate Dehydrogenase Complexes. Proc. Natl. Acad. Sci. USA. 1999, 96, 1240–1245. [Google Scholar] [CrossRef]
- Dalmau, M.; Lim, S.; Chen, H.C.; Ruiz, C.; Wang, S.W. Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol. Bioeng. 2008, 101, 654–664. [Google Scholar] [CrossRef]
- Peng, T.; Lee, H.; Lim, S. Design of a reversible inversed pH-responsive caged protein. Biomater. Sci. 2015, 3, 627–635. [Google Scholar] [CrossRef]
- Salinas, N.D.; Ma, R.; Dickey, T.H.; McAleese, H.; Ouahes, T.; Long, C.A.; Miura, K.; Lambert, L.E.; Tolia, N.H. A Potent and Durable Malaria Transmission-Blocking Vaccine Designed from a Single-Component 60-Copy Pfs230D1 Nanoparticle. NPJ Vaccines 2023, 8, 124. [Google Scholar] [CrossRef]
- Van Montfort, R.; Slingsby, C.; Vierling, E. Structure and Function of the Small Heat Shock Protein/Alpha-Crystallin Family of Molecular Chaperones. Adv. Protein Chem. 2001, 59, 105–156. [Google Scholar] [PubMed]
- Biswas, S.; Garg, P.; Dutta, S.; Suguna, K. Multiple nanocages of a cyanophage small heat shock protein with icosahedral and octahedral symmetries. Sci. Rep. 2021, 11, 21023. [Google Scholar] [CrossRef]
- Kim, K.K.; Kim, R.; Kim, S.H. Crystal Structure of a Small Heat-Shock Protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.E.; Servid, A.E.; Harmsen, A.L.; Rynda-Apple, A.; Han, S.; Wiley, J.A.; Douglas, T.; Harmsen, A.G. A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine 2012, 30, 3653–3665. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Schiller, S.M. Nanobiotechnology of protein-based compartments: Steps toward nanofactories. Bioinspired Biomim. Nanobiomater. 2013, 2, 154–172. [Google Scholar] [CrossRef]
- Kim, D.R.; Lee, I.; Ha, S.C.; Kim, K.K. Activation Mechanism of HSP16.5 from Methanococcus jannaschii. Biochem. Biophys. Res. Commun. 2003, 307, 991–998. [Google Scholar] [CrossRef]
- Carra, S.; Alberti, S.; Benesch, J.L.P.; Boelens, W.; Buchner, J.; Carver, J.A.; Cecconi, C.; Ecroyd, H.; Gusev, N.; Hightower, L.E.; et al. Small Heat Shock Proteins: Multifaceted Proteins with Important Implications for Life. Cell Stress. Chaperones 2019, 24, 295–308. [Google Scholar] [CrossRef]
- Gabashvili, A.N.; Chmelyuk, N.S.; Efremova, M.V.; Malinovskaya, J.A.; Semkina, A.S.; Abakumov, M.A. Encapsulins—Bacterial Protein Nanocompartments: Structure, Properties, and Application. Biomolecules 2020, 10, 966. [Google Scholar] [CrossRef]
- Brune, K.D.; Howarth, M. New Routes and Opportunities for Modular Construction of Particulate Vaccines: Stick, Click, and Glue. Front. Immunol. 2018, 9, 1432. [Google Scholar] [CrossRef]
- Hatlem, D.; Trunk, T.; Linke, D.; Leo, J.C. Catching a SPY: Using the SpyCatcher-SpyTag and Related Systems for Labeling and Localizing Bacterial Proteins. Int. J. Mol. Sci. 2019, 20, 2129. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Chen, H.; Li, X.; Qian, P. A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 2020, 38, 5647–5652. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Deng, X.; Wang, J.; Zhang, M.; Liu, Y.; Tang, P.; Liu, H.; Zhou, Y.; Tong, G.; et al. Nanoparticle vaccines based on the receptor binding domain of porcine deltacoronavirus elicit robust protective immune responses in mice. Front. Immunol. 2024, 15, 1328266. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhao, C.; Zhu, X. Engineering Escherichia Coli-Derived Nanoparticles for Vaccine Development. Vaccines 2024, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Q.; Patão, S.; Thomaz, M.; Nunes, T.; Alves, P.M.; Roldão, A. Tyrosinase-Mediated Conjugation for Antigen Display on Ferritin Nanoparticles. Bioconjug. Chem. 2024, 35, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Weidenbacher, P.; Musunuri, S.; Powell, A.E.; Tang, S.; Do, J.; Sanyal, M.; Kim, P.S. Simplified Purification of Glycoprotein-Modified Ferritin Nanoparticles for Vaccine Development. Biochemistry 2023, 62, 292–299. [Google Scholar] [CrossRef]
- Tu, Z.; Timashev, P.; Chen, J.; Liang, X.J. Ferritin-based drug delivery system for tumor therapy. BMEMat 2023, 1, e12022. [Google Scholar] [CrossRef]
- Wu, J.; Liang, J.; Li, S.; Lu, J.; Li, Y.; Zhang, B.; Gao, M.; Zhou, J.; Zhang, Y.; Chen, J. Cancer Vaccine Designed from Homologous Ferritin-Based Fusion Protein with Enhanced DC-T Cell Crosstalk for Durable Adaptive Immunity against Tumors. Bioact. Mater. 2025, 30, 1024–1042. [Google Scholar] [CrossRef]
- Brun, A. (Ed.) Vaccines and Vaccination for Veterinary Viral Diseases: A General Overview. In Vaccine Technologies for Veterinary Viral Diseases; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1349, pp. 1–24. [Google Scholar]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Hassanzadeh, R.; Soltani, E. Oral DNA vaccines based on CS-TPP nanoparticles and alginate microparticles confer high protection against infectious pancreatic necrosis virus (IPNV) infection in trout. Dev. Comp. Immunol. 2017, 74, 178–189. [Google Scholar] [CrossRef]
- Cao, S.; Ma, D.; Ji, S.; Zhou, M.; Zhu, S. Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications. Molecules. 2024, 29, 4221. [Google Scholar] [CrossRef]
- Tesarova, B.; Musilek, K.; Rex, S.; Heger, Z. Taking advantage of cellular uptake of ferritin nanocages for targeted drug delivery. J. Control Release. 2020, 325, 176–190. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) from Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: A phase 1 trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Hofstetter, A.R.; Houser, K.V.; Awan, S.F.; Chen, G.L.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Holman, L.A.; et al. An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in healthy adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef] [PubMed]
- Casazza, J.P.; Hofstetter, A.R.; Costner, P.J.M.; Holman, L.A.; Hendel, C.S.; Widge, A.T.; Wu, R.L.; Whalen, W.R.; Cunningham, J.; Arthur, A.; et al. Phase 1 dose-escalation trial evaluating a group 2 influenza hemagglutinin stabilized stem nanoparticle vaccine. npj Vaccines 2024, 9, 171. [Google Scholar] [CrossRef]
- Zhong, D.; Lu, Z.; Xia, Y.; Wu, H.; Zhang, X.; Li, M.; Song, X.; Wang, Y.; Moon, A.; Qiu, H.-J.; et al. Ferritin Nanoparticle Delivery of the E2 Protein of Classical Swine Fever Virus Completely Protects Pigs from Lethal Challenge. Vaccines 2024, 12, 629. [Google Scholar] [CrossRef]
- Chang, X.; Ma, J.; Zhou, Y.; Xiao, S.; Xiao, X.; Fang, L. Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses. 2024, 16, 991. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Tang, P.; Cui, E.-H.; Chang, W.-C.; Yu, C.; Wang, H.; Du, E.-Q.; Wang, J.-Y. Nanoparticle-Based Bivalent Swine Influenza Virus Vaccine Induces Enhanced Immunity and Effective Protection against Drifted H1N1 and H3N2 Viruses in Mice. Viruses 2022, 14, 2443. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, Z.; Sun, J.; Wang, X.; Zhuang, G.; Jiang, D.; Wu, Y.; et al. A Candidate Nanoparticle Vaccine Comprised of Multiple Epitopes of the African Swine Fever Virus Elicits a Robust Immune Response. J. Nanobiotechnol. 2023, 21, 424. [Google Scholar] [CrossRef]
- Aebischer, A.; Wernike, K.; König, P.; Franzke, K.; Wichgers Schreur, P.J.; Kortekaas, J.; Vitikainen, M.; Wiebe, M.; Saloheimo, M.; Tchelet, R.; et al. Development of a Modular Vaccine Platform for Multimeric Antigen Display Using an Orthobunyavirus Model. Vaccines 2021, 9, 651. [Google Scholar] [CrossRef]
- Zhang, B.; Chao, C.W.; Tsybovsky, Y.; Abiona, O.M.; Hutchinson, G.B.; Moliva, J.I.; Olia, A.S.; Pegu, A.; Phung, E.; Stewart-Jones, G.B.E.; et al. A platform incorporating trimeric antigens into self-assembling nanoparticles reveals SARS-CoV-2-spike nanoparticles to elicit substantially higher neutralizing responses than spike alone. Sci. Rep. 2020, 10, 18149. [Google Scholar] [CrossRef]
- He, L.; de Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Tacken, M.; Gutjahr, B.; Keller, M.; van Keulen, L.; Kant, J.; van de Water, S.; Lin, Y.; Eiden, M.; Rissmann, M.; et al. Vaccine Efficacy of Self-Assembled Multimeric Protein Scaffold Particles Displaying the Glycoprotein Gn Head Domain of Rift Valley Fever Virus. Vaccines 2021, 9, 301. [Google Scholar] [CrossRef]
- Choi, B.; Moon, H.; Hong, S.J.; Shin, C.; Do, Y.; Ryu, S.; Choi, K.; Kim, Y.S. Effective Delivery of Antigen-Encapsulin Nanoparticle Fusions to Dendritic Cells Leads to Antigen-Specific Cytotoxic T Cell Activation and Tumor Rejection. ACS Nano 2016, 10, 7339–7350. [Google Scholar] [CrossRef]
- Mu, Z.; Wiehe, K.; Saunders, K.O.; Henderson, R.; Cain, D.W.; Parks, R.; Martik, D.; Mansouri, K.; Edwards, R.J.; Newman, A.; et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 2022, 38, 110514. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, C.; Xing, J.; Zhang, T.; Xu, Z.; Di, Y.; Yang, S.; Jiang, R.; Tang, J.; Zhuang, X.; et al. Ferritin-binding and ubiquitination-modified mRNA vaccines induce potent immune responses and protective efficacy against SARS-CoV-2. Int. Immunopharmacol. 2024, 129, 111630. [Google Scholar] [CrossRef]
- Roier, S.; Mangala Prasad, V.; McNeal, M.M.; Lee, K.K.; Petsch, B.; Rauch, S. mRNA-based VP8* nanoparticle vaccines against rotavirus are highly immunogenic in rodents. npj Vaccines 2023, 8, 190. [Google Scholar] [CrossRef]
- Brandys, P.; Montagutelli, X.; Merenkova, I.; Barut, G.T.; Thiel, V.; Schork, N.J.; Trüeb, B.; Conquet, L.; Deng, A.; Antanasijevic, A.; et al. A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 Delta variant in transgenic K18-hACE2 mice. Front. Immunol. 2022, 13, 912898. [Google Scholar] [CrossRef]
- Shattock, R.J.; Andrianaivoarimanana, V.; McKay, P.F.; Randriantseheno, L.N.; Murugaiah, V.; Samnuan, K.; Rogers, P.; Tregoning, J.S.; Rajerison, M.; Moore, K.M.; et al. A self-amplifying RNA vaccine provides protection in a murine model of bubonic plague. Front. Microbiol. 2023, 14, 1247041. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Schmidt, C.; Schnierle, B.S. Self-amplifying RNA vaccine candidates: Alternative platforms for mRNA vaccine development. Pathogens 2023, 12, 138. [Google Scholar] [CrossRef]
- Komori, M.; Nogimori, T.; Morey, A.L.; Sekida, T.; Ishimoto, K.; Hassett, M.R.; Masuta, Y.; Ode, H.; Tamura, T.; Suzuki, R.; et al. saRNA vaccine expressing membrane-anchored RBD elicits broad and durable immunity against SARS-CoV-2 variants of concern. Nat. Commun. 2023, 14, 2810. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ip, S.; Geall, A.J. An update on self-amplifying mRNA vaccine development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Yıldız, A.; Răileanu, C.; Beissert, T. Trans-amplifying RNA: A journey from alphavirus research to future vaccines. Viruses 2024, 16, 503. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Fazel, F.; Doost, J.S.; Raj, S.; Boodhoo, N.; Karimi, K.; Sharif, S. The mRNA vaccine platform for veterinary species. Vet. Immunol. Immunopathol. 2024, 274, 110803. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, K.; Shokrpoor, S.; Rahmati-Holasoo, H.; El-Matbouli, M.; Taheri-Mirghaed, A. Cyprinid herpesvirus 3 (CyHV-3) transmission and outbreaks in Iran: Detection and characterization in farmed common carp. Microb. Pathog. 2020, 149, 104321. [Google Scholar] [CrossRef]
- Soltani, M.; Zamani, M.; Taheri-Mirghaed, A.; Ahmadivand, S.; Mohamdian, S.; Abdi, K.; Soltani, E. Incidence and Genetic Analysis of White Spot Syndrome Virus (WSSV) in Farmed Shrimps (P. indicus and L. Vannamei) in Iran. Bull. Eur. Assoc. Fish. Pathol. 2018, 38, 24–34. [Google Scholar]
- Ahmadivand, S.; Palić, D.; Weidmann, M. Molecular Epidemiology of Novirhabdoviruses Emerging in Iranian Trout Farms. Viruses 2021, 13, 448. [Google Scholar] [CrossRef]
- Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA-Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines 2024, 12, 788. [Google Scholar] [CrossRef]
- See, S.A.; Bhassu, S.; Tang, S.S.; Yusoff, K. Newly Developed mRNA Vaccines Induce Immune Responses in Litopenaeus vannamei Shrimps During Primary Vaccination. Dev. Comp. Immunol. 2024, 162, 105264. [Google Scholar] [CrossRef]
- Ozdilek, A.; Paschall, A.V.; Dookwah, M.; Tiemeyer, M.; Avci, F.Y. Host protein glycosylation in nucleic acid vaccines as a potential hurdle in vaccine design for nonviral pathogens. Proc. Natl. Acad. Sci. USA 2020, 117, 1280–1282. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular Mechanisms for Enhanced DNA Vaccine Immunogenicity. Expert. Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Soltani, E.; Hassanzadeh, R.; Ashrafi-Helan, J. VP2 (PTA motif) encoding DNA vaccine confers protection against lethal challenge with infectious pancreatic necrosis virus (IPNV) in trout. Mol. Immunol. 2018, 94, 61–67. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The Next-Generation DNA Vaccine Platforms and Delivery Systems: Advances, Challenges and Prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef]
- Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines 2023, 11, 280. [Google Scholar] [CrossRef]
- Soltani, M.; Ahmadivand, S.; Behdani, M.; Hassanzadeh, R.; Rahmati-Holasoo, H.; Taheri-Mirghaed, A. Transcription of adaptive-immune genes upon challenge with infectious pancreatic necrosis virus (IPNV) in DNA vaccinated rainbow trout. Int. J. Aquat. Biol. 2016, 4, 353–359. [Google Scholar]
- Naderi-Samani, M.; Soltani, M.; Dadar, M.; Taheri-Mirghaed, A.; Zargar, A.; Ahmadivand, S.; Hassanzadeh, R.; Moazami Goudarzi, L. Oral immunization of trout fry with recombinant Lactococcus lactis NZ3900 expressing G gene of viral hemorrhagic septicaemia virus (VHSV). Fish Shellfish. Immunol. 2020, 105, 62–70. [Google Scholar] [CrossRef]
- Almeida, A.V.; Carvalho, A.J.; Calmeiro, T.; Jones, N.C.; Hoffmann, S.V.; Fortunato, E.; Pereira, A.S.; Tavares, P. Condensation and protection of DNA by the Myxococcus xanthus encapsulin: A novel function. Int. J. Mol. Sci. 2022, 23, 7829. [Google Scholar] [CrossRef]
- Paiva, N.F.; Rios, W.M.; Antonio, G.P.; Umino, G.M.; Ferreira, M.P.; Brandão, I.T.; Del Ciampo, J.O.; Bentley, M.V.L.B.; Casagrande, R.; Verri, W.A.; et al. Ferritin-Based Systems as a Platform for DNA Construct Delivery: Preparation, Characterization, and In Vitro Evaluation. BioNanoSci. 2025, 15, 128. [Google Scholar] [CrossRef]
- Díaz-Dinamarca, D.A.; Salazar, M.L.; Castillo, B.N.; Manubens, A.; Vasquez, A.E.; Salazar, F.; Becker, M.I. Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities. Pharmaceutics 2022, 14, 1671. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Mou, M.; Li, J.; Huang, S.; Zhang, E.; Yan, H.; Yang, J.; Zhong, M. Enhanced TLR5-dependent migration and activation of antigen-loaded airway dendritic cells by flagellin. J. Leukoc. Biol. 2023, 113, 567–576. [Google Scholar] [CrossRef]
- Jones, J.A.; Benisch, R.; Giessen, T.W. Encapsulin cargo loading: Progress and potential. J. Mater. Chem. B 2023, 11, 4377–4388. [Google Scholar] [CrossRef]
- Altenburg, W.J.; Rollins, N.; Silver, P.A.; Giessen, T.W. Exploring targeting peptide-shell interactions in encapsulin nanocompartments. Sci. Rep. 2021, 11, 4951. [Google Scholar] [CrossRef]
- Afzal, H.; Murtaza, A.; Cheng, L.T. Structural Engineering of Flagellin as Vaccine Adjuvant: Quest for the Minimal Domain of Flagellin for TLR5 Activation. Mol. Biol. Rep. 2025, 52, 104. [Google Scholar] [CrossRef]
- Kim, D.; Rossi, J. RNAi Mechanisms and Applications. Biotechniques 2008, 44, 613–616. [Google Scholar] [CrossRef]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Chokwassanasakulkit, T.; Oti, V.B.; Idris, A.; McMillan, N.A.J. SiRNAs as antiviral drugs—Current status, therapeutic potential and challenges. Antivir. Res. 2024, 232, 106024. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- McAnuff, M.A.; Rettig, G.R.; Rice, K.G. Potency of siRNA versus shRNA mediated knockdown in vivo. J. Pharm. Sci. 2007, 96, 2922–2930. [Google Scholar] [CrossRef]
- Isenmann, M.; Stoddart, M.J.; Schmelzeisen, R.; Gross, C.; Della Bella, E.; Rothweiler, R.M. Basic Principles of RNA Interference: Nucleic Acid Types and In Vitro Intracellular Delivery Methods. Micromachines 2023, 14, 1321. [Google Scholar] [CrossRef]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat.Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes. Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Paddison, P.J.; Hannon, G.J. RNA interference: The new somatic cell genetics? Cancer Cell 2002, 2, 17–23. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.; Kirmani, A.G.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev Med Virol. 2018, 28, e1976. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, H.; Ari, M.M.; Alvandi, A.; Abiri, R. Principle, application and challenges of development siRNA-based therapeutics against bacterial and viral infections: A comprehensive review. Front Microbiol. 2024, 15, 1393646. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The landscape of nanoparticle-based siRNA delivery and therapeutic development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef] [PubMed]
- Charousova, M.; Kudlickova Peskova, M.; Takacsova, P.; Kapolkova, K.; Haddad, Y.; Bilek, J.; Sivak, L.; Bartejs, T.; Heger, Z.; Pekarik, V. Engineered human H-chain ferritin with reversed charge of the internal cavity exhibits RNA-mediated spongelike effect for loading RNA/DNA-binding molecules. Biomater. Sci. 2024, 12, 1249–1262. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Cao, T.; Liu, X.; Liu, X.; Yan, Y.; Shi, Y.; Wang, J.-C. Ferritin-based nanomedicine for disease treatment. Med. Rev. 2023, 3, 49–74. [Google Scholar] [CrossRef]

| SAPNs | Biological Function | Origin | Cavity Size (nm) | Outer Size (nm) | Subunits | Subunit MW | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ferritin | Iron storage and transport | Helicobacter pylori | 8 | 12 | 24 | 20 kDa | pH: 3–12; 80–100 °C | [17] |
| Lumazine Synthase (LuS) | Riboflavin biosynthesis | Aquifex aeolicus | 9 | 15.4 | 60 | 17.8 kDa | 120 °C | [38] |
| Bacillus subtilis | 16 | 30 | 180 | 16.3 kDa | Up to pH: 10 and 80 °C | [37] | ||
| Encapsulin | Oxidative stress response through encapsulation of other related proteins | Thermotoga maritima | 20 | 24 | 60 | 31 kDa | pH: 12, <3 M GuHCl; 90 °C | [8,58] |
| Pyrococcus furiosus | 22 | 32 | 180 | 39 kDa | <4 M GuHCl; 80 °C | [8,58] | ||
| Quasibacillus thermotolerance | 36 | 42 | 240 | 32.2 kDa | <1 M GuHCl; 40 °C | [8,58] | ||
| E2p | Enzyme complex for metabolic pathways | Geobacillus stearothermophilus | 12 pores of 5 nm | 27 | 60 | 41 kDa | 80 °C | [48] |
| sHSP | Response to cellular stress | Methanococcus jannaschii | 6 | 12 | 24 | 16.5 kDa | 70 °C and pH 5–11 | [56] |
| Approach | Developing Method | Key Advantages Compared to Conventional Method |
|---|---|---|
| SAPN-Subunit Vaccines | Fuse the antigen to the N- or C-termini of SAPN, express and purify the fusion protein to devlop the subunit vaccine. | Multivalent antigen display, enhanced solubility, stability and biocompatibility, cellular uptake, heightened immunogenicity, targeted delivery, and customized vaccine design. |
| SAPN-RNA Vaccines | Fuse the antigen to the N- or C-termini of SAPN, produce the antigen via RNA vaccine platforms (mRNA, saRNA, taRNA). | Promotes multivalent antigen display, enhances stability and immunogenicity of secreted proteins with prolonged expression, reduces off-target effects, and minimizes required doses. |
| SAPN-DNA Vaccines | Fuse the antigen to the N- or C-termini of SAPN, clone the fusion construct into a eukaryotic DNA plasmid. | Multivalent antigen display that stabilizes the antigenic protein and enhances expression, cellular uptake, antigen presentation, and immune responses. |
| SAPN-Adjuvant | Fuse a protein adjuvant to the N- or C-termini of SAPN, co-deliver with the antigen or encapsulate within SAPN. | Enables co-delivery of adjuvant with antigen to same cell (e.g., APC), targeted and enhanced biocompatibility, stability and immune responses with multivalent display of protein adjuvants, protection and controlled release of encapsulated small-molecule adjuvants. |
| SAPN-RNAi Delivery | Encapsulate siRNA or miRNA within SAPN using the disassembly/reassembly of SAPN. | Enables targeted gene silencing, controlled release, enhanced delivery efficiency, reduced immunogenicity, and scalable production. |
| Platform | SAPN Type | Mode of Incorporation | Diseases/Pathogen | Species/Cells | Key Finding | Ref. |
|---|---|---|---|---|---|---|
| mRNA vaccine | Ferritin | Genetic Surface Fusion | MERS-CoV | Mice; NHPs and Alpacas | This vaccine is temperature- and pH-stable, eliciting robust antibody titers in BALB/c mice and NHPs (non-human primates) with cross-clade neutralization (A, B, C). Complete protection in alpacas. | [19] |
| mRNA vaccine | Ferritin | Genetic Surface Fusion | HIV-1 Env | Mice | Induced neutralizing antibody responses in mice capable of neutralizing heterologous HIV-1 isolates. | [87] |
| mRNA vaccine | Ferritin | Genetic Surface Fusion | SARS-CoV-2 (RBD) | Mice and Hamsters | Elevated specific IgG levels and significantly higher neutralizing antibody titers correlated with improved protection in hamsters. | [88] |
| mRNA vaccine | LuS | Genetic Surface Fusion | Rotavirus (P2-VP8*) | Mice and Guinea Pigs | Induced the highest specific IgG titers compared to conventional mRNA and subunit vaccine. | [89] |
| mRNA vaccine | LuS | Genetic Surface Fusion | SARS-CoV-2 (RBD) | Mice | Strong neutralizing antibody responses and protection against the Delta variant. | [90] |
| mRNA vaccine | LuS | Genetic Surface Fusion | HIV (gp120) | Human | The vaccines were safe and induce early maturation of HIV broadly neutralizing antibody (bnAb) precursors in humans. Phase 1 clinical trial (NCT05001373 and NCT05414786). | [23] |
| saRNA vaccine | LuS | Genetic Surface Fusion | HIV (gp120) | Mice | Elicited enhanced Tfh cell activation, increased B cell responses, and stronger antibody responses in mice compared to protein vaccines | [24] |
| saRNA vaccine | Ferritin | Genetic Surface Fusion | Yersinia pestis | Mice | Induced specific IgG responses and neutralizing antibodies, conferring protection in mice against Yersinia pestis challenge. | [91] |
| DNA vaccine | Ferritin | Genetic Surface Fusion | SARS-CoV-2 (RBD) | Rat | Generates more potent neutralizing responses compared to the subunit vaccine. | [21] |
| siRNA | Ferritin | Reversible dissassembly | Cancer | hMSC and PBMCs cells | Efficient encapsulation enabling gene silencing in tumor cells and PBMCs at low concentrations without inducing immune activation. | [29] |
| siRNA | Ferritin | Reversible dissassembly | Acute myeloid leukemia | HL-60 and K-562 | The siPD-L1/HFn nanocarrier effectively silenced PD-L1 expression in HL-60 and K-562 cells. | [30] |
| siRNA | Ferritin | Reversible dissassembly | Cancer | A549 and HeLa cells | L17E-modified ferritin boosts stability, uptake, and BCL-2 silencing in A549 and HeLa cells. | [13] |
| siRNA | Ferritin (tHFn(+)) | Reversible dissassembly | Glioblastoma | Mice | Efficiently crosses the blood–brain barrier, targets glioblastoma, and demonstrates therapeutic efficacy with siTERT and siEGFR in mice. | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadivand, S. Innovation in mRNA Vaccines and RNAi via Protein Nanocages. Vaccines 2025, 13, 653. https://doi.org/10.3390/vaccines13060653
Ahmadivand S. Innovation in mRNA Vaccines and RNAi via Protein Nanocages. Vaccines. 2025; 13(6):653. https://doi.org/10.3390/vaccines13060653
Chicago/Turabian StyleAhmadivand, Sohrab. 2025. "Innovation in mRNA Vaccines and RNAi via Protein Nanocages" Vaccines 13, no. 6: 653. https://doi.org/10.3390/vaccines13060653
APA StyleAhmadivand, S. (2025). Innovation in mRNA Vaccines and RNAi via Protein Nanocages. Vaccines, 13(6), 653. https://doi.org/10.3390/vaccines13060653







