Herd Immunity to the Measles, Mumps and Rubella Viruses Among the Belgradian Population in May, 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Volunteer Cohort
2.2. Research Methods
2.2.1. Volunteer Inclusion Criteria
2.2.2. Sample Collection and Testing
2.3. Statistical Processing
3. Results
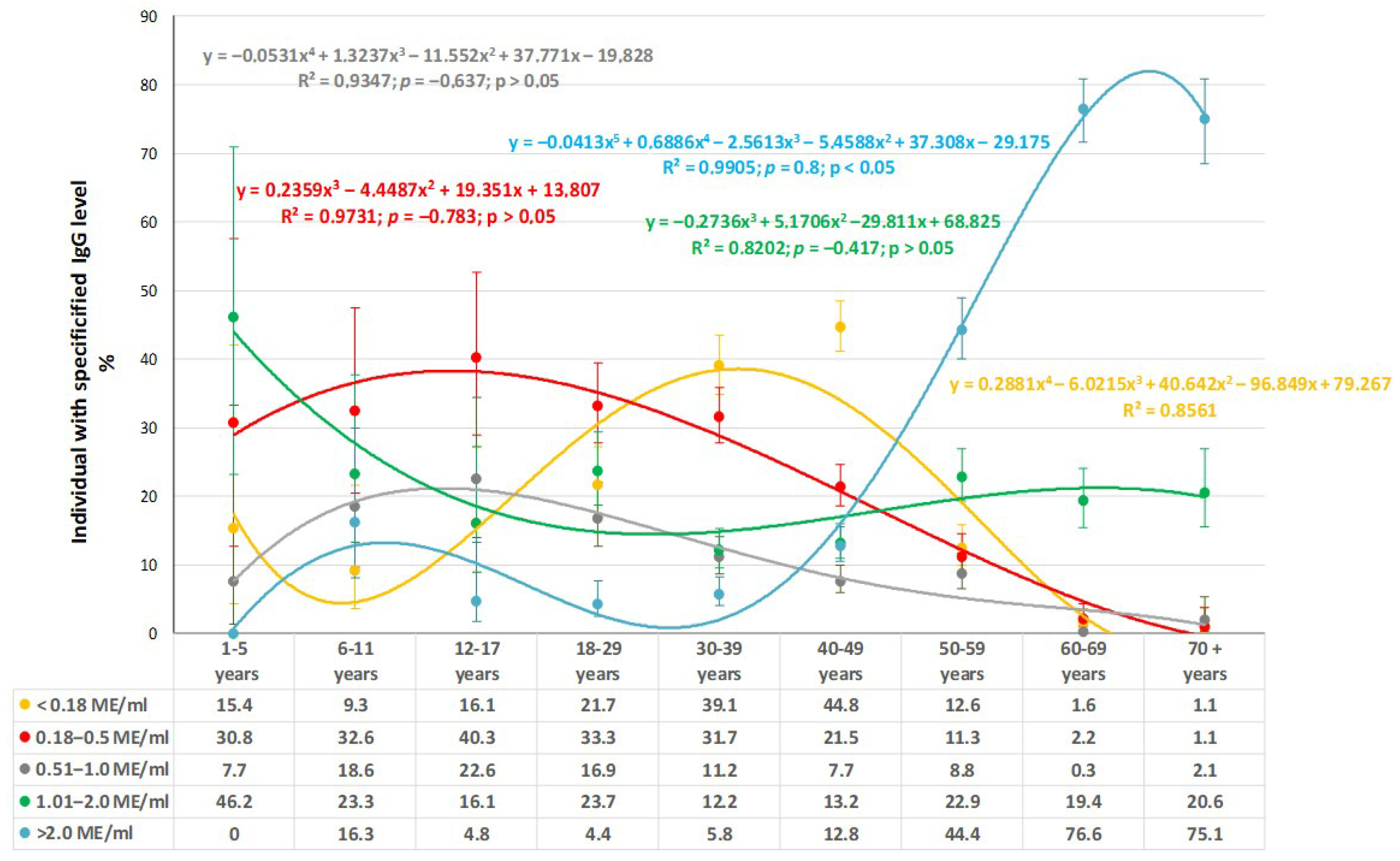
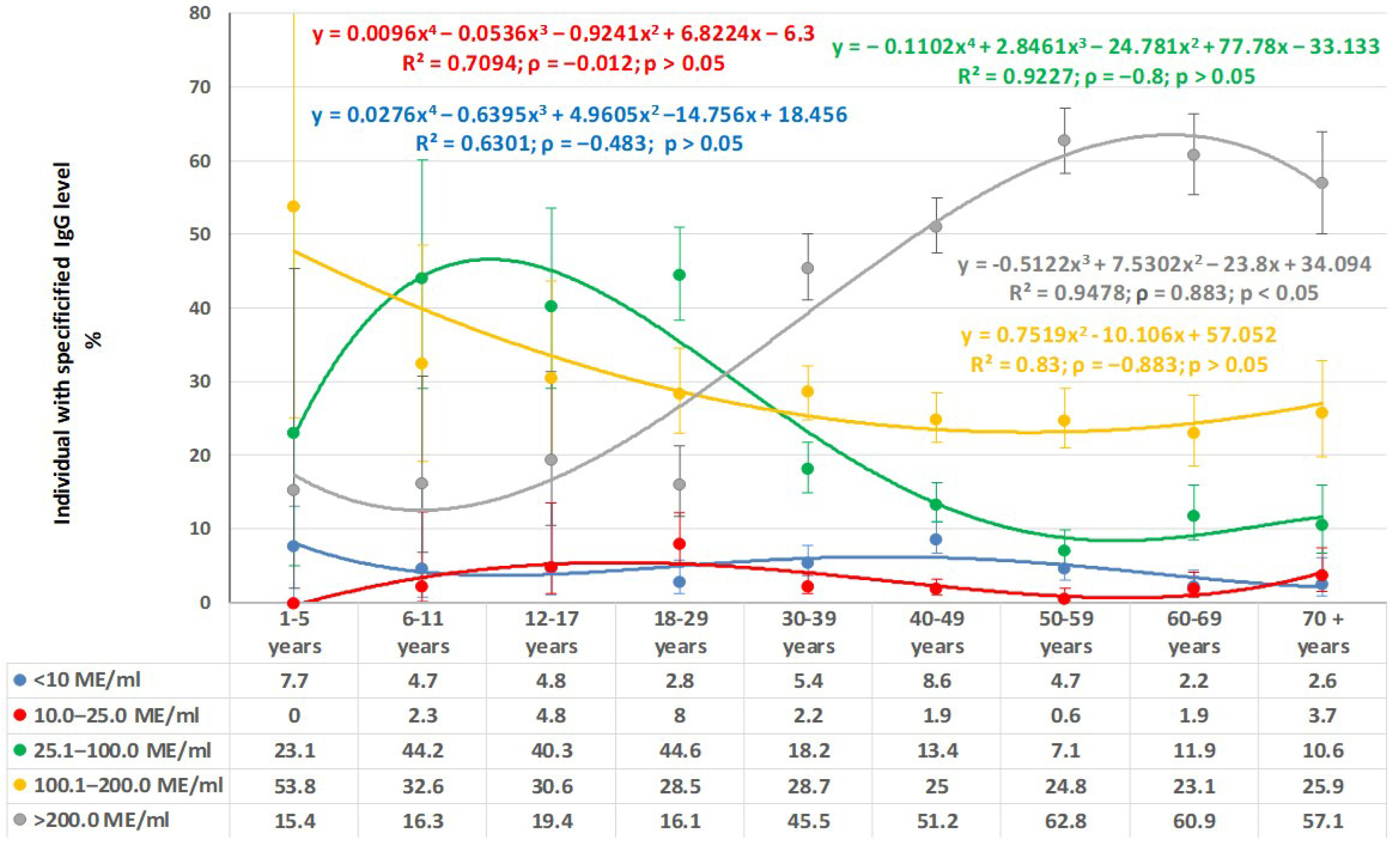
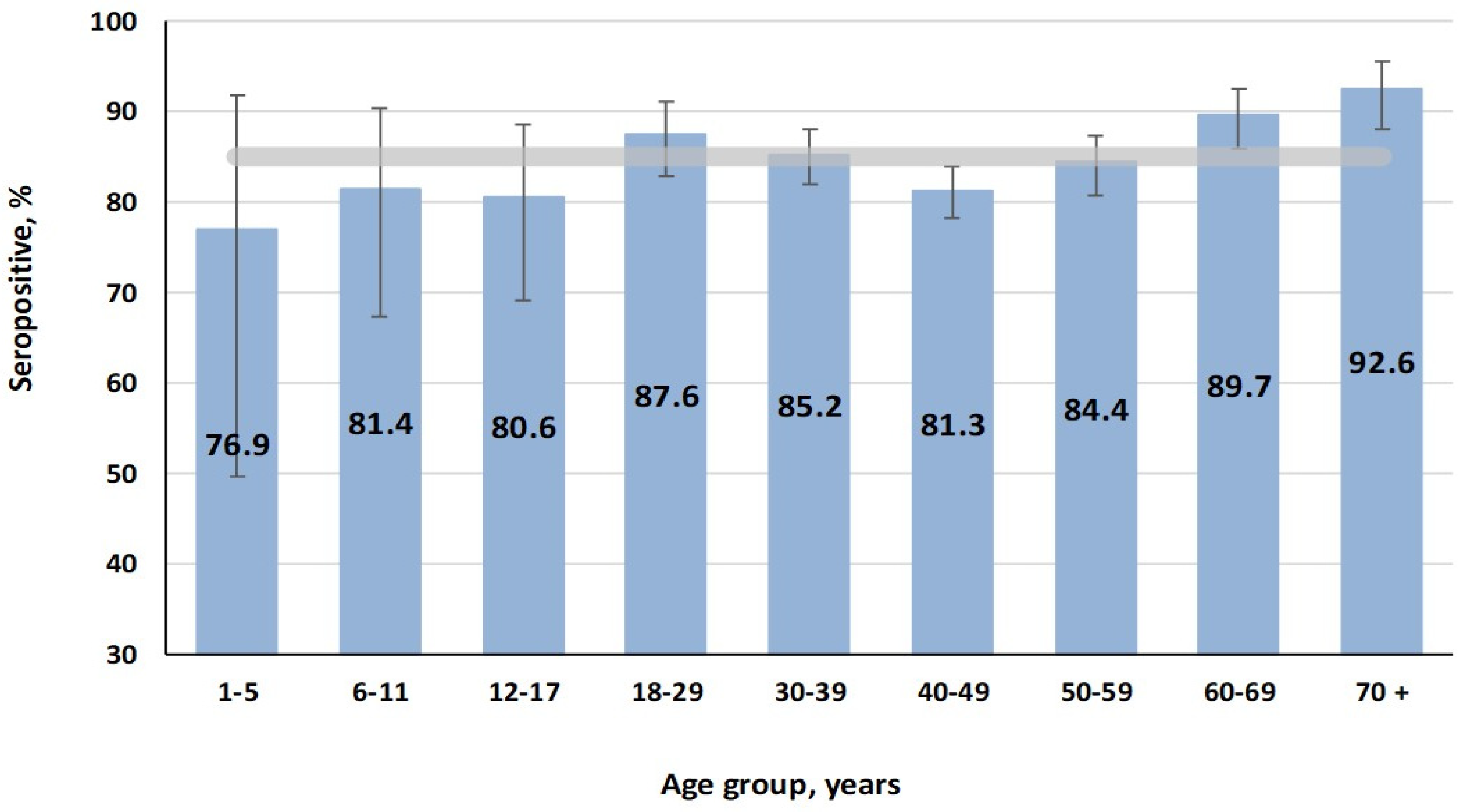
3.1. Measles
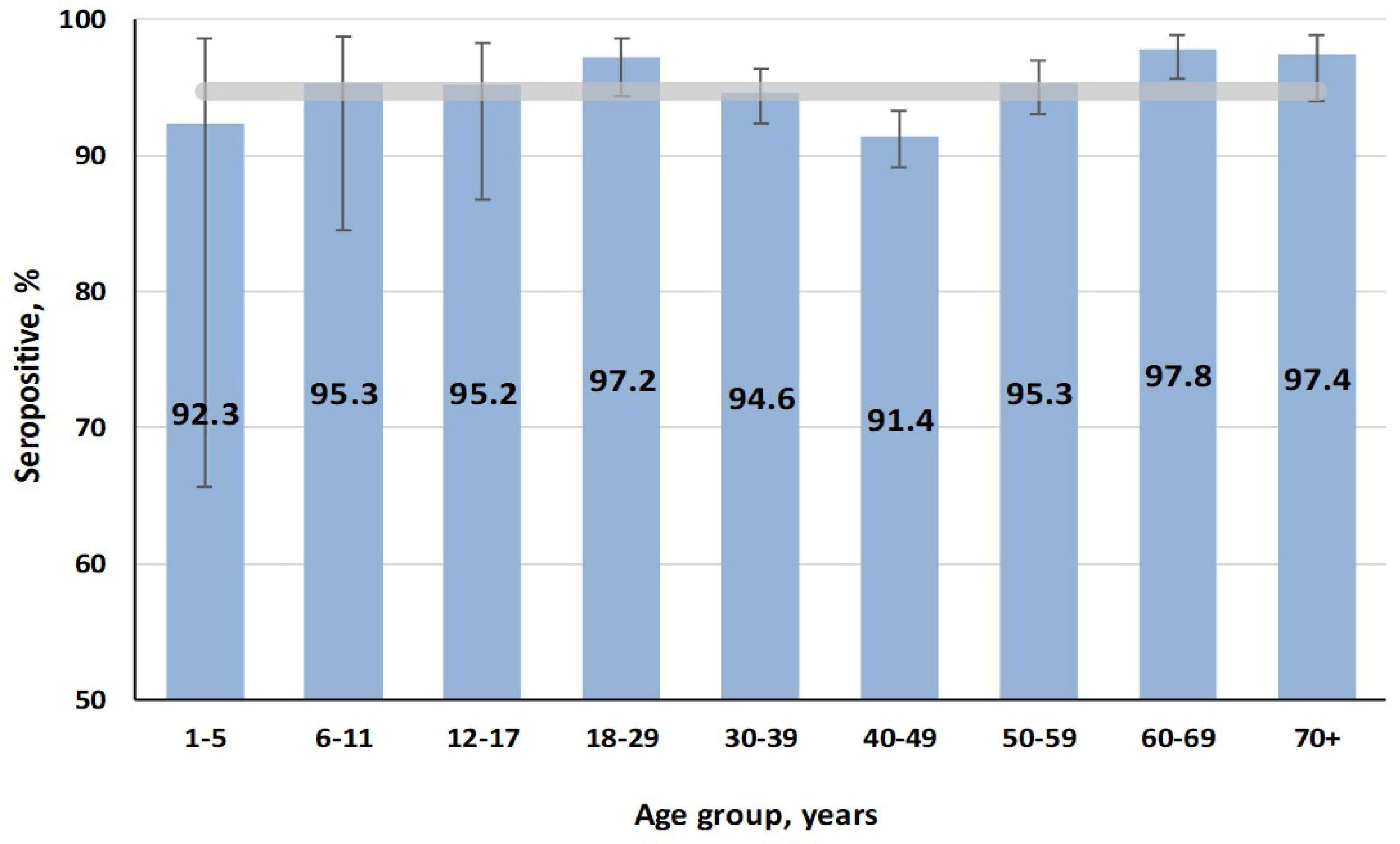
3.2. Rubella
3.3. Mumps
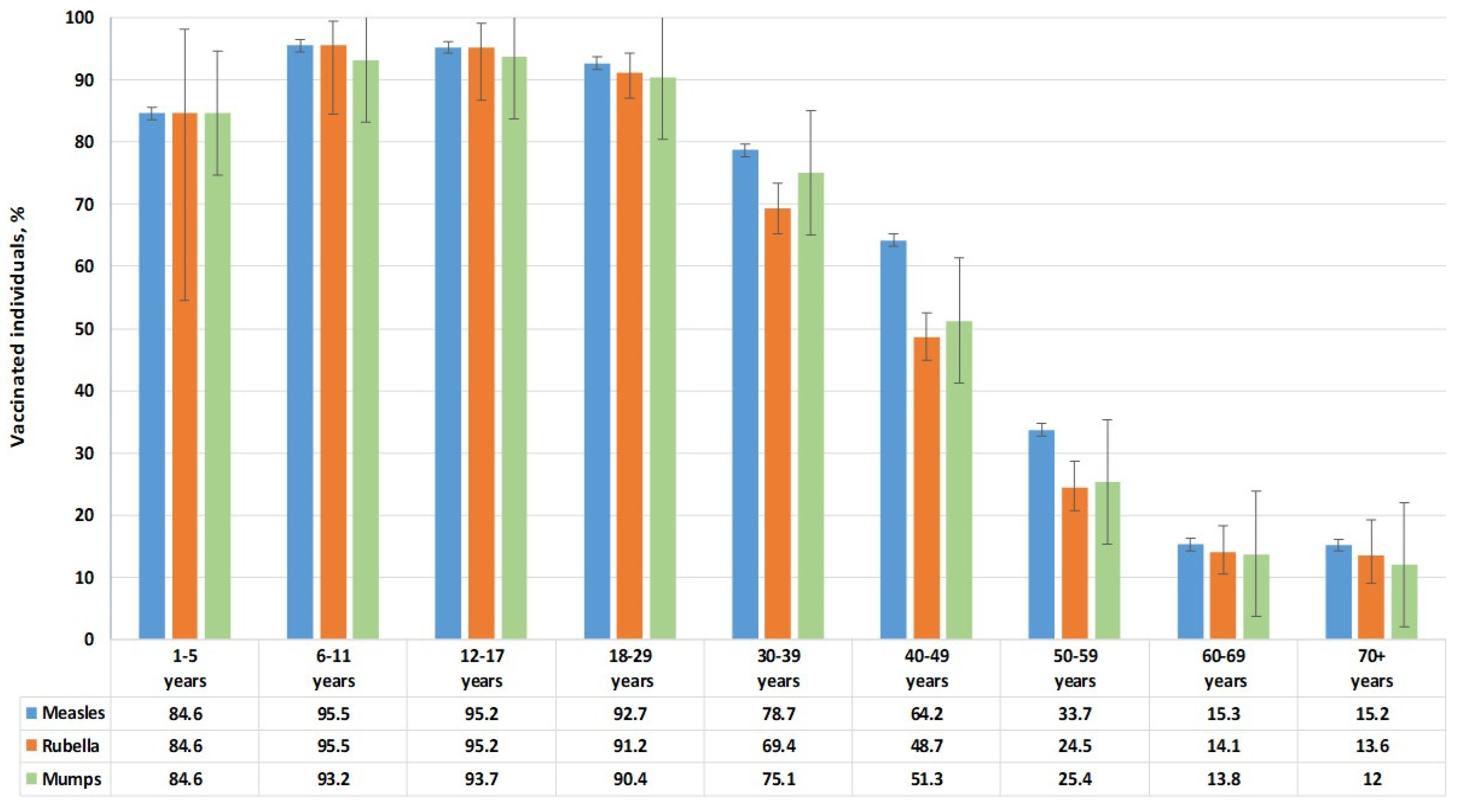
3.4. Vaccination Status
4. Discussion
4.1. Measles Immunity: Potential Need for Catch-Up Vaccination
4.2. Rubella: Sustained Immunity and Low Outbreak Risk
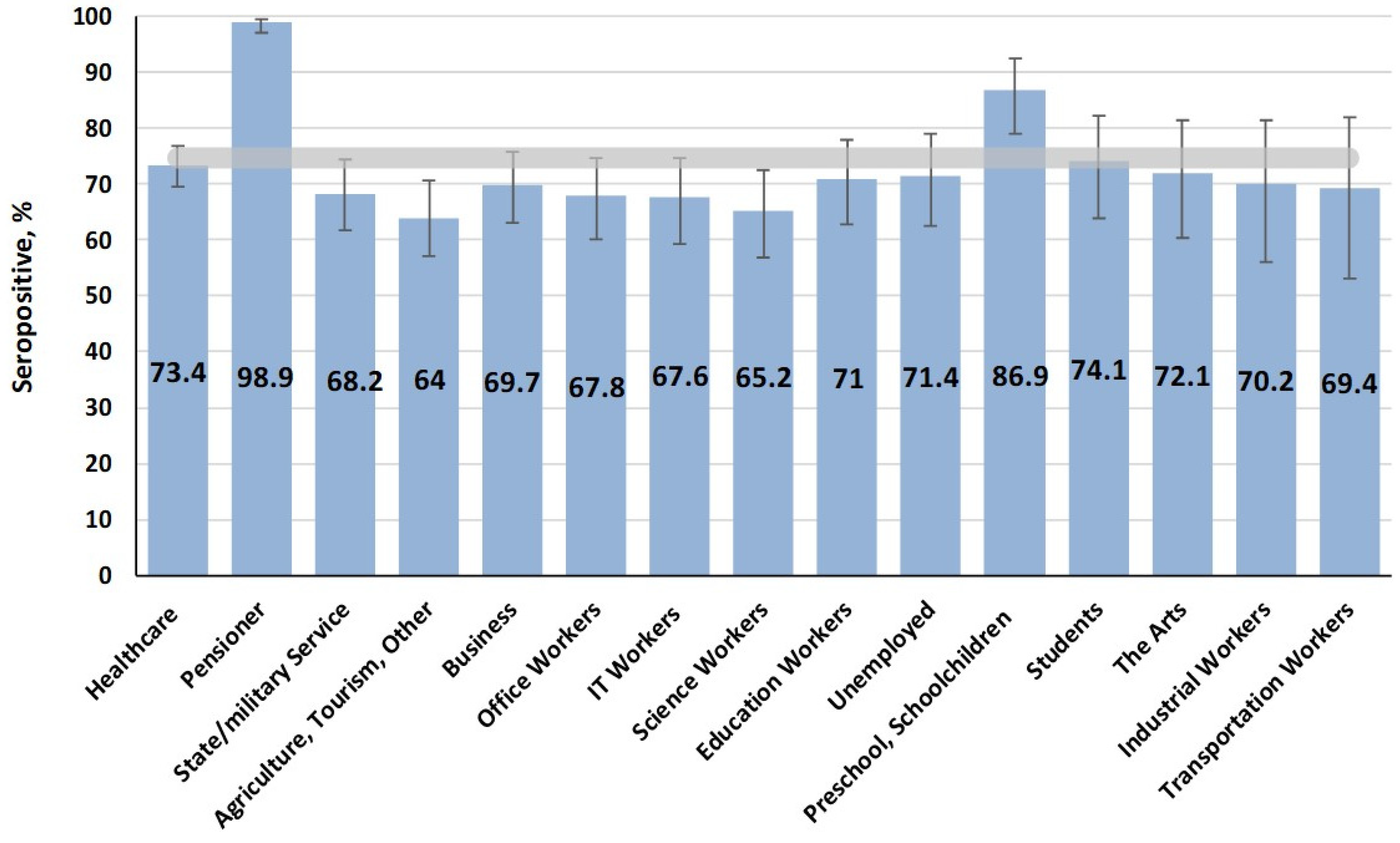
4.3. Mumps Immunity: Areas for Improvement
4.4. Conclusions Common to the Three Infections
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naim, H.Y. Measles virus. Hum. Vaccines Immunother. 2015, 11, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Su, S.B.; Chang, H.-L.; Chen, K.-T. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. Int. J. Environ. Res. Public Health 2020, 17, 1686. [Google Scholar] [CrossRef]
- Shafayi, A.; Mohammadi, A. A Review on Rubella Vaccine: Iran (1975–2010). Arch. Razi Inst. 2021, 76, 167–192. [Google Scholar] [CrossRef]
- Zverev, V.V.; Iuminova, N.V. Issues related to rubella, measles and epidemic parotiditis in the Russian Federation. Vopr. Virusol. 2004, 49, 8–11. [Google Scholar] [PubMed]
- Samoilovich, E.O.; Kapustik, L.A.; Feldman, E.V.; Yermolovich, M.A.; Svirchevskaya, A.J.; Zakharenko, D.F.; Fletcher, M.A.; Titov, L.P. The immunogenicity and reactogenicity of the trivalent vaccine, Trimovax, indicated for prevention of measles, mumps, and rubella, in 12-month-old children in Belarus. Cent. Eur. J. Public Health 2000, 8, 160–163. [Google Scholar] [PubMed]
- Vittrup, D.M.; Laursen, A.C.L.; Malon, M.; Soerensen, J.K.; Hjort, J.; Buus, S.; Svensson, J.; Stensballe, L.G. Measles-mumps-rubella vaccine at 6 months of age, immunology, and childhood morbidity in a high-income setting: Study protocol for a randomized controlled trial. Trials 2020, 21, 1015. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2021, 11, CD004407. [Google Scholar] [CrossRef]
- Semerikov, V.V.; Postanogova, N.O.; Sofronova, L.V.; Zubova, E.S.; Musikhina AYu Perminova, O.A.; Permiakova, M.A. Practical aspects of epidemiology and vaccine prevention domestic tetravaccine (measles-rubella-mumps)—Immunization of premature babies. Epidemiol. Vaccinal Prev. 2022, 21, 95–102. [Google Scholar] [CrossRef]
- O’Connor, P.; Jankovic, D.; Muscat, M.; Ben-Mamou, M.; Reef, S.; Papania, M.; Singh, S.; Kaloumenos, T.; Butler, R.; Datta, S. Measles and rubella elimination in the WHO Region for Europe: Progress and challenges. Clin. Microbiol. Infect. 2017, 23, 504–510. [Google Scholar] [CrossRef]
- Dotevall, L. The return of measles to Europe highlights the need to regain confidence in immunization. Acta Paediatr. 2019, 108, 8–9. [Google Scholar] [CrossRef]
- López-Perea, N. Measles again? Med. Clin. 2024, 163, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Bichurina, M.; Timofeeva, E.; Zheleznova, N.; Ignatyeva, N.; Shulga, R.; Lyalina, L.; Degtyarev, O. Measles outbreak in a children’s hospital in Saint Petersburg in 2012. J. Infectol. 2013, 5, 96–102. [Google Scholar] [CrossRef]
- Bichurina, M.A.; Filipović-Vignjević, S.; Antipova, A.Y.; Bančević, M.; Lavrentieva, I.N. A herd immunity to measles and rubella viruses in the population of the Republic of Serbia. Russ. J. Infect. Immun. 2021, 11, 171–176. [Google Scholar] [CrossRef]
- Bogusz, J.; Augustynowicz, E.; Wnukowska, N.; Paradowska-Stankiewicz, I. Measles in Poland in 2019. Przegl Epidemiol. 2021, 75, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.D. Mislingar á Íslandi 2019. Læknafélag Reykjavikur 2019, 105, 161. [Google Scholar] [CrossRef]
- Arapović, J.; Sulaver, Ž.; Rajič, B.; Pilav, A. The 2019 measles epidemic in Bosnia and Herzegovina: What is wrong with the mandatory vaccination program? Bosn. J. Basic Med. Sci. 2019, 19, 210–212. [Google Scholar] [CrossRef]
- Keske, Ş.; Özsürekci, Y.; Ergönül, Ö. An Alarming Emergence of Measles in Europe: Gaps Future Directions. Balk. Med. J. 2024, 41, 321–323. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Jain, N.; Tanasov, A.; Schlagenhauf, P. Measles matter: Recent outbreaks highlight the need for catch-up vaccination in Europe and around the globe. New Microbes New Infect. 2024, 58, 101238. [Google Scholar] [CrossRef]
- Thornton, J. Measles cases in Europe tripled from 2017 to 2018. BMJ 2019, 364, l634. [Google Scholar] [CrossRef]
- Measles Outbreak in the WHO European Region. Available online: https://news.un.org/ru/story/2024/02/1449742 (accessed on 21 April 2025).
- European Region Reports Highest Number of Measles Cases in More Than 25 Years. Available online: https://www.who.int/europe/news/item/13-03-2025-european-region-reports-highest-number-of-measles-cases-in-more-than-25-years---unicef--who-europe (accessed on 21 April 2025).
- Lanke, R.; Chimurkar, V. Measles Outbreak in Socioeconomically Diverse Sections: A Review. Cureus 2024, 16, e62879. [Google Scholar] [CrossRef]
- Graf, W.; Bertram, F.; Dost, K.; Brennecke, A.; Kowalski, V.; van Rüth, V.; Nörz, D.S.; Wulff, B.; Ondruschka, B.; Püschel, K.; et al. Immunity against measles, mumps, rubella, and varicella among homeless individuals in Germany—A nationwide multi-center cross-sectional study. Front. Public Health 2024, 12, 1375151. [Google Scholar] [CrossRef] [PubMed]
- El Zarif, T.; Kassir, M.F.; Bizri, N.; Kassir, G.; Musharrafieh, U.; Bizri, A.R. Measles and mumps outbreaks in Lebanon: Trends and links. BMC Infect. Dis. 2020, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Gadroen, K.; Dodd, C.N.; Muscle, G.M.C.; de Ridder, M.A.J.; Weibel, D.; Mina, M.J.; Grenfell, B.T.; Sturkenboom, M.C.J.M.; van de Vijver, D.A.M.C.; de Swart, R.L. Impact and longevity of measles-associated immune suppression: A matched cohort study using data from the THIN general practice database in the UK. BMJ Open 2018, 8, e021465. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Govindaraj, G. Infections in Inborn Errors of Immunity with Combined Immune Deficiency: A Review. Pathogens 2023, 12, 272. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Tan, C.; Wu, Y.; Zhang, Z.; Ding, J.; Li, Y. The impact of temperature, humidity and closing school on the mumps epidemic: A case study in the mainland of China. BMC Public Health 2024, 24, 1632. [Google Scholar] [CrossRef]
- Siberry, G.K.; Patel, K.; Bellini, W.J.; Karalius, B.; Purswani, M.U.; Burchett, S.K.; Meyer, W.A., III; Sowers, S.B.; Ellis, A.; Van Dyke, R.B. Immunity to Measles, Mumps, and Rubella in US Children With Perinatal HIV Infection or Perinatal HIV Exposure Without Infection. Clin. Infect. Dis. 2015, 61, 988–995. [Google Scholar] [CrossRef]
- Laue, T.; Junge, N.; Leiskau, C.; Mutschler, M.; Ohlendorf, J.; Baumann, U. Diminished measles immunity after paediatric liver transplantation—A retrospective, single-centre, cross-sectional analysis. PLoS ONE 2024, 19, e0296653. [Google Scholar] [CrossRef]
- İnce, T.; Gürocak, Ö.T.; Totur, G.; Yılmaz, Ş.; Ören, H.; Aydın, A. Waning of Humoral Immunity to Vaccine-Preventable Diseases in Children Treated for Acute Lymphoblastic Leukemia: A Single-Center Retrospective Cross-Sectional Analysis. Turk. J. Haematol. 2024, 41, 160–166. [Google Scholar] [CrossRef]
- Schweitzer, L.; Miko, B.A.; Marcus RPereira, M.B. Infectious Disease Prophylaxis During and After Immunosuppressive Therapy. Kidney Int. Rep. 2024, 9, 2337–2352. [Google Scholar] [CrossRef]
- Measles on the Rise Again in Europe: Time to Check Your Vaccination Status. Available online: https://www.ecdc.europa.eu/en/news-events/measles-rise-again-europe-time-check-your-vaccination-status (accessed on 21 April 2025).
- Petrović, V.; Šeguljev, Z.; Radovanović, Z. Imunizacija Protiv Zaraznih Bolesti; Novi Sad (Medicinski Fakultet): Novi Sad, Serbia, 2015; p. 168. [Google Scholar]
- Prévot-Monsacré, P.; Hamaide-Defrocourt, F.; Guyonvarch, O.; Masse, S.; Souty, C.; Mamou, T.; Hamel, J.; Anton, D.; Mathieu, P.; Vasseur, P.; et al. What is the relevancy of a surveillance of mumps without a systematic laboratory confirmation in highly immunized populations? Epidemiology of suspected and biologically confirmed mumps cases seen in general practice in France between 2014 and 2020. Vaccine 2024, 42, 1065–1070. [Google Scholar] [CrossRef]
- Veljkovic, M. (Institute of Public Health of Serbia “Dr Milan Jovanović Batut”, Belgrade, Serbia). Personal communication. 2025. [Google Scholar]
- Ristić, M.; Milošević, V.; Medić, S.; Malbaša, J.D.; Rajčević, S.; Boban, J.; Petrović, V. Sero-epidemiological study in prediction of the risk groups for measles outbreaks in Vojvodina, Serbia. PLoS ONE 2019, 14, e0216219. [Google Scholar] [CrossRef] [PubMed]
- Patić, A.; Štrbac, M.; Petrović, V.; Milošević, V.; Ristić, M.; Hrnjaković Cvjetković, I.; Medić, S. Seroepidemiological study of rubella in Vojvodina, Serbia: 24 years after the introduction of the MMR vaccine in the national immunization programme. PLoS ONE 2020, 15, e0227413. [Google Scholar] [CrossRef] [PubMed]
- Rodriges Del Águila, M.; González-Ramírez, A. Sample size calculation. Allergol. Immunopathol. 2014, 42, 485–492. [Google Scholar] [CrossRef]
- Popova, A.Y.; Totolian, A.A. Methodology for assessing population immunity to the SARS-CoV-2 virus in the context of the COVID-19 pandemic. Russ. J. Infect. Immun. 2021, 11, 609–616. [Google Scholar] [CrossRef]
- Wald, A.; Wolfowitz, J. Confidence limits for continuous distribution functions. Ann. Math. Statist. 1939, 10, 105–118. Available online: https://www.jstor.org/stable/2235689 (accessed on 20 April 2025). [CrossRef]
- Agresti, A.; Coull, B.A. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
- Radar Calculators. Available online: https://radar-research.ru/instruments/calculators (accessed on 20 April 2025).
- Neugebauer, M.; Ebert, M.; Vogelmann, R. Beurteilung des neuen Masernschutzgesetzes in Deutschland: Ergebnisse einer deutschlandweiten Befragung. Z. Evid. Fortbild. Qual. Gesundhwes. 2020, 158, 74–80. [Google Scholar] [CrossRef]
- Black, F.L. The role of herd immunity in control of measles. Yale J. Biol. Med. 1982, 55, 351–360. [Google Scholar] [PubMed]
- Friedrich, N.; Poethko-Müller, C.; Kuhnert, R.; Matysiak-Klose, D.; Koch, J.; Wichmann, O.; Santibanez, S.; Mankertz, M. Seroprevalence of Measles-, Mumps-, and Rubella-specific antibodies in the German adult population—cross-sectional analysis of the German Health Interview and Examination Survey for Adults (DEGS1). Lancet Reg. Health Eur. 2021, 7, 100128. [Google Scholar] [CrossRef]
- WHO Immunization Agenda 2030: A Glibal Strategy to Leave No One Behind. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 (accessed on 20 April 2025).
- Lewnard, J.A.; Grad, Y.H. Vaccine waning and mumps re-emergence in the United States. Sci. Transl. Med. 2018, 10, eaao5945. [Google Scholar] [CrossRef]
- Ferrari, C.; Somma, G.; Treglia, M.; Pallocci, M.; Passalacqua, P.; Di Giampaolo, L.; Coppeta, L. Questionable Immunity to Mumps among Healthcare Workers in Italy-A Cross-Sectional Serological Study. Vaccines 2024, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning immunity and re-emergence of measles and mumps in the vaccine era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Henle, G.; Henle, W.; Wendell, K.K.; Rosenberg, P. Isolation of mumps virus from human beings with induced apparent or inapparent infections. J. Exp. Med. 1948, 88, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.Y.; Egorova, S.A.; Smirnov, V.S.; Ezhlova, E.B.; Milichkina, A.M.; Melnikova, A.A.; Bashketova, N.S.; Istorik, O.A.; Buts, L.V.; Ramsay, E.S.; et al. Herd immunity to vaccine preventable infections in Saint Petersburg and the Leningrad region: Serological status of measles, mumps, and rubella. Russ. J. Infect. Immun. 2024, 14, 1187–1208. [Google Scholar] [CrossRef]
- Popova, A.Y.; Smirnov, V.S.; Egorova, S.A.; Nurmatov, Z.S.; Milichkina, A.M.; Drozd, I.V.; Dadanova, G.S.; Zhumagulova, G.D.; Danilova, E.M.; Kasymbekov, Z.O.; et al. Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population. Vaccines 2025, 13, 249. [Google Scholar] [CrossRef]
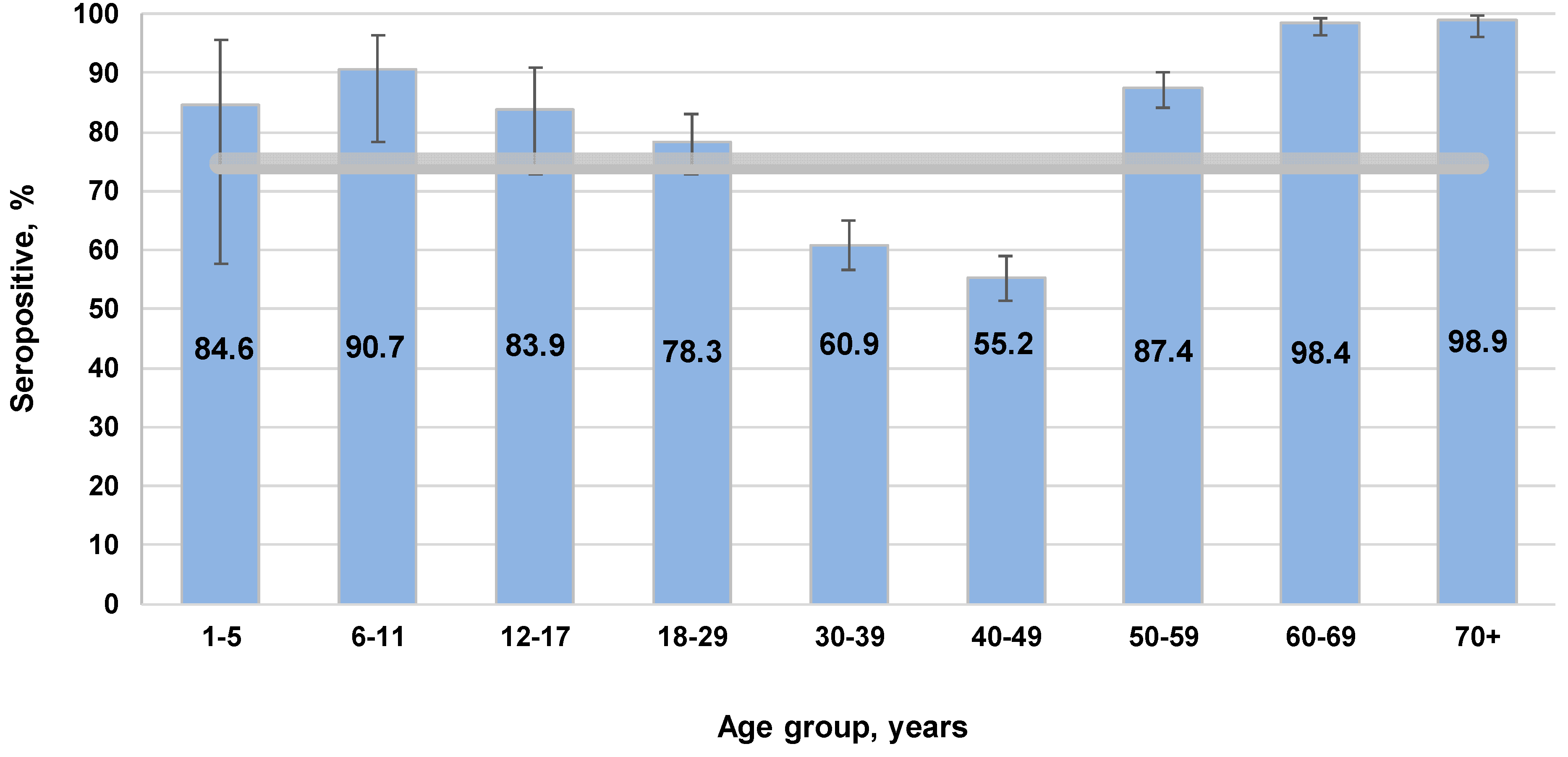
| Year | Vaccination Coverage (M-M-RVaxPro) | |
|---|---|---|
| Initial Immunization a, % | Revaccination b, % | |
| 2020 | 78.1 | 84.1 |
| 2021 | 74.8 | 85.8 |
| 2022 | 81.3 | 89.5 |
| 2023 | 84.5 | 91.0 |
| Age Group, Years | Individuals, N | Share, % | 95% CI | Males | Females | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| 1–5 | 13 | 0.5 | 0.3–0.9 | 6 | 46.2 | 7 | 53.8 |
| 6–11 | 43 | 1.7 | 1.2–2.3 | 25 | 58.1 | 18 | 41.9 |
| 12–17 | 62 | 2.4 | 1.9–3.1 | 35 | 56.4 | 27 | 43.6 |
| 18–29 | 249 | 9.8 | 8.7–11.0 | 61 | 24.5 | 188 | 75.5 |
| 30–39 | 501 | 19.8 | 18.3–21.4 | 161 | 32.1 | 340 | 67.9 |
| 40–49 | 688 | 27.2 | 25.5–28.9 | 200 | 29.1 | 488 | 70.9 |
| 50–59 | 468 | 18.5 | 17.0–20.0 | 151 | 32.3 | 317 | 67.7 |
| 60–69 | 320 | 12.6 | 9.4–14.0 | 104 | 32.5 | 216 | 67.5 |
| 70+ | 189 | 7.5 | 6.5–8.6 | 76 | 40.2 | 113 | 59.8 |
| Total | 2533 | 100.0 | - | 819 | 32.3 | 1714 | 67.7 |
| Activity | Individuals, N | Share, % |
|---|---|---|
| Healthcare Workers | 541 | 21.4 |
| Pensioners | 363 | 14.3 |
| State-military Service | 209 | 8.3 |
| Other | 200 | 7.9 |
| Business | 198 | 7.8 |
| Office Workers | 152 | 6.0 |
| Internet Technology | 139 | 5.5 |
| Science | 138 | 5.4 |
| Education | 138 | 5.4 |
| Unemployed | 112 | 4.4 |
| Preschoolers/Schoolchildren | 107 | 4.2 |
| Students | 85 | 3.4 |
| The Arts | 68 | 2.7 |
| Industrial Workers | 47 | 1.9 |
| Transportation workers | 36 | 1.4 |
| Year | Age Group, Years | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 | 5–9 | 10–14 | 15–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60+ | ||
| 2021 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 4 |
| 2022 | 2 | 1 | 3 | 1 | 0 | 0 | 0 | 1 | 3 | 11 |
| 2023 | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 6 |
| Infection | N | Vaccine, % (95% CI) | |||
|---|---|---|---|---|---|
| Priorix | M-M-R II | Other | Name Unknown | ||
| Measles | 112 | 3.6 (1.4–8.8) | 15.2 (9.1–23.0) | 8.0 (4.3–14.6) | 73.2 (64.3–80.5) |
| Rubella | 85 | 5.9 (2.5–13.0) | 12.9 (7.4–21.7) | 9.4 (4.8–17.5) | 71.8 (61.4–80.2) |
| Mumps | 91 | 5.5 (2.4–12.2) | 13.2 (7.7–21.6) | 9.9 (5.3–17.7) | 71.4 (61.4–79.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, A.Y.; Smirnov, V.S.; Egorova, S.A.; Dragačević, L.; Milichkina, A.M.; Protić, J.; Danilova, E.M.; Drozd, I.V.; Petrušić, M.; Zhimbaeva, O.B.; et al. Herd Immunity to the Measles, Mumps and Rubella Viruses Among the Belgradian Population in May, 2024. Vaccines 2025, 13, 652. https://doi.org/10.3390/vaccines13060652
Popova AY, Smirnov VS, Egorova SA, Dragačević L, Milichkina AM, Protić J, Danilova EM, Drozd IV, Petrušić M, Zhimbaeva OB, et al. Herd Immunity to the Measles, Mumps and Rubella Viruses Among the Belgradian Population in May, 2024. Vaccines. 2025; 13(6):652. https://doi.org/10.3390/vaccines13060652
Chicago/Turabian StylePopova, Anna Y., Vyacheslav S. Smirnov, Svetlana A. Egorova, Luka Dragačević, Angelica M. Milichkina, Jelena Protić, Ekaterina M. Danilova, Irina V. Drozd, Marija Petrušić, Ojuna B. Zhimbaeva, and et al. 2025. "Herd Immunity to the Measles, Mumps and Rubella Viruses Among the Belgradian Population in May, 2024" Vaccines 13, no. 6: 652. https://doi.org/10.3390/vaccines13060652
APA StylePopova, A. Y., Smirnov, V. S., Egorova, S. A., Dragačević, L., Milichkina, A. M., Protić, J., Danilova, E. M., Drozd, I. V., Petrušić, M., Zhimbaeva, O. B., Glazkova, E. S., Gutić, N., Ivanov, V. A., Ramsay, E. S., Kotsar, O. V., Smolensky, V. Y., & Totolian, A. A. (2025). Herd Immunity to the Measles, Mumps and Rubella Viruses Among the Belgradian Population in May, 2024. Vaccines, 13(6), 652. https://doi.org/10.3390/vaccines13060652






