Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Proteomic Analysis
2.2. Gene Expression Datasets for Breast Tumors and Normal Mammary Tissues
2.3. Triple-Negative Breast Cancer Cell Model
2.4. Immunohistochemistry (IHC)
2.5. Immunization with ENO1 Peptides Followed by Anti-PD1 Therapy
2.6. In Vivo Imaging System
2.7. Skin, Lymph Node, and Tumor Processing
2.8. Ex Vivo ELISpot Assay
2.9. Toxicology
2.10. Flow Cytometry
2.11. Statistics
3. Results
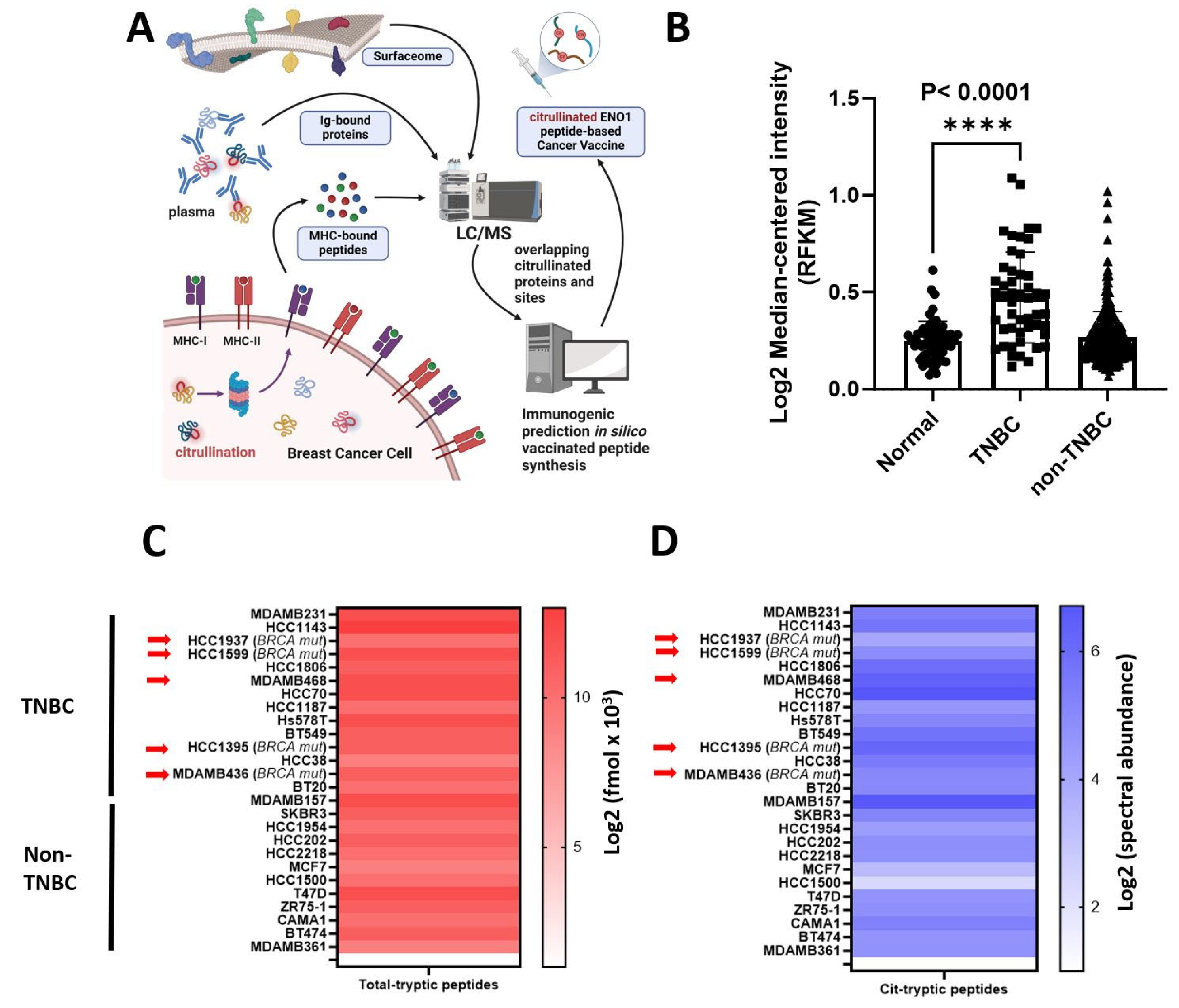
3.1. Citrullinated ENO1 Peptide-Based Vaccine
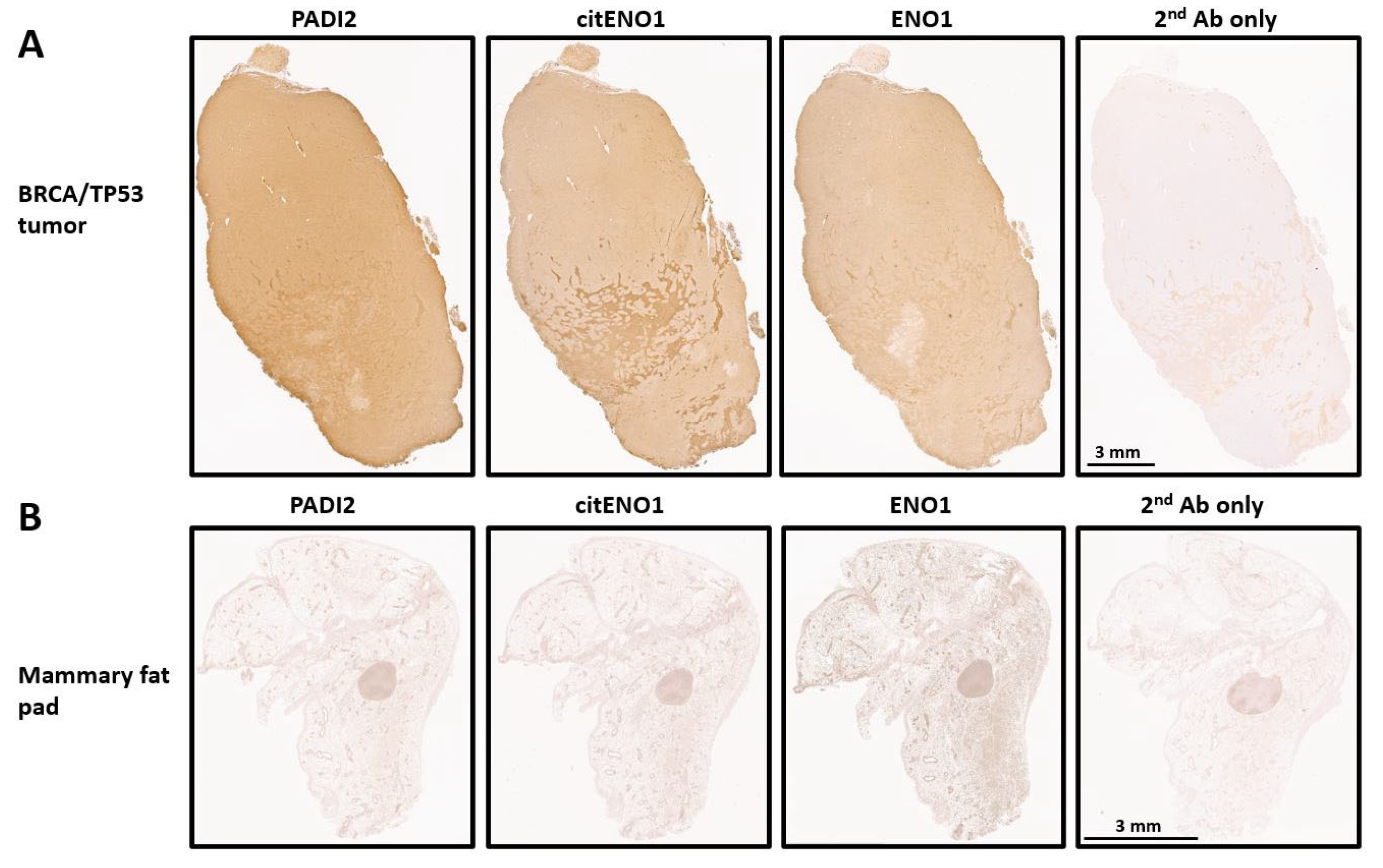
3.2. PADI2 Is Correlated with Citrullination of ENO1 in Murine Breast Tumors
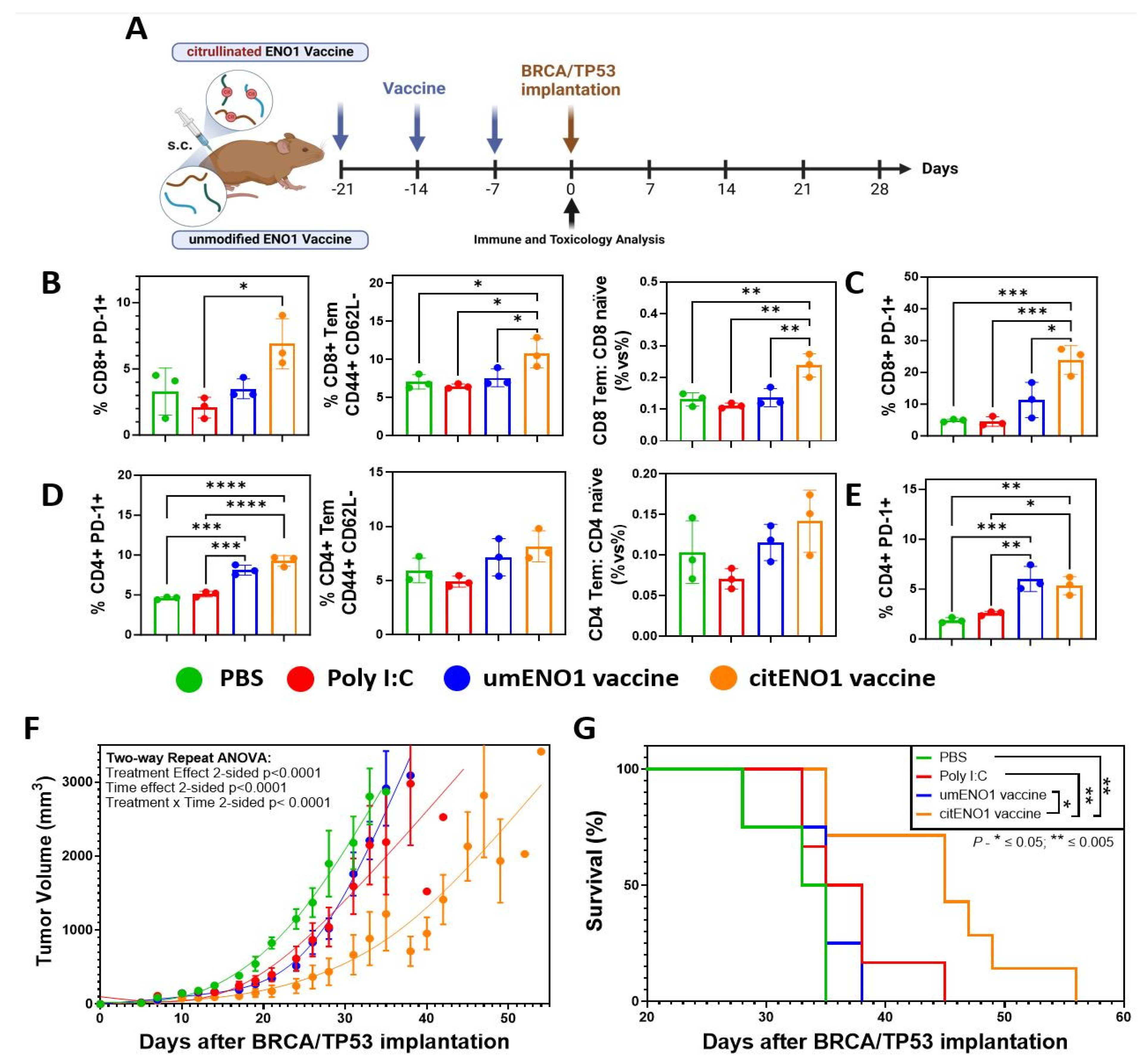
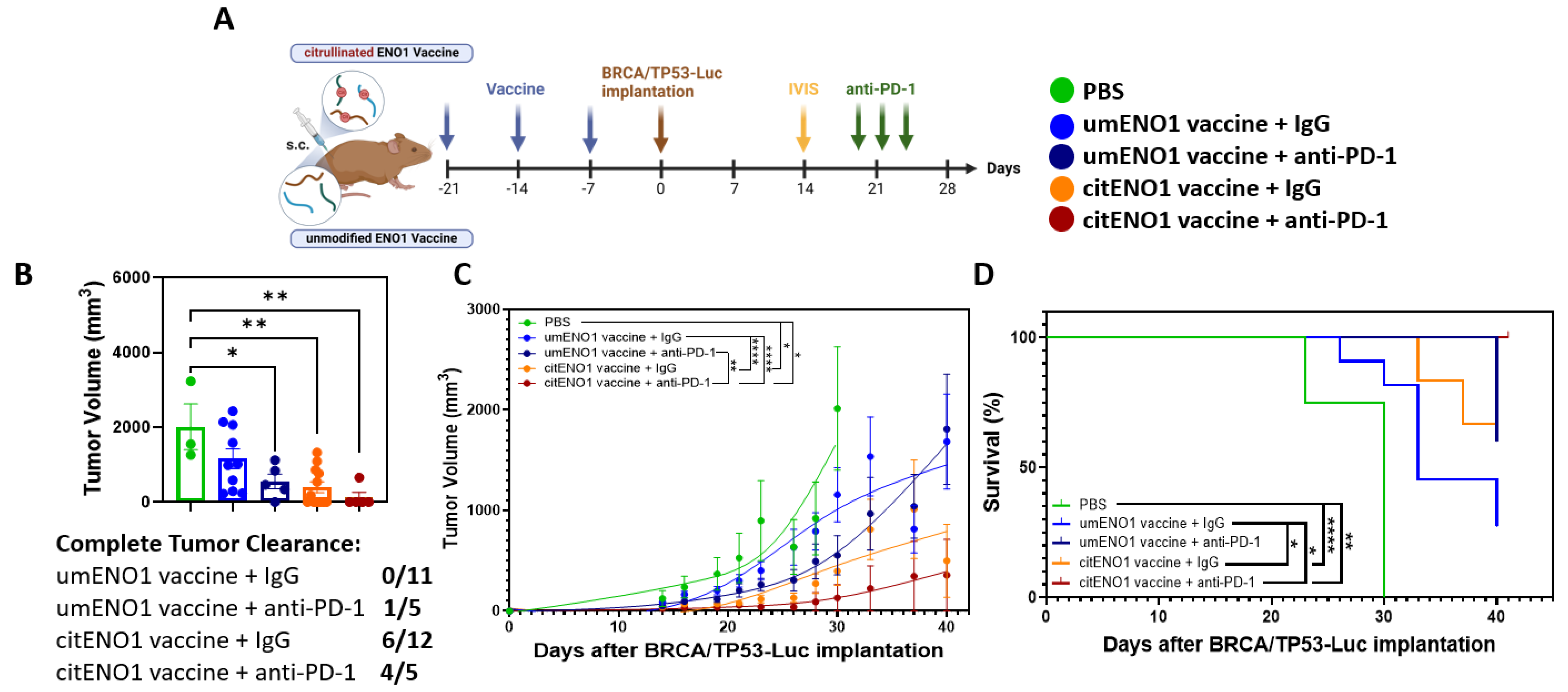
3.3. citENO1 Vaccine Impacts Tumor Growth and Survival
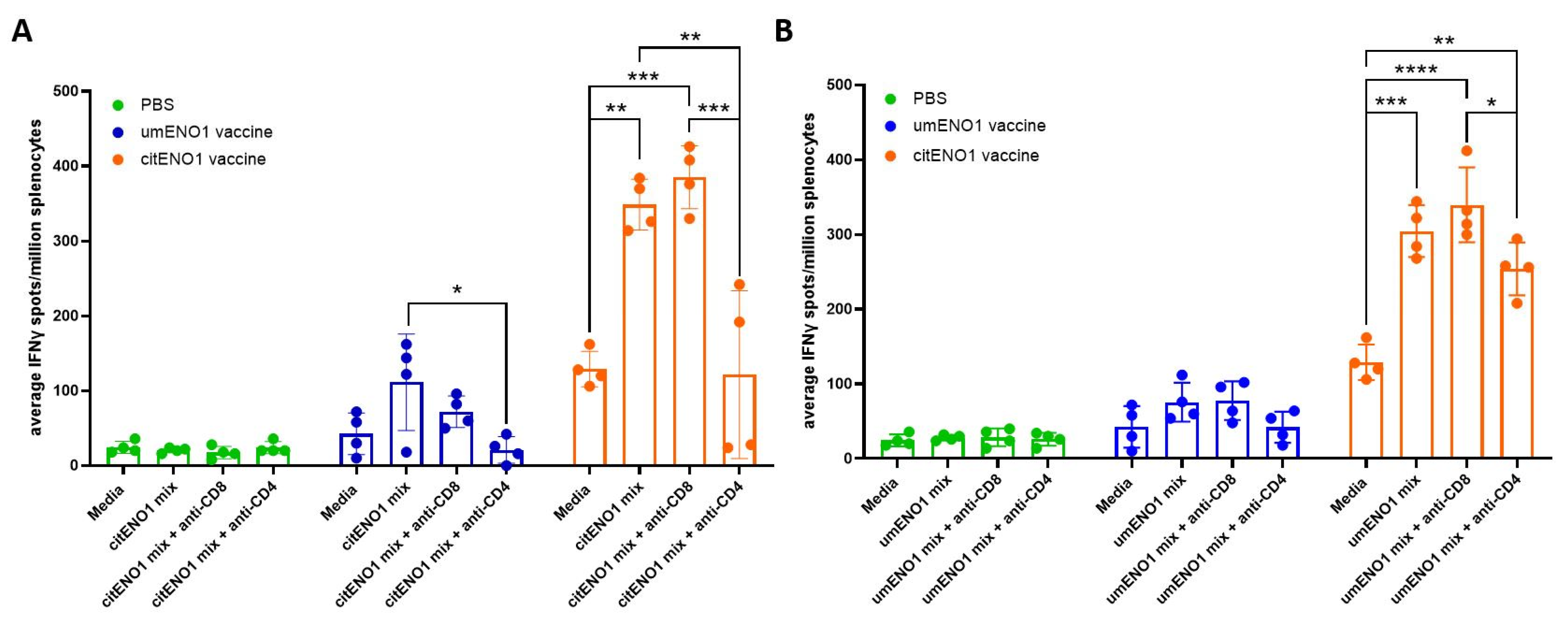
3.4. citENO1 Vaccination Induces citENO1-Specific IFNγ-Producing CD4+ T Cells
3.5. citENO1 Vaccine Enhances Immunotherapy with PD-1 Blockade
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Mani, S.A.; Moulder, S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist 2017, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.R.; Chen, M.; Yao, S.Y.; Chen, J.X.; Jin, K.T. Novel immunotherapies for breast cancer: Focus on 2023 findings. Int. Immunopharmacol. 2024, 128, 111549. [Google Scholar] [CrossRef]
- Costa, B.; Amorim, I.; Gartner, F.; Vale, N. Understanding Breast cancer: From conventional therapies to repurposed drugs. Eur. J. Pharm. Sci. 2020, 151, 105401. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Sellars, M.C.; Wu, C.J.; Fritsch, E.F. Cancer vaccines: Building a bridge over troubled waters. Cell 2022, 185, 2770–2788. [Google Scholar] [CrossRef]
- Cappello, P.; Rolla, S.; Chiarle, R.; Principe, M.; Cavallo, F.; Perconti, G.; Feo, S.; Giovarelli, M.; Novelli, F. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology 2013, 144, 1098–1106. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, O.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef]
- Cook, K.; Daniels, I.; Symonds, P.; Pitt, T.; Gijon, M.; Xue, W.; Metheringham, R.; Durrant, L.; Brentville, V. Citrullinated alpha-enolase is an effective target for anti-cancer immunity. Oncoimmunology 2018, 7, e1390642. [Google Scholar] [CrossRef] [PubMed]
- Brentville, V.A.; Metheringham, R.L.; Gunn, B.; Symonds, P.; Daniels, I.; Gijon, M.; Cook, K.; Xue, W.; Durrant, L.G. Citrullinated Vimentin Presented on MHC-II in Tumor Cells Is a Target for CD4+ T-Cell-Mediated Antitumor Immunity. Cancer Res. 2016, 76, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E. Citrullination in Cancer. Cancer Res. 2019, 79, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Witalison, E.E.; Thompson, P.R.; Hofseth, L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets 2015, 16, 700–710. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef]
- Ghislat, G.; Lawrence, T. Autophagy in dendritic cells. Cell Mol. Immunol. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Munz, C. Autophagy proteins in antigen processing for presentation on MHC molecules. Immunol. Rev. 2016, 272, 17–27. [Google Scholar] [CrossRef]
- Katayama, H.; Kobayashi, M.; Irajizad, E.; Sevillarno, A.; Patel, N.; Mao, X.; Rusling, L.; Vykoukal, J.; Cai, Y.; Hsiao, F.; et al. Protein citrullination as a source of cancer neoantigens. J. Immunother. Cancer 2021, 9, e002549. [Google Scholar] [CrossRef]
- Capello, M.; Ferri-Borgogno, S.; Riganti, C.; Chattaragada, M.S.; Principe, M.; Roux, C.; Zhou, W.; Petricoin, E.F.; Cappello, P.; Novelli, F. Targeting the Warburg effect in cancer cells through ENO1 knockdown rescues oxidative phosphorylation and induces growth arrest. Oncotarget 2016, 7, 5598–5612. [Google Scholar] [CrossRef]
- Principe, M.; Ceruti, P.; Shih, N.-Y.; Chattaragada, M.S.; Rolla, S.; Conti, L.; Bestagno, M.; Zentilin, L.; Yang, S.-H.; Migliorini, P.; et al. Targeting of surface alpha-enolase inhibits the invasiveness of pancreatic cancer cells. Oncotarget 2015, 6, 11098–11113. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, C.; Dubnovitsky, A.; Sandin, C.; Kozhukh, G.; Uchtenhagen, H.; James, E.A.; Rönnelid, J.; Ytterberg, A.J.; Pieper, J.; Reed, E.; et al. Functional and Structural Characterization of a Novel HLA-DRB1*04:01-Restricted α-Enolase T Cell Epitope in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 494. [Google Scholar] [CrossRef]
- Katayama, H.; Boldt, C.; Ladd, J.J.; Johnson, M.M.; Chao, T.; Capello, M.; Suo, J.; Mao, J.; Manson, J.E.; Prentice, R.; et al. An Autoimmune Response Signature Associated with the Development of Triple-Negative Breast Cancer Reflects Disease Pathogenesis. Cancer Res. 2015, 75, 3246–3254. [Google Scholar] [CrossRef]
- Katayama, H.; Tsou, P.; Kobayashi, M.; Capello, M.; Wang, H.; Esteva, F.; Disis, M.L.; Hanash, S. A plasma protein derived TGFbeta signature is a prognostic indicator in triple negative breast cancer. NPJ Precis. Oncol. 2019, 3, 10. [Google Scholar] [CrossRef]
- Zybailov, B.; Mosley, A.L.; Sardiu, M.E.; Coleman, M.K.; Florens, L.; Washburn, M.P. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 2006, 5, 2339–2347. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, R.; Song, S.; Hao, D.; Han, G.; Song, X.; Zhang, J.; Pizzi, M.P.; Shanbhag, N.; Futreal, A.; et al. Proteogenomic landscape of gastric adenocarcinoma peritoneal metastases. iScience 2023, 26, 106913. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sater, A.H.A.; Fahrmann, J.F.; Irajizad, E.; Cai, Y.; Katayama, H.; Vykoukal, J.; Kobayashi, M.; Dennison, J.B.; Garcia-Manero, G.; et al. Novel UHRF1-MYC Axis in Acute Lymphoblastic Leukemia. Cancers 2022, 14, 4262. [Google Scholar] [CrossRef] [PubMed]
- Capello, M.; Katayama, H.; Hanash, S.M. Proteomic Profiling of the Tumor Microenvironment. Methods Mol. Biol. 2022, 2435, 157–167. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Peters, H.L.; Taguchi, A.; Katayama, H.; Wang, H.; Momin, A.; Jolly, M.K.; Celiktas, M.; Rodriguez-Canales, J.; Liu, H.; et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. USA 2016, 113, E1555–E1564. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved Peptide-MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Deng, C.X.; Xu, X. Generation and analysis of Brca1 conditional knockout mice. Methods Mol. Biol. 2004, 280, 185–200. [Google Scholar] [CrossRef]
- Kobayashi, M.; Katayama, H.; Irajizad, E.; Vykoukal, J.V.; Fahrmann, J.F.; Kundnani, D.L.; Yu, C.Y.; Cai, Y.; Hsiao, F.C.; Yang, W.L.; et al. Proteome Profiling Uncovers an Autoimmune Response Signature That Reflects Ovarian Cancer Pathogenesis. Cancers 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, B.; Cappello, P.; Capello, M.; Fredolini, C.; Sperduti, I.; Migliorini, P.; Salacone, P.; Novarino, A.; Giacobino, A.; Ciuffreda, L.; et al. Circulating autoantibodies to phosphorylated alpha-enolase are a hallmark of pancreatic cancer. J. Proteome Res. 2011, 10, 105–112. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Designing vaccines to prevent breast cancer recurrence or invasive disease. Immunotherapy 2015, 7, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Brentville, V.A.; Metheringham, R.L.; Daniels, I.; Atabani, S.; Symonds, P.; Cook, K.W.; Vankemmelbeke, M.; Choudhury, R.; Vaghela, P.; Gijon, M.; et al. Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses. J. Immunother. Cancer 2020, 8, e000560. [Google Scholar] [CrossRef]
- Shah, S.; Cook, K.W.; Symonds, P.; Weisser, J.; Skinner, A.; Al Omari, A.; Paston, S.J.; Pike, I.; Durrant, L.G.; Brentville, V.A. Vaccination with post-translational modified, homocitrullinated peptides induces CD8 T-cell responses that mediate antitumor immunity. J. Immunother. Cancer 2023, 11, e006966. [Google Scholar] [CrossRef]
- Desch, A.N.; Gibbings, S.L.; Clambey, E.T.; Janssen, W.J.; Slansky, J.E.; Kedl, R.M.; Henson, P.M.; Jakubzick, C. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat. Commun. 2014, 5, 4674. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, L.; Kroemer, G.; Kepp, O. Conventional type 1 dendritic cells (cDC1) in cancer immunity. Biol. Direct 2023, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Mattiuz, R.; Brousse, C.; Ambrosini, M.; Cancel, J.C.; Bessou, G.; Mussard, J.; Sanlaville, A.; Caux, C.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; et al. Type 1 conventional dendritic cells and interferons are required for spontaneous CD4(+) and CD8(+) T-cell protective responses to breast cancer. Clin. Transl. Immunol. 2021, 10, e1305. [Google Scholar] [CrossRef]
- Dillon, P.M.; Petroni, G.R.; Smolkin, M.E.; Brenin, D.R.; Chianese-Bullock, K.A.; Smith, K.T.; Olson, W.C.; Fanous, I.S.; Nail, C.J.; Brenin, C.M.; et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 2017, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Tsang, K.Y.; Arlen, P.M. Generation of the therapeutic monoclonal antibody NEO-201, derived from a cancer vaccine, which targets human malignancies and immune suppressor cells. Expert. Rev. Vaccines 2024, 23, 812–829. [Google Scholar] [CrossRef]
- Dubsky, P.; Jackisch, C.; Im, S.A.; Hunt, K.K.; Li, C.F.; Unger, S.; Paluch-Shimon, S. BRCA genetic testing and counseling in breast cancer: How do we meet our patients’ needs? NPJ Breast Cancer 2024, 10, 77. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Brunet, J.S.; Stefansson, I.M.; Straume, O.; Chappuis, P.O.; Begin, L.R.; Hamel, N.; Goffin, J.R.; Wong, N.; Trudel, M.; et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004, 64, 830–835. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O.; Begin, L.R.; Goffin, J.R.; Wong, N.; Trudel, M.; Akslen, L.A. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl. Cancer Inst. 2003, 95, 1482–1485. [Google Scholar] [CrossRef]
- Nikolaienko, O.; Eikesdal, H.P.; Ognedal, E.; Gilje, B.; Lundgren, S.; Blix, E.S.; Espelid, H.; Geisler, J.; Geisler, S.; Janssen, E.A.M.; et al. Prenatal BRCA1 epimutations contribute significantly to triple-negative breast cancer development. Genome Med. 2023, 15, 104. [Google Scholar] [CrossRef]
- Wang, Y.; Dackus, G.; Rosenberg, E.H.; Cornelissen, S.; de Boo, L.W.; Broeks, A.; Brugman, W.; Chan, T.W.S.; van Diest, P.J.; Hauptmann, M.; et al. Long-term outcomes of young, node-negative, chemotherapy-naive, triple-negative breast cancer patients according to BRCA1 status. BMC Med. 2024, 22, 9. [Google Scholar] [CrossRef]
- Elmakaty, I.; Abdo, R.; Elsabagh, A.; Elsayed, A.; Malki, M.I. Comparative efficacy and safety of PD-1/PD-L1 inhibitors in triple negative breast cancer: A systematic review and network meta-analysis of randomized controlled trials. Cancer Cell Int. 2023, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Leon-Letelier, R.A.; Bonifaz, L.C.; Fuentes-Panana, E.M. OMIC signatures to understand cancer immunosurveillance and immunoediting: Melanoma and immune cells interplay in immunotherapy. J. Leukoc. Biol. 2019, 105, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Abdou, Y.; Goudarzi, A.; Yu, J.X.; Upadhaya, S.; Vincent, B.; Carey, L.A. Immunotherapy in triple negative breast cancer: Beyond checkpoint inhibitors. NPJ Breast Cancer 2022, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Pinto, L.; Almeida-Santos, T. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: A systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2022, 20, 34. [Google Scholar] [CrossRef]
| Discovered Cit-Peptides and Conversion to Unmodified Sequence | Sequence Position | Cit-R Position | Discovery Source | Predicted Immunocore by NetMHCIIpan v3.2 | Predicted Immunocore by NetMHCpan v4.1 | Vaccine Peptides | Designed Vaccine Peptides | Designed Vaccine Peptides | Human to Mouse Homology | Designed Vaccine Peptides |
|---|---|---|---|---|---|---|---|---|---|---|
| ENO1-Human (P06733) | Human ENO1, unmodified form | Mouse Eno1, unmodified form | Mouse Eno1 cit-form | |||||||
| Step (i) | Step (ii) | Step (ii) | Step (iii) | Step (iv) | Step (v) | |||||
| EIFDS(cit-R)GNPTVEVDLFTSK | 10–28 | 15 | Cell surface trypsin | DSRGNPTVE, RGNPTVEVD | SRGNPTVEV | Unused | Unused | Unused | Unused | |
| FTSKGLF(cit-R)AAVPSGASTGIYE | 25–45 | 32 | Mild acid elution peptidome-no trypsin | RAAVPSGAS, AAVPSGAST, AVPSGASTG, VPSGASTGI, PSGASTGIY, GASTGIYEA, SGASTGIYE, STGIYEALE, TGIYEALEL, ASTGIYEAL, GIYEALELR | PSGASTGIY, VPSGASTGI, RAAVPSGAS | Vaccine peptide 1, 32–56 | RAAVPSGASTGIYEALELRDNDKTR | RAAVPSGASTGIYEALELRDNDKTR | 100% | (cit-R)AAVPSGASTGIYEALEL(cit-R)DNDKT(cit-R) |
| SKGLF(cit-R)AAVPSGASTGIYEAL | 27–47 | 32 | Mild acid elution peptidome-no trypsin | RAAVPSGAS, AAVPSGAST, AVPSGASTG, VPSGASTGI, PSGASTGIY, GASTGIYEA, SGASTGIYE, STGIYEALE, TGIYEALEL, ASTGIYEAL, GIYEALELR, RAAVPSGAS, AAVPSGAST, AVPSGASTG, VPSGASTGI, PSGASTGIY | PSGASTGIY, VPSGASTGI, RAAVPSGAS | Vaccine peptide 1, 32–56 | RAAVPSGASTGIYEALELRDNDKTR | RAAVPSGASTGIYEALELRDNDKTR | 100% | (cit-R)AAVPSGASTGIYEALEL(cit-R)DNDKT(cit-R) |
| AAVPSGASTGIYEALEL(cit-R)DNDK | 33–54 | 50 | Cell surface trypsin | GASTGIYEA, SGASTGIYE, STGIYEALE, TGIYEALEL, ASTGIYEAL, GIYEALELR | GIYEALELR, PSGASTGIY, VPSGASTGI | Vaccine peptide 1, 32–56 | RAAVPSGASTGIYEALELRDNDKTR | RAAVPSGASTGIYEALELRDNDKTR | 100% | (cit-R)AAVPSGASTGIYEALEL(cit-R)DNDKT(cit-R) |
| AVEKGVPLY(cit-R)HIADLAGNSE | 123–142 | 132 | Mild acid elution peptidome-no trypsin | LYRHIADLA | AVEKGVPLY | Unused | Unused | Unused | Unused | |
| HIADLAGNSEVILPVPAFNVINGGSHAGNKLAMQEFMILPVGAANF(cit-R)EAMR | 133–183 | 179 | Plasma Ig-bound trypsin | AMQEFMILP, EFMILPVGA, FMILPVGAA, MILPVGAAN, ILPVGAANF, MQEFMILPV, QEFMILPVG, PVGAANFRE, VGAANFREA | FMILPVGAA, GAANFREAM, ILPVGAANF | Vaccine peptide 2, 164–186 | AMQEFMILPVGAANFREAMRIGA | AMQEFMILPVGASSFREAMRIGA | 176 A, 177 N (Human) to 176 S, 177 S (Mouse) | AMQEFMILPVGASSF(cit-R)EAM(cit-R)IGA |
| SGKYDLDFKSPDDPS(cit-R)YISPDQLADLYK | 254–281 | 269 | Cell surface trypsin | FKSPDDPSR, SRYISPDQL, RYISPDQLA, ISPDQLADL, LADLYKFKS | KSPDDPSRY, RYISPDQLA, SPDDPSRYI, SPDQLADLY, SRYISPDQL | Vaccine peptide 3, 262–284 | KSPDDPSRYISPDQLADLYKSFV | KSPDDPSRYITPDQLADLYKSFV | 272 S (Human) to 272 T (Mouse) | KSPDDPS(cit-R)YITPDQLADLYKSFV |
| SPDDPS(cit-R)YISPDQLADLYK | 263–281 | 269 | Cell surface trypsin | FKSPDDPSR, SRYISPDQL, RYISPDQLA, ISPDQLADL, LADLYKFKS | RYISPDQLA, SPDDPSRYI, SPDQLADLY, SRYISPDQL | Vaccine peptide 3, 262–284 | KSPDDPSRYISPDQLADLYKSFV | KSPDDPSRYITPDQLADLYKSFV | 272 S (Human) to 272 T (Mouse) | KSPDDPS(cit-R)YITPDQLADLYKSFV |
| FKSPDDPS(cit-R)YISPDQLADL | 261–279 | 269 | Mild acid elution peptidome-no trypsin | FKSPDDPSR, SRYISPDQL, RYISPDQLA, ISPDQLADL, LADLYKFKS | KSPDDPSRY, RYISPDQLA, SPDDPSRYI, SRYISPDQL | Vaccine peptide 3, 262–284 | KSPDDPSRYISPDQLADLYKSFV | KSPDDPSRYITPDQLADLYKSFV | 272 S (Human) to 272 T (Mouse) | KSPDDPS(cit-R)YITPDQLADLYKSFV |
| KSPDDPS(cit-R)YISPDQLADL | 262–279 | 269 | Mild acid elution peptidome-no trypsin | FKSPDDPSR, SRYISPDQL, RYISPDQLA, ISPDQLADL, LADLYKFKS | RYISPDQLA, SPDDPSRYI, SRYISPDQL | Vaccine peptide 3, 262–284 | KSPDDPSRYISPDQLADLYKSFV | KSPDDPSRYITPDQLADLYKSFV | 272 S (Human) to 272 T (Mouse) | KSPDDPS(cit-R)YITPDQLADLYKSFV |
| LAQANGWGVMVSH(cit-R)SGETEDTF | 359–380 | 372 | Mild acid elution peptidome-no trypsin | ANGWGVMVS, NGWGVMVSH, WGVMVSHRS, GVMVSHRSG | AQANGWGVM | Unused | Unused | Unused | Unused | |
| SGETEDTFIADLVVGLCTGQIKTGAPCRSE(cit-R) | 373–403 | 403 | Cell surface trypsin | TEDTFIADL, TFIADLVVG, FIADLVVGL, IADLVVGLC, VVGLCTGQI, CTGQIKTGA | FIADLVVGL, TEDTFIADL, GETEDTFIA | Unused | Unused | Unused | Unused | |
| YNQLL(cit-R)IEEELGSK | 407–420 | 412 | Cell surface trypsin | No hit | QLLRIEEEL | Unused | Unused | Unused | Unused | |
| IEEELGSKAKFAGRNF(cit-R)NPLAK (C-terminal) | 413–434 | 429 | Mild acid elution peptidome-no trypsin | AKFAGRNFR, GRNFRNPLA, GSKAKFAGR, AGRNFRNPL | AGRNFRNPL, EELGSKAKF, GRNFRNPLA, IEEELGSKA, KAKFAGRNF, RNFRNPLAK | Unused | Unused | Unused | Unused |
| Position | Cit-Sites | Length | Synthesized Vaccination Peptide Sequence | |
|---|---|---|---|---|
| Peptide 1_wt | 32–56 | 32, 50, 56 | 24 | RAAVPSGASTGIYEALELRDNDKTR |
| Peptide 1_cit | 32–56 | 32, 50, 56 | 24 | (cit-R)AAVPSGASTGIYEALEL(cit-R)DNDKT(cit-R) |
| Peptide 2_wt | 164–186 | 179, 183 | 22 | AMQEFMILPVGASSFREAMRIGA |
| Peptide 2_cit | 32–56 | 179, 183 | 22 | AMQEFMILPVGASSF(cit-R)EAM(cit-R)IGA |
| Peptide 3_wt | 262–284 | 269 | 22 | KSPDDPSRYITPDQLADLYKSFV |
| Peptide 3_cit | 32–56 | 269 | 22 | KSPDDPS(cit-R)YITPDQLADLYKSFV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Letelier, R.A.; Sevillano-Mantas, A.M.; Chen, Y.; Park, S.; Vykoukal, J.; Fahrmann, J.F.; Ostrin, E.J.; Garrett, C.; Dou, R.; Cai, Y.; et al. Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells. Vaccines 2025, 13, 629. https://doi.org/10.3390/vaccines13060629
León-Letelier RA, Sevillano-Mantas AM, Chen Y, Park S, Vykoukal J, Fahrmann JF, Ostrin EJ, Garrett C, Dou R, Cai Y, et al. Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells. Vaccines. 2025; 13(6):629. https://doi.org/10.3390/vaccines13060629
Chicago/Turabian StyleLeón-Letelier, Ricardo A., Alejandro M. Sevillano-Mantas, Yihui Chen, Soyoung Park, Jody Vykoukal, Johannes F. Fahrmann, Edwin J. Ostrin, Candace Garrett, Rongzhang Dou, Yining Cai, and et al. 2025. "Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells" Vaccines 13, no. 6: 629. https://doi.org/10.3390/vaccines13060629
APA StyleLeón-Letelier, R. A., Sevillano-Mantas, A. M., Chen, Y., Park, S., Vykoukal, J., Fahrmann, J. F., Ostrin, E. J., Garrett, C., Dou, R., Cai, Y., Hsiao, F.-C., Dennison, J. B., Vilar, E., Arun, B. K., Hanash, S., & Katayama, H. (2025). Citrullinated ENO1 Vaccine Enhances PD-1 Blockade in Mice Implanted with Murine Triple-Negative Breast Cancer Cells. Vaccines, 13(6), 629. https://doi.org/10.3390/vaccines13060629









