Targeting Azole-Resistant Candida albicans: Tetrapeptide Tuftsin-Modified Liposomal Vaccine Induces Superior Immune Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida albicans
2.2. In Vitro Analysis of Fluconazole’s Antifungal Potency
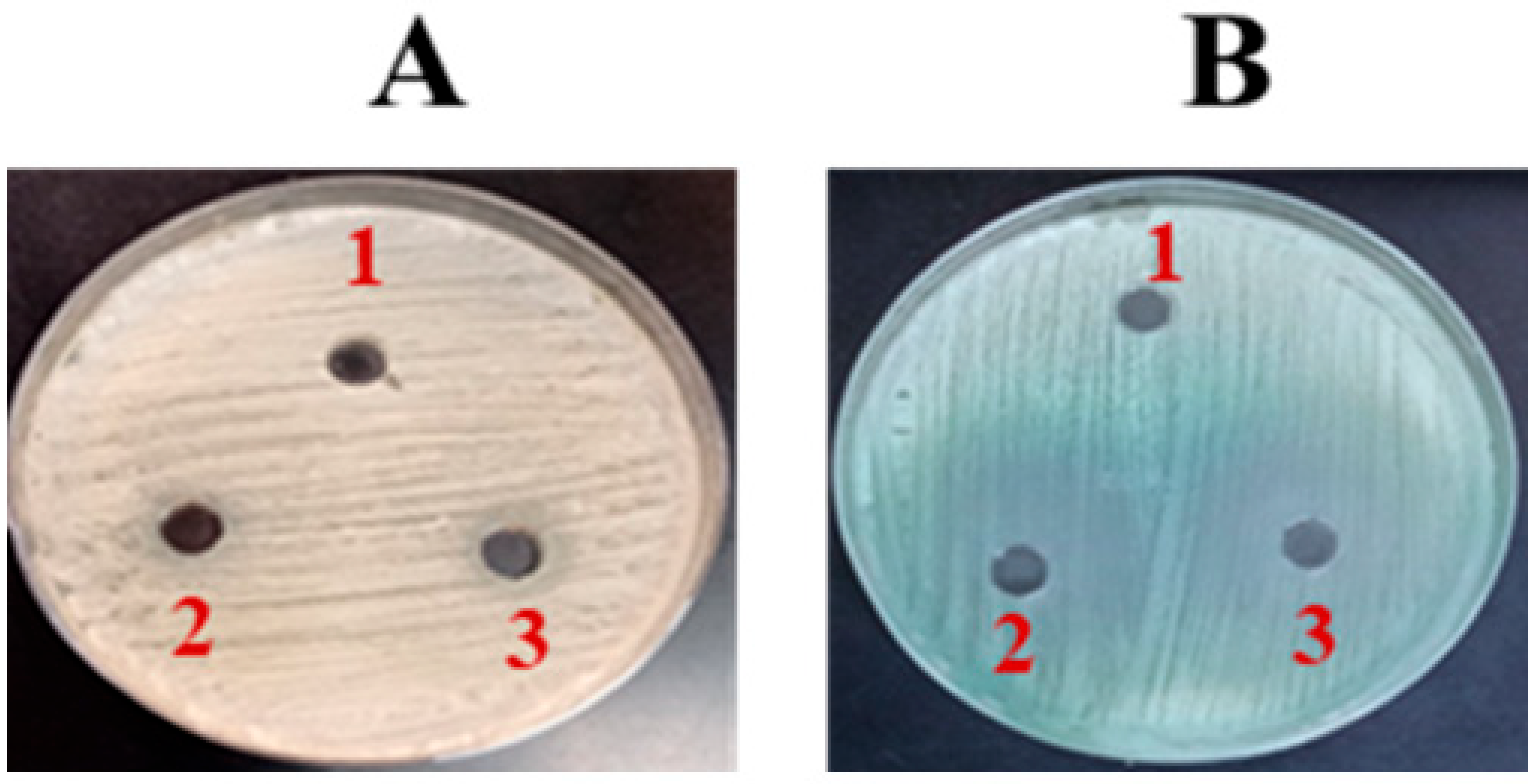
2.3. Determining C. albicans Susceptibility Using the VITEK System
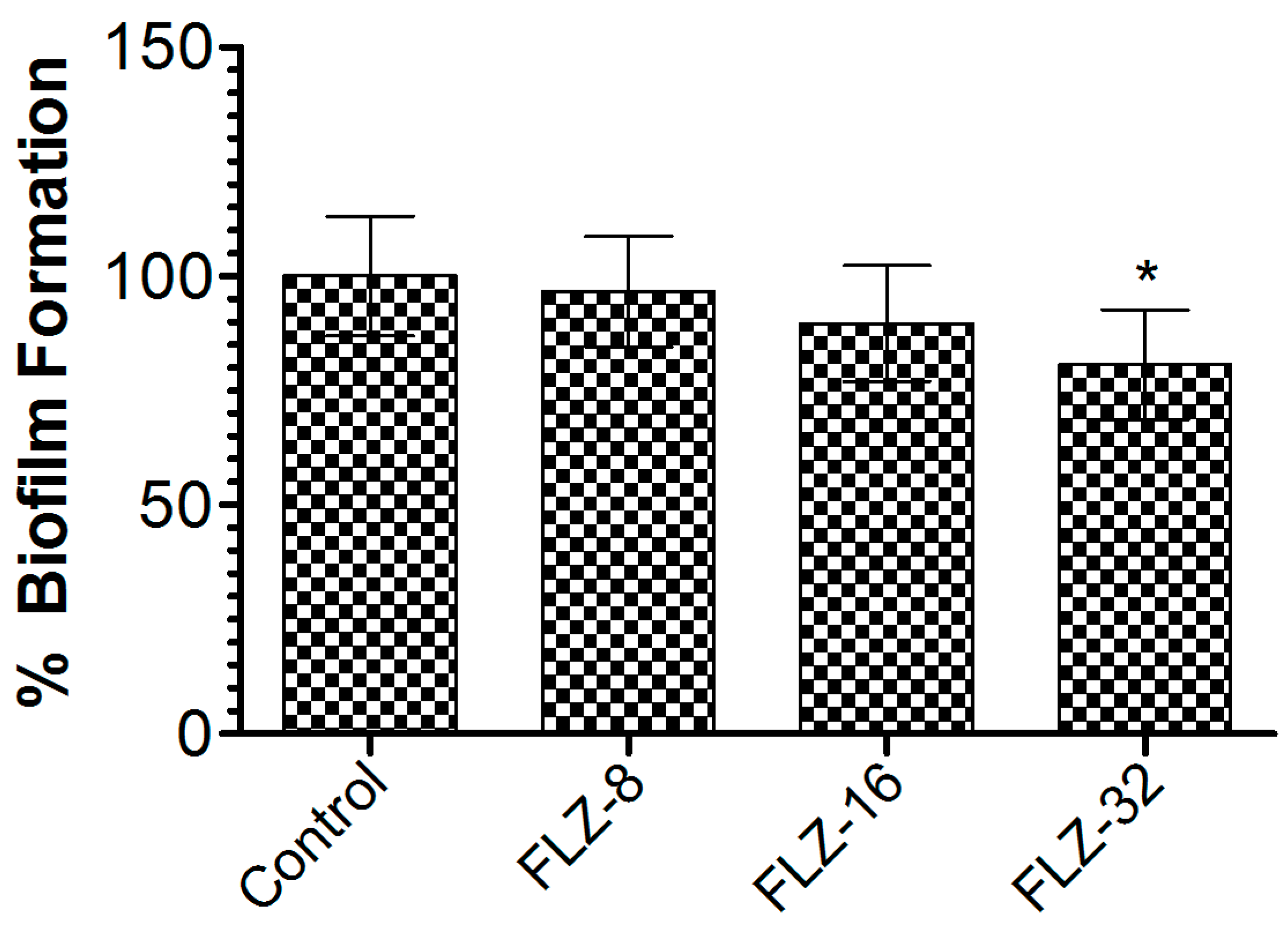
2.4. Determination of FLZ Efficacy Against Biofilm Formation
2.5. Mice
2.6. FLZ Intervention in Mice Infected with C. albicans
2.7. Whole Cell Antigens (WCAgs) of C. albicans
2.8. Preparation and Characterization of Dried-Reconstituted Vesicles (DRVs)
2.9. Estimation of WCAgs Encapsulated in Tuft-Free and Tuft-Bearing Liposomes
2.10. In Vitro Release of WCAgs from Tuftsin-Free and Tuftsin-Bearing Liposomes
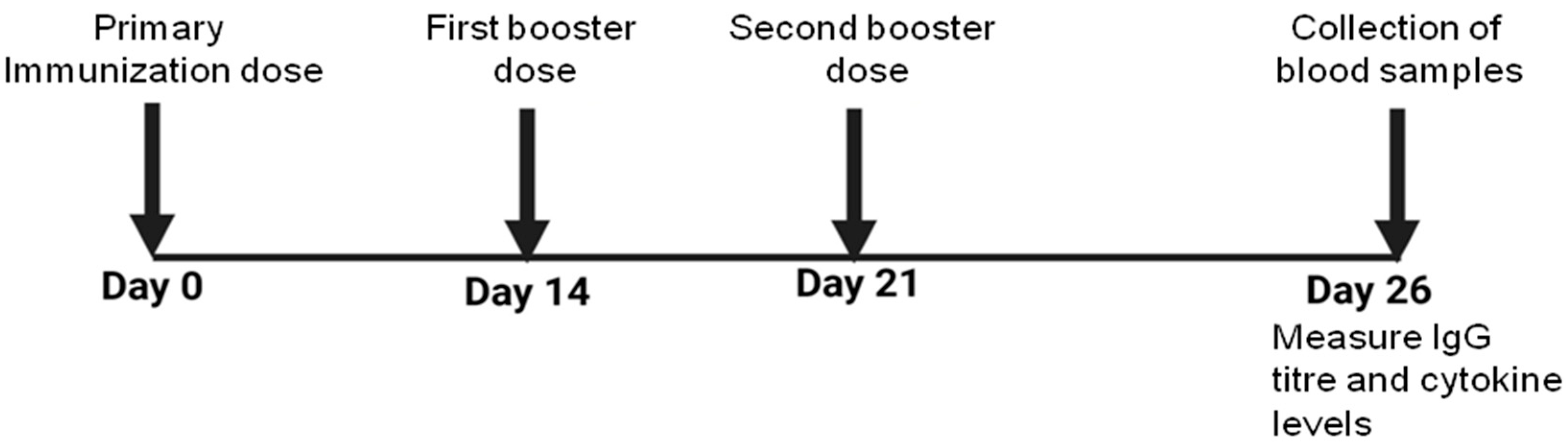
2.11. Immunization
2.12. Status of WCAg-Specific Antibody Level and IgG Isotypes
2.13. BrdU-Based T Cell Proliferation Assay
2.14. Analysis of IFN-γ and IL-4 Cytokine Profiles
2.15. Infection of Vaccinated Mice with C. albicans
2.16. To Assess the Prophylactic Effectiveness of Tutf-Lip-WCAg or Lip-WCAg Vaccine Formulations Against the Systemic Candidiasis
2.17. Statistical Analyses
3. Results
3.1. FLZ Exhibited No Effective Activity Against C. albicans
3.2. FLZ Did Not Eradicate the Preformed Biofilm of C. albicans
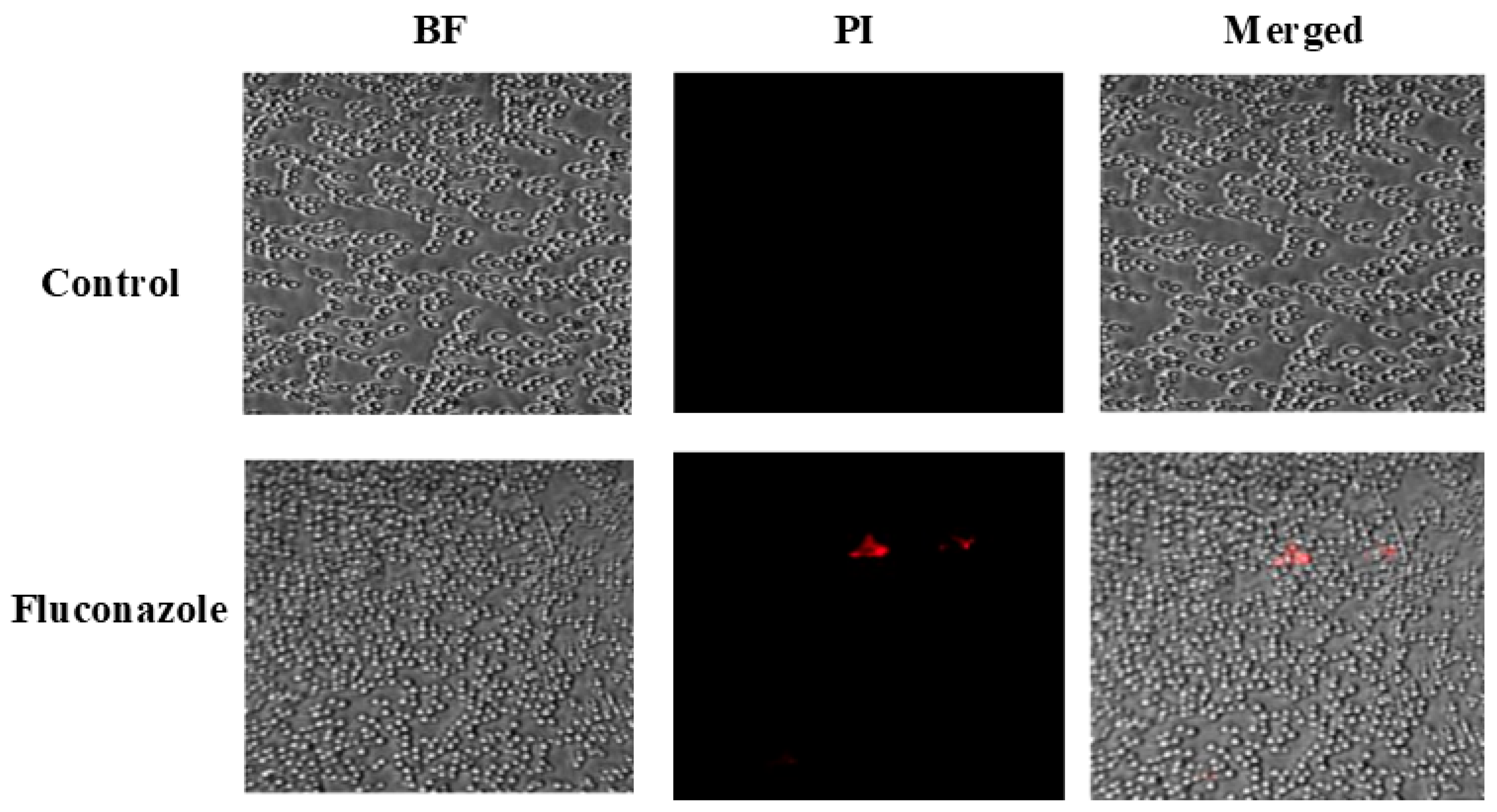
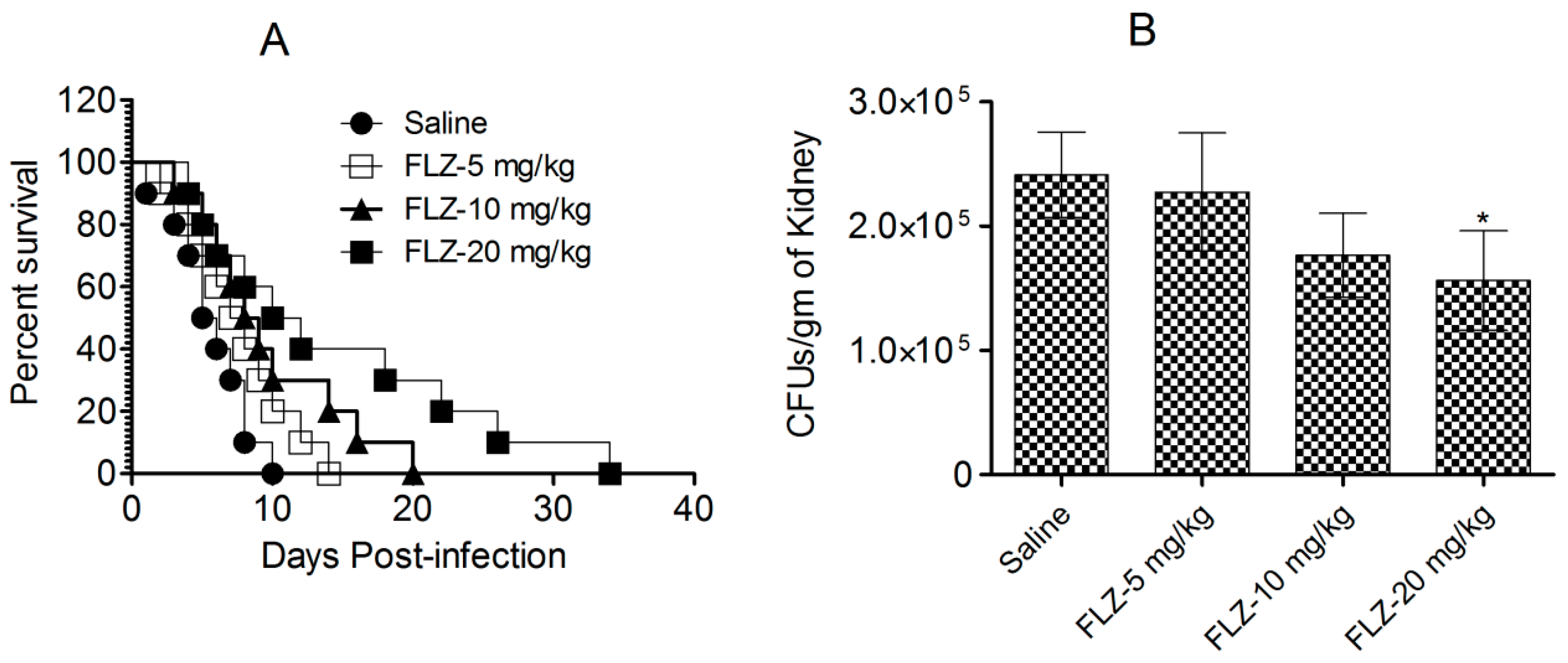
3.3. FLZ Does Not Effectively Treat C. albicans Infection in Mice
3.4. Characterization and Entrapment Efficiency of WCAgs in Tuft-Bearing and Tuftsin-Free Liposomes
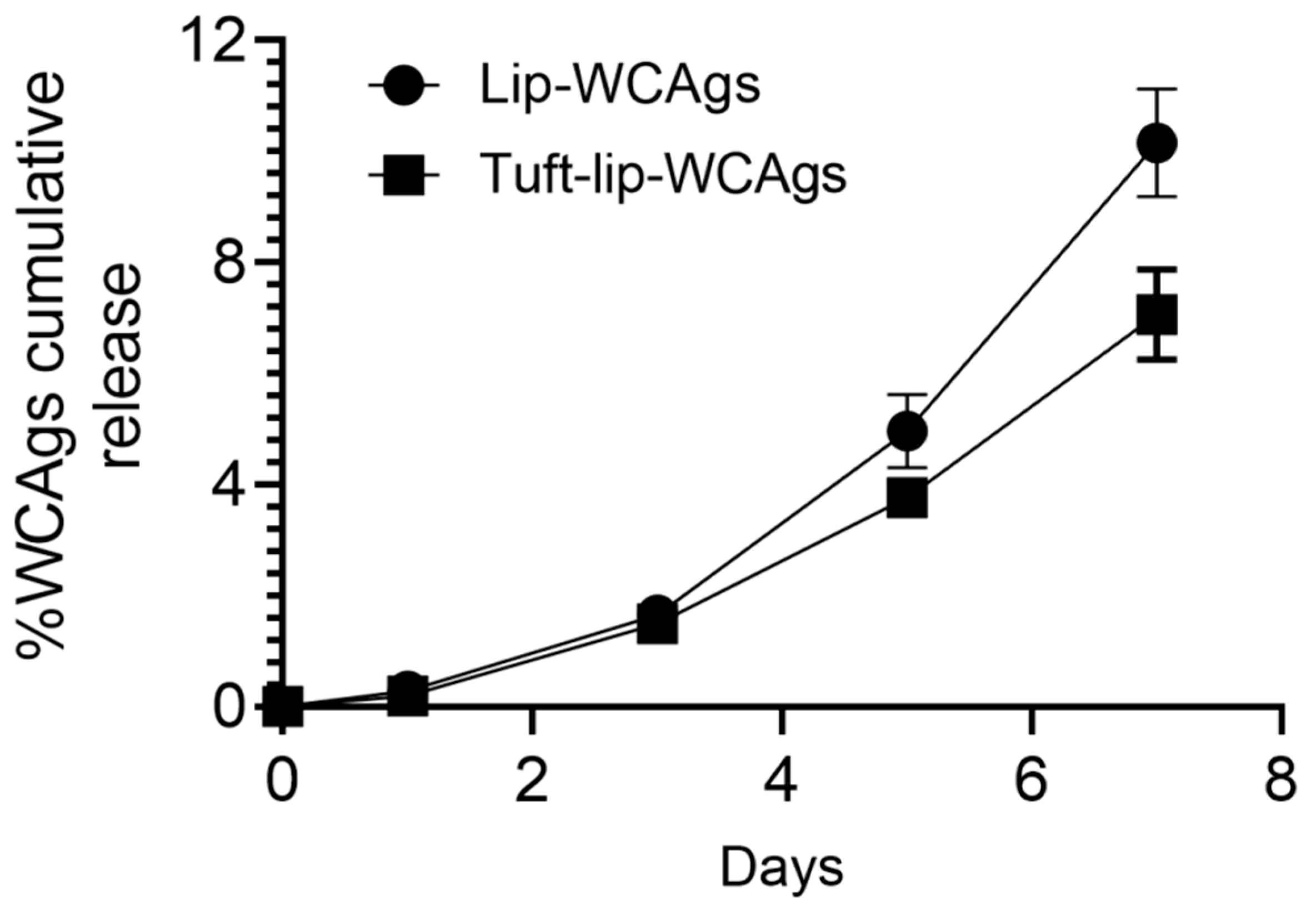
3.5. Tuftsin-Bearing Liposomes Exhibited Reduced Release of WCAgs at 4 °C
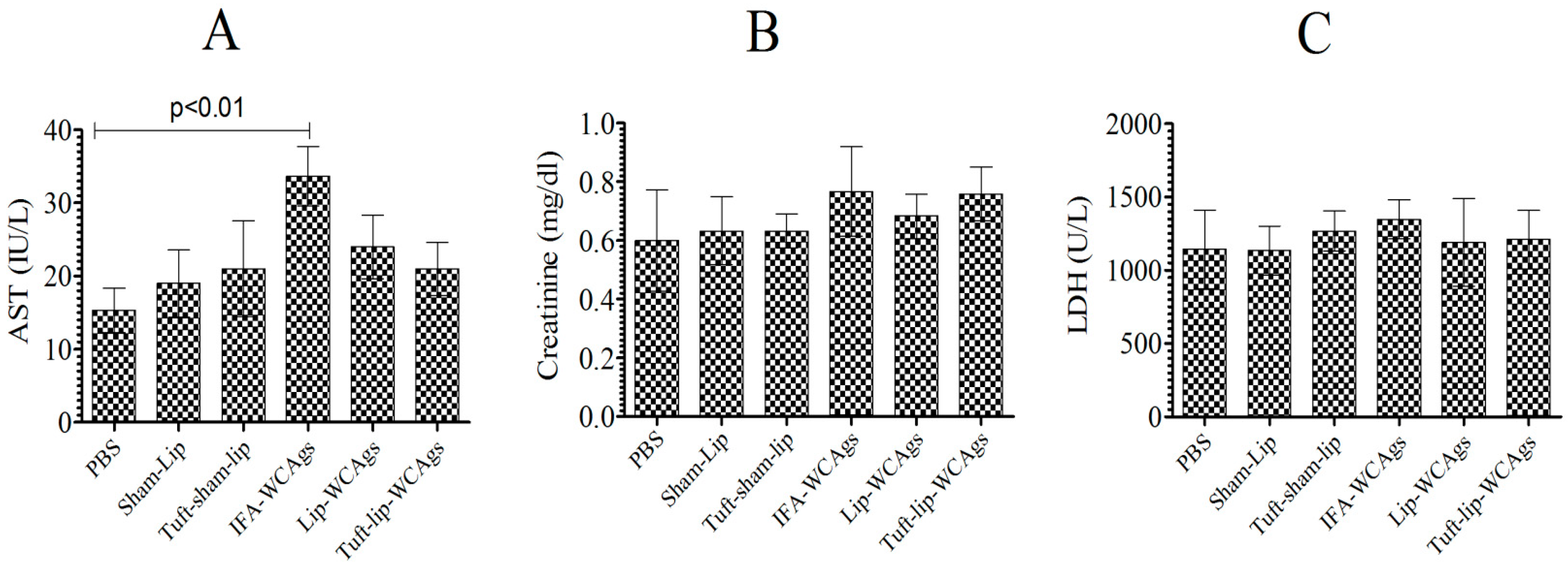
3.6. Tuft-Lip-WCAg or Lip-WCAg Vaccine Formulations Exhibited No Detectable Toxicity
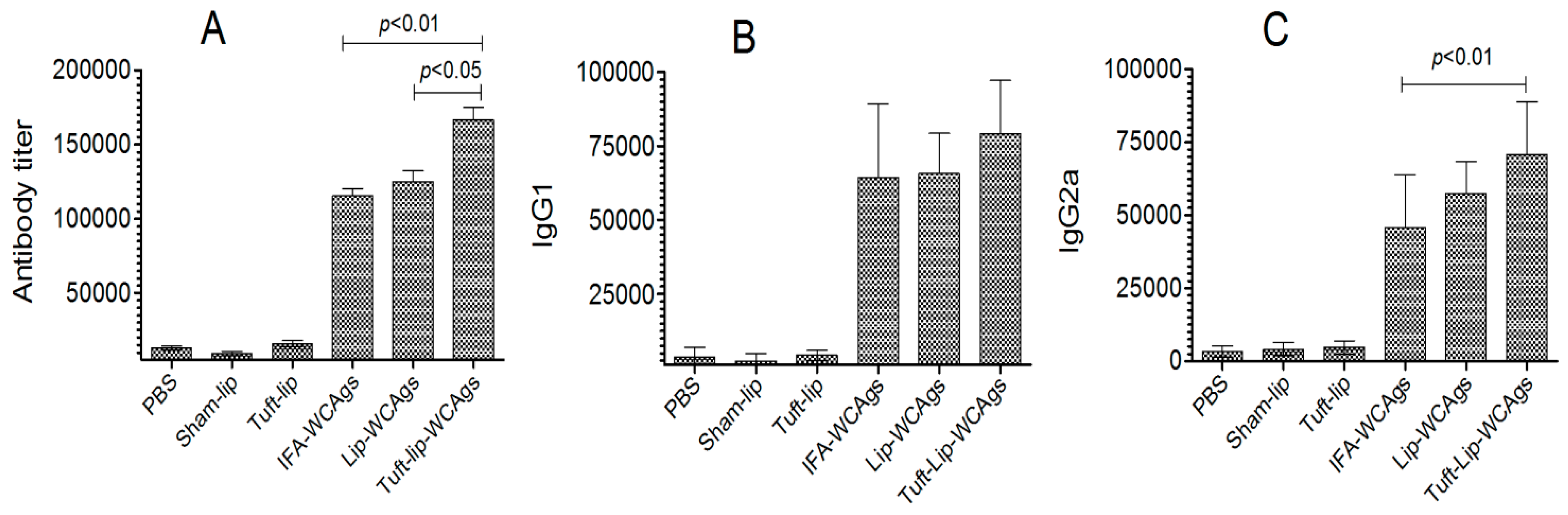
3.7. Superior Antigen-Specific Immune Responses Were Induced by Tuft-Lip-WCAgs
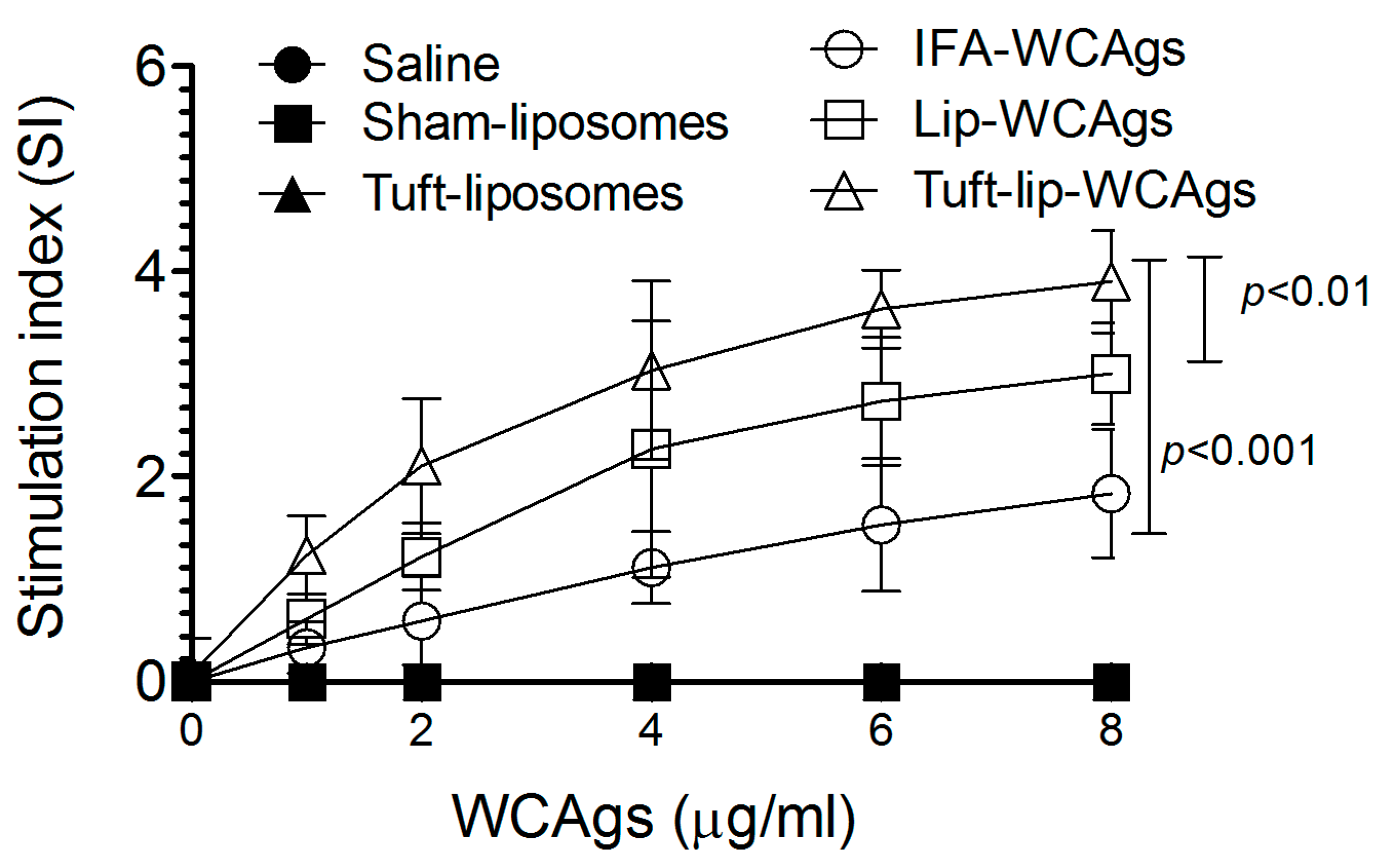
3.8. Tuft-Lip-WCAgs Induced Higher Proliferation of T Lymphocytes
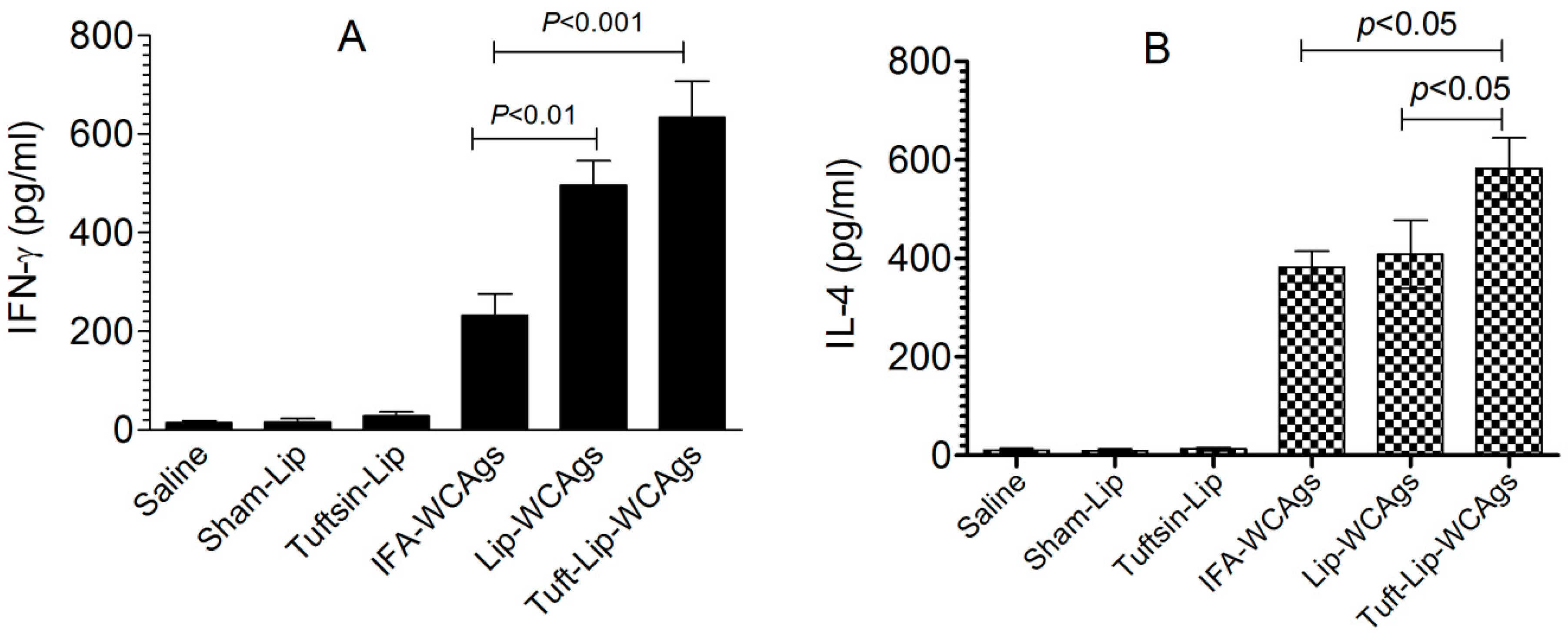
3.9. Immunization with Tuft-Lip-WCAgs Elicited Higher IFN-γ and IL-4 Secretion
3.10. Immunization with Tuft-Lip-WCAg Imparted Highest Resistance to Mice Against C. albicans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | Minimum Inhibitory Concentration |
| WCAgs | Whole Cell Antigens |
| Tuft-lip-WCAgs | Tuftsin-Bearing Liposomes Loaded with Whole Cell Antigens |
| FLZ | Fluconazole |
| PBS | Phosphate-Buffered Saline |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IFN-γ | Interferon-gamma |
| IL-4 | Interleukin-4 |
| AST | Aspartate Transaminase |
| LDH | Lactate Dehydrogenase |
| DLS | Dynamic Light Scattering |
References
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Vonk, A.G.; Netea, M.G.; van der Meer, J.W.; Kullberg, B.J. Host defence against disseminated Candida albicans infection and implications for antifungal immunotherapy. Expert Opin. Biol. Ther. 2006, 6, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Poulain, D.; Jouault, T. Candida albicans cell wall glycans, host receptors and responses: Elements for a decisive crosstalk. Curr. Opin. Microbiol. 2004, 7, 342–349. [Google Scholar] [CrossRef]
- Qian, Q.; Jutila, M.A.; Van Rooijen, N.; Cutler, J.E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 1994, 152, 5000–5008. [Google Scholar] [CrossRef]
- Wurster, S.; Watowich, S.S.; Kontoyiannis, D.P. Checkpoint inhibitors as immunotherapy for fungal infections: Promises, challenges, and unanswered questions. Front. Immunol. 2022, 13, 1018202. [Google Scholar] [CrossRef]
- Hamad, M. Antifungal immunotherapy and immunomodulation: A double-hitter approach to deal with invasive fungal infections. Scand. J. Immunol. 2008, 67, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Yang, Z.; Li, D.; Zhang, N.; Chen, B.; Shi, D. Distinct host immune responses in recurrent vulvovaginal candidiasis and vulvovaginal candidiasis. Front. Immunol. 2022, 13, 959740. [Google Scholar] [CrossRef]
- Ahmad, E.; Fatima, M.T.; Saleemuddin, M.; Owais, M. Plasma beads loaded with Candida albicans cytosolic proteins impart protection against fungal infection in BALB/c mice. Vaccine 2012, 30, 6851–6858. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Lupo, P.; Rachini, A.; Sandini, S.; Ciervo, A.; Perito, S.; Bistoni, F.; Vecchiarelli, A. A Candida albicans mannoprotein deprived of its mannan moiety is efficiently taken up and processed by human dendritic cells and induces T-cell activation without stimulating proinflammatory cytokine production. Infect. Immun. 2008, 76, 4359–4367. [Google Scholar] [CrossRef] [PubMed]
- Paulovičová, L.; Paulovičová, E.; Karelin, A.A.; Tsvetkov, Y.E.; Nifantiev, N.E.; Bystrický, S. Immune cell response to Candida cell wall mannan-derived branched α-oligomannoside conjugates in mice. J. Microbiol. Immunol. Infect. 2015, 48, 9–19. [Google Scholar] [CrossRef][Green Version]
- Nelson, R.D.; Shibata, N.; Podzorski, R.P.; Herron, M.J. Candida mannan: Chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin. Microbiol. Rev. 1991, 4, 1–19. [Google Scholar] [CrossRef]
- Ashley, C.; Morhart, M.; Rennie, R.; Ziola, B. Release of Candida albicans yeast antigens upon interaction with human neutrophils in vitro. J. Med. Microbiol. 1997, 46, 747–750. [Google Scholar] [CrossRef]
- Levy, D.A.; Bohbot, J.M.; Catalan, F.; Normier, G.; Pinel, A.M.; Dussourd d’Hinterland, L. Phase II study of D.651, an oral vaccine designed to prevent recurrences of vulvovaginal candidiasis. Vaccine 1989, 7, 337–340. [Google Scholar] [CrossRef]
- Levy, R.; Segal, E.; Barr-Nea, L. Systemic candidiasis in mice immunized with Candida albicans ribosomes. Mycopathologia 1985, 91, 17–22. [Google Scholar] [CrossRef]
- Edwards, J.E., Jr.; Schwartz, M.M.; Schmidt, C.S.; Sobel, J.D.; Nyirjesy, P.; Schödel, F.; Marchus, E.; Lizakowski, M.; DeMontigny, E.A.; Hoeg, J.; et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis: A phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2018, 66, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Alqarihi, A.; Singh, S.; Edwards, J.E., Jr.; Ibrahim, A.S.; Uppuluri, P. NDV-3A vaccination prevents Candida albicans colonization of jugular vein catheters in mice. Sci. Rep. 2019, 9, 6194. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Rohatgi, S. Vaccination with secreted aspartyl proteinase 2 protein from Candida parapsilosis can enhance survival of mice during Candida tropicalis-mediated systemic candidiasis. Infect. Immun. 2020, 88, e00312-20. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Karasaki, M.; Tafuku, S.; Aoki, W.; Sewaki, T.; Ueda, M. Oral immunization against candidiasis using Lactobacillus casei displaying enolase 1 from Candida albicans. Sci. Pharm. 2014, 82, 697–708. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans antifungal resistance and tolerance in bloodstream infections: The triad yeast–host–antifungal. Microorganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Song, J.L.; Harry, J.B.; Eastman, R.T.; Oliver, B.G.; White, T.C. The Candida albicans lanosterol 14-alpha-demethylase (ERG11) gene promoter is maximally induced after prolonged growth with antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 1136–1144. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, F.M.; Abid, M. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Kaur, G.; Chawla, S.; Kumar, P.; Singh, R. Advancing vaccine strategies against Candida infections: Exploring new frontiers. Vaccines 2023, 11, 1658. [Google Scholar] [CrossRef]
- Khan, M.A.; Allemailem, K.S.; Maswadeh, H.; Younus, H. Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice. Molecules 2022, 27, 7790. [Google Scholar] [CrossRef]
- Dwivedi, V.; Vasco, A.; Vedi, S.; Dangi, A.; Arif, K.; Bhattacharya, S.M.; Owais, M. Adjuvanticity and protective immunity of Plasmodium yoelii nigeriensis blood-stage soluble antigens encapsulated in fusogenic liposome. Vaccine 2009, 27, 473–482. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, Y.; Lovell, J.F. Vaccine approaches for antigen capture by liposomes. Expert Rev. Vaccines 2023, 22, 1022–1040. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A. Targeted drug delivery using tuftsin-bearing liposomes: Implications in the treatment of infectious diseases and tumors. Curr. Drug Targets 2021, 22, 770–778. [Google Scholar] [PubMed]
- Siemion, I.Z.; Kluczyk, A. Tuftsin: On the 30-year anniversary of Victor Najjar’s discovery. Peptides 1999, 20, 645–674. [Google Scholar] [CrossRef]
- Murugesan, K.; Srinivasan, P.; Mahadeva, R.; Gupta, C.M.; Haq, W. Tuftsin-Bearing Liposomes Co-Encapsulated with Doxorubicin and Curcumin Efficiently Inhibit EAC Tumor Growth in Mice. Int. J. Nanomed. 2020, 15, 10547–10559. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; Alhumaydhi, F.A.; Ahmad, H.I.; Khan, M.U.; Khan, M.A. Coadministration of ginger extract and fluconazole shows a synergistic effect in the treatment of drug-resistant vulvovaginal candidiasis. Infect. Drug Resist. 2021, 14, 1585–1599. [Google Scholar] [CrossRef]
- Antimisiaris, S.G. Preparation of DRV Liposomes. Methods Mol. Biol. 2017, 1522, 23–47. [Google Scholar]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Tanaka, Y.; Taneichi, M.; Kasai, M.; Kakiuchi, T.; Uchida, T. Liposome-coupled antigens are internalized by antigen-presenting cells via pinocytosis and cross-presented to CD8 T cells. PLoS ONE 2010, 5, e15225. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Gao, Y.L.; Chai, Y.F.; Dong, N.; Han, S.; Zhu, X.M.; Zhang, Q.H.; Yao, Y.M. Tuftsin-derived T-peptide prevents cellular immunosuppression and improves survival rate in septic mice. Sci. Rep. 2015, 5, 16725. [Google Scholar] [CrossRef]
- Gao, Y.; Su, Q.; Yi, Y.; Jia, Z.; Wang, H.; Lu, X.; Qiu, F.; Bi, S. Enhanced mucosal immune responses induced by a combined candidate mucosal vaccine based on hepatitis A virus and hepatitis E virus structural proteins linked to tuftsin. PLoS ONE 2015, 10, e0123400. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Li, Y.; Wang, Z.; Chu, M.; Wang, Y. Tuftsin: A natural molecule against SARS-CoV-2 infection. Front. Mol. Biosci. 2022, 9, 859162. [Google Scholar] [CrossRef] [PubMed]
- Gozalbo, D.; Maneu, V.; Gil, M.L. Role of IFN-γ in immune responses to Candida albicans infections. Front. Biosci.(Landmark Ed.) 2014, 19, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Chakma, C.R.; Good-Jacobson, K.L. Requirements of IL-4 during the generation of B cell memory. J. Immunol. 2023, 210, 1853–1860. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Khan, A.; Alnuqaydan, A.M.; Albutti, A.; Alharbi, B.F.; Owais, M. Targeting Azole-Resistant Candida albicans: Tetrapeptide Tuftsin-Modified Liposomal Vaccine Induces Superior Immune Protection. Vaccines 2025, 13, 630. https://doi.org/10.3390/vaccines13060630
Khan MA, Khan A, Alnuqaydan AM, Albutti A, Alharbi BF, Owais M. Targeting Azole-Resistant Candida albicans: Tetrapeptide Tuftsin-Modified Liposomal Vaccine Induces Superior Immune Protection. Vaccines. 2025; 13(6):630. https://doi.org/10.3390/vaccines13060630
Chicago/Turabian StyleKhan, Masood A., Arif Khan, Abdullah M. Alnuqaydan, Aqel Albutti, Basmah F. Alharbi, and Mohammad Owais. 2025. "Targeting Azole-Resistant Candida albicans: Tetrapeptide Tuftsin-Modified Liposomal Vaccine Induces Superior Immune Protection" Vaccines 13, no. 6: 630. https://doi.org/10.3390/vaccines13060630
APA StyleKhan, M. A., Khan, A., Alnuqaydan, A. M., Albutti, A., Alharbi, B. F., & Owais, M. (2025). Targeting Azole-Resistant Candida albicans: Tetrapeptide Tuftsin-Modified Liposomal Vaccine Induces Superior Immune Protection. Vaccines, 13(6), 630. https://doi.org/10.3390/vaccines13060630








