Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria’s Routine Immunization Program: An Implementation Hybrid Trial
Abstract
1. Introduction {6a: Background and Rationale}
Objectives {7: Objectives}
- To evaluate the effectiveness of Tropis for fIPV delivery as compared to the standard of care (SoC, 0.5 mL IM injection with a traditional needle/syringe) in regard to improving IPV2 coverage among children aged less than one year;
- To assess the incremental cost in regard to the immunization program of using Tropis for fIPV delivery as compared to standard vaccination practice;
- To understand the feasibility and acceptability of fIPV delivery using Tropis.
2. Materials and Methods
2.1. Study Design {8: Trial Design}
- A cluster randomized trial involving a coverage survey of the target population (children 3–12 months old) to assess the coverage of IPV using Tropis compared to the SoC;
- A pre- and post-implementation micro-costing study, with primary and secondary data collection from the health facilities implementing fIPV using Tropis, to estimate the immunization program costs for fIPV versus the SoC;
- Mixed methods assessments (post-training assessment, provider survey, key informant interviews (KIIs), and focus group discussions (FGDs)) to assess the feasibility and acceptability of fIPV delivery using Tropis.
2.2. Study Settings {9: Study Setting}
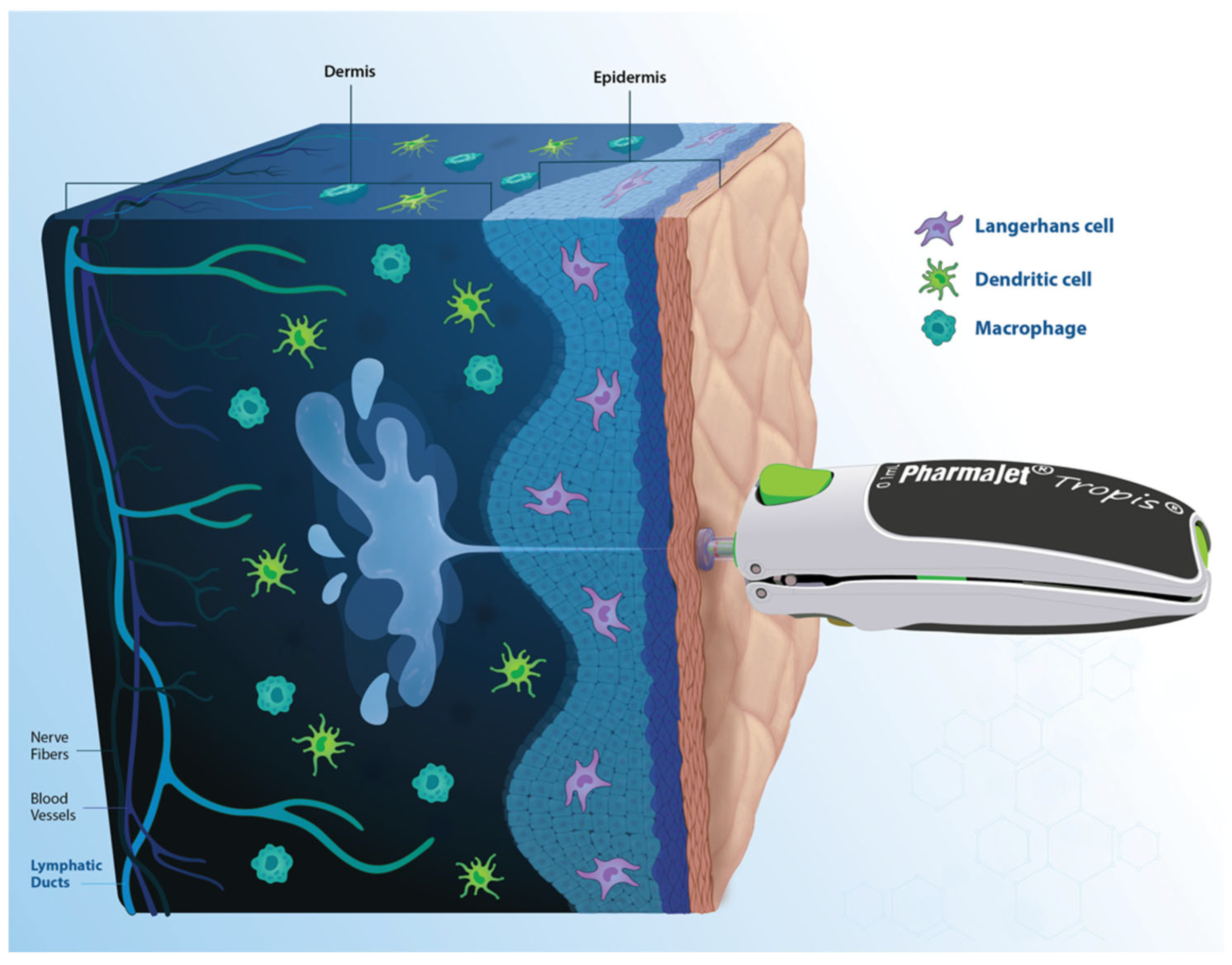
2.3. Intervention Description: Tropis {11a: Intervention Description}
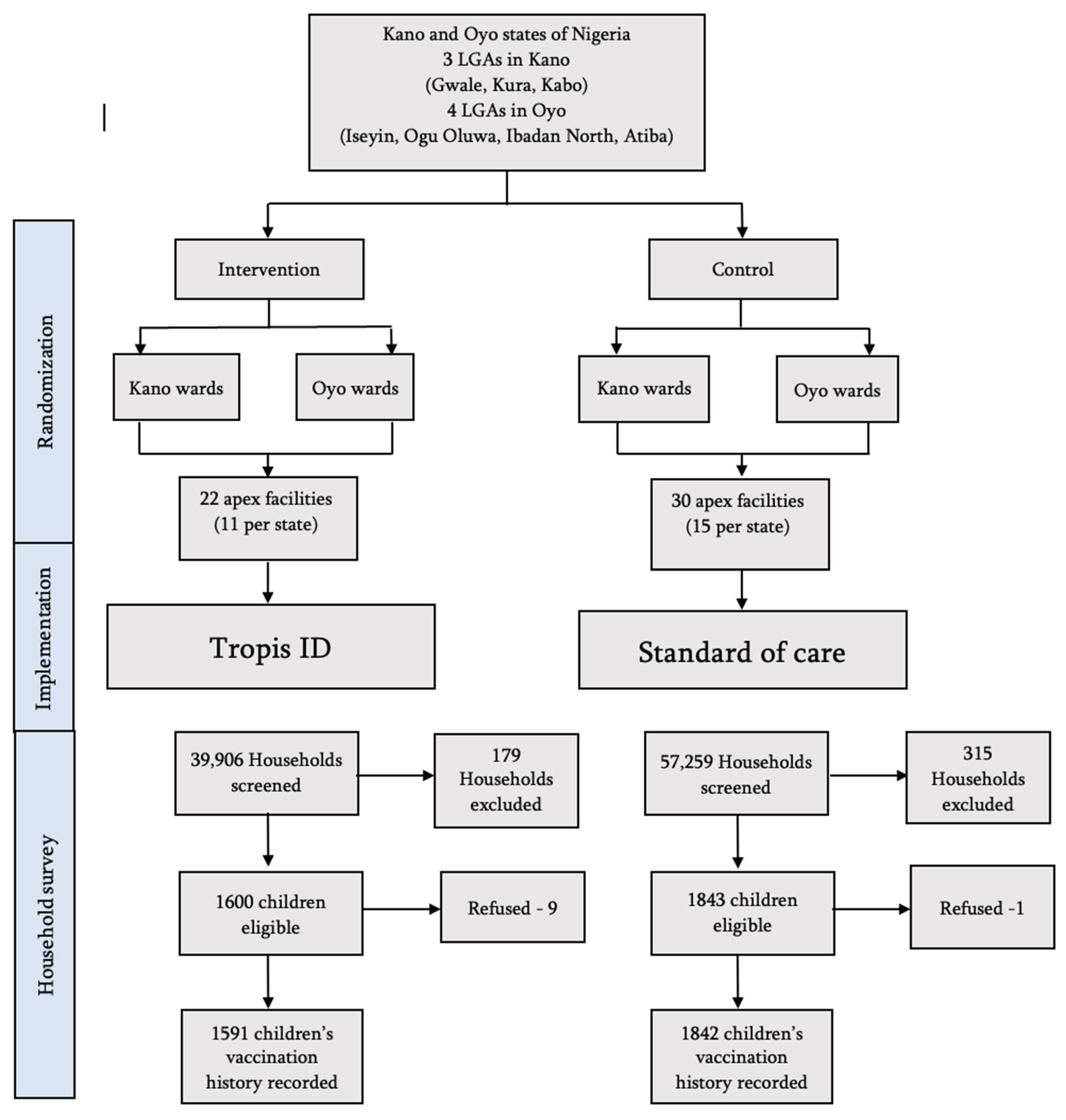
2.4. Randomization and Intervention Allocation
2.5. Description of the Implementation
2.6. Survey to Measure Immunization Coverage
2.6.1. Survey Procedures {8a: Plans for Assessment and Collection of Outcomes. 19: Data Management}
2.6.2. Sample Size and Power {14: Sample Size}
2.6.3. Outcomes {12: Outcomes}
2.6.4. Data Analysis of Coverage Survey {20a: Statistical Methods for Primary and Secondary Outcomes}
2.7. Costing Analysis
2.8. Feasibility and Acceptability Assessment
3. Results
3.1. Intention-to-Treat (ITT) Estimate
3.2. Per-Protocol Estimate
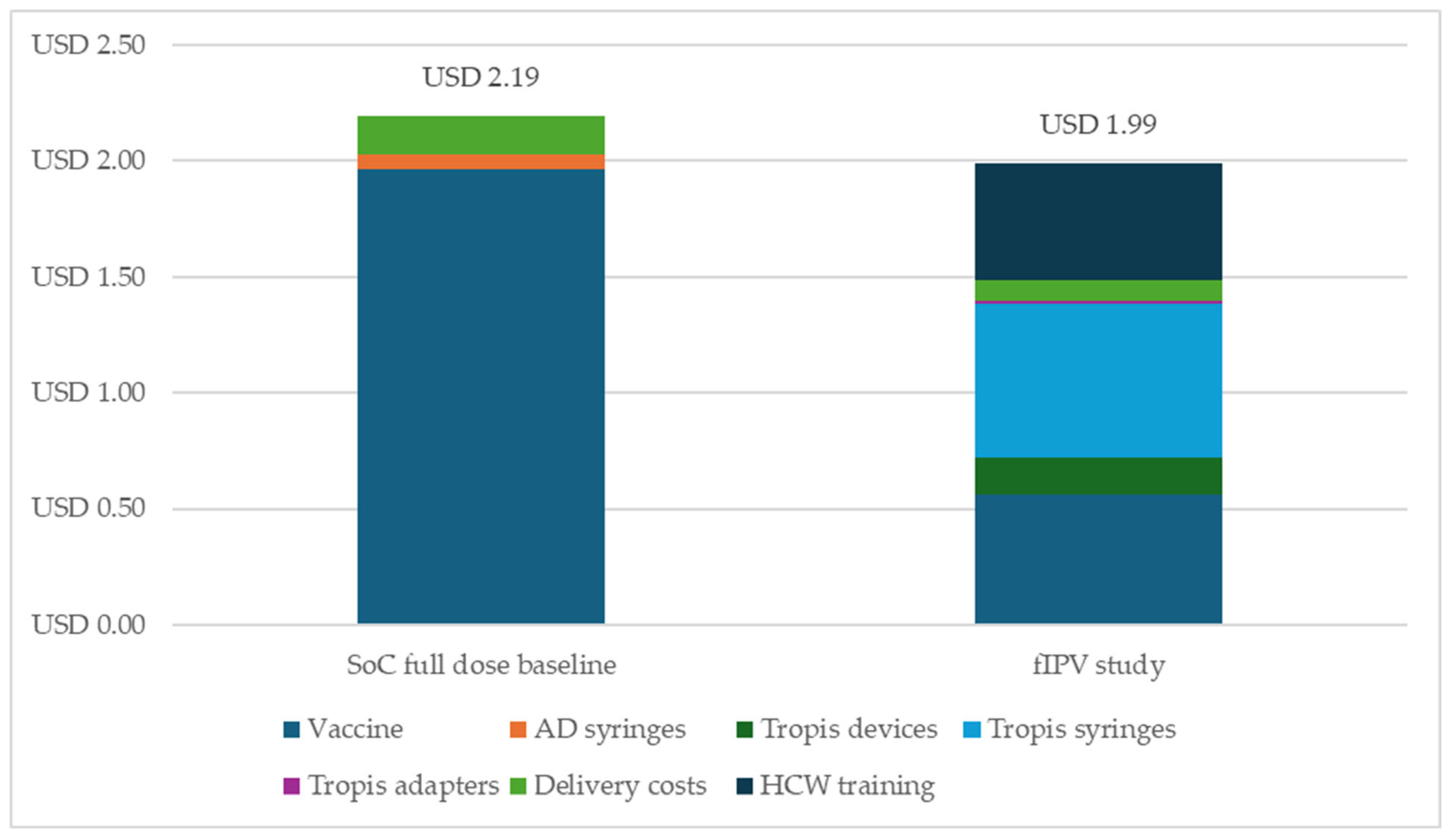
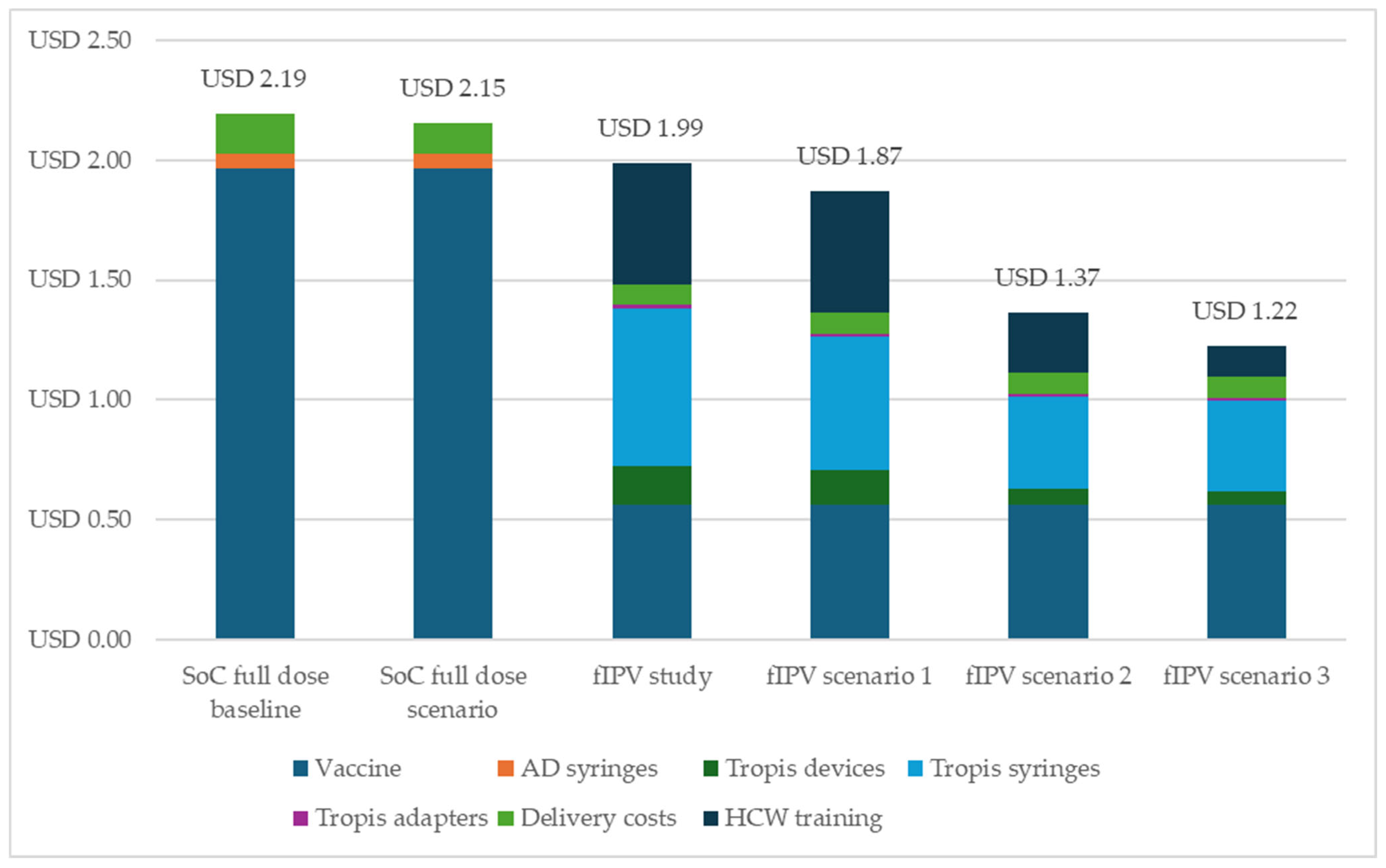
3.3. Costing Analysis
3.4. Feasibility and Acceptability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Autodisable |
| AT | As treated |
| CI | Confidence interval |
| cVDPV | Circulating vaccine-derived poliovirus |
| fIPV | Fractional inactivated poliovirus vaccine |
| FGDs | Focus group discussions |
| HCWs | Healthcare workers |
| ICC | Intraclass correlation coefficient |
| ID | Intradermal |
| IM | Intramuscular |
| IPV | Inactivated poliovirus vaccine |
| IPV2 | 2nd dose of inactivated poliovirus vaccine |
| ITT | Intention to treat |
| KIIs | Key informant interviews |
| LGAs | Local Government Areas |
| NPHCDA | National Primary Health Care Development Agency |
| OPV | Oral poliovirus vaccine |
| PP | Per protocol |
| PPS | Probability proportional to size |
| RI | Routine immunization |
| SAGE | Strategic Advisory Group of Experts |
| SoC | Standard of Care |
| USAID | U.S. Agency for International Development |
| WHO | World Health Organization |
| WPV | Wild Poliovirus |
References
- Poliomyelitis. WHO. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 21 March 2025).
- Where we work. Global Polio Eradication Initiative. 2025. Available online: https://polioeradication.org/about-polio/where-we-work/ (accessed on 21 March 2025).
- Polio transition in a snapshot: Nigeria WHO. Available online: https://cdn.who.int/media/docs/default-source/polio-transition/who-polio-transition-snapshot-nigeria.pdf (accessed on 21 March 2025).
- Statement of the fortieth meeting of the Polio IHR Emergency Committee. WHO. 2024. Available online: https://www.who.int/news/item/03-12-2024-statement-of-the-fortieth-meeting-of-the-polio-ihr-emergency-committee (accessed on 21 March 2025).
- Sutter, R.W.; Eisenhawer, M.; Molodecky, N.A.; Verma, H.; Okayasu, H. Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance. Pathogens 2024, 13, 224. [Google Scholar] [CrossRef]
- World Health Organization. (3 January 2014). Meeting of the Strategic Advisory Group of Experts on Immunization, November 5–7, 2013–conclusions and recommendations. Available online: https://www.who.int/publications/i/item/WER8901 (accessed on 12 May 2025).
- WHO. (31 April 2020). Meeting of the Strategic Advisory Group of Experts on Immunization, 31 March–1 April 2020–conclusions and recommendations. Available online: https://www.who.int/news-room/events/detail/2020/03/31/default-calendar/strategic-advisory-group-of-experts-on-immunization-(sage)---april-2020 (accessed on 12 May 2025).
- Mashunye, T.R.; Ndwandwe, D.E.; Dube, K.R.; Shey, M.; Shelton, M.; Wiysonge, C.S. Fractional dose compared with standard dose inactivated poliovirus vaccine in children: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Trueba, G.; Jeyaseelan, V.; Lopez, L.; Mainou, B.A.; Zhang, Y.; Whittembury, A.; Valarezo, A.J.O.; Baquero, G.; de Aguinaga, R.R.; Salinas, L.J.Z.; et al. Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey. Lancet Reg. Heal. Am. 2022, 11, 100235. [Google Scholar] [CrossRef]
- Mvundura, M.; Hsu, J.S.; Frivold, C.; Kristensen, D.; Boyle, S.; Zehrung, D.; Jarrahian, C. Evaluating the cost per child vaccinated with full versus fractional-dose inactivated poliovirus vaccine. Vaccine: X 2019, 2, 100032. [Google Scholar] [CrossRef]
- 2023: A Critical Year for Polio Eradication Efforts in Northern Nigeria. Global Polio Eradication Initiative. 2023. Available online: https://polioeradication.org/news-post/2023-a-critical-year-for-polio-eradication-efforts-in-northern-nigeria (accessed on 21 March 2025).
- Presentation of Evidence by the Polio Disease Working Group. In Proceedings of the Meeting of the Nigerian Immunization Technical Advisory Group, Abuja, Nigeria, 24 July 2018.
- Bakunawa, G.; (National Primary Health Care Development Agency (NPHCDA), Abuja, Nigeria). Personal Communication, 4 March 2022.
- Biya, O.; Manu, J.I.; Forbi, J.C.; Wa Nganda, G.; Ikwe, H.; Sule, A.; Edukugho, A.; Shehu, A.; Aliyu, N.; Barau, N.D.; et al. Notes from the Field: House-to-House Campaign Administration of Inactivated Poliovirus Vaccine-Sokoto State, Nigeria, November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Nouh, K.; Haga, A.; Sumaili, K.; Farid, M.; Alin, M.; Shube, M.; Abshir, A.; Hiirad, M.; Ahmed, M.; Bile, A. Use of a fractional dose of inactivated polio vaccine (fIPV) to increase IPV coverage among children under 5 years of age in Somalia. BMC Glob. Public Health 2024, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Molodecky, N.A.; Sreevatsava, M.; Belayneh, A.D.; Chandio, S.A.; Partridge, J.; Shaikh, A.; Laghari, M.; Agbor, J.; Safdar, R.M.; et al. Needle-free injectors for mass administration of fractional dose inactivated poliovirus vaccine in Karachi, Pakistan: A survey of caregiver and vaccinator acceptability. Vaccine 2020, 38, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Resik, S.; Lopez Cavestany, R.; Tejeda, A.; Díaz, M.; García, G.; Alemañy, N.; Mesa, I.; Rivero, M.; Fonseca, M.; Hong, L.H.; et al. Tropis needle-free injector for fractional-dose IPV administration: A pilot study for integration into routine immunization services in Cuba. Vaccine 2025, 52, 126903. [Google Scholar] [CrossRef]
- City Population Nigeria. Nigeria: Administrative Division (States and Local Government Areas). Available online: https://www.citypopulation.de/en/nigeria/admin (accessed on 3 April 2024).
- Nigeria Health System. Severe Malaria Observatory. Available online: https://www.severemalaria.org/countries/nigeria/nigeria-health-system (accessed on 24 March 2025).
- Office for the Coordination of Humanitarian Affairs (OCHA). Nigeria: Health facilities. Available online: https://data.humdata.org/dataset/nigeria-health-facilities/resource/4658aa59-0554-4fac-8473-377da4b7a0e9 (accessed on 3 April 2024).
- Kano State: The Center of Commerce. Available online: https://kanostate.gov.ng/lgas/ (accessed on 13 May 2025).
- Nigerian Investment Promotion Commission. Oyo State. 2019. Available online: https://www.nipc.gov.ng/nigeria-states/oyo-state/ (accessed on 3 April 2024).
- WHO. (4 June 2018). PharmaJet Tropis Prequalification, PQS Code E008/071, Single Use Auto-Disable Needle-Free Jet Injector. Available online: https://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/categorypage.aspx?id_cat=37 (accessed on 30 December 2024).
- PharmaJet. Needle-Free Injection Technology. 2024. Available online: https://pharmajet.com/needle-free-technology (accessed on 3 April 2024).
- Hentschel, B.; Haas, P.J.; Tian, Y. Exact PPS sampling with bounded sample size. Inf. Process. Lett. 2023, 182, 106382. [Google Scholar] [CrossRef]
- Smith, V.A.; Coffman, C.J.; Hudgens, M.G. Interpreting the Results of Intention-to-Treat, Per-Protocol, and As-Treated Analyses of Clinical Trials. JAMA 2021, 326, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, M.T.; Saleem, A.F.; Mach, O.; Baig, A.; Sutter, R.W.; Zaidi, A.K.M. Feasibility of conducting intradermal vaccination campaign with inactivated poliovirus vaccine using Tropis intradermal needle free injection system, Karachi, Pakistan. Heliyon 2017, 3, e00395. [Google Scholar] [CrossRef] [PubMed]
- Bullo, U.F.; Mehraj, J.; Raza, S.M.; Rasool, S.; Ansari, N.N.; Shaikh, A.A.; Phul, Z.A.; Memon, S.A.; Baloch, R.I.; Baloch, Z.A.; et al. An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh. Pak. BMC Public Health 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
| Input | Full-Dose IPV (SoC) | fIPV Using Tropis | Data Source |
|---|---|---|---|
| IPV price per dose | USD 1.90 | USD 0.38 | UNICEF price for 10-dose IPV (2023 price) |
| Autodisable syringe price | USD 0.06 | NA | |
| Tropis device price (reusable) | NA | USD 415, USD 374, USD 291 | PharmaJet |
| Tropis syringe price (per dose) | NA | USD 0.59, USD 0.50, USD 0.34 | PharmaJet |
| Tropis adapter price (one per vial) | NA | USD 0.45, USD 0.41, USD 0.38 | PharmaJet |
| Shipping and clearance rates for vaccines | 3.51% | 3.51% | UNICEF |
| Shipping and clearance rates for autodisable syringes | 3.51% | NA | UNICEF |
| Shipping and clearance rates for Tropis devices | NA | 6% | PharmaJet |
| Shipping and clearance rates for Tropis syringes | NA | 9% | PharmaJet |
| Shipping and clearance rates for Tropis adapters | NA | 18% | PharmaJet |
| Wastage rate | 15% | 20%, 30%, 40% | Assumption: wastage rates for fIPV are incrementally more than those for the SoC |
| Control | Intervention | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | Number of Children | n | % | 95% CI | Number of Children | ||||
| Total | 609 | 63.8% | 58.9% | 68.8% | 1842 | 982 | 61.7% | 56.4% | 67.0% | 1591 | 3433 |
| Kano | 326 | 68.0% | 61.5% | 74.4% | 1018 | 559 | 63.2% | 56.9% | 69.4% | 885 | 1903 |
| Oyo | 283 | 58.7% | 52.4% | 65.0% | 824 | 423 | 59.9% | 50.9% | 68.9% | 706 | 1530 |
| Control | Intervention | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | Number of Children | n | % | 95% CI | Number of Children | ||||
| Total | 1009 | 72.5% | 67.6% | 77.3% | 1392 | 516 | 82.3% | 76.4% | 88.2% | 627 | 2019 |
| Kano | 600 | 76.7% | 71.5% | 82.0% | 782 | 239 | 89.2% | 82.7% | 95.7% | 268 | 1050 |
| Oyo | 409 | 67.0% | 60.1% | 74.0% | 610 | 277 | 77.2% | 69.9% | 84.4% | 359 | 969 |
| Intention to Treat (N = 3433) | Per Protocol (N = 2019) | |||
|---|---|---|---|---|
| Adjusted Coverage | Difference | Adjusted Coverage | Difference | |
| Control | 65.1% (61.5%,68.8%) | −5% (−11.7%, 1.7%) | 71.9% (69.2%,74.7%) | 11.2% (6.4%, 16.1%) |
| Intervention | 60.1% (55.3%,65.0%) | 83.2% (79.3%,87.1%) | ||
| Total over a Five-Year Period | |
|---|---|
| Full-dose IPV | |
| Vaccines | USD 125,965,983 |
| Autodisable syringes | USD 3,580,086 |
| Total cost of full dose | USD 129,546,069 |
| Fractional dose IPV | |
| Vaccines | USD 35,990,281 |
| Tropis devices | USD 11,590,163 |
| Tropis adapters | USD 719,806 |
| Tropis syringes | USD 32,201,830 |
| Total cost of fractional dose | USD 80,502,079 |
| Difference (savings from fractional dose) | USD 49,043,990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, D.; Mvundura, M.; Sampson, S.; Adepoju, V.A.; Bakunawa, G.B.; Umebido, C.; Ekeh, A.; Little, J.; Daly, C.; Morgan, C.; et al. Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria’s Routine Immunization Program: An Implementation Hybrid Trial. Vaccines 2025, 13, 533. https://doi.org/10.3390/vaccines13050533
Mohan D, Mvundura M, Sampson S, Adepoju VA, Bakunawa GB, Umebido C, Ekeh A, Little J, Daly C, Morgan C, et al. Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria’s Routine Immunization Program: An Implementation Hybrid Trial. Vaccines. 2025; 13(5):533. https://doi.org/10.3390/vaccines13050533
Chicago/Turabian StyleMohan, Diwakar, Mercy Mvundura, Sidney Sampson, Victor Abiola Adepoju, Garba Bello Bakunawa, Chidinma Umebido, Adachi Ekeh, Joe Little, Catherine Daly, Christopher Morgan, and et al. 2025. "Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria’s Routine Immunization Program: An Implementation Hybrid Trial" Vaccines 13, no. 5: 533. https://doi.org/10.3390/vaccines13050533
APA StyleMohan, D., Mvundura, M., Sampson, S., Adepoju, V. A., Bakunawa, G. B., Umebido, C., Ekeh, A., Little, J., Daly, C., Morgan, C., Atobatele, S., LaBarre, P., & Oliveras, E., on behalf of the Nigeria Tropis® Evaluation Team. (2025). Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria’s Routine Immunization Program: An Implementation Hybrid Trial. Vaccines, 13(5), 533. https://doi.org/10.3390/vaccines13050533






