Emerging Immunotherapies in Lung Cancer: The Latest Advances and the Future of mRNA Vaccines
Abstract
1. Introduction
The Importance of the Immune System
2. The Beginning of Vaccines and the Transition to Cancer
Vaccines for Cancer Treatment
Vaccines for LC Treatment
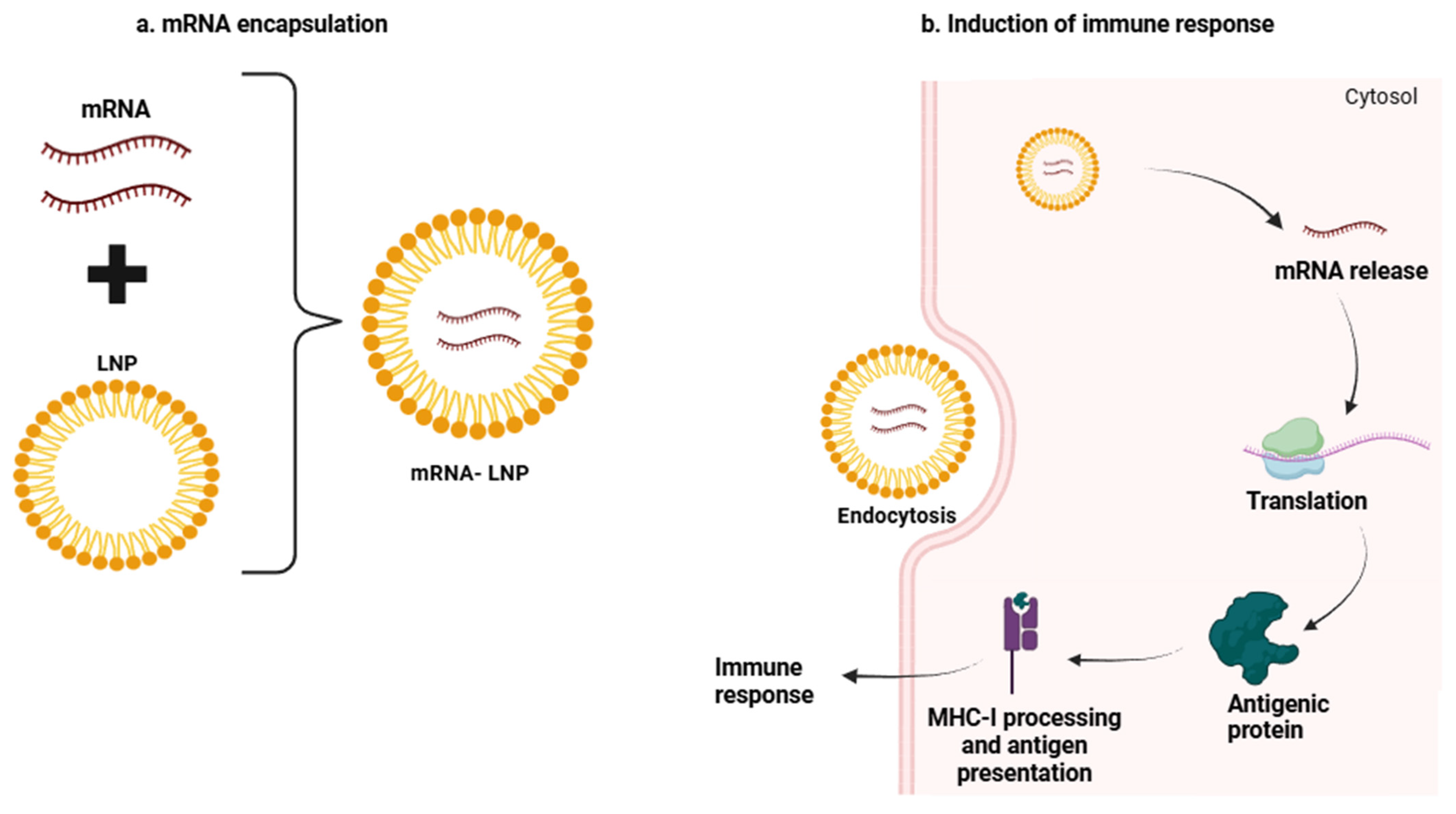
3. mRNA Vaccines
4. mRNA Vaccines for LC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| DCs | Dendritic cells |
| ICIs | Immune checkpoint inhibitors |
| LC | Lung cancer |
| LUAD | Lung adenocarcinoma |
| NSCLC | Non-small cell lung cancer |
| SCLC | Small cell lung cancer |
| TAAs | Tumor-associated antigens |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, A.; Peake, M.D.; Gilligan, D.; Cosford, P. Lung cancer in never-smokers: A hidden disease. J. R. Soc. Med. 2019, 112, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Joubert, P.; Ansari-Pour, N.; Zhao, W.; Hoang, P.H.; Lokanga, R.; Moye, A.L.; Rosenbaum, J.; Gonzalez-Perez, A.; Martínez-Jiménez, F.; et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat. Genet. 2021, 53, 1348–1359. [Google Scholar] [CrossRef]
- Ruano-Raviña, A.; Provencio, M.; Calvo de Juan, V.; Carcereny, E.; Moran, T.; Rodriguez-Abreu, D.; López-Castro, R.; Cuadrado Albite, E.; Guirado, M.; Gómez González, L.; et al. Lung cancer symptoms at diagnosis: Results of a nationwide registry study. ESMO Open 2020, 5, e001021. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.C.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef]
- Kauczor, H.-U.; Von Stackelberg, O.; Nischwitz, E.; Chorostowska-Wynimko, J.; Hierath, M.; Mathonier, C.; Prosch, H.; Zolda, P.; Revel, M.-P.; Horváth, I.; et al. Strengthening lung cancer screening in Europe: Fostering participation, improving outcomes, and addressing health inequalities through collaborative initiatives in the SOLACE consortium. Insights Imaging 2024, 15, 252. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational Implications of Tumor Heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef]
- Marino, F.Z.; Bianco, R.; Accardo, M.; Ronchi, A.; Cozzolino, I.; Morgillo, F.; Rossi, G.; Franco, R. Molecular heterogeneity in lung cancer: From mechanisms of origin to clinical implications. Int. J. Med. Sci. 2019, 16, 981–989. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Ganti, A.K.P.; Loo, B.W.; Bassetti, M.; Blakely, C.; Chiang, A.; D’Amico, T.A.; D’Avella, C.; Dowlati, A.; Downey, R.J.; Edelman, M.; et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1441–1464. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef]
- Ramos, R.; Moura, C.S.; Costa, M.; Lamas, N.J.; Correia, R.; Garcez, D.; Pereira, J.M.; Sousa, C.; Vale, N. Enhancing Lung Cancer Care in Portugal: Bridging Gaps for Improved Patient Outcomes. J. Pers. Med. 2024, 14, 446. [Google Scholar] [CrossRef]
- Cheng, J.; Kang, W.; Chen, Y.; Pan, L.; Han, H.; Lv, T. Continuous immunotherapy beyond disease progression in patients with advanced non-small cell and small cell lung cancer. Cancer Immunol. Immunother. 2025, 74, 124. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Z.; Chen, Y.; Shi, X.; Dong, S.; Wang, B. Candidate Biomarker of Response to Immunotherapy In Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2025, 26, 73–83. [Google Scholar] [CrossRef]
- Dolkar, T.; Gates, C.; Hao, Z.; Munker, R. New developments in immunotherapy for SCLC. J. Immunother. Cancer 2025, 13, e009667. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic cell maturation and cross-presentation: Timing matters! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef]
- Balan, S.; Radford, K.J.; Bhardwaj, N. Unexplored horizons of cDC1 in immunity and tolerance. Adv. Immunol. 2020, 148, 49–91. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, W.; Ly, D.; Xu, H.; Qu, L.; Zhang, L. Tumor-infiltrating B cells: Their role and application in anti-tumor immunity in lung cancer. Cell. Mol. Immunol. 2019, 16, 6–18. [Google Scholar] [CrossRef]
- Mundhara, N.; Sadhukhan, P. Cracking the Codes behind Cancer Cells’ Immune Evasion. Int. J. Mol. Sci. 2024, 25, 8899. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; de Souza, W.R.; Sande Loureiro, M.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Jeon, H.; Wang, S.; Song, J.; Gill, H.; Cheng, H. Update 2025: Management of Non-Small-Cell Lung Cancer. Lung 2025, 203, 53. [Google Scholar] [CrossRef]
- Caliendo, F.; Dukhinova, M.; Siciliano, V. Engineered Cell-Based Therapeutics: Synthetic Biology Meets Immunology. Front. Bioeng. Biotechnol. 2019, 7, 43. [Google Scholar] [CrossRef]
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef]
- Cuppens, K.; Vansteenkiste, J. Vaccination therapy for non-small-cell lung cancer. Curr. Opin. Oncol. 2014, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Cancer vaccines: Between the idea and the reality. Nat. Rev. Immunol. 2003, 3, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Giaccone, G. Lung Cancer Vaccines. Cancer J. 2011, 17, 302–308. [Google Scholar] [CrossRef]
- Moore, Z.S.; Seward, J.F.; Lane, J.M. Smallpox. Lancet 2006, 367, 425–435. [Google Scholar] [CrossRef]
- Gail, M.H. A placebo-controlled randomized double-blind study of adjuvant intrapleural BCG in patients with resected T1N0, T1N1, or T2N0 squamous cell carcinoma, adenocarcinoma, or large cell carcinoma of the lung. LCSG Protocol 771. Chest 1994, 106, 287s–292s. [Google Scholar]
- Gardner, T.; Elzey, B.; Hahn, N.M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum. Vaccines Immunother. 2012, 8, 534–539. [Google Scholar] [CrossRef]
- Gupta, D.S.; Gupta, D.S.; Abjani, N.K.; Dave, Y.; Apte, K.; Kaur, G.; Kaur, D.; Saini, A.K.; Sharma, U.; Haque, S.; et al. Vaccine-based therapeutic interventions in lung cancer management: A recent perspective. Med. Oncol. 2024, 41, 249. [Google Scholar] [CrossRef]
- Hoover, H.C., Jr.; Surdyke, M.G.; Dangel, R.B.; Peters, L.C.; Hanna, M.G., Jr. Prospectively randomized trial of adjuvant active-specific immunotherapy for human colorectal cancer. Cancer 1985, 55, 1236–1243. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Zielinski, M.; Linder, A.; Dahabreh, J.; Gonzalez, E.E.; Malinowski, W.; Lopez-Brea, M.; Vanakesa, T.; Jassem, J.; Kalofonos, H.; et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: Phase II randomized study results. J. Clin. Oncol. 2013, 31, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.-Y. Therapeutic Cancer Vaccines; Elsevier: Amsterdam, The Netherlands, 2013; pp. 421–475. [Google Scholar]
- Faghfuri, E.; Pourfarzi, F.; Faghfouri, A.H.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Baradaran, B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 201–218. [Google Scholar] [CrossRef]
- Van Nuffel, A.M.T.; Wilgenhof, S.; Thielemans, K.; Bonehill, A. Overcoming HLA restriction in clinical trials. OncoImmunology 2012, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. Toward rational vaccine engineering. Adv. Drug Deliv. Rev. 2022, 183, 114142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, F.; Li, J.; Pu, Y.; Yang, C.; Wang, Y.; Lei, Y.; Huang, Y. CAR-T cell therapy for lung cancer: Potential and perspective. Thorac. Cancer 2022, 13, 889–899. [Google Scholar] [CrossRef]
- Luo, L.; Lv, M.; Zhuang, X.; Zhang, Q.; Qiao, T. Irradiation increases the immunogenicity of lung cancer cells and irradiation-based tumor cell vaccine elicits tumor-specific T cell responses in vivo. OncoTargets Ther. 2019, 12, 3805–3815. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, S.; Cai, J.; Liu, Z.; Li, H.; Wang, P.; Li, Y.; Yang, F.; Chen, K.; Qiu, M. The current therapeutic cancer vaccines landscape in non-small cell lung cancer. Int. J. Cancer 2024, 155, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; Dipiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Weissman, D.; Karikó, K. mRNA: Fulfilling the Promise of Gene Therapy. Mol. Ther. 2015, 23, 1416–1417. [Google Scholar] [CrossRef]
- Son, S.; Nam, J.; Zenkov, I.; Ochyl, L.J.; Xu, Y.; Scheetz, L.; Shi, J.; Farokhzad, O.C.; Moon, J.J. Sugar-Nanocapsules Imprinted with Microbial Molecular Patterns for mRNA Vaccination. Nano Lett. 2020, 20, 1499–1509. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef]
- Alameh, M.G.; Weissman, D.; Pardi, N. Messenger RNA-Based Vaccines Against Infectious Diseases. Mrna Vaccines 2022, 440, 111–145. [Google Scholar] [CrossRef]
- Anindita, J.; Tanaka, H.; Yamakawa, T.; Sato, Y.; Matsumoto, C.; Ishizaki, K.; Oyama, T.; Suzuki, S.; Ueda, K.; Higashi, K.; et al. The Effect of Cholesterol Content on the Adjuvant Activity of Nucleic-Acid-Free Lipid Nanoparticles. Pharmaceutics 2024, 16, 181. [Google Scholar] [CrossRef]
- Chaudhary, N.; Kasiewicz, L.N.; Newby, A.N.; Arral, M.L.; Yerneni, S.S.; Melamed, J.R.; LoPresti, S.T.; Fein, K.C.; Strelkova Petersen, D.M.; Kumar, S.; et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng. 2024, 8, 1483–1498. [Google Scholar] [CrossRef]
- Ramadan, E.; Ahmed, A.; Naguib, Y.W. Advances in mRNA LNP-Based Cancer Vaccines: Mechanisms, Formulation Aspects, Challenges, and Future Directions. J. Pers. Med. 2024, 14, 1092. [Google Scholar] [CrossRef]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef]
- Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Rezvani, A.; Zaboli, E.; Salari, S.; Masjedi, M.R.; Bashash, D. Lung cancer vaccination from concept to reality: A critical review of clinical trials and latest advances. Life Sci. 2024, 346, 122652. [Google Scholar] [CrossRef]
- Kiousi, E.; Lyraraki, V.; Mardiki, G.L.; Stachika, N.; Damianou, A.K.; Malainou, C.P.; Syrigos, N.; Gomatou, G.; Kotteas, E. Progress and Challenges of Messenger RNA Vaccines in the Therapeutics of NSCLC. Cancers 2023, 15, 5589. [Google Scholar] [CrossRef]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Schröder, A.; Scheel, B.; Hong, H.S.; Muth, A.; Von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Ramirez, K.A.; Schwarzenberger, P.; Ricciardi, T.; Macri, M.J.; Ryan, A.; Venhaus, R.R. Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2018, 36, TPS9107. [Google Scholar] [CrossRef]
- Forde, P.M.; Atmaca, A.; Brück, P.; Munshi, N.; Kaczorowska, M.A.; Schell, T.; Türeci, Ö.; Sahin, U. 1196TiP LuCa-MERIT-1: First-in-human, open label, phase I dose confirmation trial evaluating the safety, tolerability, and preliminary efficacy of BNT116 alone and in combinations in patients with advanced non-small cell lung cancer. Ann. Oncol. 2022, 33, S1095. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Bian, F. The progress of tumor vaccines clinical trials in non-small cell lung cancer. Clin. Transl. Oncol. 2024, 27, 1062–1074. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Li, Y.; Tan, X.; Li, W.; Tan, S.; Liu, G. Discovery of lung adenocarcinoma tumor antigens and ferroptosis subtypes for developing mRNA vaccines. Sci. Rep. 2024, 14, 3219. [Google Scholar] [CrossRef]

| Immunotherapy Type | Description | Refs. |
|---|---|---|
| Immune checkpoint inhibitors (ICIs) | CTLA-4, PD-1, and PD-L1 inhibitors represent the most successful immunotherapeutic strategy in NSCLC | [12] |
| Oncolytic virus therapy (Ovs) | Genetically-modified viruses selectively target and destroy cancer cells, triggering an immune response and inhibiting tumor progression | [26] |
| CAR-T | Genetically-engineered autologous or allogeneic T cells are modified in vitro to express specific T-cell receptors that recognize cancer antigens | [27,29] |
| Bispecific T-cell engagers [BiTEs] | Antibody-based therapy that connects the patient’s own T cells to cancer cells, activating the T cells’ cytotoxic function without the need for genetic modification or external manipulation of the T cells | [30] |
| Therapeutic vaccines | Enhance adaptive anti-tumor immune responses by introducing tumor-specific antigens to activate the host immune system | [31,32,33] |
| Vaccine Type | Formulation | Trial Phase | Trail Identification and Target |
|---|---|---|---|
| Cancer cell vaccine | Inactivated tumor cells or lysated | Clinical trial | NCT00676507—Stages III or IV of NSCLC |
| Dendritic cell vaccine | Protein/peptide; DNA; RNA; chemokines | Clinical trial | NCT03546361—Stage IV of NSCLC |
| Protein/peptide vaccine | Recombinant protein; peptide | Clinical trial | NCT04298606—preventing NSCLC recurrence in IB–IIIA stages (production of antibodies against EGF) |
| DNA vaccine | DNA | Clinical trial | NCT05242965—Stage IV of NSCL (given with GM-CSF to help create a stronger immune response |
| mRNA vaccine | mRNA | Clinical trial | NCT05557591—Advanced NSCLC with tumors expressing PD-L1 ≥ 50% |
| circRNA 1 vaccine | circRNA | Preclinical trial | - |
| Neoantigen vaccine | Peptide; protein; mRNA | Clinical trial | NCT03948763—Advanced or metastatic NSCLC with KRAS mutant |
| Cancer Type | Trial Phase | Combination | CLINICALTRIALS.GOV Identifier |
|---|---|---|---|
| Solid tumors | Phase I/Phase II | Chimeric antigen receptor therapy | NCT04503278 |
| Solid tumors | Phase I | Atezolizumab | NCT03289962 |
| Resected solid tumors | Phase I | Pembrolizumab | NCT03313778 |
| Melanoma | Phase II | Pembrolizumab | NCT03897881 |
| Melanoma | Phase II | Cemiplimab | NCT04526899 |
| NSCLC 1 | Phase I | Cemiplimab + docetaxel | NCT05142189 |
| Pancreatic cancer | Phase I | Oxaliplatin, irinotecan, fluorouracil, leucovorin, and atezolizumab | NCT04161755 |
| Head and neck squamous cell carcinoma | Phase II | Pembrolizumab | NCT04534205 |
| CRC 2 | Phase II | None | NCT04486378 |
| Glioblastoma | Phase I | None | NCT04573140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, R.; Vale, N. Emerging Immunotherapies in Lung Cancer: The Latest Advances and the Future of mRNA Vaccines. Vaccines 2025, 13, 476. https://doi.org/10.3390/vaccines13050476
Ramos R, Vale N. Emerging Immunotherapies in Lung Cancer: The Latest Advances and the Future of mRNA Vaccines. Vaccines. 2025; 13(5):476. https://doi.org/10.3390/vaccines13050476
Chicago/Turabian StyleRamos, Raquel, and Nuno Vale. 2025. "Emerging Immunotherapies in Lung Cancer: The Latest Advances and the Future of mRNA Vaccines" Vaccines 13, no. 5: 476. https://doi.org/10.3390/vaccines13050476
APA StyleRamos, R., & Vale, N. (2025). Emerging Immunotherapies in Lung Cancer: The Latest Advances and the Future of mRNA Vaccines. Vaccines, 13(5), 476. https://doi.org/10.3390/vaccines13050476







