Venous Thromboembolic Risk Does Not Increase After a Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine in Cancer Patients Receiving Active Systemic Therapies: Updated Results from the Vax-On-Third-Profile Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Diagnostic Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Occurrence of Thromboembolic Events and SARS-CoV-2 Infections
3.3. Analysis of Immune Correlates
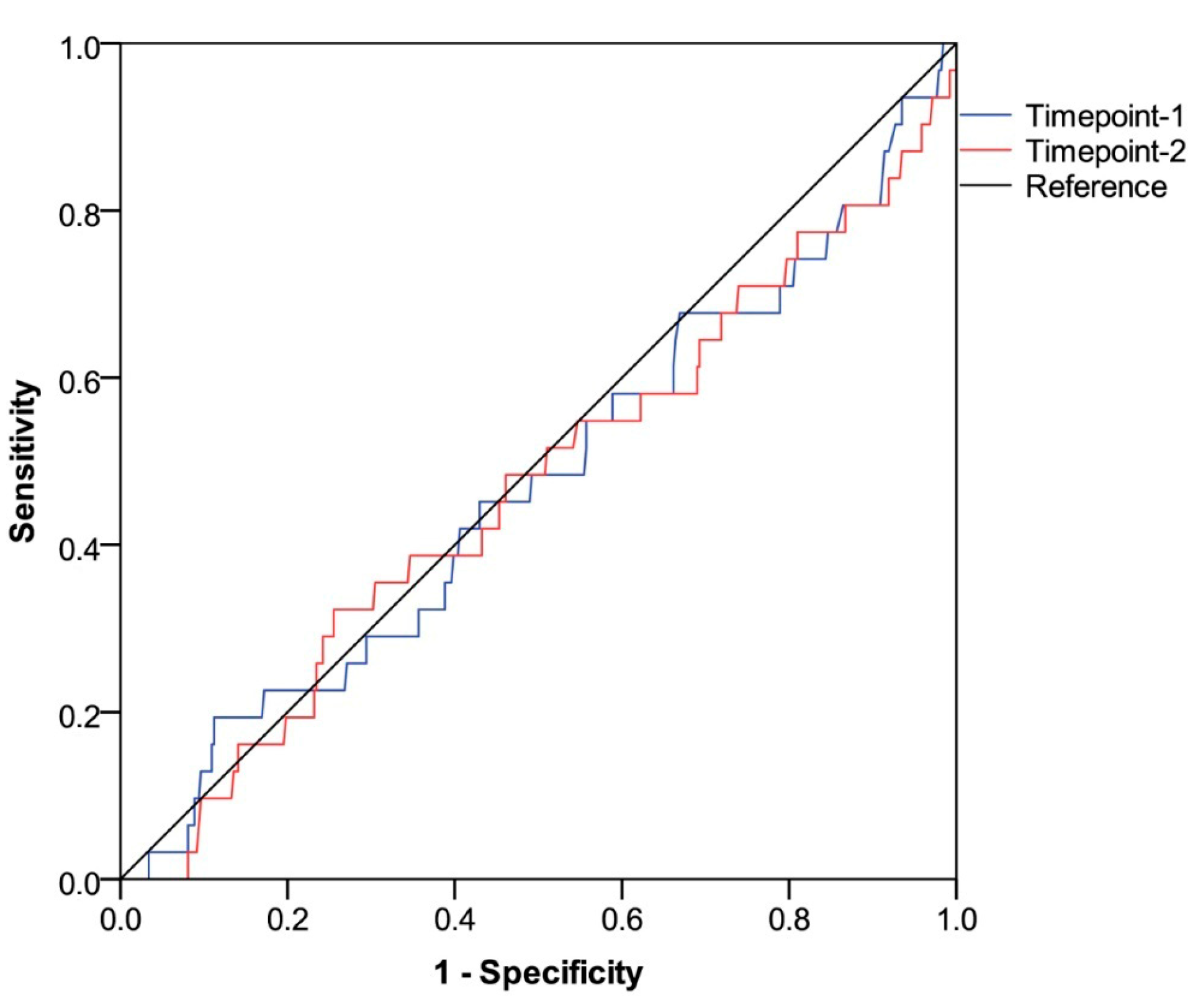
3.4. Assessment of Thromboembolic Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 31 January 2025).
- World Health Organization. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2023. Available online: https://www.who.int/europe/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 31 January 2025).
- COVID-19 Advice for the Public: Getting Vaccinated. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 31 January 2025).
- Hua, T.; Fan, R.; Fan, Y.; Chen, F. Immune response of COVID-19 vaccines in solid cancer patients: A meta-analysis. Hum. Vaccin. Immunother. 2024, 20, 2357424. [Google Scholar] [CrossRef]
- Hosseini-Moghaddam, S.M.; Shepherd, F.A.; Swayze, S.; Kwong, J.C.; Chan, K.K.W. SARS-CoV-2 Infection, Hospitalization, and Mortality in Adults With and Without Cancer. JAMA Netw. Open 2023, 6, 2331617. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Bates, B.; Shao, Y.R.; Hsu, F.C.; Liu, F.; Madhira, V.; Mitra, A.K.; Bergquist, T.; Kavuluru, R.; Li, X.; et al. Risk and Outcome of Breakthrough COVID-19 Infections in Vaccinated Patients With Cancer: Real-World Evidence From the National COVID Cohort Collaborative. J. Clin. Oncol. 2022, 40, 1414–1427. [Google Scholar] [CrossRef]
- Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 31 January 2025).
- Kamboj, M.; Bohlke, K.; Baptiste, D.M.; Dunleavy, K.; Fueger, A.; Jones, L.; Kelkar, A.H.; Law, L.Y.; LeFebvre, K.B.; Ljungman, P.; et al. Vaccination of Adults With Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Prevention and Treatment of Cancer-Related Infections. Version 3.2024 Released 23 September 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf (accessed on 31 January 2025).
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Fors Connolly, A.M. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Buhl, C.; Jacobsen, R.; Almarsdóttir, A.B.; Abtahi, S.; Andersen, A.; Deligianni, E.; Dermiki-Gkana, F.; Kontogiorgis, C.; Oikonomou, C.; Kursite, M.; et al. Public’s perspective on COVID-19 adenovirus vector vaccines after thrombosis with thrombocytopenia syndrome (TTS) reports and associated regulatory actions—A cross-sectional study in six EU member states. Vaccine 2024, 42, 556–563. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Dag Berild, J.; Bergstad Larsen, V.; Myrup Thiesson, E.; Lehtonen, T.; Grøsland, M.; Helgeland, J.; Wolhlfahrt, J.; Vinsløv Hansen, J.; Palmu, A.A.; Hviid, A. Analysis of Thromboembolic and Thrombocytopenic Events After the AZD1222, BNT162b2, and MRNA-1273 COVID-19 Vaccines in 3 Nordic Countries. JAMA Netw. Open 2022, 5, 2217375. [Google Scholar] [CrossRef]
- Yousaf, Z.; Ata, F.; Hammamy, R.A.M. Thrombosis post-mRNA-based SARS-CoV-2 vaccination (BNT162b2)—Time to think beyond thrombosis with thrombocytopenia syndrome (TTS). Thromb. Update 2022, 7, 100104. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [PubMed]
- Aid, M.; Stephenson, K.E.; Collier, A.Y.; Nkolola, J.P.; Michael, J.V.; McKenzie, S.E.; Barouch, D.H. Activation of coagulation and proinflammatory pathways in thrombosis with thrombocytopenia syndrome and following COVID-19 vaccination. Nat. Commun. 2023, 14, 6703. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 2021, 6, e210–e221. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Chin, B.S.; Blann, A.D. Cancer and the prothrombotic state. Lancet Oncol. 2002, 3, 27–34. [Google Scholar]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Lyman, G.H. Risk factors for chemotherapy associated venous thromboembolism in a prospective observational study. Cancer 2005, 104, 2822–2829. [Google Scholar]
- Ruggeri, E.M.; Nelli, F.; Giannarelli, D.; Fabbri, A.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Mazzotta, M.; Schirripa, M.; Signorelli, C.; et al. Dynamic changes in peripheral lymphocytes and antibody response following a third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients. Sci. Rep. 2022, 12, 21908. [Google Scholar]
- Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). Available online: https://www.strobe-statement.org (accessed on 31 January 2025).
- BD FACSCanto™ Software. Available online: https://www.bdbiosciences.com/en-eu/products/software/instrument-software/bd-facscanto-clinical-software (accessed on 31 January 2025).
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- AdviseDx SARS-CoV-2 IgG II. Package Insert. Abbott Laboratories. 2021. Available online: https://www.fda.gov/media/146371/download (accessed on 31 January 2025).
- COVID-19 Integrated Surveillance Data in Italy. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard (accessed on 31 January 2025).
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biomed. J. 2005, 47, 458–472. [Google Scholar]
- Whitaker, H.J.; Farrington, C.P.; Spiessens, B.; Musonda, P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 2006, 25, 1768–1797. [Google Scholar] [CrossRef]
- Tran, H.N.Q.; Risk, M.; Nair, G.B.; Zhao, L. Risk benefit analysis to evaluate risk of thromboembolic events after mRNA COVID-19 vaccination and COVID-19. NPJ Vaccines 2024, 9, 166. [Google Scholar] [PubMed]
- Nicholson, M.; Goubran, H.; Chan, N.; Siegal, D. No apparent association between mRNA COVID-19 vaccination and venous thromboembolism. Blood Rev. 2022, 56, 100970. [Google Scholar]
- Houghton, D.E.; Wysokinski, W.; Casanegra, A.I.; Padrnos, L.J.; Shah, S.; Wysokinska, E.; Pruthi, R.; Ashrani, A.; Sridharan, M.; Baumann-Kreuziger, L.; et al. Risk of venous thromboembolism after COVID-19 vaccination. J. Thromb. Haemost. 2022, 20, 1638–1644. [Google Scholar] [PubMed]
- Mahajan, A.; Brunson, A.; Adesina, O.; Keegan, T.H.M.; Wun, T. The incidence of cancer-associated thrombosis is increasing over time. Blood Adv. 2022, 11, 307–320. [Google Scholar]
- Martens, K.L.; Li, A.; La, J.; May, S.B.; Swinnerton, K.N.; Tosi, H.; Elbers, D.C.; Do, N.V.; Brophy, M.T.; Gaziano, J.M.; et al. Epidemiology of Cancer-Associated Venous Thromboembolism in Patients With Solid and Hematologic Neoplasms in the Veterans Affairs Health Care System. JAMA Netw. Open 2023, 6, e2317945. [Google Scholar]
- Cohen, A.T.; Katholing, A.; Rietbrock, S.; Bamber, L.; Martinez, C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb. Haemost. 2017, 117, 57–65. [Google Scholar]
- Verso, M.; Moik, F.; Graziani, M.; Cohen, A.T. Targeted anti-cancer agents and risk of venous thromboembolism. Haematologica 2024, 109, 3868–3878. [Google Scholar] [PubMed]
- Li, H.; Sun, X.; Sun, D. Thromboembolic events associated with immune checkpoint inhibitors: A real-world study of data from the food and drug administration adverse event reporting system (FAERS) database. Int. Immunopharmacol. 2021, 98, 107818. [Google Scholar]
- Khorana, A.A.; Palaia, J.; Rosenblatt, L.; Pisupati, R.; Huang, N.; Nguyen, C.; Barron, J.; Gallagher, K.; Bond, T.C. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J. Immunother. Cancer 2023, 11, e006072. [Google Scholar]
- Kuter, D.J. Thrombotic complications of central venous catheters in cancer patients. Oncologist 2004, 9, 207–216. [Google Scholar]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [PubMed]
- Alhajjat, A.M.; Redden, C.R.; Langereis, M.; Papastefan, S.T.; Ito, J.A.S.; Ott, K.C.; Turner, L.E.; Kang, H.K.; Shaaban, A.F. CD4 and IL-2 mediated NK cell responses after COVID-19 infection and mRNA vaccination in adults. Immunobiology 2023, 228, 152304. [Google Scholar]
- Mele, D.; Ottolini, S.; Lombardi, A.; Conteianni, D.; Bandera, A.; Oliviero, B.; Mantovani, S.; Cassaniti, I.; Baldanti, F.; Gori, A. Long-term dynamics of natural killer cells in response to SARS-CoV-2 vaccination: Persistently enhanced activity postvaccination. J. Med. Virol. 2024, 96, e29585. [Google Scholar]
- Cuapio, A.; Boulouis, C.; Filipovic, I.; Wullimann, D.; Kammann, T.; Parrot, T.; Chen, P.; Akber, M.; Gao, Y.; Hammer, Q.; et al. NK cell frequencies, function and correlates to vaccine outcome in BNT162b2 mRNA anti-SARSCoV-2 vaccinated healthy and immunocompromised individuals. Mol. Med. 2022, 28, 20. [Google Scholar] [PubMed]
- Graydon, E.K.; Conner, T.L.; Dunham, K.; Olsen, C.; Goguet, E.; Coggins, S.A.; Rekedal, M.; Samuels, E.; Jackson-Thompson, B.; Moser, M.; et al. Natural killer cells and BNT162b2 mRNA vaccine reactogenicity and durability. Front. Immunol. 2023, 14, 1225025. [Google Scholar]
- Nelli, F.; Giannarelli, D.; Fabbri, A.; Virtuoso, A.; Giron Berrios, J.R.; Marrucci, E.; Fiore, C.; Schirripa, M.; Signorelli, C.; Chilelli, M.G.; et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 3217–3228. [Google Scholar]
- Nelli, F.; Fabbri, A.; Virtuoso, A.; Giannarelli, D.; Marrucci, E.; Fiore, C.; Giron Berrios, J.R.; Schirripa, M.; Signorelli, C.; Chilelli, M.G.; et al. Herpes zoster after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in actively treated cancer patients: A prospective study. Clin. Exp. Med. 2024, 24, 13. [Google Scholar]
- Huang, S.L.; Xin, H.Y.; Wang, X.Y.; Feng, G.G.; Wu, F.Q.; Feng, Z.P.; Xing, Z.; Zhang, X.H.; Xin, H.W.; Luo, W.Y. Recent Advances on the Molecular Mechanism and Clinical Trials of Venous Thromboembolism. J. Inflamm. Res. 2023, 16, 6167–6178. [Google Scholar] [CrossRef]
- Bertin, F.R.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar]
- Zhang, X.; Wang, Q.; Shen, Y.; Song, H.; Gong, Z.; Wang, L. Compromised natural killer cells in pulmonary embolism. Int. J. Clin. Exp. Pathol. 2015, 8, 8244–8251. [Google Scholar]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Upadhyaya, B.; Tuballes, K.; Kappes, K.; Gleason, C.R.; Beach, K.; Agte, S.; Srivastava, K.; PVI/Seronet Study Group; Van Oekelen, O.; et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell 2021, 39, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients, N = 429 (100%) |
|---|---|
| Median age, years (SD) | 67.0 (±11.2) |
| - <65 years | 200 (46.6%) |
| - ≥65 years | 229 (53.4%) |
| Sex | |
| - female | 252 (58.7%) |
| - male | 177 (41.3%) |
| Primary tumor diagnosis | |
| - breast | 131 (30.5%) |
| - lung | 82 (19.1%) |
| - kidney | 13 (3.0%) |
| - prostate | 11 (2.6%) |
| - colorectal | 85 (19.8%) |
| - urothelial | 17 (4.0%) |
| - pancreatic | 14 (3.3%) |
| - gastric | 17 (4.0%) |
| - skin (Melanoma, Merkel-cell) | 10 (2.3%) |
| - gynecological | 15 (3.5%) |
| - head and neck | 5 (1.2%) |
| - brain | 15 (3.5%) |
| - others a | 14 (3.3%) |
| Smoking habit | |
| - never | 200 (46.6%) |
| - current or former | 229 (53.4%) |
| BMI | |
| - median (SD) | 25.3 (4.8) |
| - ≥30 kg/m2 | 95 (22.2%) |
| Disease staging | |
| - localized or locally advanced | 173 (40.3%) |
| - metastatic | 256 (59.7%) |
| Treatment setting | |
| - (neo)adjuvant | 176 (41.0%) |
| - metastatic (first or later line) | 253 (59.0%) |
| ECOG PS | |
| - 0 | 220 (51.3%) |
| - 1 | 187 (46.3%) |
| - 2 | 22 (5.1%) |
| Corticosteroid therapy b | 62 (14.5%) |
| Active systemic therapy | |
| - none (reference subgroup) | 119 (27.7%) |
| - targeted therapy | 109 (25.4%) |
| - cytotoxic chemotherapy | 111 (25.9%) |
| - immune checkpoint inhibitors | 39 (9.1%) |
| - hormonal therapy | 21 (4.9%) |
| - cytotoxic chemotherapy and targeted agents | 30 (7.0%) |
| Khorana score c | |
| - high risk | 161 (37.5%) |
| - low risk | 268 (62.5%) |
| Presence of CVC | 104 (24.2%) |
| Previous VTE | |
| - any | 47 (11.0%) |
| - after the first or second of vaccine | 30 (7.0%) |
| - before the start of vaccination | 17 (3.9%) |
| - none | 382 (89.0%) |
| Antiplatelet therapies d | 112 (26.1%) |
| Therapeutic indication for antiplatelet agents | |
| - heart disease | 57 (13.3%) |
| - cerebrovascular disease | 23 (5.4%) |
| - peripheral artery disease | 15 (3.5%) |
| - previous VTE | 12 (2.8%) |
| - hematologic disease | 3 (0.7%) |
| - others | 2 (0.5%) |
| Anticoagulation therapies e | 80 (18.7%) |
| Therapeutic indication for anticoagulant agents | |
| - heart disease | 49 (11.4%) |
| - previous VTE | 12 (2.8%) |
| - primary prophylaxis of cancer-related VTE | 9 (2.1%) |
| - cerebrovascular disease | 7 (1.6%) |
| - others | 3 (0.7%) |
| Parameter | Timepoint-1 | Timepoint-2 | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (N = 429) | No VTE (N = 384) | Any VTE (N = 31) | p Value | All Patients (N = 415) | No VTE (N = 289) | Any VTE (N = 31) | p Value | |
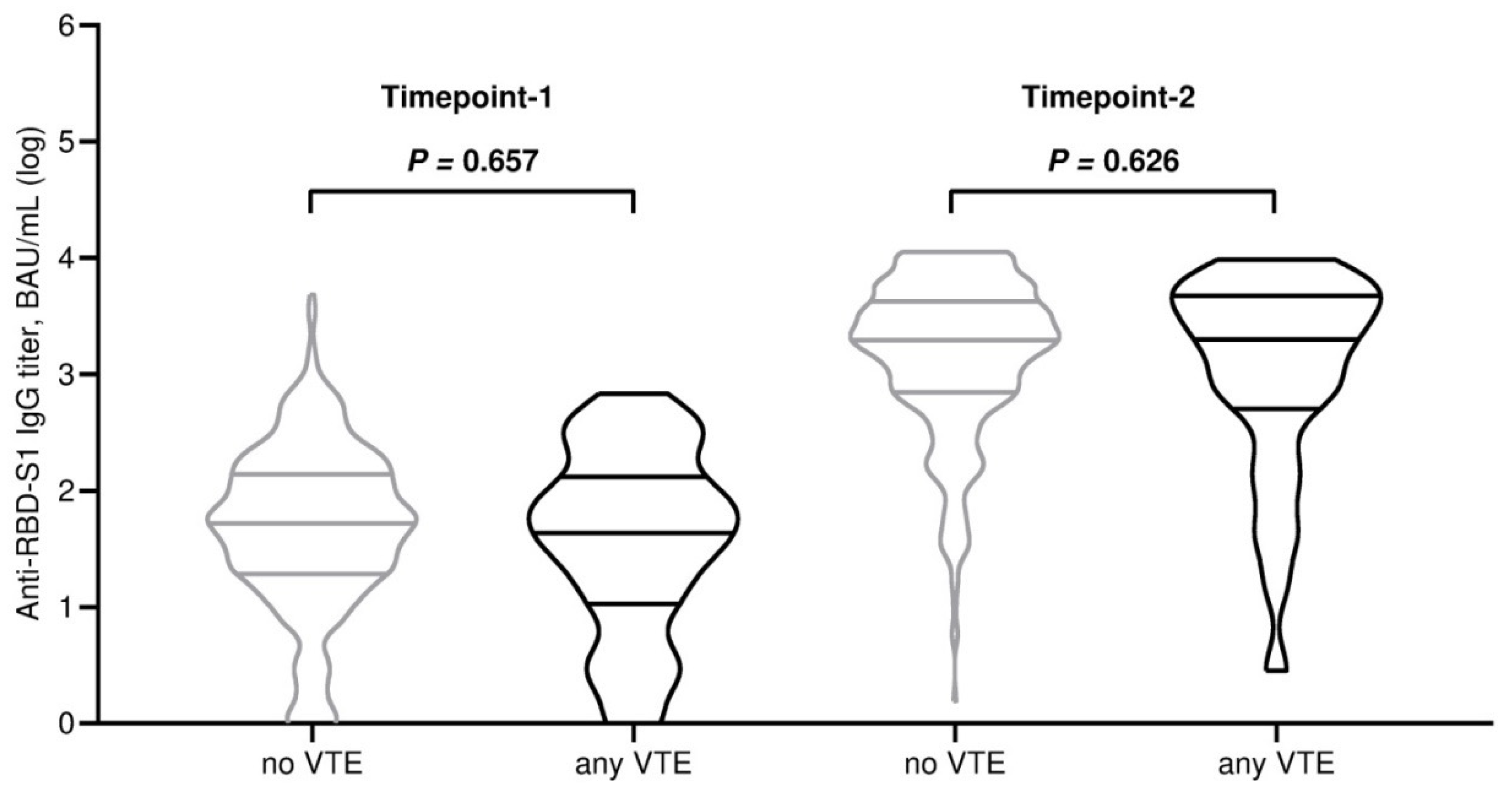
| Anti-RDB-S1 antibody titer (BAU/mL), median (95% CI) | 53 (44–61) | 53 (45–62) | 43 (27–80) | 0.609 | 2064 (1768–2257) | 2066 (1755–2282) | 1981 (809–3570) | 0.566 |
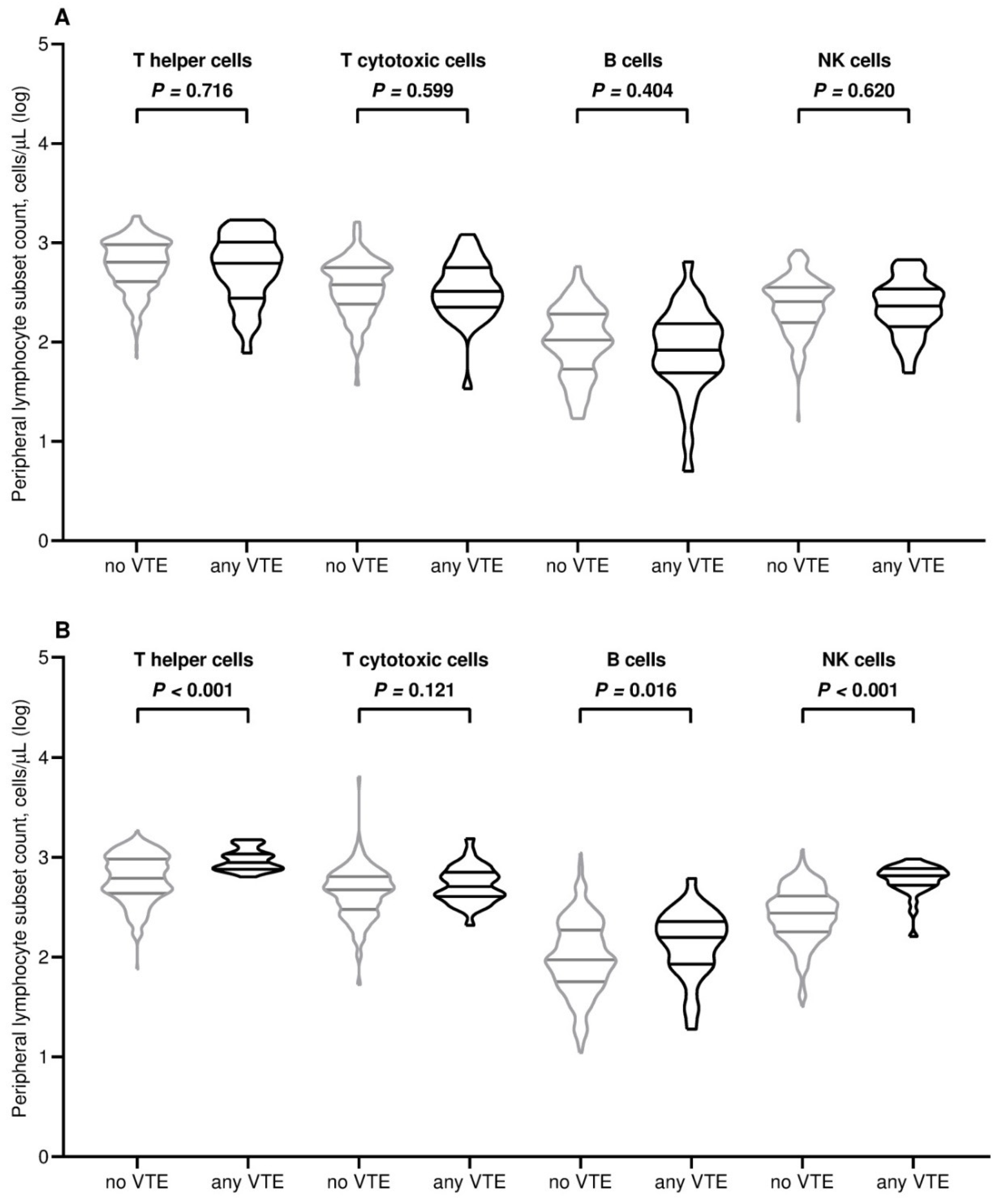
| T helper cell count/µL, median (95% CI) | 639 (601–680) | 639 (591–688) | 623 (439–792) | 0.742 | 680 (610–737) | 615 (583–689) | 882 (786–1040) | <0.001 |
| T cytotoxic cell count/µL, median (95% CI) | 376 (343–392) | 345 (338–393) | 325 (252–394) | 0.601 | 471 (452–507) | 471 (452–507) | 509 (419–639) | 0.121 |
| B cell/µL, median (95% CI) | 100 (95–106) | 100 (96–106) | 83 (57–118) | 0.421 | 97 (92–107) | 94 (90–103) | 157 (111–222) | 0.016 |
| NK cell count/µL, median (95% CI) | 256 (230–274) | 256 (230–274) | 231 (181–313) | 0.611 | 292 (266–311) | 275 (252–299) | 653 (561–692) | <0.001 |
| Covariates | No TEEs (N = 384) | Any TEEs (N = 31) | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age | ||||
| - <65 years (N = 198) | 179 (90.4%) | 19 (9.6%) | 1.00 | - |
| - ≥65 years (N = 217) | 205 (94.5%) | 12 (5.5%) | 0.53 (0.25–1.13) | 0.102 |
| Sex | ||||
| - female (N = 244) | 229 (93.9%) | 15 (6.1%) | 1.00 | - |
| - male (N = 171) | 155 (90.6%) | 16 (9.4%) | 1.57 (0.75–3.21) | 0.224 |
| Tumor type | ||||
| - breast cancer (N = 128) | 118 (92.2%) | 10 (7.8%) | 1.00 | - |
| - lung cancer (N = 79) | 72 (91.1%) | 7 (8.9%) | 1.14 (0.41–3.14) | 0.790 |
| - colorectal cancer (N = 41) | 37 (90.2%) | 4 (9.8%) | 1.27 (0.37–4.30) | 0.695 |
| - genitourinary cancer (N = 86) | 80 (93.0%) | 6 (7.0%) | 0.88 (0.30–2.53) | 0.820 |
| - others (N = 81) | 77 (95.1%) | 4 (4.9%) | 0.61 (0.18–2.02) | 0.422 |
| ECOG PS | ||||
| - 0 (N = 215) | 198 (91.2%) | 17 (7.9%) | 1.00 | - |
| - 1 (N = 179) | 165 (92.2%) | 14 (7.8%) | 0.98 (0.47–2.06) | 0.975 |
| - 2 (N = 21) | 21 (100%) | - | 1.00 (NA) | 0.998 |
| Smoking habits | ||||
| - never (N = 193) | 178 (92.2%) | 15 (7.8%) | 1.00 | - |
| - former or current (N = 222) | 206 (92.8%) | 16 (7.2%) | 0.92 (.044–1.91) | 0.827 |
| BMI | ||||
| - <30 (N = 326) | 303 (93.0%) | 23 (7.0%) | 1.00 | - |
| - ≥30 (N = 89) | 81 (91.0%) | 8 (9.0%) | 1.31 (0.56–3.05) | 0.521 |
| Disease staging | ||||
| - early stage (N = 166) | 156 (94.0%) | 10 (6.0%) | 1.00 | - |
| - advanced stage (N = 249) | 228 (91.6%) | 21 (8.4%) | 1.43 (0.65–3.13) | 0.362 |
| Therapeutic setting | ||||
| - adjuvant or neoadjuvant (N = 169) | 158 (93.5%) | 11 (6.5%) | 1.00 | - |
| - metastatic, any line of therapy (N = 246) | 226 (91.9%) | 20 (8.1%) | 1.27 (0.59–2.72) | 0.538 |
| Type of active treatment | ||||
| - none (N = 118) | 114 (96.6%) | 4 (3.4%) | 1.00 | - |
| - targeted therapies (N = 106) | 94 (88.7%) | 12 (11.3%) | 3.63 (1.13–11.65) | 0.030 |
| - cytotoxic chemotherapy (N = 105) | 98 (93.3%) | 7 (6.7%) | 2.03 (0.57–7.16) | 0.268 |
| - immune checkpoint inhibitors (N = 37) | 31 (83.8%) | 6 (16.2%) | 5.51 (1.46–20.77) | 0.012 |
| - hormonal therapies (N = 20) | 19 (95.0%) | 1 (5.0%) | 1.50 (0.15–14.15) | 0.723 |
| - chemotherapy and targeted agents (N = 29) | 28 (96.5%) | 1 (3.5%) | 1.01 (0.10–9.46) | 0.988 |
| Corticosteroid therapy a | ||||
| - none (N = 354) | 329 (92.9%) | 25 (7.1%) | 1.00 | - |
| - any (N = 61) | 55 (90.2%) | 6 (9.8%) | 1.43 (0.56–3.65) | 0.449 |
| Khorana score b | ||||
| - low risk (N = 260) | 238 (91.5%) | 22 (8.5%) | 1.00 | - |
| - high risk (N = 155) | 146 (94.2%) | 9 (5.8%) | 0.66 (0.29–1.48) | 0.322 |
| Previous VTE | ||||
| - none (N = 369) | 361 (97.8%) | 8 (2.2%) | 1.00 | - |
| - any (N = 46) | 23 (50.0%) | 23 (50.0%) | 14.78 (7.77–28.10) | <0.001 |
| CVC | ||||
| - no (N = 316) | 303 (95.9%) | 13 (4.1%) | 1.00 | - |
| - yes (N = 99) | 81 (81.8%) | 18 (18.2%) | 5.17 (2.43–11.01) | <0.001 |
| Antiplatelet agents c | ||||
| - no (N = 306) | 283 (92.5%) | 23 (7.5%) | 1.00 | - |
| - yes (N = 109) | 101 (92.7%) | 8 (7.3%) | 0.97 (0.42–2.24) | 0.952 |
| Anticoagulation therapy d | ||||
| - no (N = 336) | 313 (93.2%) | 23 (6.8%) | 1.00 | - |
| - yes (N = 78) | 70 (89.7%) | 8 (10.3%) | 1.55 (0.66–3.62) | 0.306 |
| Previous SARS-CoV-2 infection | ||||
| - no (N = 405) | 383 (94.6%) | 22 (5.4%) | 1.00 | - |
| - yes (N = 10) | 1 (10.0%) | 9 (90.0%) | 35.65 (8.1–>100) | <0.001 |
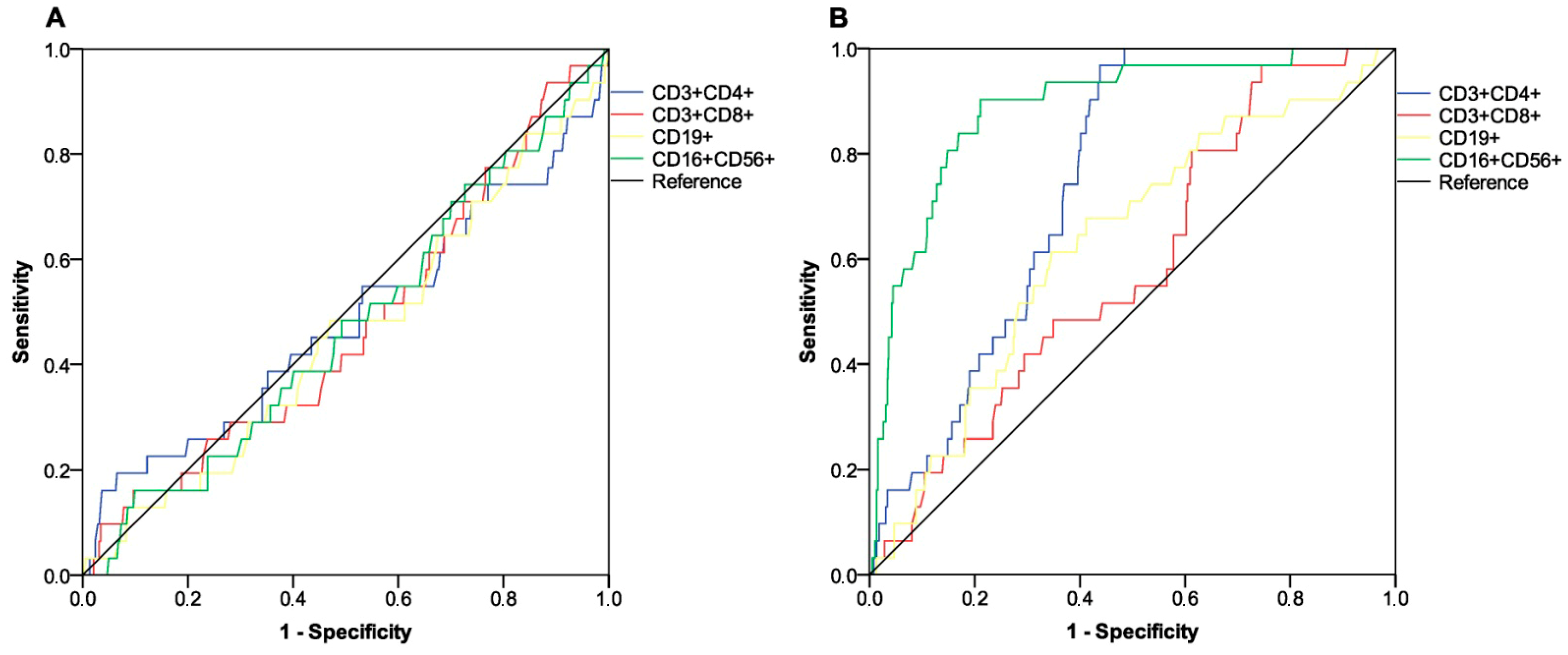
| NK cell count e | ||||
| - low responders (N = 306) | 303 (99.0%) | 3 (1.0%) | 1.00 | - |
| - high responders (N = 108) | 81 (74.1%) | 28 (25.9%) | 12.33 (5.22–29.13) | <0.001 |
| Covariates | OR (95% CI) | p Value |
|---|---|---|
| Type of active treatment | ||
| - none (reference) | 1.00 | - |
| - targeted therapies | 1.23 (0.18–8.45) | 0.830 |
| - cytotoxic chemotherapy | 0.73 (0.11–4.73) | 0.748 |
| - immune checkpoint inhibitors | 0.63 (0.10–3.92) | 0.624 |
| - hormonal therapies | 0.79 (0.13–4.71) | 0.796 |
| - chemotherapy and targeted agents | 1.01 (0.23–4.32) | 0.990 |
| Previous VTE | ||
| - none (reference) | 1.00 | - |
| - any | 9.81 (3.99–24.13) | <0.001 |
| CVC | ||
| - no (reference) | 1.00 | - |
| - yes | 5.02 (1.84–13.67) | 0.002 |
| Previous SARS-CoV-2 infection | ||
| - no (reference) | 1.00 | - |
| - yes | 1.19 (0.40–3.09) | 0.828 |
| NK cell count a | ||
| - low responders (reference) | 1.00 | - |
| - high responders | 6.10 (2.16–17.21) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, F.; Ruggeri, E.M.; Virtuoso, A.; Giannarelli, D.; Barbuta, J.; Chegai, F.; Raso, A.; Panichi, V.; Giron Berrios, J.R.; Schirripa, M.; et al. Venous Thromboembolic Risk Does Not Increase After a Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine in Cancer Patients Receiving Active Systemic Therapies: Updated Results from the Vax-On-Third-Profile Study. Vaccines 2025, 13, 392. https://doi.org/10.3390/vaccines13040392
Nelli F, Ruggeri EM, Virtuoso A, Giannarelli D, Barbuta J, Chegai F, Raso A, Panichi V, Giron Berrios JR, Schirripa M, et al. Venous Thromboembolic Risk Does Not Increase After a Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine in Cancer Patients Receiving Active Systemic Therapies: Updated Results from the Vax-On-Third-Profile Study. Vaccines. 2025; 13(4):392. https://doi.org/10.3390/vaccines13040392
Chicago/Turabian StyleNelli, Fabrizio, Enzo Maria Ruggeri, Antonella Virtuoso, Diana Giannarelli, Jona Barbuta, Fabrizio Chegai, Armando Raso, Valentina Panichi, Julio Rodrigo Giron Berrios, Marta Schirripa, and et al. 2025. "Venous Thromboembolic Risk Does Not Increase After a Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine in Cancer Patients Receiving Active Systemic Therapies: Updated Results from the Vax-On-Third-Profile Study" Vaccines 13, no. 4: 392. https://doi.org/10.3390/vaccines13040392
APA StyleNelli, F., Ruggeri, E. M., Virtuoso, A., Giannarelli, D., Barbuta, J., Chegai, F., Raso, A., Panichi, V., Giron Berrios, J. R., Schirripa, M., Fiore, C., Schietroma, F., Strusi, A., Signorelli, C., Chilelli, M. G., Primi, F., & Fabbri, A. (2025). Venous Thromboembolic Risk Does Not Increase After a Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine in Cancer Patients Receiving Active Systemic Therapies: Updated Results from the Vax-On-Third-Profile Study. Vaccines, 13(4), 392. https://doi.org/10.3390/vaccines13040392





