Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
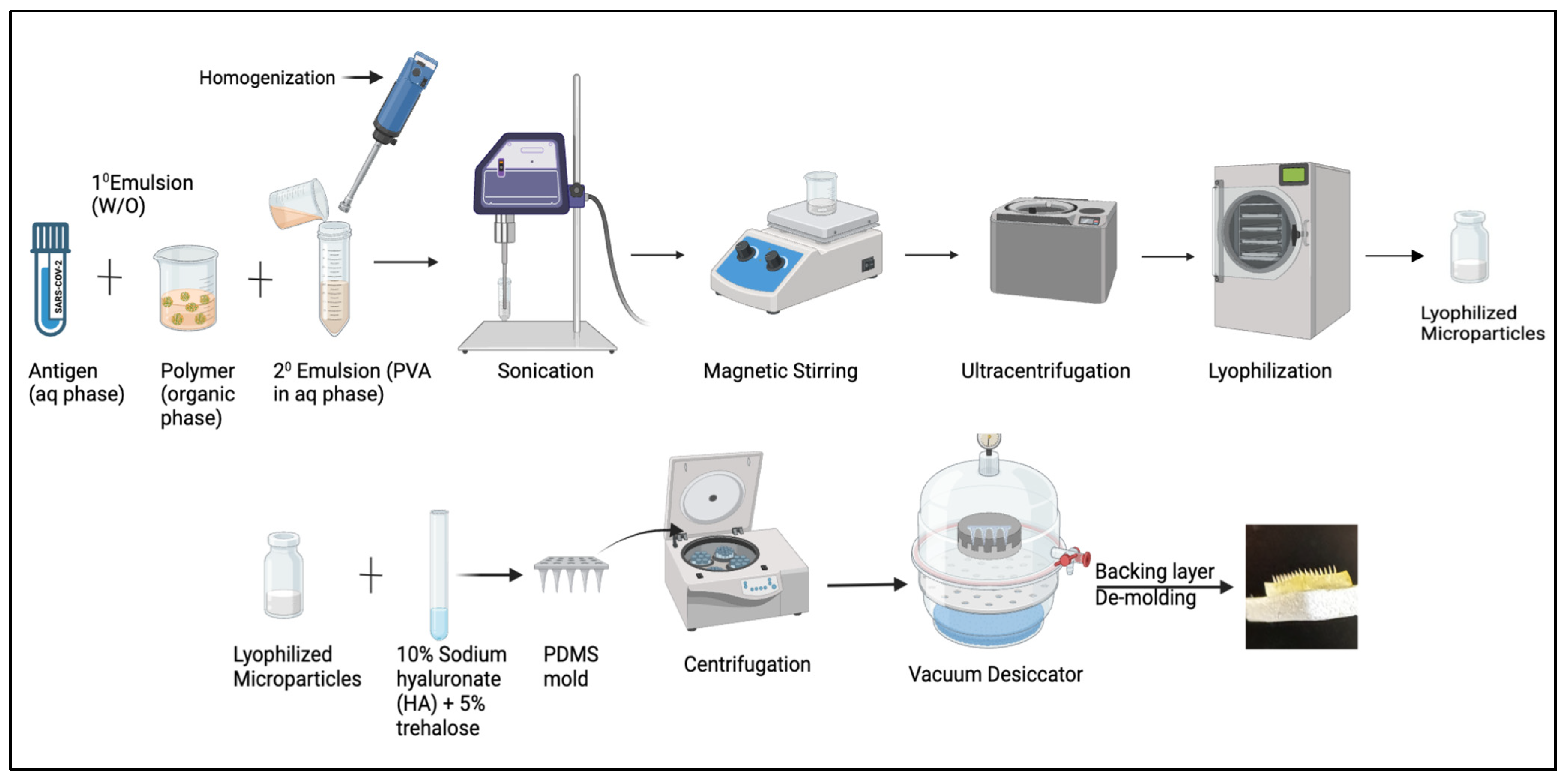
2.2. Formulating Microparticulate Microneedle Vaccine
2.3. Characterization of Vaccine Microparticles
2.3.1. Percent Recovery Yield
2.3.2. Scanning Electron Microscopy
2.3.3. Particle Size, Count, Zeta Potential, and Poly-Dispersibility Index (PDI)
2.3.4. Encapsulation Efficacy
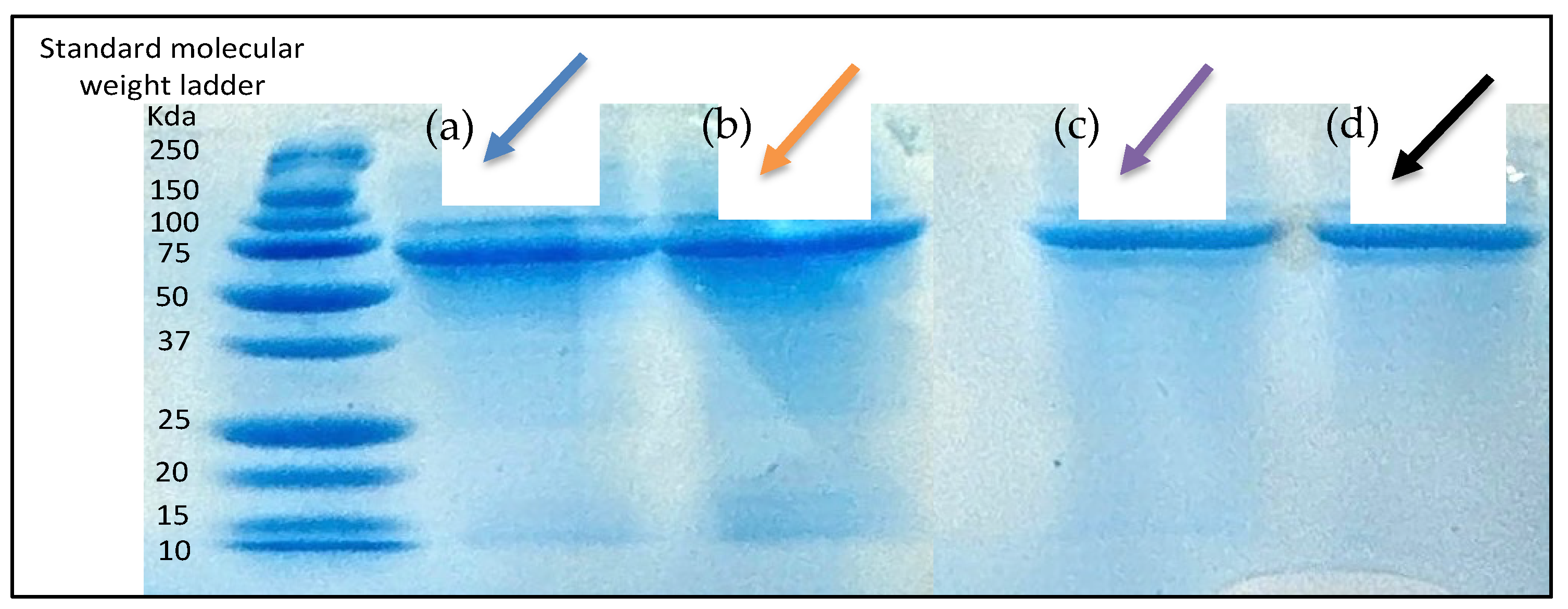
2.3.5. Assessment of Antigen Integrity
2.4. In Vitro Assessment of Microparticles
2.4.1. Cytotoxicity Assessment Using MTT Assay
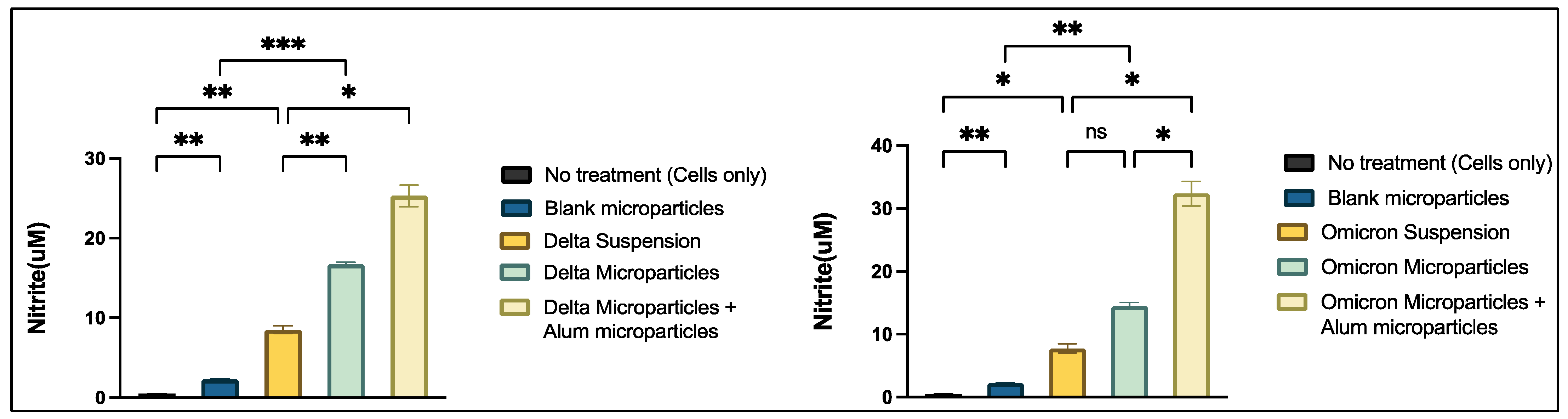
2.4.2. Griess’s Assay
2.5. In Vivo Administration
2.6. Detection of Antibody Response Using Enzyme-Linked Immunosorbent Assay
2.7. Statistical Analysis
3. Results
3.1. Characterization of Microparticulate Microneedles
3.2. SEM Imaging
3.3. In Vitro Assesssment of Microparticles
3.3.1. In-Vitro Cytotoxicity Assessment Using MTT Assay
3.3.2. Determination of Antigen Integrity
3.3.3. Griess Assay
3.4. Analyzing Antibody Responses Using Enzyme-Linked Immunosorbent Assay (ELISA)
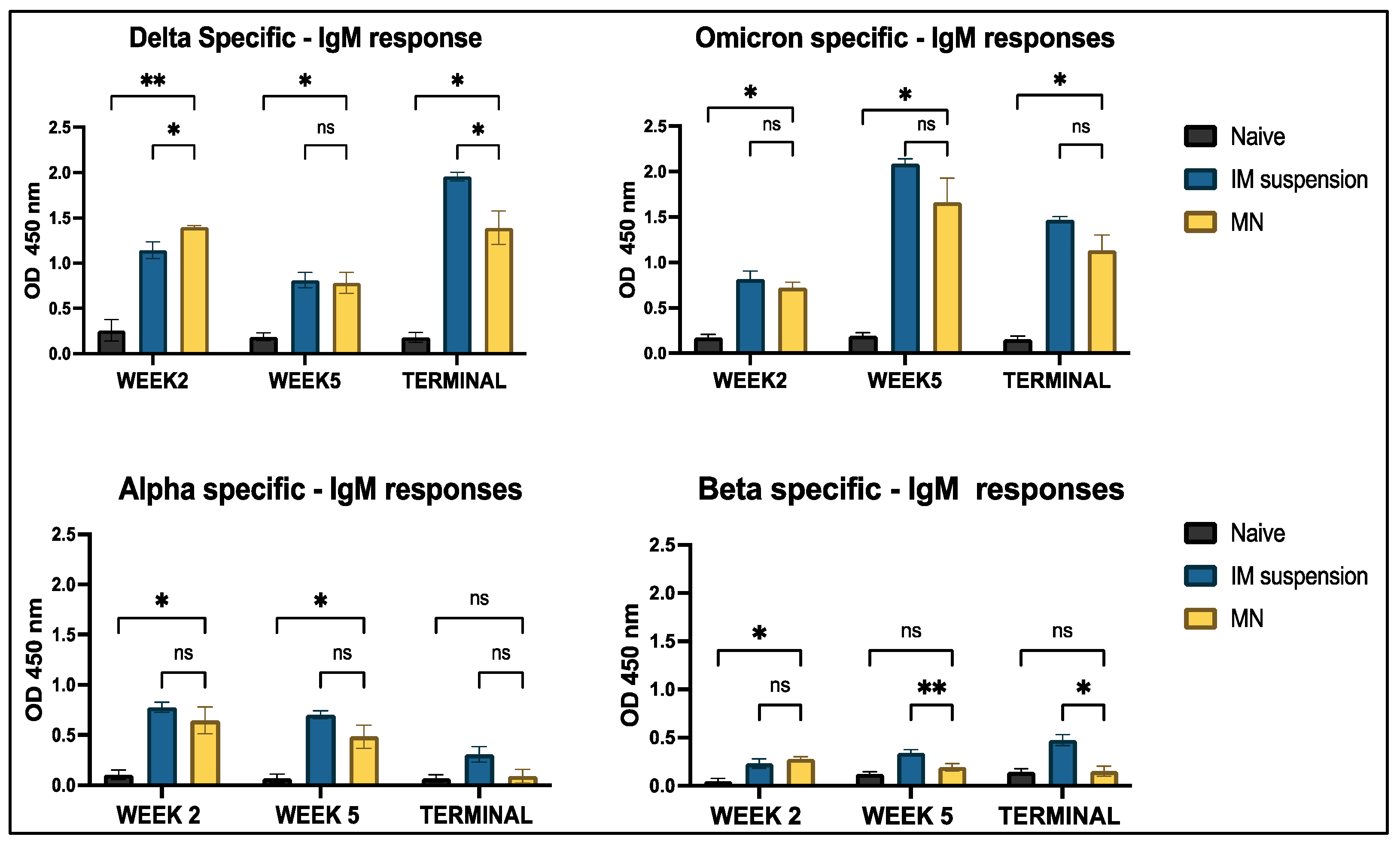
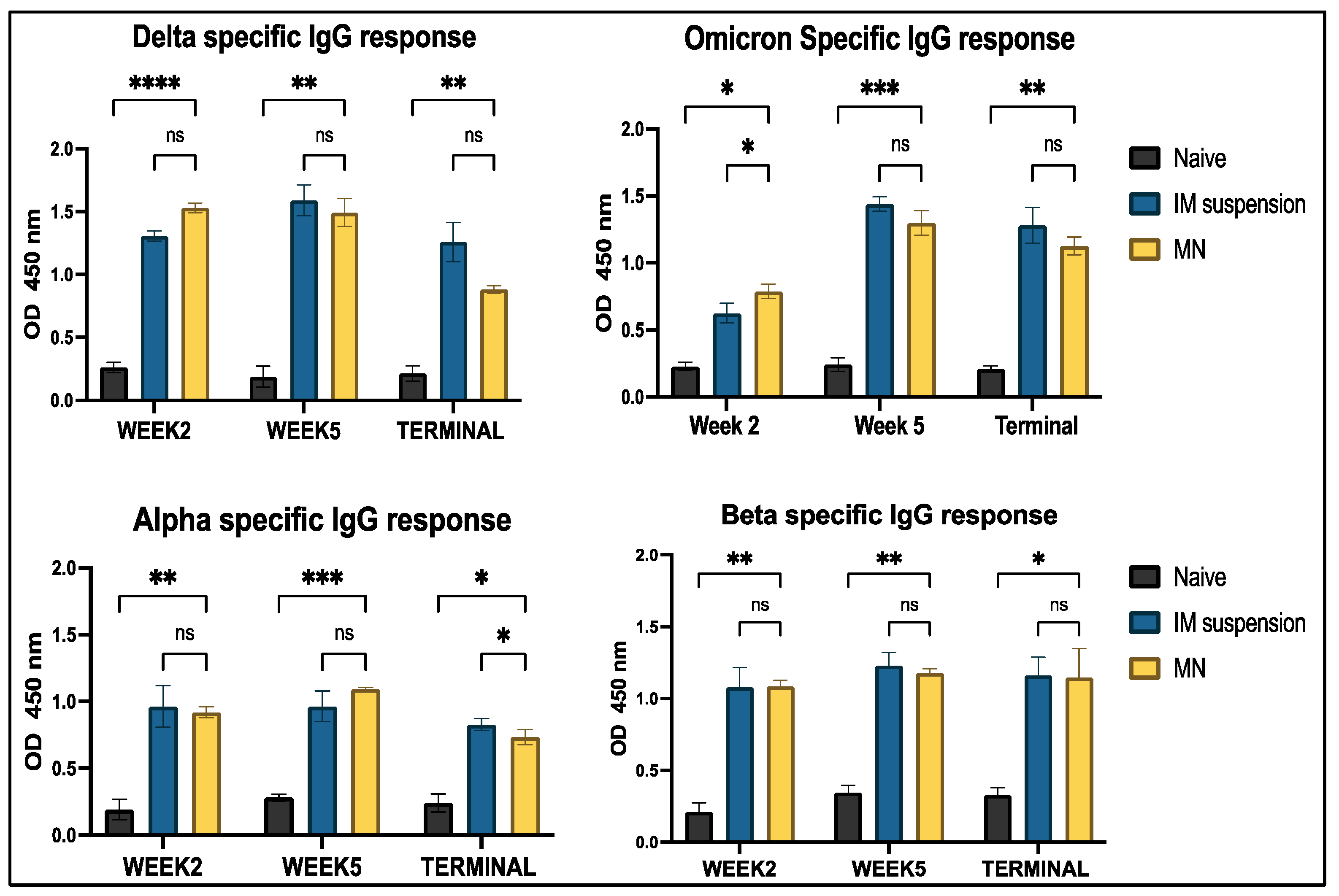
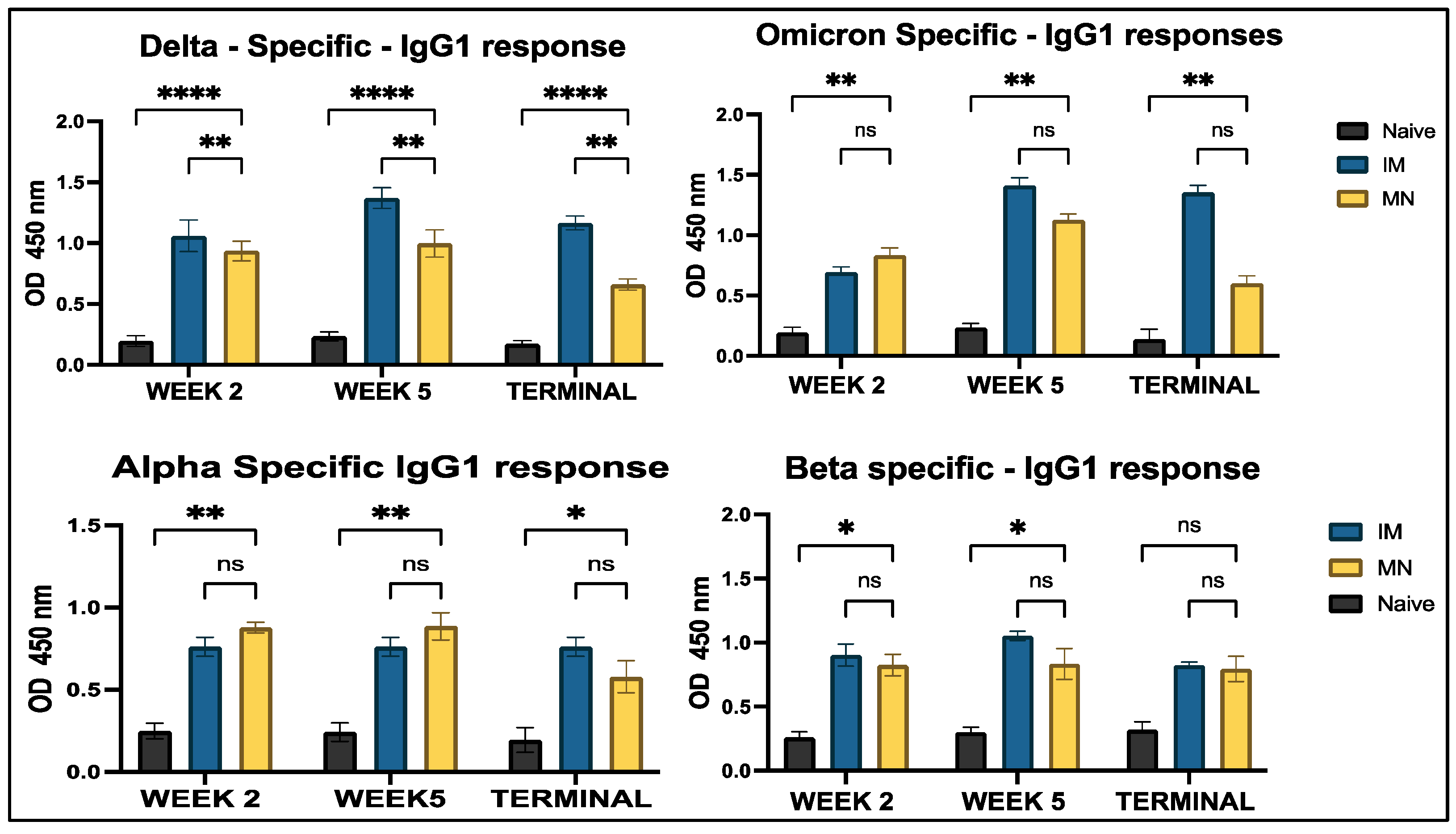
3.4.1. Serum Antibody Response to In Vivo Administration
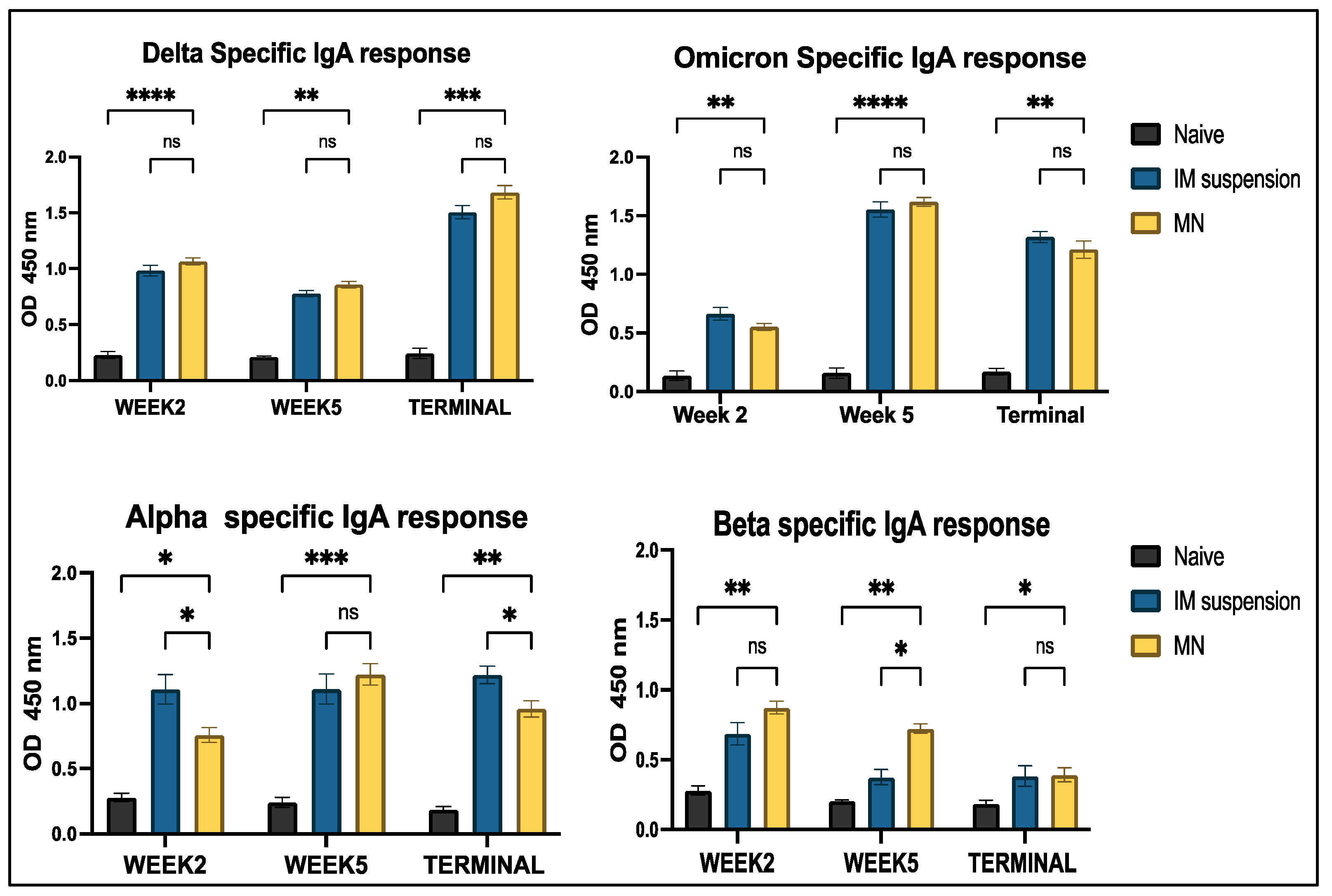
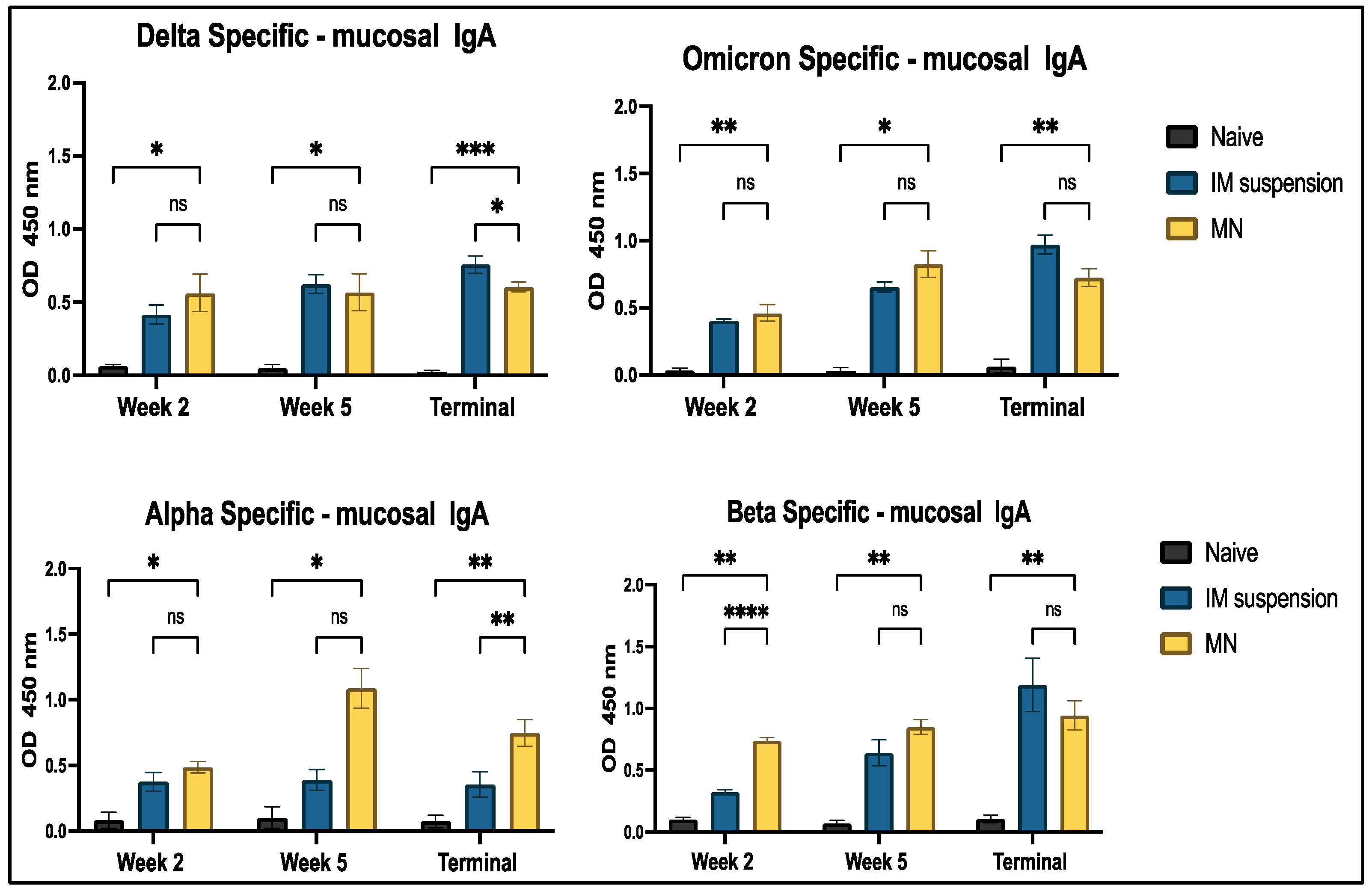
3.4.2. Mucosal Antibody IgA Response to In Vivo Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 27 March 2025).
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Hussham Arshad, M.; et al. COVID-19 Pandemic: From Origins to Outcomes. A Comprehensive Review of Viral Pathogenesis, Clinical Manifestations, Diagnostic Evaluation, and Management. Infez. Med. 2021, 29, 20–36. [Google Scholar] [PubMed]
- Roknuzzaman, A.; Sarker, R.; Nazmunnahar; Shahriar, M.; Mosharrafa, R.A.; Islam, M.R. The WHO Has Declared COVID-19 Is No Longer a Pandemic-Level Threat: A Perspective Evaluating Potential Public Health Impacts. Clin. Med. Insights Pathol. 2024, 17, 2632010X241228053. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations and Immune Escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef]
- Dhawan, M.; Sharma, A.; Priyanka; Thakur, N.; Rajkhowa, T.K.; Choudhary, O.P. Delta Variant (B.1.617.2) of SARS-CoV-2: Mutations, Impact, Challenges and Possible Solutions. Hum. Vaccines Immunother. 2022, 18, 2068883. [Google Scholar] [CrossRef]
- CDC Museum COVID-19 Timeline. 2024. Available online: https://www.cdc.gov/museum/timeline/covid19.html (accessed on 27 March 2025).
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef]
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) Variant, Salient Features, High Global Health Concerns and Strategies to Counter It amid Ongoing COVID-19 Pandemic. Environ. Res. 2022, 209, 112816. [Google Scholar] [CrossRef]
- Chi, W.-Y.; Li, Y.-D.; Huang, H.-C.; Chan, T.E.H.; Chow, S.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. COVID-19 Vaccine Update: Vaccine Effectiveness, SARS-CoV-2 Variants, Boosters, Adverse Effects, and Immune Correlates of Protection. J. Biomed. Sci. 2022, 29, 82. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Kim, K.-H.; Subbiah, J.; Muhammad-Worsham, S.; Park, B.R.; Liu, R.; Grovenstein, P.; Wang, B.-Z.; Kang, S.-M. Heterologous Prime-Boost Vaccination with Inactivated Influenza Viruses Induces More Effective Cross-Protection than Homologous Repeat Vaccination. Vaccines 2023, 11, 1209. [Google Scholar] [CrossRef]
- Pliasas, V.C.; Neasham, P.J.; Naskou, M.C.; Neto, R.; Strate, P.G.; North, J.F.; Pedroza, S.; Chastain, S.D.; Padykula, I.; Tompkins, S.M.; et al. Heterologous Prime-Boost H1N1 Vaccination Exacerbates Disease Following Challenge with a Mismatched H1N2 Influenza Virus in the Swine Model. Front. Immunol. 2023, 14, 1253626. [Google Scholar] [CrossRef]
- Polania, G.; Sunil, M.; Intramuscular Injection. In StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556121/ (accessed on 27 March 2025).
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Alawi, Z.A.; Al Mutair, A. COVID-19 Intranasal Vaccines: Current Progress, Advantages, Prospects, and Challenges. Hum. Vaccines Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, O.P.; Priyanka; Mohammed, T.A.; Singh, I. Intranasal COVID-19 Vaccines: Is It a Boon or Bane? Int. J. Surg. 2021, 94, 106119. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, S.; Patil, S.; Joshi, D.; Menon, I.; Braz Gomes, K.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus. Vaccines 2022, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Braz Gomes, K.; Uddin, M.; D’Souza, M. Zika Vaccine Microparticles (MPs)-Loaded Dissolving Microneedles (MNs) Elicit a Significant Immune Response in a Pre-Clinical Murine Model. Vaccines 2023, 11, 583. [Google Scholar] [CrossRef]
- Patil, S.; Vijayanand, S.; Menon, I.; Gomes, K.B.; Kale, A.; Bagwe, P.; Yacoub, S.; Uddin, M.N.; D’Souza, M.J. Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice. Pharmaceuticals 2023, 16, 1131. [Google Scholar] [CrossRef]
- Bagwe, P.; Bajaj, L.; Gala, R.P.; D’Souza, M.J.; Zughaier, S.M. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022, 10, 983. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A Microneedle Patch for Measles and Rubella Vaccination: A Game Changer for Achieving Elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Delivery 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine Adjuvants: Mechanisms and Platforms. Sig. Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in Aluminum Hydroxide-Based Adjuvant Research and Its Mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Braz Gomes, K.; D’Souza, B.; Vijayanand, S.; Menon, I.; D’Souza, M.J. A Dual-Delivery Platform for Vaccination Using Antigen-Loaded Nanoparticles in Dissolving Microneedles. Int. J. Pharm. 2022, 613, 121393. [Google Scholar] [CrossRef]
- Phoka, T.; Thanuthanakhun, N.; Visitchanakun, P.; Dueanphen, N.; Wanichwecharungruang, N.; Leelahavanichkul, A.; Palaga, T.; Ruxrungtham, K.; Wanichwecharungruang, S. Detachable-Dissolvable-Microneedle as a Potent Subunit Vaccine Delivery Device That Requires No Cold-Chain. Vaccine X 2023, 15, 100398. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Kaurav, M.; Minz, S.; Sahu, K.; Kumar, M.; Madan, J.; Pandey, R.S. Nanoparticulate Mediated Transcutaneous Immunization: Myth or Reality. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1063–1081. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.-T.; Huang, Q. Applications and Delivery Mechanisms of Hyaluronic Acid Used for Topical/Transdermal Delivery—A Review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef]
- Shang, L.; Li, M.; Xu, A.; Zhuo, F. Recent Applications and Molecular Mechanisms of Hyaluronic Acid in Skin Aging and Wound Healing. Med. Nov. Technol. Devices 2024, 23, 100320. [Google Scholar] [CrossRef]
- Figueiredo, M.; Esenaliev, R. PLGA Nanoparticles for Ultrasound-Mediated Gene Delivery to Solid Tumors. J. Drug Deliv. 2012, 2012, 767839. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zuo, X.; Zhou, Z.; Gu, Y.; Zheng, H.; Wang, X.; Wang, G.; Xu, C.; Wang, F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 4333. [Google Scholar] [CrossRef] [PubMed]
- Braz Gomes, K.; Vijayanand, S.; Bagwe, P.; Menon, I.; Kale, A.; Patil, S.; Kang, S.-M.; Uddin, M.N.; D’Souza, M.J. Vaccine-Induced Immunity Elicited by Microneedle Delivery of Influenza Ectodomain Matrix Protein 2 Virus-like Particle (M2e VLP)-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2023, 24, 10612. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Xia, C.Q.; Clare-Salzler, M. Multifunctional Dendritic Cell-Targeting Polymeric Microparticles: Engineering New Vaccines for Type 1 Diabetes. Hum. Vaccines 2011, 7, 37–44. [Google Scholar] [CrossRef][Green Version]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Matsumoto, H.; Haniu, H.; Komori, N. Determination of Protein Molecular Weights on SDS-PAGE. In Electrophoretic Separation of Proteins; Kurien, B.T., Scofield, R.H., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2019; Volume 1855, pp. 101–105. ISBN 978-1-4939-8792-4. [Google Scholar] [CrossRef]
- Buranaamnuay, K. The MTT Assay Application to Measure the Viability of Spermatozoa: A Variety of the Assay Protocols. Open Vet. J. 2021, 11, 251. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture; Cree, I.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 237–245. ISBN 978-1-61779-079-9. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Serrador, J.M. Nitric Oxide Signaling in T Cell-Mediated Immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef]
- Angel, A.; Vaillant, J.; Jamal, Z.; Patel, P. Kamleshun Ramphul Immunoglobulin. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513460 (accessed on 27 March 2025).
- Assaid, N.; Arich, S.; Charoute, H.; Akarid, K.; Anouar Sadat, M.; Maaroufi, A.; Ezzikouri, S.; Sarih, M. Kinetics of SARS-CoV-2 IgM and IgG Antibodies 3 Months after COVID-19 Onset in Moroccan Patients. Am. J. Trop. Med. Hyg. 2023, 108, 145–154. [Google Scholar] [CrossRef]
- Duarte, J.H. Functional Switching. Nat. Immunol. 2016, 17, S12. [Google Scholar] [CrossRef]
- Davis, S.K.; Selva, K.J.; Kent, S.J.; Chung, A.W. Serum IgA Fc Effector Functions in Infectious Disease and Cancer. Immunol. Cell Biol. 2020, 98, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 Variant of Concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Hannan, S.B.; Saikat, T.A.; Limon, M.B.H.; Topu, M.R.; Rana, M.J.; Salauddin, A.; Bosu, S.; Rahman, M.Z. Beta, Delta, and Omicron, Deadliest Among SARS-CoV-2 Variants: A Computational Repurposing Approach. Evol. Bioinform. Online 2023, 19, 11769343231182258. [Google Scholar] [CrossRef]
- Tang, H.; Gao, L.; Wu, Z.; Meng, F.; Zhao, X.; Shao, Y.; Shi, X.; Qiao, S.; An, J.; Du, X.; et al. Corrigendum: Characterization of SARS-CoV-2 Variants N501Y.V1 and N501Y.V2 Spike on Viral Infectivity. Front. Cell. Infect. Microbiol. 2021, 11, 813645. [Google Scholar] [CrossRef]
- Shkunnikova, S.; Mijakovac, A.; Sironic, L.; Hanic, M.; Lauc, G.; Kavur, M.M. IgG Glycans in Health and Disease: Prediction, Intervention, Prognosis, and Therapy. Biotechnol. Adv. 2023, 67, 108169. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Lin, B.; Cheng, L.; Zhang, J.; Yang, M.; Zhang, Y.; Liu, J.; Qin, X. Immunology of SARS-CoV-2 Infection and Vaccination. Clin. Chim. Acta 2023, 545, 117390. [Google Scholar] [CrossRef]
- Berger, A. Science Commentary: Th1 and Th2 Responses: What Are They? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Alqahtani, S.A.M. Mucosal Immunity in COVID-19: A Comprehensive Review. Front. Immunol. 2024, 15, 1433452. [Google Scholar] [CrossRef]
- Andre, M.; Lau, L.-S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Akkaoui, J.; Ales, E.; Brown, H.; Shil, R.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef]




| Study Groups | Route of Administration | Dose of Whole Inactivated Virus and Adjuvant |
|---|---|---|
| Naïve (No Treatment) | NA | NA |
| Intramuscular Suspension | Intramuscular Injection | Prime Dose: 40 µg WIV Delta Antigen + 30 µg Alum Booster Dose: 40 µg WIV Omicron Antigen + 30 µg Alum |
| Microparticulate Microneedle | Intradermal Microneedle | PLGA-Based Microparticles in Dissolving Microneedles Prime Dose: 40 µg WIV Delta Antigen + 30 µg Alum Booster Dose: 40 µg WIV Omicron Antigen + 30 µg Alum |
| Whole Inactivated Virus —Delta Variant | Whole Inactivated Virus —Omicron Variant | |
|---|---|---|
| Average particle size | 692.7 nm ± 38.7 | 731 nm ± 21.3 |
| Particle count | 1197 ± 49.16 | 1227.3 ± 28.9 |
| Zeta potential | −52.9 ± 19.9 | −43 mV ± 15.2 |
| Encapsulation efficacy | 86.5 ± 0.9 | 89.12 ± 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arte, T.M.; Patil, S.R.; Adediran, E.; Singh, R.; Bagwe, P.; Gulani, M.A.; Pasupuleti, D.; Ferguson, A.; Zughaier, S.M.; D’Souza, M.J. Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice. Vaccines 2025, 13, 380. https://doi.org/10.3390/vaccines13040380
Arte TM, Patil SR, Adediran E, Singh R, Bagwe P, Gulani MA, Pasupuleti D, Ferguson A, Zughaier SM, D’Souza MJ. Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice. Vaccines. 2025; 13(4):380. https://doi.org/10.3390/vaccines13040380
Chicago/Turabian StyleArte, Tanisha Manoj, Smital Rajan Patil, Emmanuel Adediran, Revanth Singh, Priyal Bagwe, Mahek Anil Gulani, Dedeepya Pasupuleti, Amarae Ferguson, Susu M. Zughaier, and Martin J. D’Souza. 2025. "Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice" Vaccines 13, no. 4: 380. https://doi.org/10.3390/vaccines13040380
APA StyleArte, T. M., Patil, S. R., Adediran, E., Singh, R., Bagwe, P., Gulani, M. A., Pasupuleti, D., Ferguson, A., Zughaier, S. M., & D’Souza, M. J. (2025). Microneedle Delivery of Heterologous Microparticulate COVID-19 Vaccine Induces Cross Strain Specific Antibody Levels in Mice. Vaccines, 13(4), 380. https://doi.org/10.3390/vaccines13040380









