Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods and Data Sources
2.2. Screening
3. Results
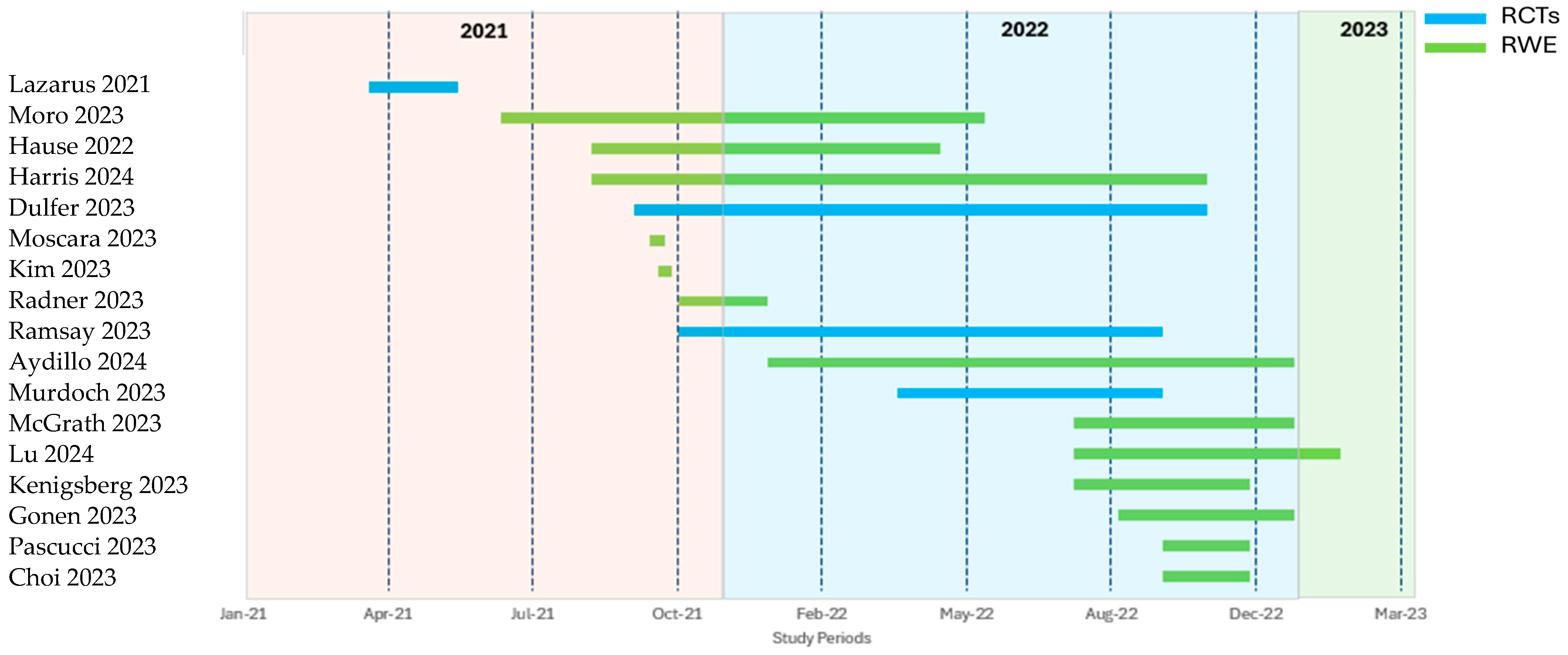
3.1. Study Characteristics
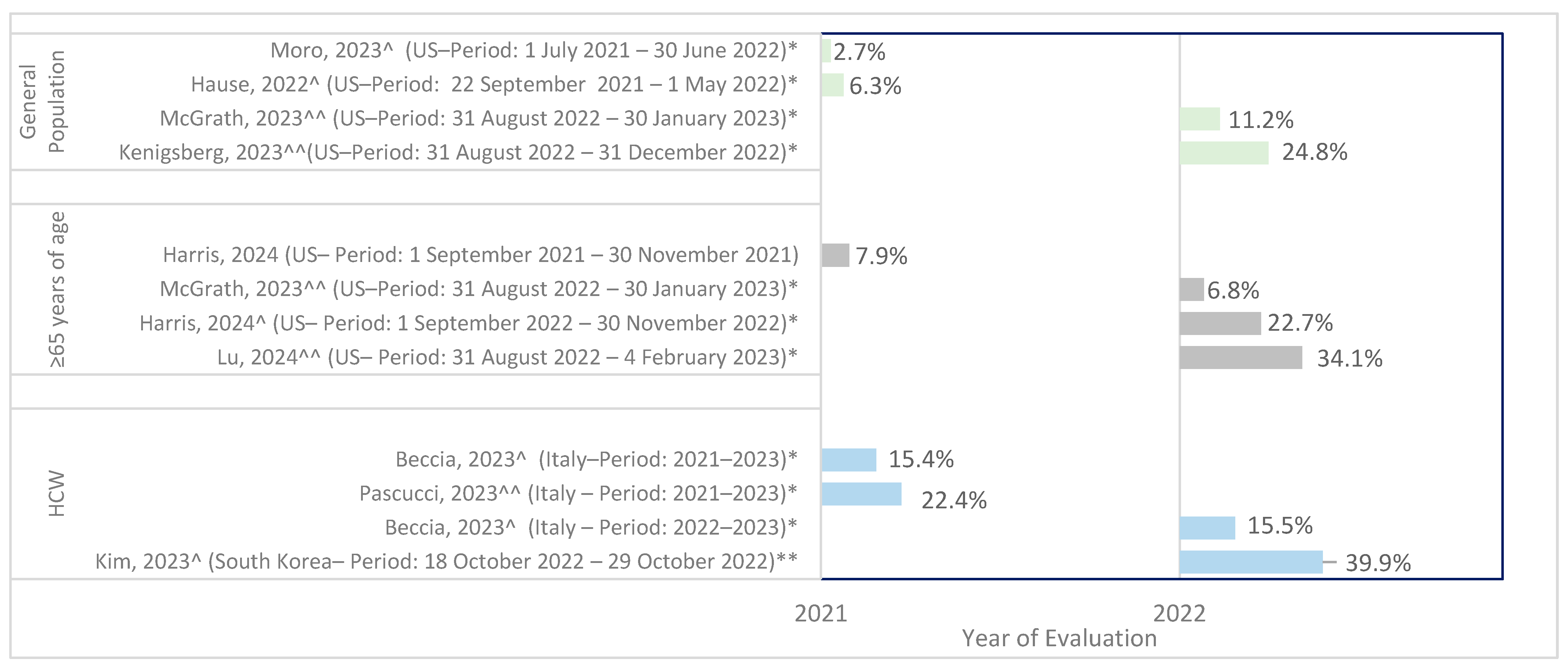
3.2. Prevalence of Co-Administration
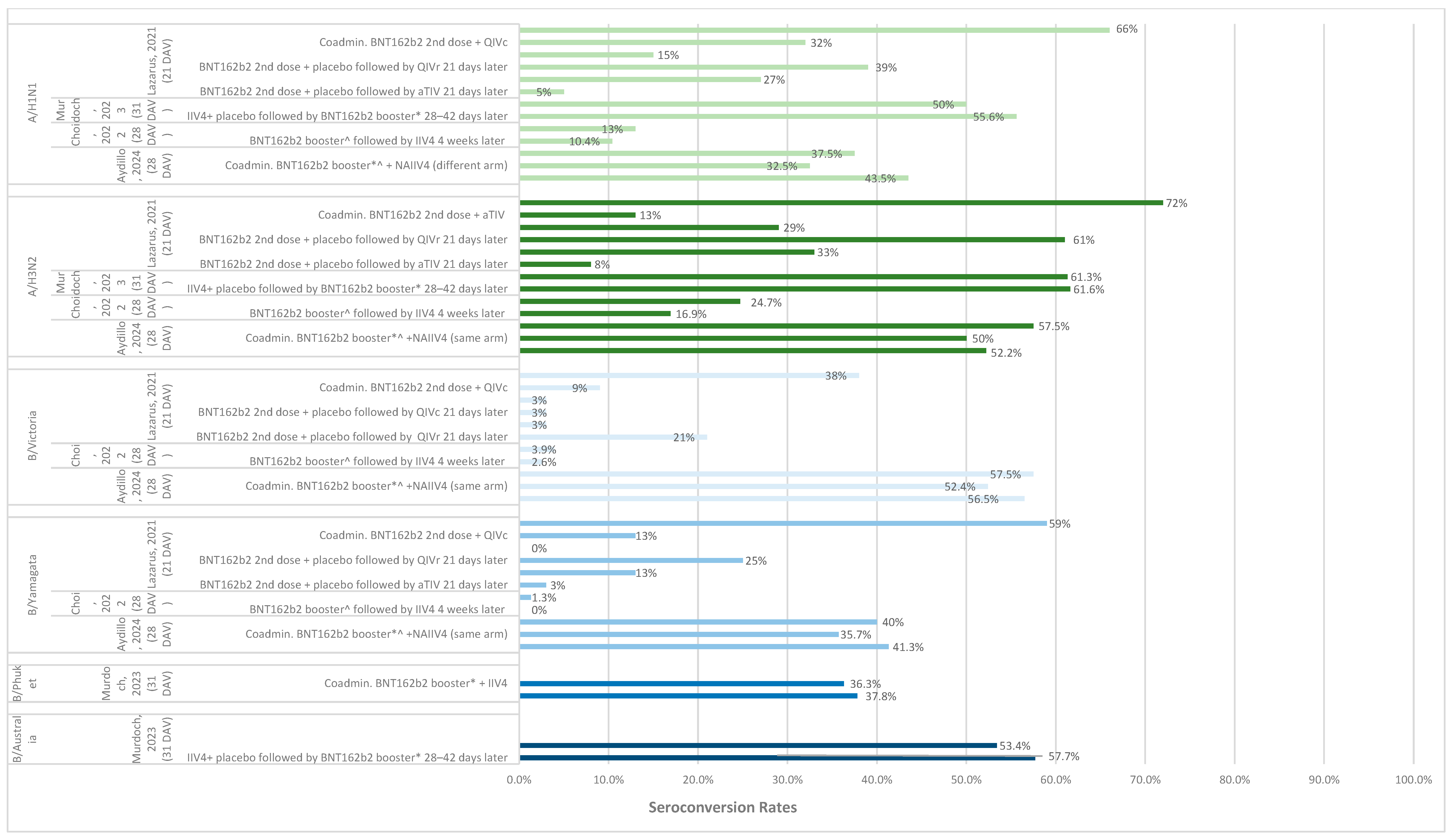
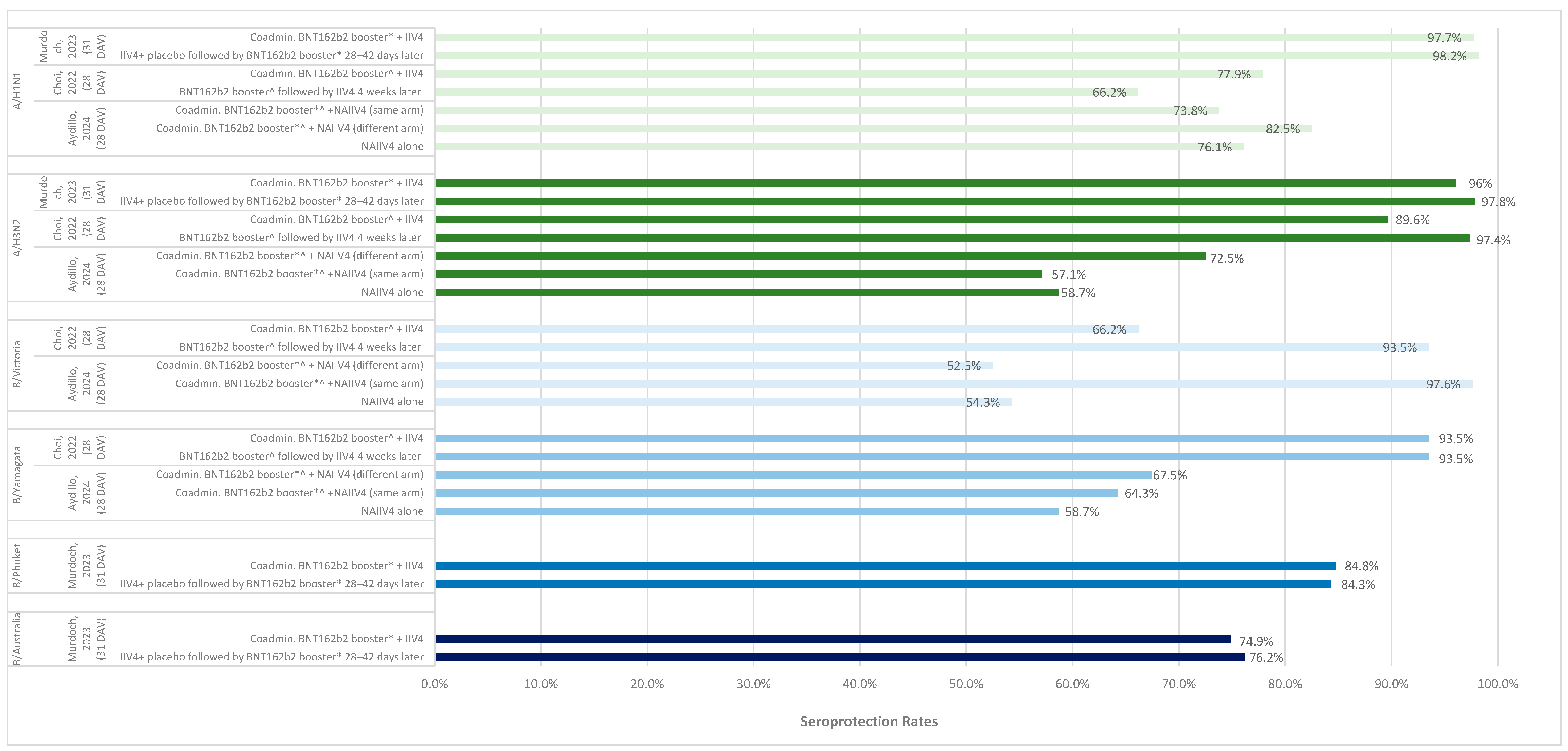
3.3. Immunogenicity of Same-Day Co-Administration
3.3.1. Seroconversion and Seroprotection
3.3.2. Antibody Response Metrics
3.3.3. Cell-Mediated Immunity
3.4. Efficacy and Effectiveness of Same-Day Co-Administration
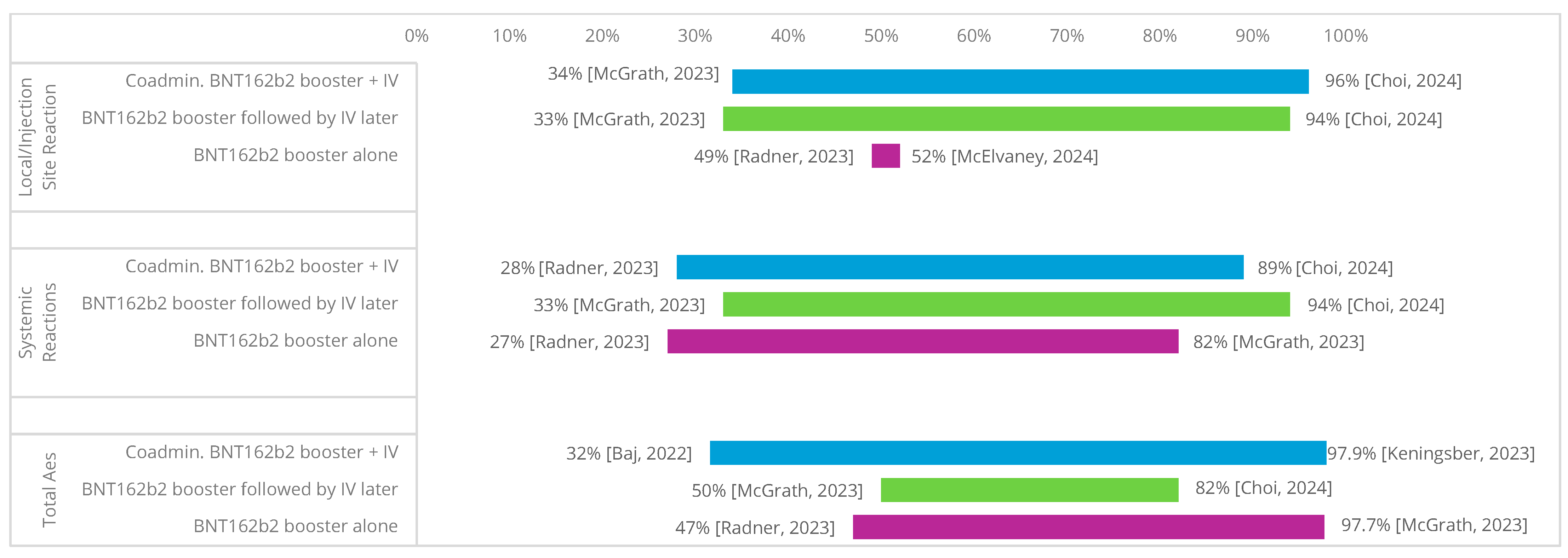
3.5. Safety/Reactogenicity of Same-Day Co-Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author, Year | Study Design | Population Description | Country; Center/Setting | Sample Size | Intervention | Comparator | Length of Follow-Up (Days) | Prevalence | Efficacy/ EFF | IG | Safety/ RTG |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lazarus, 2021 [34] | RCT | General population | UK Multi-center | 679 | 1. Coadmin. BNT162b2 second dose with QIVc (Flucelvax, Seqirus) same day 2. Coadmin. BNT162b2 second dose with MF59C aTIV (FluAd, Seqirus, Maidenhead), same day 3. Coadmin. BNT162b2 second dose with QIVr (Flublok, Sanofi), same day | 1. BNT162b2 second dose with placebo followed by MF59C aTIV (FluAd-Seqirus, Maidenhead) 21 days later 2. BNT162b2 second dose with placebo followed by QIVc (Flucelvax, Seqirus) 21 days later 3. BNT162b2 second dose with placebo followed by QIVr (Flublok, Sanofi) 21 days later | 42 days | No | No | Yes | Yes |
| Dulfer, 2023 [33] | RCT | Patients ≥60 years of age | Netherlands Single center | 154 | 1. Coadmin. BNT162b2 booster with IIV4 (Vaxigrip Tetra, Sanofi), same day | 1. IIV4 (Vaxigrip Tetra, Sanofi) with placebo followed by BNT162b2 booster 21 days later 2. BNT162b2 booster with placebo followed by IIV4 (Vaxigrip Tetra), Sanofi 21 days later 3. BNT162b2 booster with placebo followed by placebo 21 days later | Visits each 21 days apart | No | No | Yes | Yes |
| Murdoch, 2023 [35] | RCT | General population | Australia and New Zealand Multi-center | 564 | 1. Coadmin. BNT162b2 second booster with IIV4 (Afluria Quad, Seqirus), same day | 1. IIV4 (Afluria Quad, Seqirus) with placebo followed by BNT162b2 second booster 28–42 days later | 30 days | No | No | Yes | Yes |
| Ramsay, 2023 [36] | RCT | General population | Australia Multi-center | 71 | 1. Coadmin. BNT162b2 first booster with SIV (including Fluarix Tetra, GlaxoSmithKline, FluQuadri, Sanofi, Fluad Quad, Seqirus), same day 2. Coadmin. BNT162b2 first booster (fourth dose) with SIV (including Fluarix Tetra, GlaxoSmithKline, FluQuadri, Sanofi, Fluad Quad, Seqirus), same day | 1. BNT162b2 first booster with placebo followed by SIV (including Fluarix Tetra, GlaxoSmithKline, FluQuadri, Sanofi, Fluad Quad, Seqirus) 7–14 days later 2. BNT162b2 second booster with placebo followed by SIV (including Fluarix Tetra, GlaxoSmithKline, FluQuadri, Sanofi, Fluad Quad, Seqirus) 7–14 days later | 7 days | No | No | No | Yes |
| Choi, 2024 [37] | Non-randomized trials | General population | Korea Single center | 77 | 1. Coadmin. BNT162b2 bivalent BA.5 second booster with IIV4 (GC flu, GC Biopharma Corp), same day | 1. BNT162b2 bivalent BA.5 second booster followed by IIV4 4 weeks later | 28 days | No | Yes | Yes | Yes |
| Aydillo, 2024 [18] | Observational, Prospective | General population | US, Spain Multi-center | 128 | 1. Coadmin. BNT162b2 booster with NAIIV4 (QIV, Sanofi Pasteur), same arm in the same day 2. Coadmin. BNT162b2 booster with NAIIV4 (QIV, Sanofi Pasteur), different arm on the same day | 1. NAIIV4 alone | 28 days | No | No | Yes | No |
| Harris, 2024 [21] | Observational, Cross-sectional | Medicare beneficiaries ≥66 years of age | US Multi-center | Period 1: 6,292,777 Period 2: 4,757,501 | 1. Coadmin. BNT162b2 booster with SIV (Fluzone High-Dose Quad, Sanofi Pasteur, FluMist Quadrivalent, AstraZeneca, Flucelvax Quadrivalent, Seqirus, Flublok Quadrivalent, Sanofi Pasteur, Fluad Trivalent, CSL Seqirus), same day | NA | 7 | Yes | No | No | No |
| Lu, 2024 [25] | Observational, Case series | Patients ≥65 years of age | US Multi-center | 9,040,176 | 1. Coadmin. BNT162b2 bivalent BA.4/5 booster with QIV (Fluzone, Sanofi Pasteur, Fluad, Seqirus), same day | NA | 90 days | Yes | No | No | No |
| McElvaney, 2024 [26] | Observational, Prospective | Patients with alpha-1 antitrypsin deficiency | Ireland Multi-center | 170 | 1. Coadmin. BNT162b2 BA.4/5 bivalent second booster with QIV (NR), same day | NA | 2 to 8 days | No | No | No | Yes |
| Gonen, 2023 [20]; Moss, 2023 [41] | Observational, Prospective | General population | Israel Single center | 649 | 1. Coadmin. BNT162b2 BA.4/5 bivalent booster with IIV4 (Influvac Tetra, Abbott), same day | 1. BNT162b2 BA.4/5 bivalent booster alone 2. IIV4 alone | 60 days | No | No | Yes | Yes |
| Kenigsberg, 2023 [23] | Observational, Retrospective | General population | US Multi-center | 2,301,876 | 1. Coadmin. BNT162b2 BA.4/5 bivalent booster with QIV (NR), same day | NA | NR | Yes | No | No | No |
| Kim, 2023 [24,39] | Observational, Prospective | Healthcare workers | Korea Single center | 2061 | 1. IIV4 (Boryung FLU Vaccine VIII-TFinj®, Boryung Biopharma) followed by BNT162b2 first booster 1 week later | NA | 7 days | Yes | No | No | No |
| McGrath, 2023 [27] | Observational, Retrospective | General population | US Multi-center | 3,442,996 | 1. Coadmin. BNT162b2 BA.4/5 bivalent booster + SIV (Flublok, Sanofi Pasteur; Fluzone, Sanofi Pasteur, Fluad, Seqirus, Flucelvax, Seqirus, FluMist, AstraZeneca), same day | 1. BNT162b2 BA.4/5 bivalent booster alone 2. SIV alone (Flublok, Sanofi Pasteur, Fluzone, Sanofi Pasteur, Fluad, Seqirus, Flucelvax, Seqirus, FluMist, AstraZeneca) | Median: 109 days (IQR, 89–125) for the co-administration of BNT162b2-biv and SIV Median: 51 days (IQR, 17–99) for the BNT162b2-biv alone Median: 90 days (IQR, 49–112) for SIV alone | Yes | Yes | No | No |
| Moro, 2023 [28] | Observational, Retrospective | General population | US Multi-center | 2449 | 1. Coadmin. BNT162b2 first booster with SIV (Fluzone, Sanofi Pasteur, Afluria, Seqirus, Fluzone high dose, Sanofi Pasteur), same medical visit | NA | NR | Yes | No | No | No |
| Moscara, 2023 [29] | Observational, Prospective | Healthcare workers | Italy Single center | 942 | 1. Coadmin. BNT162b2 booster with QIVc (Flucelvax Tetra, Seqirus), same day | 1. BNT162b2 booster alone 2. QIVc followed by BNT162b2 booster after 22 October 2021 | 280 days | No | Yes | No | Yes |
| Pascucci, 2023 [30,38] | Observational, Retrospective | Healthcare workers | Italy Single center | 7399 | 1. Coadmin. BNT162b2 BA.4/5 bivalent booster with QIV (Vaxigrip Tetra, Sanofi Pasteur, Flucelvax Tetra, Seqirus), same day | NA | NR | Yes | No | No | No |
| Radner, 2023 [31] | Observational, Prospective | Healthcare workers | Austria Multi-center | 838 | 1. Coadmin. BNT162b2 first booster with QIV (Vaxigrip Tetra, Sanofi Pasteur, Flucelvax Tetra, Seqirus), same day | 1. BNT162b2 first booster alone 2. QIV (Vaxigrip Tetra, Sanofi Pasteur, Flucelvax Tetra, Seqirus) alone | 28 days | No | No | No | Yes |
| Baj, 2022 [19] | Observational, Prospective | Healthcare workers | Italy Single center | 64 | 1. Coadmin. BNT162b2 first booster with IIV4 (Vaxigrip Tetra, Sanofi), same day | NA | 14 days | No | No | No | Yes |
| Hause, 2022 [22] | Observational, Retrospective | General population | US NR | 61,390 | 1. Coadmin. BNT162b2 first booster with IV (NR), same day | 1. BNT162b2 first booster alone | 0 to 7 days post-vaccination | Yes | No | No | Yes |
| Venuto, 2022 [32] | Observational, Retrospective | Healthcare workers | Italy Single center | 64 | 1. Coadmin. BNT162b2 first booster + QIV (Vaxigrip Tetra, Sanofi, Flucelvax Tetra, Seqirus), same day | 1. BNT162b2 first booster alone | 30 days | No | No | No | Yes |
| Trial, Reference | Bias From | Overall Bias | ||||
|---|---|---|---|---|---|---|
| Randomization Process | Deviations From Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | ||
| Dulfer, 2023 [33] |  |  |  |  |  |  |
| Ramsay, 2023 [36] |  |  |  |  |  |  |
| Lazarus, 2021 [34] |  |  |  |  |  |  |
| Murdoch, 2023 [35] |  |  |  |  |  |  |
 | ||||||
| Trial, Reference | Bias From | Overall Bias | ||
|---|---|---|---|---|
| Selection | Comparability | Outcome | ||
| Harris, 2024 [21] | 3 | 0 | 3 | 6 |
| Baj, 2022 [19] | 3 | 0 | 3 | 6 |
| Gonen, 2023 [20] | 3 | 0 | 3 | 6 |
| Choi, 2024 [37] | 3 | 0 | 3 | 6 |
| Hause, 2022 [22] | 4 | 1 | 2 | 7 |
| Kenigsberg, 2023 [23] | 4 | 0 | 3 | 7 |
| Kim, 2023 [24] | 3 | 0 | 3 | 6 |
| Lu, 2024 [25] | 3 | 0 | 3 | 6 |
| McElvaney, 2024 [26] | 3 | 0 | 3 | 6 |
| McGrath, 2023 [27] | 4 | 0 | 3 | 7 |
| Aydillo, 2024 [18] | 3 | 0 | 3 | 6 |
| Moro, 2023 [28] | 3 | 0 | 3 | 6 |
| Moscara, 2023 [29] | 3 | 1 | 3 | 7 |
| Venuto, 2022 [32] | 3 | 0 | 3 | 6 |
| Pascucci, 2023 [30] | 3 | 0 | 3 | 6 |
| Radner, 2023 [31] | 3 | 0 | 3 | 6 |
| Outcomes | Moscara, 2023 [29] | McGrath, 2023 [27] | Choi, 2024 [37] | |||||
| Coadmin. BNT162b2 Booster with QIV, Same Day | BNT162b2 Booster Alone | QIVc Followed by BNT162b2 Booster after the 22nd of October 2021 | Coadmin.BNT162b2 BA.4/5 Bivalent Booster with SIV, Same Day | BNT162b2 BA.4/5 Bivalent Booster Alone | SIV Alone | Coadmin. BNT162b2 Bivalent BA.5 Second Booster with IIV4, Same Day | BNT162b2 Bivalent BA.5 Second Booster Followed by IIV4 4 Weeks Later | |
| Influenza vaccines | ||||||||
| Influenza-like infection (ILI), n (%) | NR | NR | NR | NR | NR | NR | 1 (1.3%) | 0 (0) |
| Hospitalizations for influenza | NR | NR | NR | 18 to 64 years: 0.03% ≥65 years: 0.07% | NR | 18 to 64 years: 0.03% ≥65 years: 0.09% | NR | NR |
| Influenza-related medical encounters (including emergency department or urgent care), % | NR | NR | NR | 18 to 64 years: 0.09% ≥65 years: 0.22% | NR | 18 to 64 years: 0.08% ≥65 years: 0.23% | NR | NR |
| Influenza-related medical encounters (including outpatient visits), % | NR | NR | NR | 18 to 64 years: 0.70% ≥65 years: 0.45% | NR | 18 to 64 years: 0.94% ≥65 years: 0.52% | NR | NR |
| BNT162b2 vaccines | ||||||||
| Laboratory-confirmed SARS-CoV-2 infection (regardless of presence of symptoms) | 40.00 per 100 subjects (95% CI: 36.11–43.89) | 44.96 per 100 subjects (95% CI: 38.64–51.28) | 42.55 per 100 subjects (95% CI: 32.56/52.55) | NR | NR | NR | NR | NR |
| Laboratory-confirmed symptomatic COVID-19 | 32.62 per 100 subjects (95% CI: 28.90–36.34) | 37.82 per 100 subjects (95% CI: 31.65–43.98) | 36.17 per 100 subjects (95% CI: 26.46–48.88) | NR | NR | NR | NR | NR |
| COVID-19--like infection (CLI), n (%) | NR | NR | NR | NR | NR | NR | 0 (0) | 0 (0) |
| COVID-19-related hospitalizations, % | NR | NR | NR | 18 to 64 years: 0.03% ≥65 years: 0.13% | 18 to 64 years: 0.02% ≥65 years: 0.12% | NR | NR | NR |
| COVID-19-related medical encounters (including emergency department or urgent care), % | NR | NR | NR | 18 to 64 years: 0.06% ≥65 years: 0.50% | 18 to 64 years: 0.04% ≥65 years: 0.42% | NR | NR | NR |
| COVID-19-related medical encounters (including outpatient visits), % | NR | NR | NR | 18 to 64 years: 2.06% ≥65 years: 1.94% | 18 to 64 years: 1.71% ≥65 years: 1.76% | NR | NR | NR |
| Author, Year | Population Description | Treatment Name | Sample Size | Timepoint | Local/Injection Site Reaction, n (%) | Systemic Reactions, n (%) | Total AEs, n (%) |
|---|---|---|---|---|---|---|---|
| Baj, 2022 [19] | Healthcare workers | Coadmin. BNT162b2 booster + IIV4 | 36 | 14 days after vaccination | NR | NR | 18 (50.0) |
| BNT162b2 booster alone | 28 | NR | NR | 20 (71.0) | |||
| Choi, 2024 [37] | General population | Coadmin. BNT162b2 BA.4/5 bivalent booster + IIV4 | 77 | 7 days after vaccination | 36 (46.8) | 47 (61.0) | NR |
| BNT162b2 BA.4/5 bivalent booster + IIV4 at least 4 weeks apart | 77 | 43 (55.8) | 50 (64.9) | NR | |||
| Dulfer, 2023 [33] | Patients ≥60 years of age | Coadmin. BNT162b2 booster + IIV4 | 38 | 14 days after vaccination | Redness at injection site: 5 (13.2) Pain at injection site: 33 (86.8) Swollen at injection site: 3 (7.9) | Fever: 1 (2.6) Fatigue: 9 (23.7) Myalgia: 13 (34.2) Joint pain: 7 (18.4) Headache: 12 (31.6) Chills: 5 (13.2) Nausea: 0 (0.0) | NR |
| IIV4 + placebo followed by BNT162b2 booster 21 days later | 38 | Redness at injection site: 3 (7.9) Pain at injection site: 8 (21.1) Swollen at injection site: 1 (2.6) | Fever: 0 (0.0) Fatigue: 7 (18.4) Myalgia: 3 (7.9) Joint pain: 4 (10.5) Headache: 8 (21.1) Chills: 2 (5.3) Nausea: 0 (0.0) | NR | |||
| BNT162b2 booster + placebo followed by IIV4 21 days later | 38 | Redness at injection site: 5 (13.2) Pain at injection site: 24 (63.2) Swollen at injection site: 9 (23.7) | Fever: 2 (5.3) Fatigue: 8 (21.1) Myalgia: 7 (18.4) Joint pain: 3 (7.9) Headache: 6 (15.8) Chills: 4 (10.5) Nausea: 1 (2.6) | NR | |||
| BNT162b2 booster + placebo followed by placebo 21 days later | 38 | Redness at injection site: 3 (7.9) Pain at injection site: Swollen at injection site: 4 (10.5) | Fever: 3 (7.9) Fatigue: 6 (15.8) Myalgia: 10 (26.3) Joint pain: 3 (7.9) Headache: 9 (23.7) Chills: 5 (13.2) Nausea: 3 (7.9) | NR | |||
| Gonen, 2023 [20] | General population | Coadmin. BNT162b2 BA.4/5 bivalent booster + IIV4 | 146 | Mean 28.9 days after vaccination | 76 (52.0) | 40 (28.0) | NR |
| BNT162b2 booster alone | 85 | 42 (49.0) | 23 (27.0) | NR | |||
| Hause, 2022 [22] | General population | Coadmin. BNT162b2 booster + influenza vaccine | 61,390 | 7 days after vaccination | 39,818 (64.9) | 422,637 (58.9) | NR |
| BNT162b2 booster alone | 466,439 | 298,529 (64.0) | 274,539 (58.9) | NR | |||
| Lazarus, 2021 [34] | General population | BNT162b2 2nd dose + placebo followed by QIVc 21 days later | 71 | 7 days after vaccination | 67 (94) | 54/67 (81) | NR |
| Coadmin. BNT162b2 2nd dose + QIVc | 68 | 65 (96) | 59 (87) | NR | |||
| BNT162b2 2nd dose + placebo followed by MF59C aTIV 21 days later | 38 | 30 (79) | 25/35 (71) | NR | |||
| Coadmin. BNT162b2 2nd dose + MF59C aTIV | 41 | 31 (76) | 24 (59) | NR | |||
| BNT162b2 2nd dose + placebo followed by QIVr 21 days later | 29 | 24/27 (89) | 23/28 (82) | NR | |||
| Coadmin. BNT162b2 2nd dose + QIVr | 29 | 25/26 (96) | 24/27 (89) | NR | |||
| McElvaney, 2024 [26] | Patients with alpha-1 antitrypsin deficiency | Coadmin. BNT162b2 BA.4/5 bivalent booster + QIV | 44 | 8 days after vaccination | 15 (34.0) | 22 (50.0) | NR |
| BNT162b2 BA.4/5 bivalent booster followed by QIV one week later | 40 | 13 (33.0) | 20 (50) | ||||
| Moscara, 2023 [29] | Healthcare workers | Coadmin. BNT162b2 booster + QIVc | 610 | 7 days after vaccination | NR (57.0) | NR | 474 (78.0) |
| BNT162b2 booster alone | 238 | NR (52.0) | NR | 190 (80.0) | |||
| QIVc followed by BNT162b2 booster | 94 | NR (43.0) | NR | 53 (56.0) | |||
| Murdoch, 2023 [35] | General population | Coadmin. BNT162b2 booster + IIV4 | 564 | 7 days after vaccination | Redness: NR (31.6) Swelling: NR (6.2) Pain at injection site: NR (86.2) | Fatigue: NR (64.0) Headache: NR (47.2) Chills: NR (19.9) New or worsened muscle pain: NR (27.7) | NR (31.6) * |
| IIV4 + placebo followed by BNT162b2 booster 28–42 days later | 564 | Redness: NR (29.0) Swelling: NR (0.4) Pain at injection site: NR (13.9) | Fatigue: NR (42.1) Headache: NR (34.3) Chills: NR (6.2) New or worsened muscle pain: NR (11.4) | NR (29.0) * | |||
| Radner, 2023 [31] | Healthcare workers | Coadmin. BNT162b2 booster + QIV | 240 | 7 days after vaccination | NR | NR | 235 (97.9) |
| BNT162b2 booster alone | 558 | NR | NR | 525 (97.9) | |||
| QIV alone | 33 | NR | NR | 26 (78.8) | |||
| Ramsay, 2023 [36] | General population | Coadmin. BNT162b2 first booster + SIV | 70 | 7 days after vaccination | Pain at COVID-19 injection site: 29 (41) Pain at SIV/placebo injection site: 5 (7) Swelling at COVID-19 injection site: 2 (3) Swelling at SIV/placebo injection site: 0 (0) Redness at COVID-19 injection site: 0 (0) Redness at SIV/placebo injection site: 0 (0) | Headache: 13 (19) Fatigue: 27 (39) Chills: 5 (7) Myalgia: 13 (19) Joint pain: 5 (7) Nausea: 2 (3) Diarrhea: 0 (0) Fever: 8 (11) | 45/71 (63) |
| BNT162b2 first booster+ placebo | 72 | Pain at COVID-19 injection site: 25 (35) Pain at SIV/placebo injection site: 1 (1) Swelling at COVID-19 injection site: 2 (3) Swelling at SIV/placebo injection site: 1 (1) Redness at COVID-19 injection site: 1 (1) Redness at SIV/placebo injection site: 0 (0) | Headache: 15 (21) Fatigue: 22 (31) Chills: 6 (8) Myalgia: 15 (21) Joint pain: 7 (10) Nausea: 5 (7) Diarrhea: 1 (1) Fever: 12 (17) | 36/76 (47) | |||
| Coadmin. BNT162b2 second booster + SIV | 22 | Pain at COVID-19 injection site: 4 (18) Pain at SIV/placebo injection site: 2 (9) Swelling at COVID-19 injection site: 0 (0) Swelling at SIV/placebo injection site: 0 (0) Redness at COVID-19 injection site: 0 (0) Redness at SIV/placebo injection site: 0 (0) | Headache: 3 (14) Fatigue: 5 (23) Chills: 0 (0) Myalgia: 1 (5) Joint pain: 3 (14) Nausea: 0 (0) Diarrhea: 0 (0) Fever: 1 (5) | 8/23 (35) | |||
| BNT162b2 second booster + placebo | 21 | Pain at COVID-19 injection site: 6 (29) Pain at SIV/placebo injection site: 0 (0) Swelling at COVID-19 injection site: 0 (0) Swelling at SIV/placebo injection site: 0 (0) Redness at COVID-19 injection site: 0 (0) Redness at SIV/placebo injection site: 0 (0) | Headache: 3 (14) Fatigue: 5 (24) Chills: 1 (5) Myalgia: 0 (0) Joint pain: 0 (0) Nausea: 0 (0) Diarrhea: 1 (5) Fever: 1 (5) | 11/21 (52) | |||
| Venuto, 2022 [32] | Healthcare workers | Coadmin. BNT162b2 booster + QIV | 64 | NR | Local pain: NR (50.6) | Headache: NR (18.8) Chills: NR (8.7) Myalgia: NR (17.3) Joint pain: NR (11.5) Lethargy: NR (7.2) Fever: NR (27.4) | NR |
| BNT162b2 booster alone | 64 | Local pain: NR (53.1) | Headache: NR (17.2) Chills: NR (6.5) Myalgia: NR (15.2) Joint pain: NR (13.3) Lethargy: NR (6.8) Fever: NR (24.0) | NR |
References
- Bonanni, P.; Steffen, R.; Schelling, J.; Balaisyte-Jazone, L.; Posiuniene, I.; Zatoński, M.; Van Damme, P. Vaccine co-administration in adults: An effective way to improve vaccination coverage. Hum. Vaccines Immunother. 2023, 19, 2195786. [Google Scholar] [CrossRef] [PubMed]
- Privor-Dumm, L.A.; Poland, G.A.; Barratt, J.; Durrheim, D.N.; Knoll, M.D.; Vasudevan, P.; Jit, M.; Bonvehí, P.E.; Bonanni, P. A global agenda for older adult immunization in the COVID-19 era: A roadmap for action. Vaccine 2021, 39, 5240–5250. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Nowalk, M.P.; Pavlik, V.N.; Brown, A.E.; Zhang, S.; Raviotta, J.M.; Moehling, K.K.; Hawk, M.; Ricci, E.M.; Middleton, D.B. Using the 4 pillars™ practice transformation program to increase adult influenza vaccination and reduce missed opportunities in a randomized cluster trial. BMC Infect. Dis. 2016, 16, 623. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Vaccine Schedules in All Countries in the EU/EEA. Available online: https://vaccine-schedule.ecdc.europa.eu/ (accessed on 11 June 2024).
- National Foundation for Infectious Diseases. Call to Action: Strategies to Improve Adult Immunization in the US. Available online: https://www.nfid.org/resource/call-to-action-strategies-to-improve-adult-immunization-in-the-us/ (accessed on 1 November 2024).
- World Health Organization. The Burden of Influenza. Available online: https://www.who.int/news-room/feature-stories/detail/the-burden-of-influenza#:~:text=Nevertheless%2C%20WHO%20estimates%20that%20there,650%20000%20respiratory%20deaths%20annually.&text=On%20the%20other%20hand%2C%20pandemic,from%20another%20animal%20and%20spreads (accessed on 13 May 2024).
- World Health Organization. COVID-19 Epidemiological Update–15 March 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update-15-march-2024 (accessed on 1 April 2024).
- Lafond, K.E.; Porter, R.M.; Whaley, M.J.; Suizan, Z.; Ran, Z.; Aleem, M.A.; Thapa, B.; Sar, B.; Proschle, V.S.; Peng, Z.; et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Med. 2021, 18, e1003550. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Number of COVID-19 Cases Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/cases (accessed on 13 May 2024).
- World Health Organization. Number of COVID-19 Deaths Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 13 May 2024).
- Janssen, C.; Mosnier, A.; Gavazzi, G.; Combadière, B.; Crépey, P.; Gaillat, J.; Launay, O.; Botelho-Nevers, E. Coadministration of seasonal influenza and COVID-19 vaccines: A systematic review of clinical studies. Hum. Vaccin. Immunother. 2022, 18, 2131166. [Google Scholar] [CrossRef] [PubMed]
- Achterbergh, R.C.A.; McGovern, I.; Haag, M. Co-administration of influenza and COVID-19 vaccines: Policy review and vaccination coverage trends in the European Union, UK, US, and Canada between 2019 and 2023. Vaccines 2024, 12, 216. [Google Scholar] [CrossRef]
- World Health Organization. Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines: Interim Guidance, 21 October 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Aydillo, T.; Balsera-Manzanero, M.; Rojo-Fernandez, A.; Escalera, A.; Salamanca-Rivera, C.; Pachon, J.; Del Mar Munoz-Garcia, M.; Sanchez-Cordero, M.J.; Sanchez-Cespedes, J.; Garcia-Sastre, A.; et al. Concomitant administration of seasonal influenza and COVID-19 mRNA vaccines. Emerg. Microbes Infect. 2024, 13, 2292068. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Gasperina, D.D.; Focosi, D.; Forlani, G.; Ferrante, F.D.; Novazzi, F.; Azzi, L.; Maggi, F. Safety and immunogenicity of synchronous COVID19 and influenza vaccination. J. Clin. Virol. Plus 2022, 2, 100082. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Barda, N.; Asraf, K.; Joseph, G.; Weiss-Ottolenghi, Y.; Doolman, R.; Kreiss, Y.; Lustig, Y.; Regev-Yochay, G. Immunogenicity and reactogenicity of coadministration of COVID-19 and influenza vaccines. JAMA Netw. Open 2023, 6, e2332813. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Chachlani, P.; Hayes, K.N.; McCarthy, E.P.; Wen, K.J.; Deng, Y.; Zullo, A.R.; Djibo, D.A.; McMahill-Walraven, C.N.; Smith-Ray, R.L.; et al. COVID-19 and influenza vaccine coadministration among older U.S. adults. Am. J. Prev. Med. 2024, 67, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Zhang, B.; Yue, X.; Marquez, P.; Myers, T.R.; Parker, C.; Gee, J.; Su, J.; Shimabukuro, T.T.; Shay, D.K. Reactogenicity of simultaneous COVID-19 mRNA booster and influenza vaccination in the US. JAMA Netw. Open 2022, 5, e2222241. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, T.A.; Goddard, K.; Hanson, K.E.; Lewis, N.; Klein, N.; Irving, S.A.; Naleway, A.L.; Crane, B.; Kauffman, T.L.; Xu, S.; et al. Simultaneous administration of mRNA COVID-19 bivalent booster and influenza vaccines. Vaccine 2023, 41, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Kim, S.M.; Song, J.E.; Hwang, S.; Nam, E.; Kwon, K.T. Adverse reactions after BNT162b2 messenger RNA vaccination for Coronavirus Disease 2019 in healthcare workers compared with influenza vaccination. Vaccines 2023, 11, 363. [Google Scholar] [CrossRef]
- Lu, Y.; Matuska, K.; Nadimpalli, G.; Ma, Y.; Duma, N.; Zhang, H.T.; Chiang, Y.; Lyu, H.; Chillarige, Y.; Kelman, J.A.; et al. Stroke risk after COVID-19 bivalent vaccination among US older adults. JAMA 2024, 331, 938–950. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Cleary, B.; Fraughen, D.D.; Kelly, G.; McElvaney, O.F.; Murphy, M.P.; Branagan, P.; Gunaratnam, C.; Carroll, T.P.; Goss, C.H.; et al. Safety and reactogenicity of COVID-19 vaccination in severe alpha-1 antitrypsin deficiency. Chronic Obstr. Pulm. Dis. 2024, 11, 3–12. [Google Scholar] [CrossRef]
- McGrath, L.J.; Malhotra, D.; Miles, A.C.; Welch, V.L.; Di Fusco, M.; Surinach, A.; Barthel, A.; Alfred, T.; Jodar, L.; McLaughlin, J.M. Estimated effectiveness of coadministration of the BNT162b2 BA.4/5 COVID-19 vaccine with influenza vaccine. JAMA Netw. Open 2023, 6, e2342151. [Google Scholar] [CrossRef]
- Moro, P.L.; Zhang, B.; Ennulat, C.; Harris, M.; McVey, R.; Woody, G.; Marquez, P.; McNeil, M.M.; Su, J.R. Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the vaccine adverse event reporting system (VAERS) during July 1, 2021–June 30, 2022. Vaccine 2023, 41, 1859–1863. [Google Scholar] [CrossRef]
- Moscara, L.; Venerito, V.; Martinelli, A.; Di Lorenzo, A.; Toro, F.; Violante, F.; Tafuri, S.; Stefanizzi, P. Safety profile and SARS-CoV-2 breakthrough infections among HCWs receiving anti-SARS-CoV-2 and influenza vaccines simultaneously: An Italian observational study. Vaccine 2023, 41, 5655–5661. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, D.; Lontano, A.; Regazzi, L.; Marziali, E.; Nurchis, M.C.; Raponi, M.; Vetrugno, G.; Moscato, U.; Cadeddu, C.; Laurenti, P. Co-administration of SARS-CoV-2 and influenza vaccines in healthcare workers: Results of two vaccination campaigns in a large teaching hospital in Rome. Human. Vaccines Immunother. 2023, 19, 2287282. [Google Scholar] [CrossRef] [PubMed]
- Radner, H.; Sieghart, D.; Jorda, A.; Fedrizzi, C.; Hasenohrl, T.; Zdravkovic, A.; Redlberger-Fritz, M.; Puchammer-Stoeckl, E.; Anderle, K.; Bergmann, F.; et al. Reduced immunogenicity of BNT162b2 booster vaccination in combination with a tetravalent influenza vaccination: Results of a prospective cohort study in 838 health workers. Clin. Microbiol. Infect. 2023, 29, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Venuto, R.; Giunta, I.; Cortese, R.; Denaro, F.; Panto, G.; Privitera, A.; D’Amato, S.; Genovese, C.; La Fauci, V.; Fedele, F.; et al. The importance of COVID-19/influenza vaccines co-administration: An essential public health tool. Infect. Dis. Rep. 2022, 14, 987–995. [Google Scholar] [CrossRef]
- Dulfer, E.A.; Geckin, B.; Taks, E.J.M.; GeurtsvanKessel, C.H.; Dijkstra, H.; van Emst, L.; van der Gaast-de Jongh, C.E.; van Mourik, D.; Koopmans, P.C.; Domínguez-Andrés, J.; et al. Timing and sequence of vaccination against COVID-19 and influenza (TACTIC): A single-blind, placebo-controlled randomized clinical trial. Lancet Reg. Health Eur. 2023, 29, 100628. [Google Scholar] [CrossRef]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Murdoch, L.; Quan, K.; Baber, J.A.; Ho, A.W.Y.; Zhang, Y.; Xu, X.; Lu, C.; Cooper, D.; Koury, K.; Lockhart, S.P.; et al. Safety and immunogenicity of the BNT162b2 vaccine coadministered with seasonal inactivated influenza vaccine in adults. Infect. Dis. Ther. 2023, 12, 2241–2258. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Jones, M.; Vande More, A.M.; Hunt, S.L.; Williams, P.C.M.; Messer, M.; Wood, N.; Macartney, K.; Lee, F.J.; Britton, W.J.; et al. A single blinded, phase IV, adaptive randomised control trial to evaluate the safety of coadministration of seasonal influenza and COVID-19 vaccines (The FluVID study). Vaccine 2023, 41, 7250–7258. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, Y.J.; Kim, J.W.; Ju, H.J.; Shin, S.Y.; Yang, Y.J.; Cheong, H.J.; Kim, W.J.; Kim, C.; Kim, H.J.; et al. Immunogenicity and safety of concomitant bivalent COVID-19 and quadrivalent influenza vaccination: Implications of immune imprinting and interference. Clin. Microbiol. Infect. 2024, 30, 653–659. [Google Scholar] [CrossRef]
- Beccia, F.; Lontano, A.; Rossi, M.F.; Marziali, E.; Pascucci, D.; Raponi, M.; Santoro, P.E.; Moscato, U.; Laurenti, P. Three-year COVID-19 and flu vaccinations among medical residents in a tertiary hospital in Italy: The threat of acceptance decline in seasonal campaigns. Human. Vaccines Immunother. 2023, 19, 2252708. [Google Scholar] [CrossRef]
- Kim, A.S.; Kim, S.M.; Song, J.; Hwang, S.; Nam, E.; Kwon, K.T. Adverse reactions following third dose of the BNT162b2 mRNA COVID-19 vaccine compared with influenza vaccine in healthcare workers. Infect. Chemother. 2022, 54, pS329. [Google Scholar] [CrossRef]
- AstraZeneca. FluMist (Influenza Vaccine Live, Intranasal). Available online: https://www.flumist.com/ (accessed on 1 December 2024).
- Moss, S.; Jurkowicz, M.; Nemet, I.; Atari, N.; Kliker, L.; Abd-Elkader, B.; Gonen, T.; Martin, E.T.; Lustig, Y.; Regev-Yochay, G.; et al. Immunogenicity of co-administered omicron BA.4/BA.5 bivalent COVID-19 and quadrivalent seasonal influenza vaccines in Israel during the 2022–2023 winter season. Vaccines 2023, 11, 1624. [Google Scholar] [CrossRef]
- World Health Organization. Interim recommendations on COVID-19 vaccination in autumn 2022 for the WHO European Region: Conclusions and recommendations of the European Technical Advisory Group of Experts on Immunization: Ad hoc virtual meeting. In Proceedings of the Interim Recommendations on COVID-19 Vaccination in Autumn 2022 for the WHO European Region: Conclusions and Recommendations of the European Technical Advisory Group of Experts on Immunization: Ad hoc Virtual Meeting, Virtual, 5 July 2022. [Google Scholar]
- Gianfredi, V.; Pennisi, F.; Lume, A.; Ricciardi, G.E.; Minerva, M.; Riccò, M.; Odone, A.; Signorelli, C. Challenges and opportunities of mass vaccination centers in COVID-19 times: A rapid review of literature. Vaccines 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Goralnick, E.; Kaufmann, C.; Gawande, A.A. Mass-vaccination sites—An essential innovation to curb the COVID-19 pandemic. N. Engl. J. Med. 2021, 384, e67. [Google Scholar]
- Haviari, S.; Bénet, T.; Saadatian-Elahi, M.; André, P.; Loulergue, P.; Vanhems, P. Vaccination of healthcare workers: A review. Hum. Vaccines Immunother. 2015, 11, 2522–2537. [Google Scholar] [CrossRef]
- Okpani, A.I.; Lockhart, K.; Barker, S.; Grant, J.M.; Yassi, A. Did the health care vaccine mandate work? An evaluation of the impact of the COVID-19 vaccine mandate on vaccine uptake and infection risk in a large cohort of Canadian health care workers. Am. J. Infect. Control 2024, 52, 1065–1072. [Google Scholar] [CrossRef]
- World Health Organization. Implementation Guide for Vaccination of Health Workers; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Al-Worafi, Y.M. Healthcare facilities in developing countries: Infrastructure. In Handbook of Medical and Health Sciences in Developing Countries: Education, Practice, and Research; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–21. [Google Scholar]

| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | General population ≥ 18 years of age, including high-risk groups (for either/or influenza and COVID-19), receiving COVID-19 and influenza vaccinations Population subgroups of interest include ages 18–49, 50–64, and ≥65 years, and other age groups reported in the literature | Population < 18 years of age Studies based on the general population with no reported COVID-19 or flu vaccination Age information not available |
| Intervention ** | To inform the efficacy, effectiveness, safety, and immunogenicity outcomes, co-administration of BNT162b2 (any formulation, including original monovalent, bivalent, and XBB.1.5-adapted) and any type of SIV (and valency, including both TIV and QIV) will be defined as on the same medical/pharmacy visit or day To inform the prevalence of administration, co-administration will first be defined as vaccines of interest administered within 3 months of each other with or without any third vaccine (e.g., pneumococcal vaccine, RSV) For all outcomes, booster refers to any BNT162b2 vaccination other than the primary two-dose series | COVID-19 vaccines other than BNT162b2 Co-administration of BNT162b2 with no vaccine against influenza a Vaccines where the time interval between co-administration is not reported |
| Comparator | Any other approved vaccine (e.g., BNT162b2 or other COVID-19 vaccination alone, SIV alone, RSV vaccine alone) No vaccination or no recent vaccination None required (for non-vaccine efficacy/effectiveness evidence) | NA |
| Outcomes | Outcomes including Prevalence: Prevalence of co-administration by time interval Factors influencing co-administration, if reported (e.g., public health guidelines, vaccine availability, healthcare provider recommendation) Efficacy/Effectiveness:
Influenza-related medical encounters (including outpatient, urgent care, and ER visits) Laboratory-confirmed influenza cases with associated ILI Hospitalizations for influenza Influenza-related ICU admission Influenza-related mortality Hospitalization for pneumonia Influenza-related medical encounters (including outpatient, urgent care, and ER visits)
Laboratory-confirmed symptomatic COVID-19 Laboratory-confirmed severe COVID-19 Laboratory-confirmed critical COVID-19 COVID-19-like infection COVID-19-related medical encounters (including outpatient, urgent care, and ER visits) COVID-19-related Hospitalizations COVID-19-related mortality COVID-19-related ICU admission Hospitalization for pneumonia Immunogenicity: Seroconversion Seroprotection GMTs for HI GMCs for anti-spike antibodies Cell-mediated immunity Safety/Reactogenicity: Total AEs Total SAEs Total grade 3+ treatment-related AEs Total AESIs Any SAEs leading to hospitalization Cardioembolic events Injection site reaction | Publications that do not report on at least one of the outcomes listed in the inclusion criteria |
| Study Design | Clinical trials phases 1–3 (randomized/non-randomized) Post-hoc analysis of trials Pooled analysis of trials Observational studies, including prospective/retrospective cohort studies, case–control studies (including case-only designs), ecological studies | SLRs/MA b Pharmacodynamic/pharmacokinetic studies Genetic studies or cellular/molecular studies Trials without results Trial protocols Case reports, case studies, or case series Narrative reviews Editorials, theses, dissertations, book chapters, news articles |
| Publication Type | Full-text publications, including preprints posted within 6 months of the search date Conference abstracts Conference posters/presentations (where available) Letters to the editor, comments, commentaries | Preprints older than 6 months c |
| Publication Dates | 2021–7 August 2024 | Publications indexed before 1 January 2021 |
| Language | English language | Non-English-language publications |
| Geography | No geographic limits | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boikos, C.; Schaible, K.; Nunez-Gonzalez, S.; Welch, V.; Hu, T.; Kyaw, M.H.; Choi, L.E.; Kamar, J.; Goebe, H.; McLaughlin, J. Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review. Vaccines 2025, 13, 381. https://doi.org/10.3390/vaccines13040381
Boikos C, Schaible K, Nunez-Gonzalez S, Welch V, Hu T, Kyaw MH, Choi LE, Kamar J, Goebe H, McLaughlin J. Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review. Vaccines. 2025; 13(4):381. https://doi.org/10.3390/vaccines13040381
Chicago/Turabian StyleBoikos, Constantina, Kassandra Schaible, Solange Nunez-Gonzalez, Verna Welch, Tianyan Hu, Moe Hein Kyaw, Laura Elizabeth Choi, Joanna Kamar, Henry Goebe, and John McLaughlin. 2025. "Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review" Vaccines 13, no. 4: 381. https://doi.org/10.3390/vaccines13040381
APA StyleBoikos, C., Schaible, K., Nunez-Gonzalez, S., Welch, V., Hu, T., Kyaw, M. H., Choi, L. E., Kamar, J., Goebe, H., & McLaughlin, J. (2025). Co-Administration of BNT162b2 COVID-19 and Influenza Vaccines in Adults: A Global Systematic Review. Vaccines, 13(4), 381. https://doi.org/10.3390/vaccines13040381






