Pre-Existing Anti-Vector Immunity to Adenovirus-Inspired VLP Vaccines Shows an Adjuvant-Dependent Antagonism
Abstract
1. Introduction
2. Materials and Methods
2.1. Baculovirus Production
2.2. Ad-VLP and Ad-VLP-S14P5 Purification
2.3. Negative-Stain Electron Microscopy
2.4. Cohort of Patients Infected by SARS-CoV-2
2.5. Vaccination Protocol and Schedule
2.6. ELISA on S14P5 Peptide or Recombinant SARS-CoV-2 Spike
2.7. Statistical Analysis
3. Results
3.1. Ad-VLP and Ad-VLP-S14P5 Recognition by Serums from COVID-19-Infected Patients
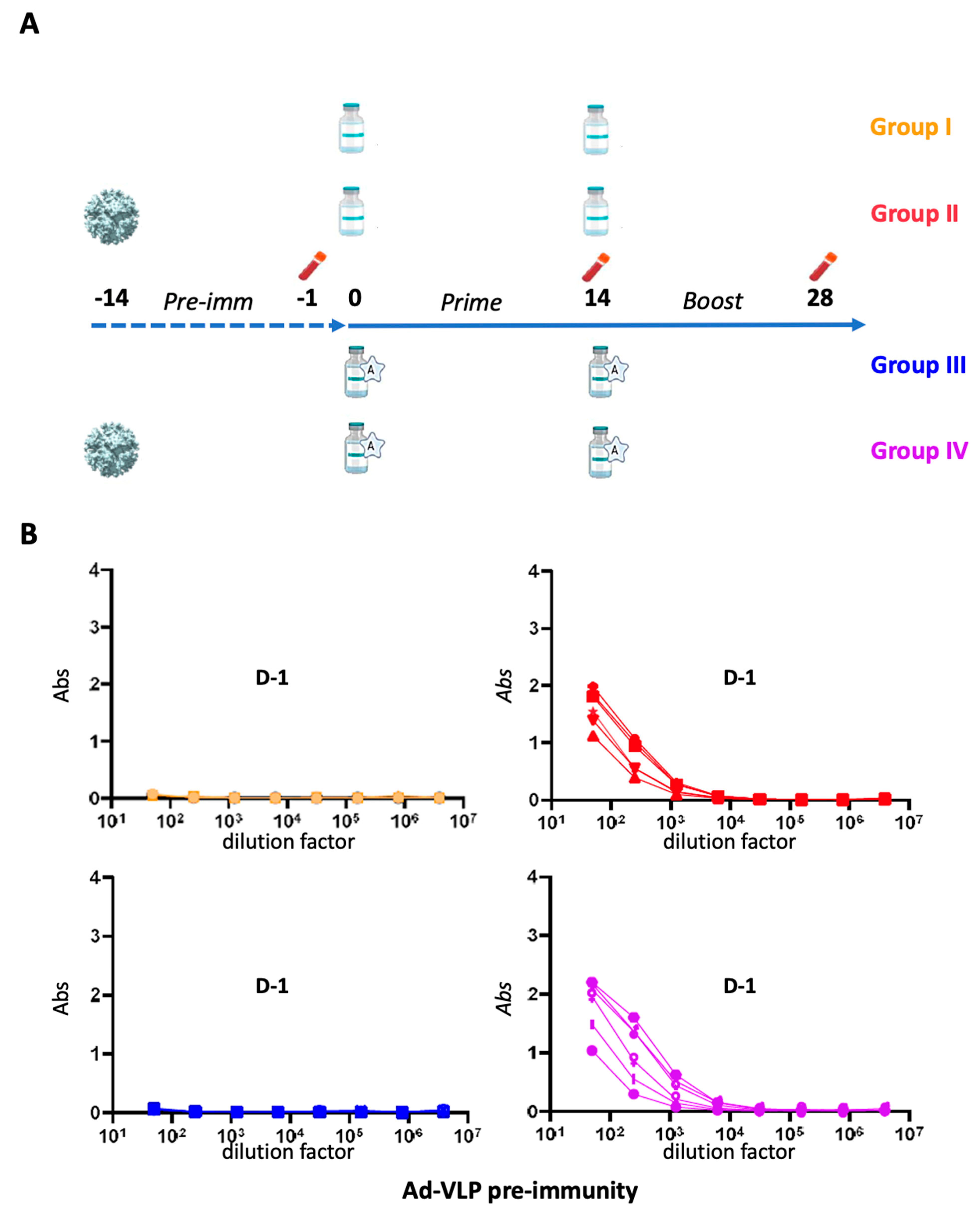
3.2. Immunization Regimen and Assessment of Adenovirus Pre-Immunity Before Ad-VLP-S14P5 Immunizations in Mice
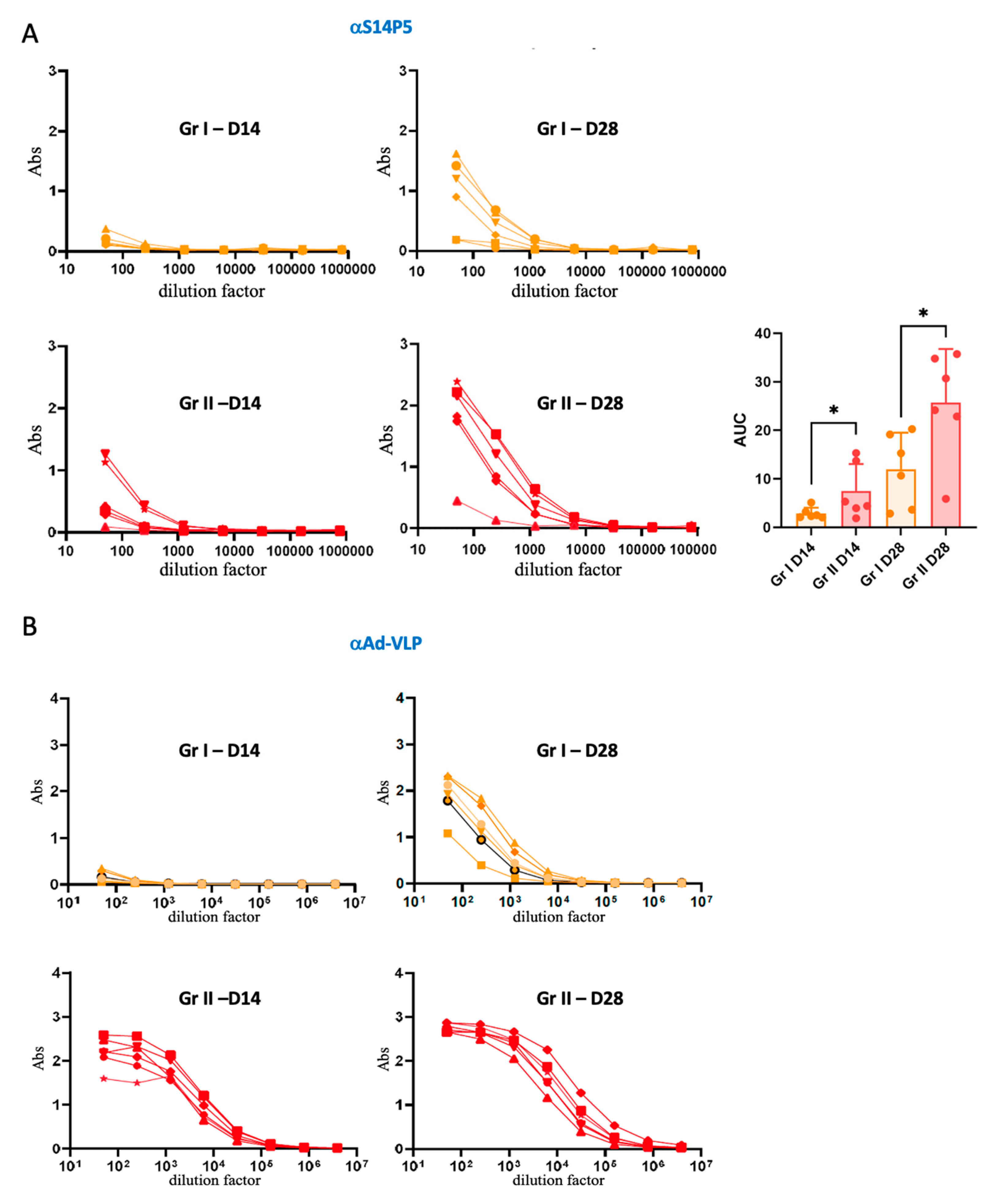
3.3. Association Between Pre-Existing Adenovirus Immunity and Anti-S14P5 Response in Absence of Adjuvant
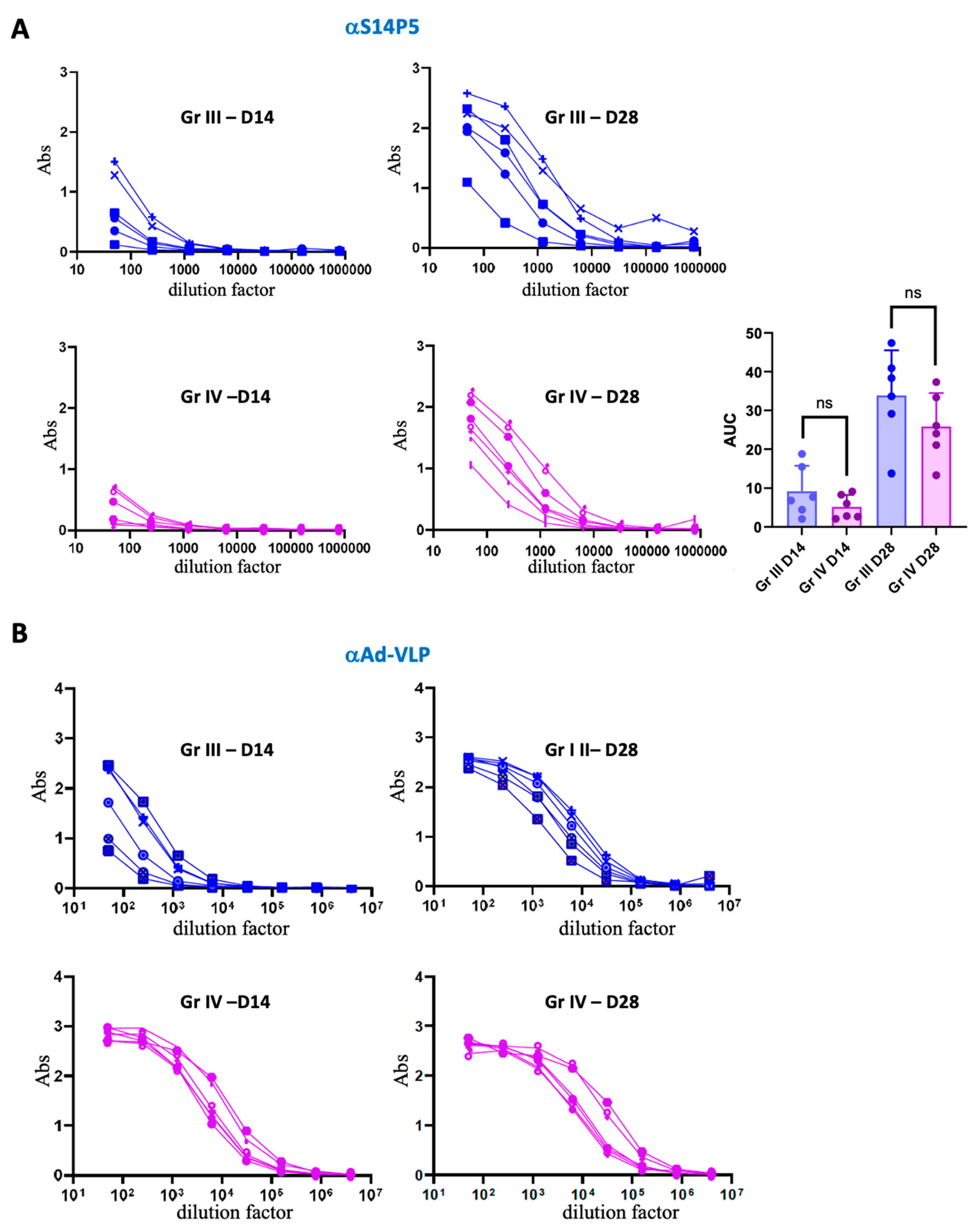
3.4. Association Between Pre-Existing Adenovirus Immunity and the Anti-S14P5 Response in the Presence of Adjuvant
3.5. Functionality of Anti-S14P5 Recognition on the Recombinant Trimeric SARS-CoV-2 Spike
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Weiner, D.; Xiao, W.; Baker, A.; Sanders, N. Molecular Therapies and Vaccines Face the Challenges of Emerging Infectious Diseases. Mol. Ther. 2022, 30, 1789–1790. [Google Scholar] [CrossRef] [PubMed]
- Setyo Utomo, D.I.; Suhaimi, H.; Muhammad Azami, N.A.; Azmi, F.; Mohd Amin, M.C.I.; Xu, J. An Overview of Recent Developments in the Application of Antigen Displaying Vaccine Platforms: Hints for Future SARS-CoV-2 VLP Vaccines. Vaccines 2023, 11, 1506. [Google Scholar] [CrossRef]
- van Riel, D.; de Wit, E. Next-Generation Vaccine Platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef]
- Nguyen, B.; Tolia, N.H. Protein-Based Antigen Presentation Platforms for Nanoparticle Vaccines. npj Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Vragniau, C.; Bufton, J.C.; Garzoni, F.; Stermann, E.; Rabi, F.; Terrat, C.; Guidetti, M.; Josserand, V.; Williams, M.; Woods, C.J.; et al. Synthetic Self-Assembling ADDomer Platform for Highly Efficient Vaccination by Genetically Encoded Multiepitope Display. Sci. Adv. 2019, 5, eaaw2853. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, Advances, and Technical Challenges in Virus-like Particles-Based Vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef]
- Lanzi, A.; Ben Youssef, G.; Perricaudet, M.; Benihoud, K. Anti-Adenovirus Humoral Responses Influence on the Efficacy of Vaccines Based on Epitope Display on Adenovirus Capsid. Vaccine 2011, 29, 1463–1471. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A Review of 65 Years of Human Adenovirus Seroprevalence. Expert. Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 nCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S Vaccine against SARS-CoV-2 Variants in Humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; De Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Poh, C.M.; Carissimo, G.; Wang, B.; Amrun, S.N.; Lee, C.Y.-P.; Chee, R.S.-L.; Fong, S.-W.; Yeo, N.K.-W.; Lee, W.-H.; Torres-Ruesta, A.; et al. Two Linear Epitopes on the SARS-CoV-2 Spike Protein That Elicit Neutralising Antibodies in COVID-19 Patients. Nat. Commun. 2020, 11, 2806. [Google Scholar] [CrossRef]
- Sari, D.; Gupta, K.; Raj, D.B.T.G.; Aubert, A.; Drncová, P.; Garzoni, F.; Fitzgerald, D.; Berger, I. The MultiBac Baculovirus/Insect Cell Expression Vector System for Producing Complex Protein Biologics. In Advanced Technologies for Protein Complex Production and Characterization; Vega, M.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 896, pp. 199–215. ISBN 978-3-319-27214-6. [Google Scholar]
- Fender, P. Use of Dodecahedron “VLPs” as an Alternative to the Whole Adenovirus. Methods Mol. Biol. 2014, 1089, 61–70. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; De Silva, T.I.; Peacock, S.J.; Barclay, W.S.; De Silva, T.I.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Lynch, J.; Kajon, A. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Wallace, R.; Bliss, C.M.; Parker, A.L. The Immune System—A Double-Edged Sword for Adenovirus-Based Therapies. Viruses 2024, 16, 973. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Rowley, T.F.; Junker, F.; Peters, S.J.; Crilly, S.; Compson, J.; Eddleston, A.; Björkelund, H.; Greenslade, K.; Parkinson, M.; et al. Multivalent Fcγ-Receptor Engagement by a Hexameric Fc-Fusion Protein Triggers Fcγ-Receptor Internalisation and Modulation of Fcγ-Receptor Functions. Sci. Rep. 2017, 7, 17049. [Google Scholar] [CrossRef]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Ma, N.; Liang, J.; Ma, X.; Feng, Q.; Liu, G.; Xu, C.; Tang, M.; Zhang, L.; Gao, X.; et al. Site-Specific Modification of Virus-Like Particles for Exogenous Tumor Antigen Display and Minimizing Preexisting Immunity. Small 2023, 19, 2300125. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Read, B.J. Shaping Humoral Immunity to Vaccines through Antigen-Displaying Nanoparticles. Curr. Opin. Immunol. 2020, 65, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Bachmann, M.F. Virus-like Particle Vaccinology, from Bench to Bedside. Cell Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Ott, G.; Barchfeld, G.L.; Chernoff, D.; Radhakrishnan, R.; Van Hoogevest, P.; Van Nest, G. MF59 Design and Evaluation of a Safe and Potent Adjuvant for Human Vaccines. In Vaccine Design; Powell, M.F., Newman, M.J., Eds.; Pharmaceutical Biotechnology; Springer: Boston, MA, USA, 1995; Volume 6, pp. 277–296. ISBN 978-1-4613-5737-7. [Google Scholar]
- Ott, G.; Radhakrishnan, R.; Fang, J.-H.; Hora, M. The Adjuvant MF59: A 10-Year Perspective. In Vaccine Adjuvants; Humana Press: Totowa, NJ, USA, 2000; Volume 42, pp. 211–228. ISBN 978-1-59259-083-4. [Google Scholar]
- Chevillard, C.; Amen, A.; Besson, S.; Hannani, D.; Bally, I.; Dettling, V.; Gout, E.; Moreau, C.J.; Buisson, M.; Gallet, S.; et al. Elicitation of Potent SARS-CoV-2 Neutralizing Antibody Responses through Immunization with a Versatile Adenovirus-Inspired Multimerization Platform. Mol. Ther. 2022, 30, 1913–1925. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; O’Connor, M.A.; Chen, C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallet, S.; Hannani, D.; Dergan-Dylon, S.; Vassal-Stermann, E.; Bally, I.; Chevillard, C.; Fenel, D.; Epaulard, O.; Poignard, P.; Fender, P. Pre-Existing Anti-Vector Immunity to Adenovirus-Inspired VLP Vaccines Shows an Adjuvant-Dependent Antagonism. Vaccines 2025, 13, 238. https://doi.org/10.3390/vaccines13030238
Gallet S, Hannani D, Dergan-Dylon S, Vassal-Stermann E, Bally I, Chevillard C, Fenel D, Epaulard O, Poignard P, Fender P. Pre-Existing Anti-Vector Immunity to Adenovirus-Inspired VLP Vaccines Shows an Adjuvant-Dependent Antagonism. Vaccines. 2025; 13(3):238. https://doi.org/10.3390/vaccines13030238
Chicago/Turabian StyleGallet, Salomé, Dalil Hannani, Sebastian Dergan-Dylon, Emilie Vassal-Stermann, Isabelle Bally, Christopher Chevillard, Daphna Fenel, Olivier Epaulard, Pascal Poignard, and Pascal Fender. 2025. "Pre-Existing Anti-Vector Immunity to Adenovirus-Inspired VLP Vaccines Shows an Adjuvant-Dependent Antagonism" Vaccines 13, no. 3: 238. https://doi.org/10.3390/vaccines13030238
APA StyleGallet, S., Hannani, D., Dergan-Dylon, S., Vassal-Stermann, E., Bally, I., Chevillard, C., Fenel, D., Epaulard, O., Poignard, P., & Fender, P. (2025). Pre-Existing Anti-Vector Immunity to Adenovirus-Inspired VLP Vaccines Shows an Adjuvant-Dependent Antagonism. Vaccines, 13(3), 238. https://doi.org/10.3390/vaccines13030238









