Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Antibody–Drug Conjugate
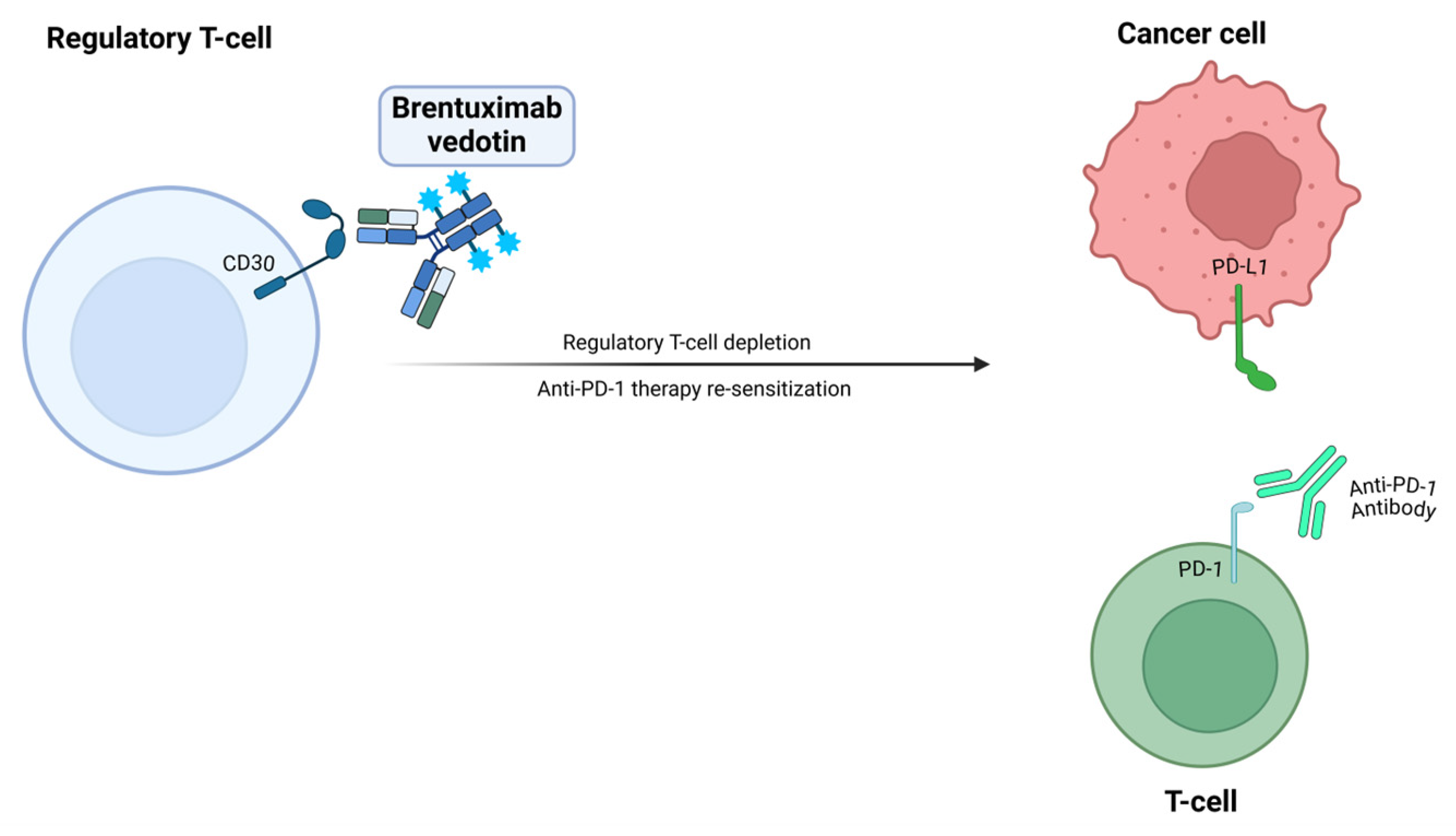
Brentuximab Vedotin
3. Bispecific Antibody
3.1. CD-16 and EGFR
3.2. PD-L1 and 4-1BB
3.3. PD-L1 and CTLA-4
3.4. PD-L1 and VEGF
3.5. CD137 and PD-L1
4. Cellular Therapy
4.1. T-Cell Receptor–Engineered T Cells
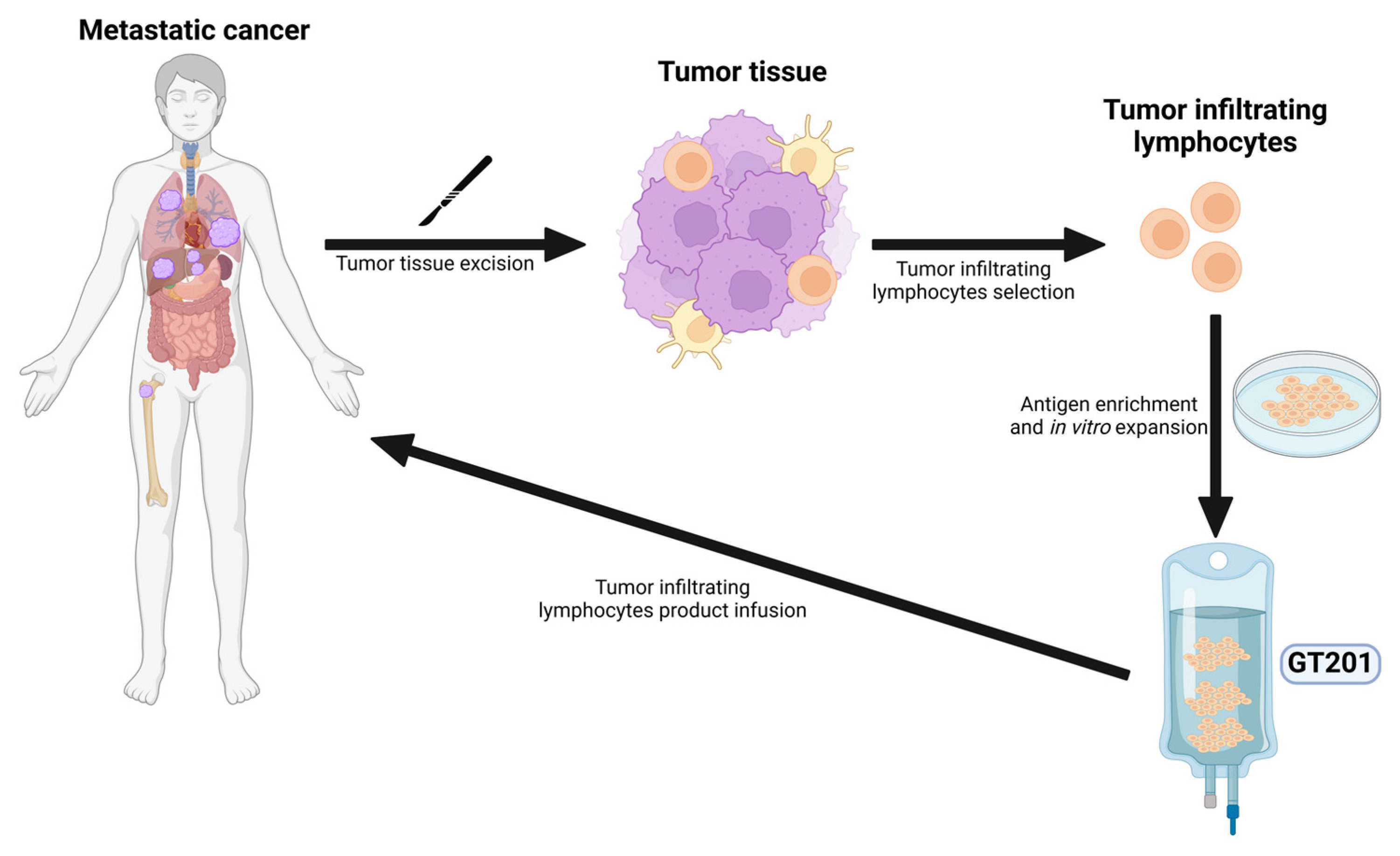
4.2. Tumor-Infiltrating Lymphocytes
5. Gut Microbiome
6. Monoclonal Antibodies
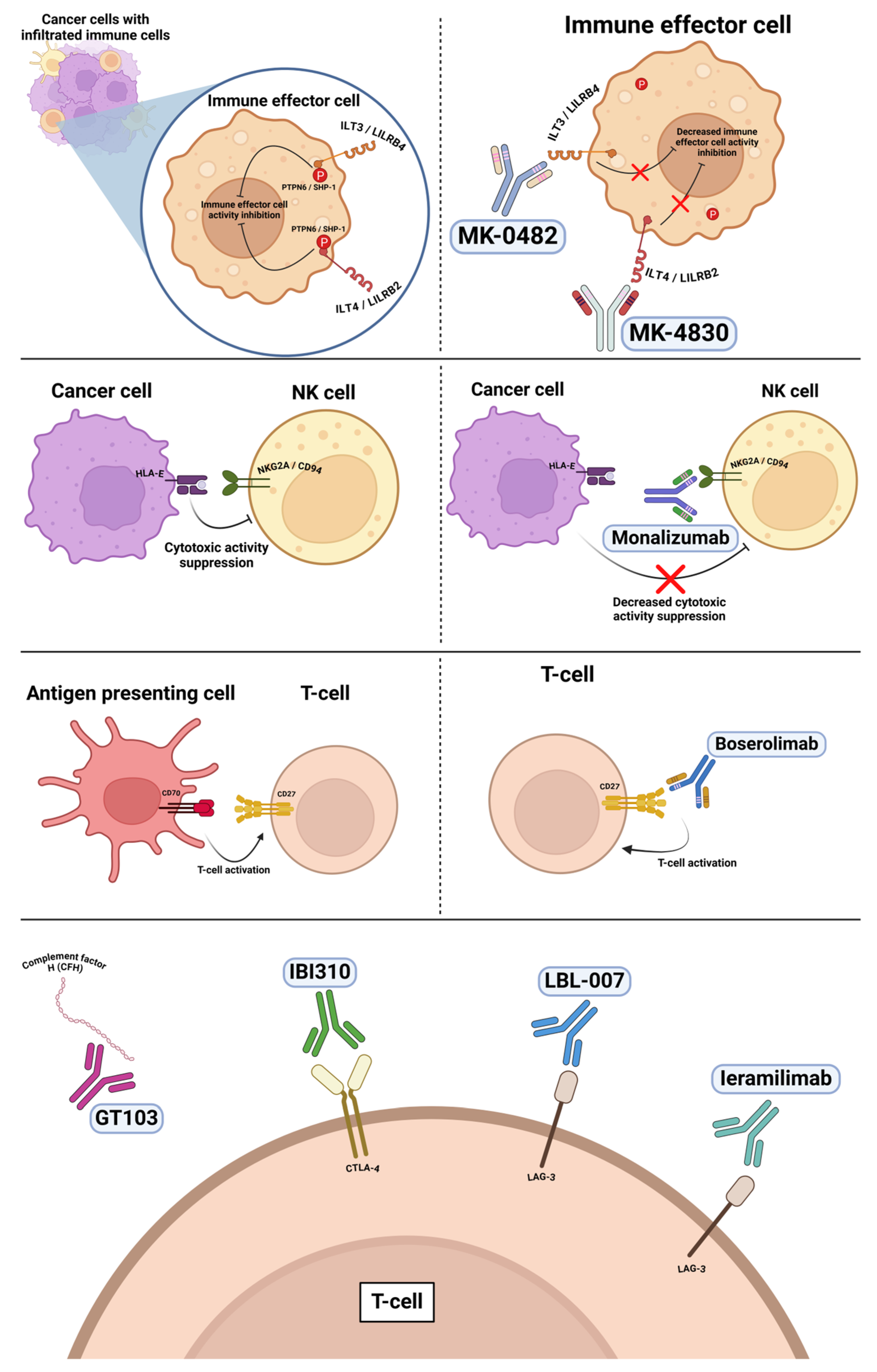
6.1. Anti-ILT4 and Anti-ILT3
6.2. Anti-CD27
6.3. Anti-NKG2A
6.4. Anti-Complement Factor H
6.5. Anti-CTLA-4
6.6. Anti-LAG-3
7. Other Molecules
7.1. CXCR2 Antagonist
7.2. TLR 7/8 Agonist
7.3. α/β IL-2R Agonist
8. Vaccines
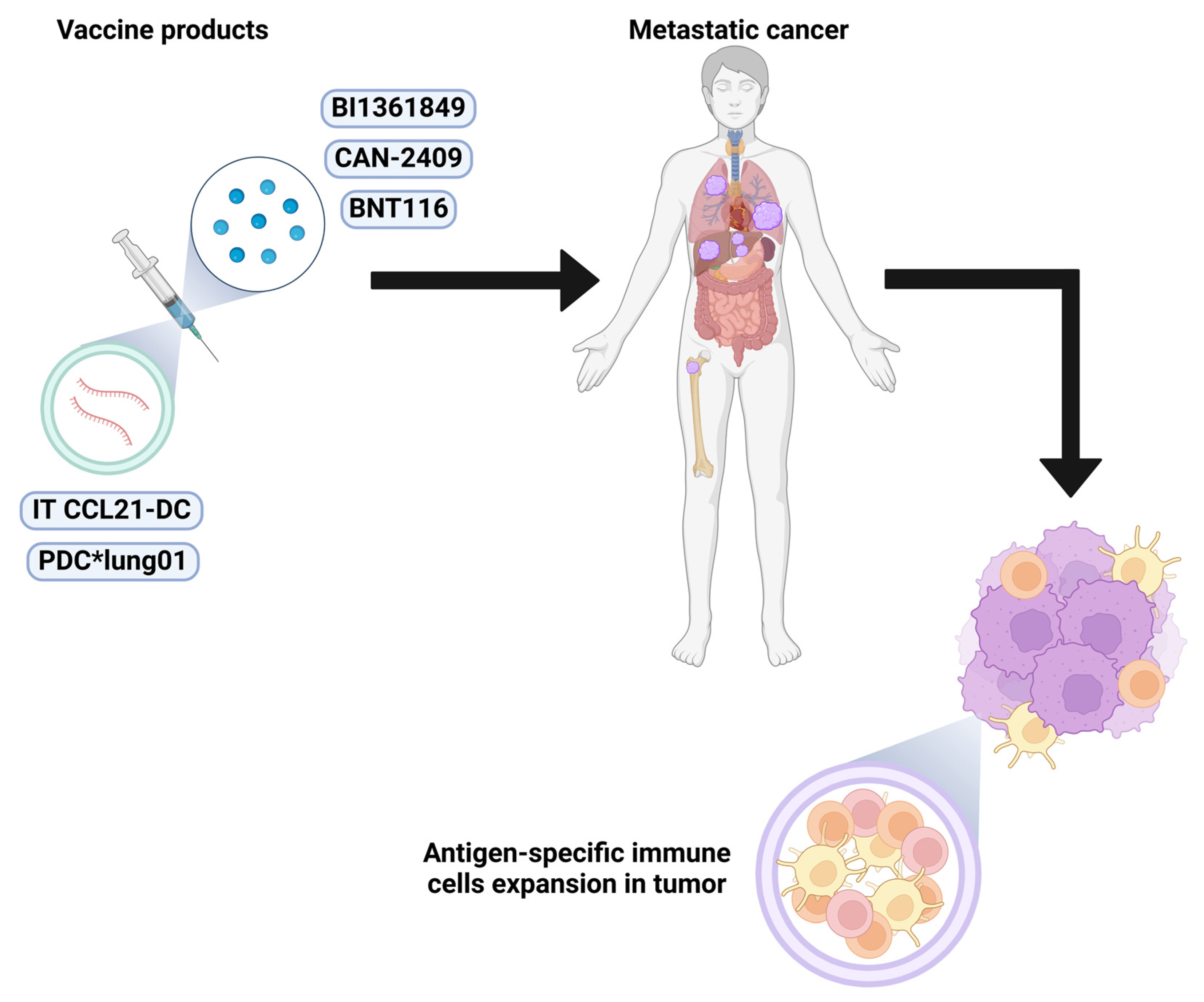
8.1. CCL21-DC
8.2. PDC*lung01
8.3. CAN-2409
8.4. BNT116
8.5. BI1361849
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLO BOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Tan, D.S. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; Angelis, F.D.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Waterhouse, D.; Lam, J.; Betts, K.A.; Yin, L.; Gao, S.; Yuan, Y.; Hartman, J.; Rao, S.; Lubinga, S.; Stenehjem, D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer 2021, 156, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Horn, L.; Borghaei, H.; Spigel, D.R.; Steins, M.; Ready, N.; Chow, L.Q.M.; Vokes, E.E.; Felip, E.; Holgado, E. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2015, 33, LBA109. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: Nivolumab versus docetaxel in previously treated non–small-cell lung cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.-E.; Lee, J.-S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J. Thorac. Oncol. 2022, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Parra, H.S.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.; De Marinis, F.; Giaccone, G.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann. Oncol. 2019, 30, v915. [Google Scholar] [CrossRef]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.-W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non–small-cell lung cancer: The phase III POSEIDON study. J. Clin. Oncol. 2023, 41, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. LBA51 EMPOWER-Lung 3: Cemiplimab in combination with platinum doublet chemotherapy for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 2021, 32, S1328. [Google Scholar] [CrossRef]
- Özgüroğlu, M.; Kilickap, S.; Sezer, A.; Gümüş, M.; Bondarenko, I.; Gogishvili, M.; Nechaeva, M.; Schenker, M.; Cicin, I.; Ho, G.F.; et al. First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 989–1001. [Google Scholar] [CrossRef]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Sterman, D.; Alesi, E.R.; Maldonado, F.; Mehra, R.; Bestvina, C.M.; Reisenauer, J.S.; Swartz, L.K.; Puri, S.; Ib-rahim, O.; et al. Overall survival after treatment with CAN-2409 plus valacyclovir in combination with continued ICI in patients with stage III/IV NSCLC with an inadequate response to ICI. J. Clin. Oncol. 2024, 42, 8634. [Google Scholar] [CrossRef]
- Öven, B.B.; Baz, D.V.; Wolf, J.; Ates, O.; Göker, E.; Brück, P.; Wenger, M.; Munshi, N.; Tsyhankova, I.; Kaczorowska, M.; et al. Abstract CT051: Preliminary results from LuCa-MERIT-1, a first-in-human Phase I trial evaluating the fixed antigen mRNA vaccine BNT116+ docetaxel in patients with advanced non-small cell lung cancer. Cancer Res. 2024, 84, CT051. [Google Scholar] [CrossRef]
- Senter, P.D.; Sievers, E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012, 30, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-T.; Sabari, J.; Thompson, J.; Niu, J.; Mamdani, H.; Thapa, R.; Thompson, Z.; Posina, T.; Ryan, A.; Venhaus, R.; et al. Abstract CT052: A phase IB study of mRNA-based active cancer vaccine, BI1361849, combined with durvalumab and tremelimumab im-munotherapy in patients with non-small cell lung cancer (NSCLC). Cancer Res. 2024, 84, CT052. [Google Scholar] [CrossRef]
- Heiser, R.A.; Grogan, B.M.; James, R.D.; Ulrich, M.L.; Berndt, J.D.; O’Connor, B.P.; Gardai, S.J.; Lu, H.; Knowles, S.M. CD30 is a marker of activated effector regulatory T cells in solid tumors providing clinical rationale for the combination of brentuximab vedotin and PD-1 inhibitors. Cancer Res. 2023, 83, 3253. [Google Scholar] [CrossRef]
- Zakharia, Y.; Lee, S.; Jotte, R.M.; Gillespie-Twardy, A.L.; Mehmi, I.; Chandra, S.; Hamid, O.; Watson, G.T.; Ward, P.J.; Chaney, M.F.; et al. Phase 2 trial of brentuximab vedotin (BV) with pembrolizumab (pembro) in patients with previously treated metastatic non-small cell lung cancer (NSCLC) or cutaneous melanoma (SGN35-033): Overall survival. J. Clin. Oncol. 2024, 42, 2617. [Google Scholar] [CrossRef]
- Wingert, S.; Reusch, U.; Knackmuss, S.; Kluge, M.; Damrat, M.; Pahl, J.; Schniegler-Mattox, U.; Mueller, T.; Fucek, I.; Ellwanger, K.; et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs 2021, 13, 1950264. [Google Scholar] [CrossRef]
- Kim, H.R.; Saavedra, O.; Cervantes, A.; Lugowska, I.A.; Oberoi, A.; El-Khoueiry, A.B.; Thomas, J.S.; Rogowski, W.; Lopez, J.S.; Shim, B.Y.; et al. Preliminary results from the phase 2 study of AFM24 in combination with atezolizumab in patients with EGFR wild-type (EGFR-WT) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2024, 42, 2522. [Google Scholar] [CrossRef]
- Aerts, J.; Paz-Ares, L.G.; Helissey, C.; Cappuzzo, F.; Quere, G.; Kowalski, D.; Benitez, J.C.; Guisier, F.; Besse, B.; Gadgeel, S.M.; et al. Acasunlimab (DuoBody-PD-L1x4-1BB) alone or in combination with pembrolizumab (pembro) in patients (pts) with previously treated metastatic non-small cell lung cancer (mNSCLC): Initial results of a randomized, open-label, phase 2 trial. J. Clin. Oncol. 2024, 42, 2533. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Li, X.; Wu, J.; Chang, B.; Hu, S.; Yang, S.; Xu, T.; Liu, Y.; Wang, N.; et al. KN046, a bispecific antibody against PD-L1 and CTLA-4, plus chemotherapy as first-line treatment for metastatic NSCLC: A multicenter phase 2 trial. Cell Rep. Med. 2024, 5, 101470. [Google Scholar] [CrossRef]
- Meng, Y.; Du, Y.; Liu, X.; Mu, Y.; Sun, M.; Wen, Q.; Liu, N.; Chen, H.; Sun, Y.; Dang, Q.; et al. Phase I summary of the HB0025 efficacy and safety to the heavily pretreated non-small cell lung cancer (NSCLC) population. J. Clin. Oncol. 2024, 42, e14600. [Google Scholar] [CrossRef]
- Wu, C.; Lv, D.; Cui, J.; Wang, Z.; Zhao, H.; Duan, H.; Duan, P.; Yin, Y.; Pan, Y.; Liu, F.-N.; et al. A phase Ib/IIa trial to evaluate the safety and efficacy of PM8002, a bispecific antibody targeting PD-L1 and VEGF-A, as a monotherapy in patients with advanced NSCLC. J. Clin. Oncol. 2024, 42, 8533. [Google Scholar] [CrossRef]
- Kyi, C.; van Dongen, M.; Rottey, S.; Bermejo, I.M.; Mittag, D.; Gouveia, D.; Bol, K.; Yan, C.; Joe, A.K.; Laus, G.; et al. Phase I study of MCLA-145, a bispecific antibody targeting CD137 and PD-L1, in solid tumors, as monotherapy or in combination with pem-brolizumab. J. Clin. Oncol. 2024, 42, 2520. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Coveler, A.; Safyan, R.; Cohen, S.; Lee, S.; Yeung, C.; Redman, M.; Schmitt, T.; Chapuis, A.; Greenberg, P.D. Abstract CT082: Phase I study of autologous CD8+ and CD4+ transgenic T cells expressing high-affinity KRAS G12V mutation-specific T cell receptors (FH-A11KRASG12V-TCR) in patients with metastatic pancreatic, colorectal and non-small cell lung cancers with KRAS G12V mutations. Cancer Res. 2024, 84, CT082. [Google Scholar] [CrossRef]
- Han, Z.; Fang, W.; Chen, K.; Chen, Y.; Wang, H.; Tang, J.; Yu, J.; Ma, L.; Liu, Y.; Lu, L.; et al. Exploring the safety and efficacy of GT201 as a first-in-class autologous tumor-infiltrating lymphocyte monotherapy in advanced solid tumors. J. Clin. Oncol. 2024, 42, 2547. [Google Scholar] [CrossRef]
- Perets, R.; Meshner, S.; Tirosh, O.; Eshar, S.; Metz-Breiner, A.; Ben-Shabat, S.K.; Ben-Yehuda, H.; Haber, E.; Cohen-Asis, M.; Maurice-Dror, C.; et al. Preliminary results from a FIH, open-label phase 1 study with BMC128, a rationally designed live bacterial consortium, in combination with nivolumab. J. Clin. Oncol. 2024, 42, 8631. [Google Scholar] [CrossRef]
- Burns, T.F.; Wang, H.; Hurd, D.; Nguyen, M.; Villaruz, L.C.; Petro, D.P.; Schwartz, M.B.; Dubner, H.M.; Zarour, H.M.; Trinchieri, G.; et al. Phase I/II trial of healthy donor fecal microbiota transplant (hdFMT) in PD-1 relapsed/refractory (R/R) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2024, 42, TPS8648. [Google Scholar] [CrossRef]
- Aggarwal, C.; Stevenson, J.; Kowalski, D.M.; Csőszi, T.; Peled, N.; Cho, B.C.; Rubio-Viqueira, B.; Niu, J.; Carbone, D.P.; Arnold, S.M.; et al. Abstract CT249: Results from KEYMAKER-U01 substudy 3: A phase 2 umbrella study of pembrolizumab plus investigational agents in patients with non-small-cell lung cancer (NSCLC) previously treated with anti-PD-(L) 1 therapy. Cancer Res. 2024, 84, CT249. [Google Scholar] [CrossRef]
- Patel, S.P.; Alonso-Gordoa, T.; Banerjee, S.; Wang, D.; Naidoo, J.; Standifer, N.E.; Palmer, D.C.; Cheng, L.-Y.; Kourtesis, P.; Ascierto, M.L.; et al. Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors. J. Immunother. Cancer 2024, 12, e007340. [Google Scholar] [CrossRef]
- Mamdani, H.; Clarke, J.M.; Gu, L.; Stinchcombe, T.; Crawford, J.; Antonia, S.J.; Campa, M.; Nixon, A.B.; Sonpavde, G.P.; Simon, G.R.; et al. Safety and efficacy of complement factor H (CFH) inhibitor GT103 in advanced non-small cell lung cancer (NSCLC): Results of a phase Ib first in human dose escalation study. J. Clin. Oncol. 2024, 42, e14588. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Yao, J.; Long, J.; Mao, Y.; Wu, D.; Zang, A.; Zhao, J.; Liu, Z.; Meng, R.; et al. A phase Ib study evaluating the safety and efficacy of IBI310 plus sintilimab in patients with advanced non-small-cell lung cancer who have progressed after anti-PD-1/L1 therapy. Cancer Med. 2024, 13, e6855. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Fang, W.; Huang, Y.; Zhang, Y.; Ba, Y.; Wang, Z.; Deng, C.; Hu, D.; Wang, W.; et al. Abstract CT227: Anti-LAG-3 antibody LBL-007 in combination with anti-PD-1 antibody toripalimab, in patients with advanced malignant tumors: A phase Ib/II, open-label, multicenter, dose escalation/expansion study. Cancer Res. 2024, 84, CT227. [Google Scholar] [CrossRef]
- Lin, C.-C.; Garralda, E.; Schöffski, P.; Hong, D.S.; Siu, L.L.; Martin, M.; Maur, M.; Hui, R.; Soo, R.A.; Chiu, J.; et al. A phase 2, mul-ticenter, open-label study of anti-LAG-3 ieramilimab in combination with anti-PD-1 spartalizumab in patients with advanced solid malignancies. Oncoimmunology 2024, 13, 2290787. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller Jr, W.H.; Hansen, A.R.; Lee, J.-S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
- Costin, D.; Chen, K.; Gralla, R.J.; Garofalo, B.; Wang, M.; Kurland, E. A phase 2 study of EIK1001, a Toll-like receptor 7/8 (TLR7/8) agonist, in combination with pembrolizumab and chemotherapy in patients with stage 4 non-small cell lung cancer. J. Clin. Oncol. 2024, 42, TPS8667. [Google Scholar] [CrossRef]
- Izar, B.; Zamarin, D.; Spigel, D.R.; Hoimes, C.J.; McDermott, D.F.; Sehgal, K.; Najjar, Y.G.; Schoenfeld, A.J.; Garon, E.B.; Sullivan, R.J.; et al. Abstract CT183: Initial results from a phase 1a/1b study of STK-012, a first-in-class α/β IL-2 receptor biased partial agonist in ad-vanced solid tumors (NCT05098132). Cancer Res. 2024, 84, CT183. [Google Scholar] [CrossRef]
- Liu, B.; Lisberg, A.; Salehi-Rad, R.; Oh, M.; Lee, J.M.; Tran, L.M.; Krysan, K.; Lim, R.J.; Dumitras, C.; Jing, Z.; et al. Abstract CT153: Phase I trial of intratumoral administration of autologous CCL21 gene-modified dendritic cells in combination with pembrolizumab for advanced NSCLC: Feasibility of repeated IT injections. Cancer Res. 2024, 84, CT153. [Google Scholar] [CrossRef]
- Theelen, W.; Perol, M.; Cuppens, K.; Demedts, I.; Borm, F.; Biesma, B.; Wauters, E.; Colinet, B.; Buchmeier, E.-L.; Althoff, F.; et al. Abstract CT021: Preliminary clinical results of a therapeutic cancer vaccine PDC* lung01 in combination with anti-PD-1 in patients (pts) with stage IV NSCLC. Cancer Res. 2024, 84, CT021. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Curran, M.A. 4-1BB agonists: Multi-potent potentiators of tumor immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Altintas, I.; Kosoff, R.; Gieseke, F.; Schödel, K.; Salcedo, T.; Burm, S.; Toker, A.; Kranz, L.; Vormehr, M.; et al. 561 Duo-Body®-PD-L1 × 4–1BB (GEN1046) induces superior immune-cell activation, cytokine production and cytotoxicity by combining PD-L1 blockade with conditional 4–1BB co-stimulation. J. ImmunoTherapy Cancer 2020, 8, A338–A339. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Cui, X.; Jia, H.; Xin, H.; Zhang, L.; Chen, S.; Xia, S.; Li, X.; Xu, W.; Chen, X.; Feng, Y.; et al. A novel bispecific antibody targeting PD-L1 and VEGF with combined anti-tumor activities. Front. Immunol. 2021, 12, 778978. [Google Scholar] [CrossRef]
- Geuijen, C.; Tacken, P.; Wang, L.-C.; Klooster, R.; van Loo, P.F.; Zhou, J.; Mondal, A.; Liu, Y.-b.; Kramer, A.; Condamine, T.; et al. A human CD137 × PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat. Commun. 2021, 12, 4445. [Google Scholar] [CrossRef]
- Timar, J.; Kashofer, K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: Progressions and challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, W.; Han, Z.; Chen, K.; Chen, Y.; Yu, J.; Liu, Y.; Ma, L.; Shi, Z.; Sun, J.; et al. Harnessing the power of tumor-infiltrating lymphocytes: A first-in-human study of GT201 as monotherapy in advanced solid tumors. J. Clin. Oncol. 2023, 41, 2551. [Google Scholar] [CrossRef]
- Lee, J.B.; Huang, Y.; Oya, Y.; Nutzinger, J.; Ang, Y.L.; Sooi, K.; Cho, B.C.; Soo, R.A. Modulating the gut microbiome in non-small cell lung cancer: Challenges and opportunities. Lung Cancer 2024, 194, 107862. [Google Scholar]
- Ren, S.; Feng, L.; Liu, H.; Mao, Y.; Yu, Z. Gut microbiome affects the response to immunotherapy in non-small cell lung cancer. Thorac. Cancer 2024, 15, 1149–1163. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Wang, S.; Wang, J.; Chen, X.; Zhou, D.; Fang, Y.; Gao, A.; Sun, Y. Overexpressed immunoglobulin-like transcript (ILT) 4 in lung adenocarcinoma is correlated with immunosuppressive T cell subset infiltration and poor patient outcomes. Biomark. Res. 2020, 8, 11. [Google Scholar] [CrossRef]
- Zuniga, L.; Joyce-Shaikh, B.; Wilson, D.; Cherwinski, H.; Chen, Y.; Jeff, G. Preclinical characterization of a first-in-class ILT4 antagonist, MK-4830. J. ImmunoTher Can. 2018, 6, 115. [Google Scholar] [CrossRef]
- Vlad, G.; Chang, C.-C.; Colovai, A.I.; Berloco, P.; Cortesini, R.; Suciu-Foca, N. Immunoglobulin-like transcript 3: A crucial regulator of dendritic cell function. Hum. Immunol. 2009, 70, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Hendriks, J.; Xiao, Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005, 17, 275–281. [Google Scholar] [CrossRef]
- Flieswasser, T.; Eynde, A.V.D.; van Audenaerde, J.; de Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef]
- Van Hall, T.; André, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; van Der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG 3 (CD 223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, Y.; Ma, Y.; Ding, C.; Zhang, H.; Lu, Z.; Gu, Z.; Zhu, C. CXC chemokine receptor 2 correlates with unfavorable prognosis and facilitates malignant cell activities via activating JAK2/STAT3 pathway in non-small cell lung cancer. Cell Cycle 2019, 18, 3456–3471. [Google Scholar] [CrossRef]
- Chi, H.; Li, C.; Zhao, F.S.; Zhang, L.; Ng, T.B.; Jin, G.; Sha, O. Anti-tumor activity of toll-like receptor 7 agonists. Front. Pharmacol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Rasco, D.W.; Johnson, M.L.; Patel, M.R.; Alistar, A.T.; Cohen, J.W.; Cho, C.; Wang, M.; Kurland, E. Safety and pre-liminary efficacy of EIK1001 in combination with pembrolizumab in participants with advanced solid tumors. J. Clin. Oncol. 2024, 42, 2521. [Google Scholar] [CrossRef]
- Hernandez, R.; Põder, J.; LaPorte, K.M.; Malek, T.R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat. Rev. Immunol. 2022, 22, 614–628. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Emmerich, J.; Bauer, M.; Semana, M.; Jayaraman, B.; Vivona, S.; McCauley, S.; Riener, R.; Malefyt, R.D.W.; Aspuria, P.-J.; Rokkam, D.; et al. STK-012, an alpha/beta selective IL-2 mutein for the activation of the antigen-activated T cells in solid tumor. Cancer Res. 2021, 81, 1744. [Google Scholar] [CrossRef]
- Yang, S.-C.; Hillinger, S.; Riedl, K.; Zhang, L.; Zhu, L.; Huang, M.; Atianzar, K.; Kuo, B.Y.; Gardner, B.; Batra, R.K.; et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin. Cancer Res. 2004, 10, 2891–2901. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.-H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J.; et al. Phase I trial of intratumoral injection of CCL21 gene–modified dendritic cells in lung cancer elicits tumor-specific immune re-sponses and CD8+ T-cell infiltration. Clin. Cancer Res. 2017, 23, 4556–4568. [Google Scholar] [CrossRef]
- Aggarwal, C.; Sterman, D.; Maldonado, F.; Bestvina, C.M.; Reisenauer, J.S.; Li, H.; Puri, S.; Ibrahim, O.; Swan, R.; Caine, A.; et al. CAN-2409 plus prodrug with standard of care immune checkpoint inhibitor for patients with stage III/IV NSCLC. J. Clin. Oncol. 2023, 41, TPS9162. [Google Scholar] [CrossRef]

| Drug | Class | Trials | Indications |
|---|---|---|---|
| Nivolumab | Anti-PD-1 | CHECKMATE-057 [12] CHECKMATE-017 [13] |
|
| Nivolumab + Ipilimumab | Anti-PD-1 Anti-CTLA-4 | CHECKMATE-227 [14] CHECKMATE-9LA [15] |
|
| Pembrolizumab | Anti-PD-1 | KEYNOTE-010 [16] KEYNOTE-042 [17] KEYNOTE-407 [18] KEYNOTE-189 [19] |
|
| Atezolizumab | Anti-PD-L1 | IMpower110 [20] IMpower150 [21] IMpower130 [22] OAK [23] |
|
| Durvalumab | Anti-PD-L1 | PACIFIC [24] |
|
| Durvalumab + Tremelimumab | Anti-PD-L1 Anti-CTLA-4 | POSEIDON [25] |
|
| Cemiplimab | Anti-PD-1 | EMPOWER-LUNG 3 [26] EMPOWER-LUNG1 [27] |
|
| Target | Drug | Trial | Phase | Type of Tumor | Preliminary Efficacy | Safety | Comments | Ref |
|---|---|---|---|---|---|---|---|---|
| Antibody–drug conjugate | ||||||||
| CD30 | Brentuximab vedotin | NCT04609566 | II | NSCLC Melanoma | mPFS was 4.1 months (mo) and mOS was 13.9 mo (NSCLC). | Grade ≥ 3 TEAEs in 56%, TESAEs in 42%. Peripheral neuropathy reported in 48% of pts.; 17% of pts discontinued treatment due to TEAEs. | In combination with pembrolizumab Recruiting | [29] |
| Bispecific antibodies | ||||||||
| CD16A and EGFR | AFM24 | NCT05109442 | I/IIa | NSCLC | 3/15 PR, 1/15 CR, 7/15 SD. | 2/17 Grade 3 toxicity infusion-related reaction. | In combination with atezolizumab EGFR wild type Ongoing study, recruiting | [30] |
| PD-L1 and 4-1BB | Acasunlimab | NCT05117242 | II | NSCLC | ORR 13%, 21%, and 22% with 6 mo PFS 0%, 18%, and 33% for monotherapy, pembrolizumab combination therapy q3 week, or pembrolizumab combination therapy q6 week. | Most common grade ≥ 3 TRAEs were asthenia, liver-related events, and anemia. | Patients progressed on ICI Monotherapy and combination therapy with pembrolizumab Active, not recruiting | [31] |
| PD-L1 and CTLA-4 | KN046 | NCT04054531 | II | NSCLC | ORR is 46.0%, and the median duration of response is 8.1 mo. The mPFS and mOS are 5.8 and 26.6 mo, respectively. | Grade ≥ 3 TRAEs was observed to be 66.7%. Treatment-related deaths occurred in 4 (4.6%) patients, with one case of immune-related pneumonia attributed to KN046. | First-line In combination with chemotherapy | [32] |
| PD-L1 and VEGF | HB0025 | NCT04678908 | I | NSCLC | ORR 25%, DCR 66.7% with 3/12 PR, 5/12 SD, and 4/12 PD. | 11/12 patients experienced TRAE. Grade 3 ≥ TRAEs occurred in 2/12. No DLT. | Monotherapy Included TKI-resistant population with EGFR/ALK mutation | [33] |
| PD-L1 and VEGF | PM8002 | NCT05918445 | Ib/Iia | NSCLC | 16/61 PR, 32/61 SD. | 11/61 experienced grade ≥ 3 TRAEs. 5/61 patients discontinued due to TRAEs. | Monotherapy Included TKI-resistant population with EGFR mutation | [34] |
| CD137 and PD-L1 | MCLA-145 | NCT03922204 | I | Advanced solid tumors | DCR 37% with monotherapy and 68% with combination. | 6/53 patients receiving monotherapy had DLTs. No DLTs occurred in the patients receiving combination therapy. | Monotherapy or in combination with pembrolizumab | [35] |
| Cellular therapy | ||||||||
| KRAS | FH-A11KRASG12V-TCR | NCT06043713 | I | mutant KRAS including pancreatic, colorectal, and NSCLC | - | - | Recruiting | [36] |
| Tumor-infiltrating lymphocytes (TILs) | GT201 | NCT05729399. | I | Advanced solid tumors | 3/7 patients PR, 2/7 patients SD. In the NSCLC subgroup, disease control was observed in 3/3 patients. | Grade ≥ 3 AEs related to lymphodepleting chemotherapy and IL-2 included decreased lymphocyte count, decreased neutrophil count, and decreased white blood cell count, pyrexia and increased heart rate. | 3/7 patients PR, 2/7 patients SD. In the NSCLC subgroup, disease control was observed in 3/3 patients Recruiting | [37] |
| Microbiome-based therapeutics | ||||||||
| Gut microbiome | BMC128 | NCT05354102 | I | RCC, melanoma, and NSCLC | 4/8 patients with SD. | No SAEs. | In combination with nivolumab | [38] |
| Gut microbiome | hdFMT | NCT05669846 | II | NSCLC | - | - | Patients with R/R NSCLC progressed on prior anti-PD-1-based therapy Not yet recruiting | [39] |
| Monoclonal antibodies | ||||||||
| anti-ILT3 | MK-0482 | NCT04165798 | II | NSCLC | ORR (95% CI) 4% (1%−15%; 0 CR, 2 PR). PFS was 2.6 (1.4−4.7) mo. | Grade ≥ 3 in 17 patients (38%). | In combination with pembrolizumab | [40] |
| anti-ILT4 | MK-4830 | NCT04165798 | II | NSCLC | ORR (95% CI) 11% (4−24%; 1 CR, 4 PR). PFS was 2.4 (1.5−2.7) mo. | Grade ≥ 3 in 20 (44%). | In combination with pembrolizumab | [40] |
| anti-NKG2A/CD94 | monalizumab | NCT02671435 | I/II | Advanced solid tumors | 2/20 PR and 6/20 SD. | No DLTs. | In combination with durvalumab | [41] |
| CD27 agonist | boserolimab | NCT04165798 | II | NSCLC | ORR (95% CI) was 8% (2−22%; 1 CR, 2 PR). PFS was 2.4 mo. | Grade ≥ 3 in 21 (57%). | In combination with pembrolizumab | [40] |
| Complement factor H (CFH) | GT103 | NCT04314089 | Ib | NSCLC | Best treatment response was SD in 9/31 patients. | 3/31 patients experienced grade ≥ g3 TRAEs | Monotherapy | [42] |
| Complement factor H (CFH) | GT103 | NCT05617313 | II | NSCLC | - | - | In combination with pembrolizumab Ongoing | |
| CTLA-4 | IBI310 | NCT05118334 | II | NSCLC | 2/30 PR, 15/30 SD, 2/20 ORR, 17/30 DCR. | TEAEs leading to treatment interruption of any treatment drug occurred in 7 (7/15, 46.7%) and 9 (9/15, 60%) patients in cohorts A and B, respectively. Three treatment-related deaths. | In combination with sintilimab (anti-PD1) | [43] |
| LAG-3 | LBL-007 | NCT05102006 | Ib/II | Advanced solid tumors | Out of 75 efficacy evaluable patients, ORR and DCR were 13.3% and 48.0%, respectively. | Treatment interruption and permanent discontinuation due to TEAEs occurred in 6 (7.5%) patients each. | In combination with toripalimab (anti-PD-1) | [44] |
| LAG-3 | Ieramilimab | NCT02460224 | II | Advanced solid tumors | In NSCLC cohort, 3/42 PR, 18/42 with SD, ORR 15%. | In the anti-PD-1/L1 naive and pretreated cohorts, 9.9% and 5.4% of patients, respectively, discontinued study treatment due to AEs regardless of study drug relationship. | In combination with startalizumab (anti-PD-1) | [45] |
| Other | ||||||||
| CXCR2 | Navarixin | NCT03473925 | II | Advanced solid tumors | mPFS was 1.8–2.4 mo without evidence of a dose–response relationship, and the study was closed at a prespecified interim analysis for lack of efficacy. | DLTs occurred in 2/48 patients (4%) receiving navarixin 30 mg and 3/48 (6%) receiving navarixin 100 mg. | In combination with pembrolizumab | [46] |
| TLR7/8 | EIK1001 | NCT06246110 | II | NSCLC | - | - | In combination with pembrolizumab Recruiting | [47] |
| α/β IL-2R | STK-012 | NCT05098132 | Ia/Ib | Advanced solid tumors | 3/38 PR. 17/38 SD. | No DLTs. | Monotherapy | [48] |
| Cancer vaccines | ||||||||
| CCL21 | IT CCL21-DC | NCT03546361 | I | NSCLC | - | - | Active, not recruiting | [49] |
| HLA-A*02:01-restricted peptides | PDC*lung01 | NCT03970746 | I/II | NSCLC | The BOR included 12 PR (63.2%) and 7 SD (36.8%) with ORR of 63.2% (80% CI 45.9–78.2%) and DCR of 94.7%. The mPFS was 10.9 months. | Only 1 severe TRAE occurred, a grade 4 allergic infusion-related reaction, leading to IMP discontinuation. | In combination with pembrolizumab HLA-A*02 positive | [50] |
| Tumor associated antigen | CAN-2409 | NCT04495153 | II | NSCLC | mOS of evaluable population was 22.0 mo (n = 44). 64% had systemic clinical response of injected and uninjected lesions. | No DLT. | Refractory or resistant to ICI Injected into tumor followed by oral prodrug (valacyclovir) | [51] |
| Tumor associated antigen | BNT116 | NCT05142189 | I | NSCLC | 7/20 (35%) PR, 10/20 (50%) SD. ORR was 35% (95% CI: 15.4–59.2%) and the DCR was 85% (95% CI: 62.1–96.8%). | TEAEs ≥ grade 3, (incidence rate ≥10%). No DLTs within the dose confirmation period or deaths under treatment were observed. | In combination with docetaxel | [52] |
| Tumor associated antigen | BI1361849 | NCT03164772 | IB | NSCLC | Arm A had 29% ORR and 71% DCR, median months of 10, 7.3 5.7, and not reached for DOR, irPFS, PFS, and OS, respectively. In contrast, arm B yielded 11% ORR and 53% DCR, median months of 6, 2.5, 2.5 and 10 for DOR, irPFS, PFS, and OS, respectively. | Both arms had comparable TRAEs (56–57%) of a generally low grade and manageable within current guidelines. Both arms had a comparable rate of treatment discontinuation (22–24%). | Arm A: BI1361849 + durvalumab and arm B: BI1361849 + durvalumab+ tremelimumab | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, E.; Sacchi de Camargo Correia, G.; Li, S.; Zhao, Y.; Manochakian, R.; Lou, Y. Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer. Vaccines 2025, 13, 128. https://doi.org/10.3390/vaccines13020128
Wolf E, Sacchi de Camargo Correia G, Li S, Zhao Y, Manochakian R, Lou Y. Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer. Vaccines. 2025; 13(2):128. https://doi.org/10.3390/vaccines13020128
Chicago/Turabian StyleWolf, Emily, Guilherme Sacchi de Camargo Correia, Shenduo Li, Yujie Zhao, Rami Manochakian, and Yanyan Lou. 2025. "Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer" Vaccines 13, no. 2: 128. https://doi.org/10.3390/vaccines13020128
APA StyleWolf, E., Sacchi de Camargo Correia, G., Li, S., Zhao, Y., Manochakian, R., & Lou, Y. (2025). Emerging Immunotherapies for Advanced Non-Small-Cell Lung Cancer. Vaccines, 13(2), 128. https://doi.org/10.3390/vaccines13020128






