Abstract
Infectious diseases continue to pose a significant global health threat. To combat these challenges, innovative vaccine technologies are urgently needed. Nanoparticles (NPs) have unique properties and have emerged as a promising platform for developing next-generation vaccines. Nanoparticles are revolutionizing the field of vaccine development, offering a new era of immunization. They allow the creation of more effective, stable, and easily deliverable vaccines. Various types of NPs, including lipid, polymeric, metal, and virus-like particles, can be employed to encapsulate and deliver vaccine components, such as mRNA or protein antigens. These NPs protect antigens from degradation, target them to specific immune cells, and enhance antigen presentation, leading to robust and durable immune responses. Additionally, NPs can simultaneously deliver multiple vaccine components, including antigens, and adjuvants, in a single formulation, simplifying vaccine production and administration. Nanovaccines offer a promising approach to combat food- and water-borne bacterial diseases, surpassing traditional formulations. Further research is needed to address the global burden of these infections. This review highlights the potential of NPs to revolutionize vaccine platforms. We explore their mechanisms of action, current applications, and emerging trends. The review discusses the limitations of nanovaccines, innovative solutions and the potential role of artificial intelligence in developing more effective and accessible nanovaccines to combat infectious diseases.
1. Introduction
The world is currently grappling with a series of critical public health challenges, including the emergence of multidrug-resistant bacteria, rapidly mutating viruses, increasing populations of superbugs, anthelmintic-resistant parasites, zoonotic pathogens, and secondary tumors. These challenges, compounded by the recent COVID-19 pandemic, pose significant threats to human health and livestock [1]. These epidemic and pandemic outbreaks have far-reaching consequences, contributing to global mortality rates and hindering socioeconomic development. Additionally, they necessitate robust biodefense strategies to protect populations from future health crises [2,3,4,5]. The overuse and misuse of antibiotics and other drugs have significantly contributed to the rise of antimicrobial resistance, a major global health crisis [6,7]. Additionally, climate change and environmental pollution have accelerated the emergence, transmission, and spread of infectious diseases, further exacerbating the challenges faced by public health systems worldwide [8,9]. Global cooperation has highlighted the need to tackle these rising resistance and infection levels. The One Health Trust has declared that vaccination is considered an integral strategy to combat antimicrobial resistance, slow the spread of superbugs, and lower the overall burden of infection [10,11,12].
The immune system is highly sophisticated and is considered the first line of defense against foreign infectious and non-infectious signals. The innate immune cells are composed of a variety of circulating cells, such as natural killer cells, granulocytes (neutrophils, basophils, eosinophils, and mast cells), and antigen-presenting cells (macrophages and dendritic cells). The immune system is responsible for the processes driving the uptake and ingestion of phagocytosed particles as well as the process associated with breaking them down [13]. The five pattern-recognition receptors that can recognize pathogens play important roles in the initiation and activation of the immune response [13,14]. The intended output of vaccination is to stimulate the innate and adaptive immune systems to induce B- or T-cell responses and provide prolonged protection [15]. When a vaccine introduces an antigen into the body, B-lymphocytes produce antibodies that fight infection, while T-cells recognize and kill cells infected with a virus or other foreign cells, thus controlling the infection transmission and spread [16].
Recently, nanotechnology has provided significant advancements in the medical sector, including the production of nanovaccines, nanotherapeutics, diagnostics, and the improvement of drug screening and gene delivery approaches [17,18,19]. Nanovaccine platforms represent a transformative avenue for vaccine delivery, offering potential solutions to challenges in traditional vaccine development [20]. These platforms use nanoparticles (NPs), such as lipids, polymers, proteins, etc. to improve the delivery of antigens to target cells, resulting in broader and more specific protection against cancers, infectious diseases, inflammatory diseases, atopic diseases, autoimmune diseases, and others [20]. Nanoparticles can be designed to increase the stability and immunogenicity of antigens. They not only serve as antigens carriers but can also function as antigens themselves [21]. NPs can efficiently target vaccine molecules to the desired cell and its receptors, thereby minimizing side effects [22]. Additionally, they have been found to facilitate antigen distribution to APCs and protect antigens and adjuvants from proteolytic and enzymatic degradation [23]. Nanovaccines can evoke both humoral and cell-mediated immune responses [22]. Nanovaccines can persist for a longer time without degradation or alteration, thereby providing enough opportunity for antigen presentation and dendritic cell (DC)-mediated antigen uptake, resulting in mature DC–T-cell interaction, which is necessary to influence the release of pro- and anti-inflammatory cytokines that promote cell-mediated immunity [24]. The NP-mediated vaccine can enhance B-cell antibody production by activating lymphocytes and monocytes. Furthermore, they can enable a targeted release of cargo into draining lymph nodes after passing through the lymphatic drainage system, generating a potent, long-lasting, and antigen-specific immune response [25,26]. These characteristics suggest that NPs might be crucial to vaccines as immune cell stimulators.
In this review, we highlight the transformative impact of NPs on vaccine platforms, focusing on their ability to enhance antigen delivery, improve immune system activation, and address the limitations of traditional vaccines. Specifically, we explore the types of NPs utilized, their mechanisms of action, current applications in vaccine development, and the potential they hold for creating more effective, stable, and accessible immunization strategies in the future. Additionally, we discuss the challenges and opportunities that lie ahead in the development and deployment of nanoparticle-based vaccines.
2. Conventional vs. Nanoparticle-Based Vaccines
2.1. Conventional Vaccines
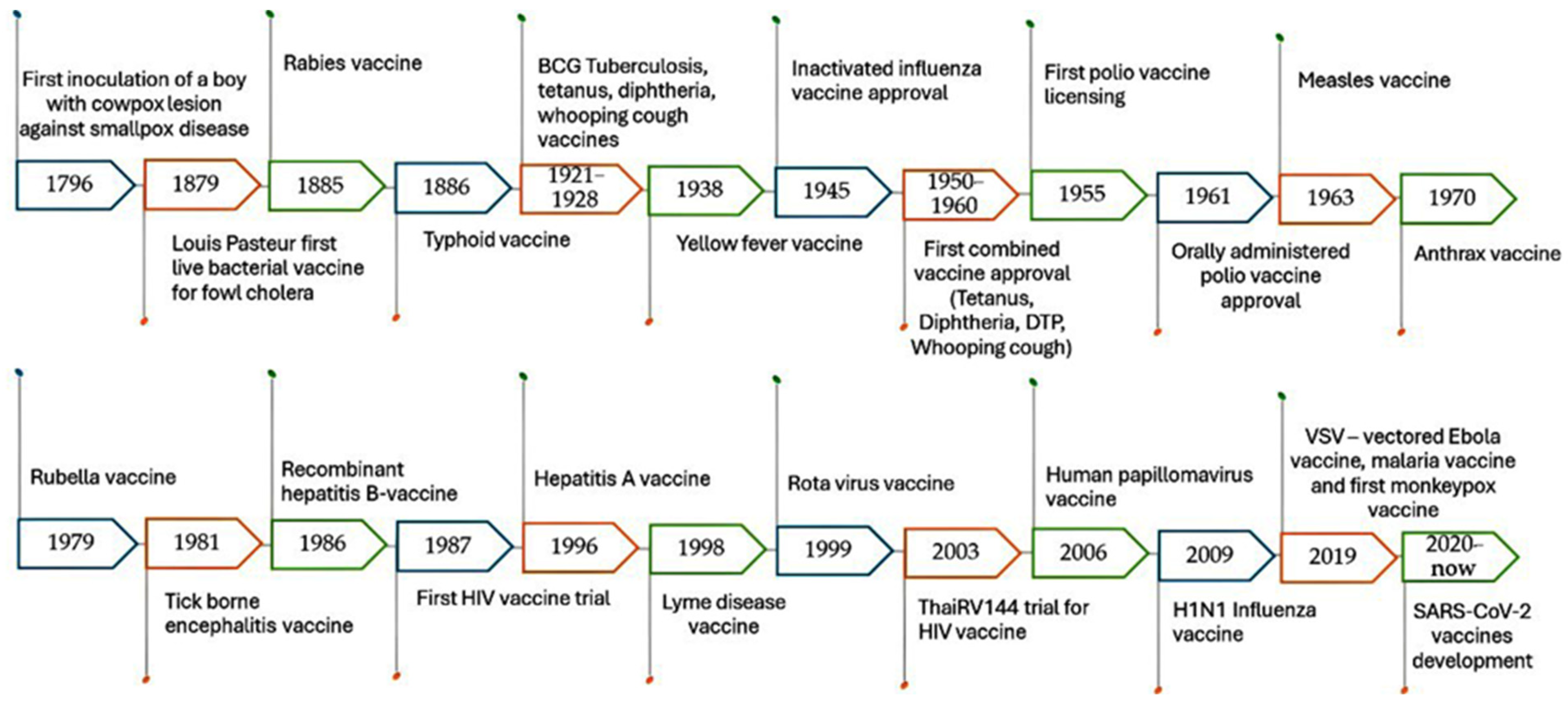
Conventional vaccines are considered one of the greatest advances in medical history against various diseases. They were discovered in the 1700s when Edward Jenner, a British physician, demonstrated that infection with mild cowpox virus can confer immunity against smallpox virus. This discovery led to the development of the smallpox vaccine, which was the first vaccine to be developed against a contagious disease. Consequently, scientists and vaccinologists began occasional and inconclusive experiments for immunization against specific pathogens, as illustrated in Figure 1 [27,28,29,30,31]. The traditional vaccine development pipeline consists of exploratory research, pre-clinical studies, and clinical trials (Phases I, II, and III), followed by regulatory evaluation and approval. However, there are many types of conventional vaccines, which are classified based on their mechanisms of action.
Figure 1.
A brief history of conventional vaccine development.
2.1.1. Inactivated Vaccines
This type contains killed pathogens such as influenza, coryza, typhoid, cholera, mycoplasma, hepatitis A, rabies, Brucella abortus, Mannheimia haemolytica, and Erysipelothrix rhusipathiae vaccines [19,32,33]. These vaccines do not offer as much protection as live vaccines do, so booster shots are often required to maintain immunity.
2.1.2. Live Vaccines
Live vaccines contain attenuated forms of pathogens such as measles, rotavirus, avian infectious bronchitis, Newcastle disease, bovine viral diarrhea, canine distemper, and fowl pox [34]. The immune system responds to this type of vaccine by producing strong and long-lasting protection. However, they may not be suitable for hosts with weakened immune systems, due to the possibility of reversion to virulence, interference with maternal immunity, and tissue damage, which may result in pathological disorders or secondary bacterial infection.
2.1.3. Subunit Vaccines
Subunit, recombinant, polysaccharide, and conjugate vaccines utilize specific microbial components, such as proteins, sugars, or capsules, to induce a targeted immune response. Examples include vaccines for hepatitis B, human papillomavirus, and shingles. While these vaccines offer several advantages, including suitability for immunocompromised individuals, they may require booster doses to maintain long-lasting immunity. Additionally, the absence of pathogen-associated molecular patterns in subunit vaccines can limit their ability to stimulate a robust immune response [35,36].
2.1.4. Toxoid Vaccines
Toxoid vaccines, such as tetanus and diphtheria vaccines [37], contain attenuated bacterial toxins, and have a more effective anti-toxin immune response [38,39]. These conventional vaccines have different administration routes, including intramuscular, subcutaneous, ocular, oral, and intranasal routes [13].
Traditional vaccines offer several advantages, including safety, proven efficacy in disease prevention, and the establishment of strong and long-lasting immunity [40,41]. However, their main limitations include a lack of genetic stability, the potential of mutations in live vaccines, and a limited ability to induce a robust immune response for long-lasting immunity for inactivated vaccines [42,43,44]. This has led to the emergence of nanovaccines as a next-generation approach.
2.2. Nanoparticle-Based Vaccines
NPs can be defined as ultrafine particles with definite dimensions that exhibit unique physicochemical characteristics, supporting their application in various biomedical and food quality fields [45,46,47]. They have emerged as promising vectors in vaccine technology development and are considered a promising alternative to conventional vaccines (Table 1) [48,49]. Studies have revealed that the physicochemical properties of NPs can influence the immune response in terms of factors such as particle size, hydrophilicity/hydrophobicity ratio, the number of antigens associated with each particle, and the ability of an antigen to raise a B- or T-cell response. Smaller-sized particles can pass biological barriers and epithelia more easily when compared with particles ranging from 20 to 50 nm in diameter [46]. The size of nanoparticle-based vaccines is similar, to some extent, to the size of intracellular components, making it easy for them to enter cells and induce robust and long-lasting immune responses against intracellular pathogens [50]. Spherical NPs induce the most effective immune responses when compared to other nanoparticle shapes [51]. Hydrophobic NPs are more efficiently taken up by specific immune cells and potentially enhance the immune response when compared with hydrophilic NPs that produce a weakened immune response [52,53]. Negatively charged NPs interact more efficiently with macrophages, while positively charged NPs interact more efficiently with dendritic cells [54]. NPs can be composed of polymers, lipids, proteins, emulsions, nano-beads, inorganic nanomaterials, and virus-like particles and have been approved for both animal and human use to prevent infectious and non-infectious diseases [55].

Table 1.
Comparison between conventional and nanoparticle-based vaccines.
The major types of NPs used for vaccinology against infectious and non-infectious diseases are illustrated in Table 2. They exhibit a wide range of properties that make them valuable humoral and cellular immune stimulators [13]. They can enhance immune responses by effectively presenting or transporting antigens and allowing precise targeting of immune cells [56,57]. They protect antigens from degradation before reaching their target, thus improving vaccine efficacy and shelf life [58]. Some NPs can act as adjuvants themselves, thus stimulating the immune response without the need for additional substances to the vaccine formula [59]. NPs can be designed to control antigen release, extending immune response duration [60]. Additionally, NPs can be tailored for specific pathogens, making them versatile tools for preventing and controlling infectious diseases [49,61,62,63,64]. The nanoparticle-based vaccines have different administration routes, including ocular, oral, nasal, sublingual, topical, intradermal, intramuscular, and subcutaneous routes. The subcutaneous route is considered the most advantageous route in stimulating the immune response, as vaccines are injected directly into a site that is rich in immune cells, resulting in the production of antibodies at high concentrations [65].

Table 2.
List of nanoparticle-based vaccines used for treating infectious and non-infectious diseases.
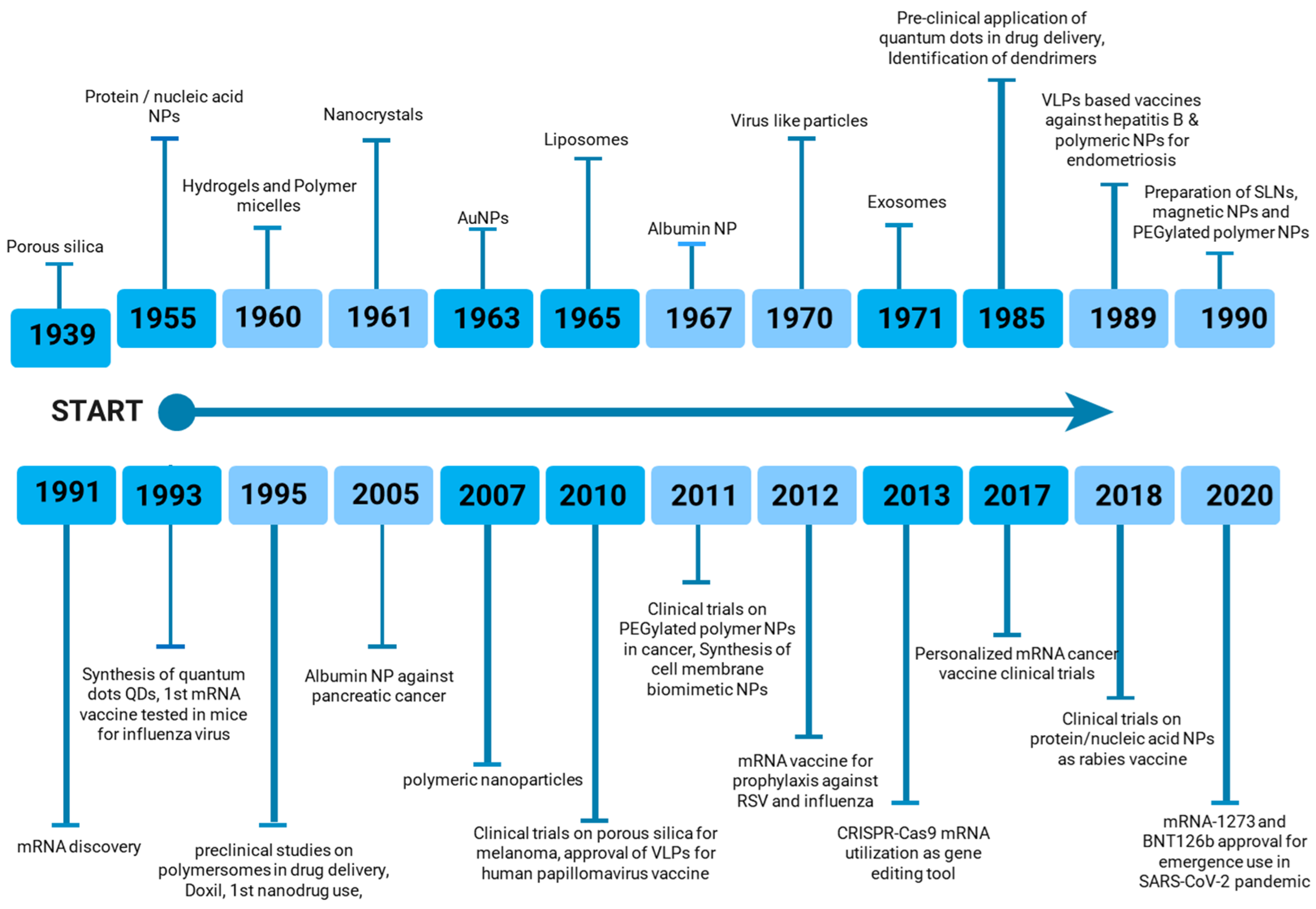
The increasing diversity of nanoparticle-based vaccines has prompted ongoing research toward the optimization of delivery methods and the maximization of immune responses against specific antigens. As depicted in Figure 2, nanoparticle-based vaccines have gained significant prominence in the pharmaceutical and biological industries over the past century. The continued development and implementation of nanoparticle-based vaccines hold immense potential to address a wide range of infectious diseases (viral, bacterial, fungal) and non-infectious diseases (autoimmune diseases, cancer) that pose substantial global health challenges [48,49].
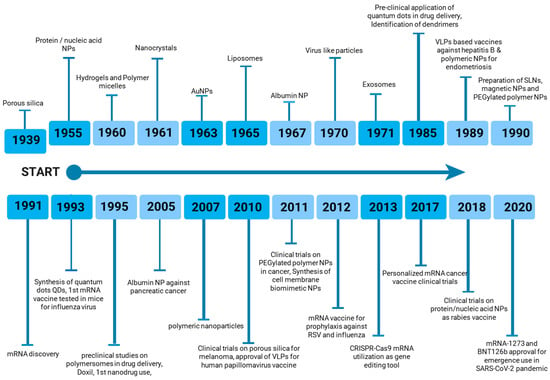
Figure 2.
Timeline of nanoparticle-based vaccine development.
Despite significant advancements in nanovaccine development for combating infectious diseases, inflammatory conditions, allergic diseases, and cancers, several challenges persist. Firstly, ensuring consistent size, shape, and composition of NPs remains a complex task, as these properties are crucial for achieving optimal pharmacological, biological, and physicochemical characteristics [149,150]. Secondly, unintended immune reactions or toxicity can arise from nanoparticle accumulation in tissues and organs [20]. Thirdly, stability and shelf-life, under various environmental conditions, pose challenges, particularly for lipid NPs that are sensitive to temperature fluctuations [151,152]. Fourthly, regulatory hurdles, production costs, and safe waste disposal are additional considerations [153]. Lastly, mRNA-based nanovaccines face specific challenges, including the selection of appropriate antigens, the synthesis and purification of mRNA, and ensuring efficient encapsulation within lipid NPs [154].
3. Types of NPs Used in Vaccines
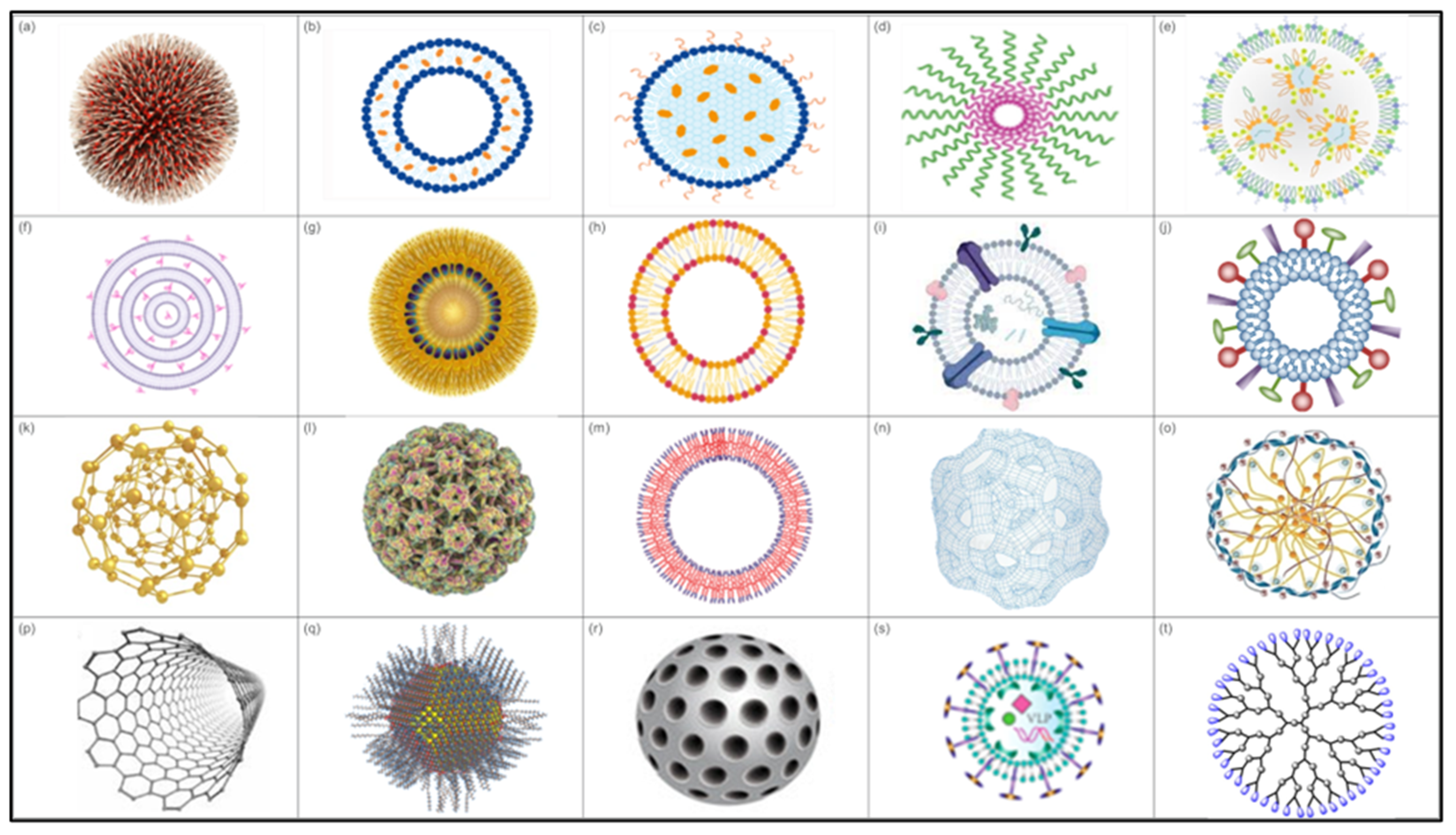
Nanoparticles exhibit specific characteristics that render them more effective for enhancing the immune system and delivering antigens in a controlled and targeted manner [57,155,156]. Several types of NPs are being explored for vaccine development, including lipid-based NPs, polymeric NPs, self-assembled protein or peptide NPs, biomimetic NPs, and inorganic NPs (Figure 3).
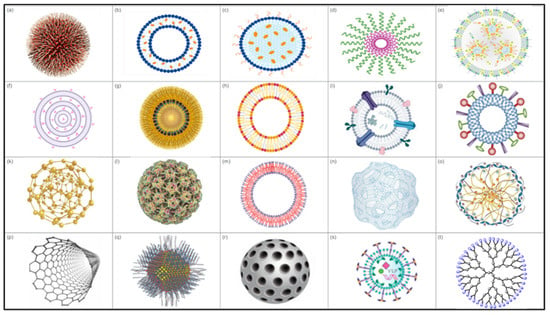
Figure 3.
Types of NPs. (a) Polymeric nanoparticle, (b) liposome, (c) solid lipid NP, (d) polymeric micelle, (e) lipid NP, (f) multilamellar lipid vesicles, (g) lipid nanoemulsion, (h) lipoprotein, (i) exosomes, (j) virosomes, (k) gold NP, (l) protein-based NP, (m) polymersomes, (n) polymeric nanosphere, (o) chitosan NP, (p) carbon nanotubes, (q) quantum dots, (r) mesorphous silica NP, (s) virus-like particle (VLP), and (t) dendrimer.
3.1. Lipid-Based NPs
Lipid-based nanoparticles (LNPs) are also known as lipid-based nanocarriers. They are spherical NPs that use ionizable lipids as their main structural component. These NPs can be categorized based on their structure and composition. In particular, LNPs offer advantages such as biodegradability, biocompatibility, and safety, which are ideal characteristics for a vaccine delivery system [157]. LPNs can facilitate the delivery of mRNA and protect it from rapid degradation by RNases [68]. Additionally, LNPs can interact with the immune system, enhancing cellular and humoral immunity. They have been pivotal in the development of vaccines and other nanomedicines [68,158]. Furthermore, there are many examples of lipid-based NP vaccines that have been widely used in preclinical and clinical studies (Table 3).

Table 3.
Examples of lipid-based NP vaccines widely used in preclinical and clinical studies.
3.1.1. Interbilayer-Crosslinked Multilamellar Vesicles (ICMVs)
ICMVs have an aqueous core surrounded by multilayers of lipids. Their robust lipid wall protects the particles from serum-mediated degradation, enabling stable delivery of vaccine components to lymphoid tissues and thus generating potent antibody responses. ICMVs can serve as a delivery system for both hydrophilic and hydrophobic antigens and adjuvants, showing promise as a vaccination strategy against cancers and intracellular pathogens [181]. Studies have demonstrated that ICMVs with both surface-conjugated and encapsulated malarial antigens offer superior vaccination protection compared to soluble antigens due to the prolonged persistence of antigens in the draining lymph nodes [182]. Despite these values, ICMVs have some disadvantages, such as the complexity of production, the risk of triggering an unintended immune response, the unpredictable release of encapsulated substances from ICMVs, and the fact that techniques used to produce ICMVs may not be easily scaled up for industrial production, limiting their widespread application [181].
3.1.2. Lipoproteins
Lipoproteins are complexes of proteins and lipids. They have been reported to transfer and target various lipids, hormones, proteins, vitamins, and endogenous microRNA to recipient cells [183]. They have a hydrophobic core and a hydrophilic surface, that can facilitate the delivery of a broad range of molecules and act as vaccine-delivery carriers [184]. Recombinant lipoproteins with built-in immune stimulators have been established for novel subunit vaccine development. This core platform technology has demonstrated safety in meningococcal group B subunit vaccine, dengue subunit vaccine, novel subunit vaccine against Clostridium difficile and HPV-based immunotherapeutic vaccines in animal model studies [185]. The main disadvantage of lipoprotein NPs is the stability issues as they can be unstable in the bloodstream, leading to premature release of their cargo [186]. Additionally, the manufacturing process can be complex and costly, requiring precise control over conditions to ensure consistency and efficacy [187]. They are potentially toxic and some lipoprotein NPs can trigger immune responses and cause adverse reactions [188,189].
3.1.3. Liposomes
Liposomes are composed of phospholipid bilayers and range in size from 50 to 500 nm in diameter. They are amphiphilic in nature, with hydrophobic surfaces and a hydrophilic core. Liposomes have gained significant attention in delivering immunostimulatory molecules, drugs, DNA, and RNA [190]. They are highly versatile, biocompatible, safe, and capable of creating different structures by modulating components of the lipid matrix. They act as carriers for vaccines and facilitate the targeted delivery of viral proteins, triggering antibody production. Liposome carriers influence antigen internalization by immunocompetent cells and immune response induction [191]. They can act as adjuvants employed in nanovaccines formulation, so prolong stability and trigger antigen-presenting cells to initiate an immune response. The impact of liposomes on antigen-presenting cells depends on many factors, such as size, charge, and lipid composition [192]. Immunostimulant components (ligands of pathogen-associated molecular pattern receptors) can be added to liposomal vaccine complexes to modulate the strength and type of immune response [192]. Furthermore, liposomes can induce immunologic reactions to antigens adsorbed on the surface or encapsulated internally [193]. Several studies have shown that liposome-based vaccines have been widely used in preclinical and clinical studies [194]. However, liposomes have some drawbacks as they can be prone to leakage and fusion with the encapsulated drug, which can reduce their effectiveness [195]. Moreover, the manufacturing process for liposomes is often expensive and complex and they may need to be stored at specific temperatures to maintain stability. They also have been reported to have a short half-life in the bloodstream, which can limit their effectiveness [196].
3.1.4. Solid Lipid Nanoparticles (SLNs)
SLNs are particulate carrier systems that are composed of a solid lipid core and a cationic lipid surface [197]. These SLNs can be used to bind DNA, forming an SLN/DNA complex or lipoplex, which shows promise as a potential vaccine [198]. SLNs offer several advantages, including the ability to encapsulate both hydrophilic and hydrophobic drugs, protecting them from degradation in the body and destruction by enzymes. These properties make SLNs suitable for delivering various active ingredients, including vaccines, thereby enhancing therapeutic efficacy [199,200]. SLNs have some limitations, as some biomolecules can be expelled from these NPs during storage. Additionally, the high water content in lipid dispersions can affect stability and drug concentration. SLNs also have a limited ability to deliver drugs through the skin [201].
3.1.5. Exosomes
Exosomes are small vesicles composed of lipophilic bilayers ranging from 50 to 100 nm in size and are used in the formation of nanovaccines. They can cross biological barriers, including the blood-brain barrier, and deliver biological and therapeutic antigens to specific tissues [202,203]. Exosomes are biocompatible and biodegradable particles, so they are safe for clinical use. Additionally, they are immunogenic and can induce the immune response to the vaccine antigen [202]. It has been reported that exosome-based vaccines can improve the therapeutic index against cancer and immunosuppressive conditions [204]. Ongoing research is evaluating the immune response of exosome-based vaccines against Ebola virus, influenza and COVID-19 [107,205].
3.1.6. Virosomes
Virosomes are structures composed of viral proteins and liposomes, ranging from 20 to 50 nm in diameter and with hydrophobic surfaces that facilitate antigen loading. The viral protein components could be glycoproteins of different viruses, such as herpes and influenza viruses [206]. Inflexal®V, an approved FDA nanovaccine, has been used as a subunit influenza vaccine that uses virosomes as carriers to enhance the immune responses against influenza H1N1, H3N2, and B [207].
3.1.7. Emulsions
Emulsions, typically consisting of oil-in-water dispersions, are widely used as delivery systems for vaccines. By incorporating antigens, surfactants, and adjuvants, these emulsions can enhance the immune response to the vaccine. Emulsion-based vaccines have shown significant potential for combating various infectious diseases, including human papillomavirus, hepatitis B virus, and influenza viruses. One notable example of an emulsion-based vaccine adjuvant is MF59, which is licensed for use in certain influenza vaccines [208,209].
3.2. Polymeric NPs
Polymeric nanoparticles (PNPs) have served as versatile carriers for active compounds, either absorbed onto their polymeric core or entrapped within the structure itself. PNPs can be synthetics that are chemically synthesized in the laboratory or natural, originating from biological sources such as polysaccharides, proteins, and lipids. Natural PNPs have more advantages over synthetic PNPs in terms of biodegradability, biocompatibility, and low toxicity [210]. There are different PNP design structures such as nanospheres, micelles, and polymerases. Poly (lactic-co-glycolic acid) (PLGA)-based NPs, chitosan, hyaluronic acid, silk fibroin, alginate, polyethyleneimine, polypropylene sulfide, and polystyrene NPs are the major types of polymeric NPs that are commonly used in vaccine adjuvant delivery systems [211,212]. The production of polymeric NPs generally follows two main strategies, the polymerization of monomers or the dispersion of preformed polymers [213].
PNPs can encapsulate multiple adjuvant materials or antigens within the polymer matrix or adsorb it onto the surface, this can protect these biomedical molecules from degradation and enhance their stability. The release of biomedical molecules can be controlled through diffusion, degradation of the polymer, or a combination of both, this allows sustained and targeted delivery. PNPs can be modified with ligands, antibodies, or other molecules that target specific cells or tissues to ensure that the biomedical molecules are delivered precisely to the desired site, reducing side effects and improving efficacy. Some PNPs are designed to release their payload in response to specific stimuli such as pH, temperature or enzymes present in the target tissue [214]. In addition, PNPs are often taken up by cells through endocytosis, a process where the cell membrane engulfs the NPs. Once inside the cell, NPs can release their biomedical molecules payload in tumors and inflamed tissues, meaning that PNP-based vaccines show significant potential as anti-tumor agents [215]. PNPs can also protect labile antigenic particles from enzymatic degradation in vivo [216,217]. Other biodegradable polymers, such as poly-lactic acid, polyethylene oxide, polyethylene glycol, and poly-ε-caprolactone are also used as adjuvants to boost vaccine immunogenicity [218,219]. Polymeric micelles are self-assembled amphiphilic structures that can efficiently deliver viral proteins. The main advantage of micelle NPs is their ability to present antigens in native-like conformations; however, their poor stability in vivo is a major drawback [220,221]. Chitosan NPs have also been used to improve pulmonary immunity against TB by increasing immune responses at mucosal sites [222]. Overall, the main advantages of PNPs include precise control of particle characteristics, payload flexibility, and ease of surface modification. However, there are potential drawbacks, including the possibility of aggregation and toxicity [223], the complexity of their production, their stability under certain conditions, and the fact that some PNPs could be cytotoxic [156,224,225]. There are different applications of PNPs, either in cancer therapy for the targeted delivery of chemotherapeutic agents or in gene therapy applications through the delivery of genetic materials such as DNA or RNA [215,226]. They have also been investigated against malaria, HIV, COVID-19, and tuberculosis diseases [227]. The main examples of polymeric-based nanovaccines that have been widely used in pre-clinical and clinical trials include NVX-CoV2373, a vaccine produced by Novavax which delivers the spike protein from the original Wuhan strain of SARS-CoV-2. The spike proteins self-assemble into 3D structures called “trimers,” which are purified and mixed with an immune-stimulating substance (adjuvant) to form NPs [86]. Clinical trials in adults given two shots of this vaccine 21 days apart demonstrated about 90% efficacy in preventing infection after seven days from the second shot [85,228]. Another example of a PNP-based vaccine is GBP510, a candidate COVID-19 vaccine that has recently undergone a combined Phase 1/2 trial. GBP510 leverages nanoparticle bioengineering technology to stimulate immune responses against COVID-19 [229,230]. Additionally, the TAK-101 vaccine, which incorporates gliadin encapsulated within PLGA nanoparticles (NPs), has been developed for celiac disease and simian immunodeficiency virus (SIV). These PLGA NPs are co-formulated with TLR4 and TLR7/8 agonists, and have demonstrated the ability to induce a prolonged, robust, and high level of protection against SIV in macaque models [231].
3.3. Inorganic NPs
Inorganic NPs are biocompatible, non-toxic, hydrophilic and highly stable particles, presenting significant advantages over their organic counterparts. They can be classified into solids (hydroxyapatite and calcium phosphate) or porous (porous silicon and mesoporous silica) particles. Inorganic NPs exhibit unique properties that hold great promise for enhancing immune responses, improving antigen delivery efficiency, and enabling applications in tumor imaging, drug delivery, vaccine development, and infectious disease prevention. These NPs offer advantages, such as enhanced stability, controlled release kinetics, and targeted delivery, which can significantly improve the efficacy of therapeutic interventions [232]. Gold nanoparticles (AuNPs), carbon nanotubes, quantum dots, and silica NPs are among the inorganic NPs.
AuNPs exhibit unique physical, chemical, and optical properties that differ significantly from bulk gold, making them valuable for a wide range of high-technological applications, such as organic photovoltaics, drug delivery, vaccine development, and catalysis [233,234]. AuNPs are commonly referred to as colloidal gold when dispersed in water. Their most pronounced properties are the intense absorbance and scattering of incident light at their surface plasmon resonance wavelength [235]. AuNPs have garnered attention in vaccine development due to their ability to enhance immune response, making them potential adjuvants for vaccines. AuNPs can be functionalized with drugs to enhance targeted delivery and reduce side effects [236,237,238]. Additionally, they provide stability to vaccine formulations during storage [239,240]. Several studies have demonstrated the potential of AuNPs in vaccine development; for example, conjugated antigenic peptides from the SARS-CoV-2 spike protein to AuNPs have shown no inherent toxicity and have been shown to induce significant IgG antibody levels in tested mice [75]. Another example is the increase in the efficacy of the influenza vaccine by over 25% in specific strains through chiral AuNPs [241]. Moreover, AuNPs can attach to and disrupt the cell membranes of bacteria and viruses, leading to cell lysis and death. Additionally, they can induce the production of reactive oxygen species, which cause oxidative stress and damage DNA, proteins, lipids, and other cellular components. They can also trigger apoptosis (programmed cell death) by activating various cellular pathways and can disrupt the metabolic processes of microbial cells through the inhibition of the activity of essential enzymes. AuNPs can induce cellular and humoral immune responses against enterohemorrhagic E. coli through increasing antigen uptake and processing [242]. Additionally, they can be loaded with platinum on copper nanosheets, and can promote the glucose oxidation process in tumor tissues, so are considered an optional therapeutic for cancer [243].
The primary limitations associated with AuNPs include (a) potential cytotoxicity at high concentrations, despite their inherent biocompatibility; (b) the requirement for specialized, often complex, and costly techniques for their synthesis and functionalization [244]; (c) their susceptibility to aggregation over time, which can compromise their stability and functionality [245]; and (d) the possibility that they might induce adverse effects like inflammation and immune responses [246].
Silica nanoparticles (SiO2 NPs), also known as nanosilica are considered another type of inorganic NP. They are typically amorphous substances with a spherical morphology, although they can be synthesized in various shapes and sizes [247]. Their surfaces can be easily modified for specific purposes, making them suitable nanocarriers for drug delivery due to their high surface area and their ability to adsorb active components [248]. SiO2 NPs possess several advantages including the following: (a) they exhibit low toxicity and are considered safe for biomedical applications; (b) they are thermally stable and can withstand high temperatures without significant degradation; (c) silica is abundant and cost-effective; and (d) their surfaces can be modified and functionalized for specific applications and have gained attention as potential adjuvants for vaccine development. For instance, mesoporous silica nanoparticles (MSNs) are nanomaterials with tunable particle size, high porosity, and versatile surface chemistry. They are commonly used for controlled drug release and delivery due to their low toxicity and biocompatibility [249]. Traditional MSNs have small pores (typically 2–6 nm), which limit their utility for immunotherapy since many antigens and immunostimulatory factors are macromolecules (e.g., proteins, nucleic acids). Recently, MSNs have been developed with extra-large pores (20–30 nm) to incorporate antigens for more effective cancer vaccines. Large-pore MSNs can efficiently load macromolecules, making them suitable for immunotherapy [77]. They target dendritic cells in lymph nodes, delivering tumor antigens and activating DCs, which leads to a cytotoxic T lymphocyte (CTL) antitumor response. Overall, silica-based nanomaterials are biodegradable, biocompatible, and easily conjugated with antigens and adjuvants [250]. They exert immunostimulatory effects, enhancing the immunogenicity of subunit vaccines and offering promise in cancer immunotherapy [78]. There are ongoing clinical trials for various cancers, including ovarian cancer, prostate cancer, and glioblastoma [251]. The primary drawbacks of SiO2 NPs include the complexity of their synthesis processes and the potential long-term environmental impacts and health risks associated with exposure. These risks may include inflammation, oxidative stress, and cytotoxicity [252,253,254].
3.4. Virus-like Particles (VLPs)
VLPs are non-infectious, non-replicating viral structural proteins that lack viral genetic material. They mimic the structure of real viruses and do not cause disease. VLPs are safe and effective immune stimulators [255], highly immunogenic, and capable of inducing both cellular and humoral immune responses. They serve as an alternative platform for developing vaccines against infectious diseases, including hepatitis B, malaria, and SARS-CoV-2. VLP-based vaccines have the advantages of efficacy, safety, stability, and diversity [82,83]. Most licensed VLP-based vaccines use classic aluminum adjuvants, which enhance antibody responses through complex cellular reactions and local antigen trapping at the injection site, ensuring slow release and prolonged stimulation of the immune system [256]. The main drawbacks of VLPs include the following: (1) they can be less stable compared to other NPs; (2) they degrade or lose their structural integrity under certain conditions, which can affect their efficacy; (3) the removal of impurities and the achievement of high purity levels of VLPs is a labor-intensive and time-consuming process; (4) their sensitivity to environmental conditions such as temperature and pH can limit their storage and handling options; and (5) VLPs produced in bacterial systems may contain endotoxins, which can cause severe immune reactions [82,255,257,258].
There are numerous examples of VLP-based vaccines in various stages of clinical and pre-clinical development. For instance, VLPs derived from the hepatitis B virus and human papillomavirus (HPV) have been successfully commercialized. Recently, VLP-based vaccines targeting emerging pathogens such as SARS-CoV-2 have shown significant promise. These include VLPs displaying the SARS-CoV-2 spike protein, which have entered clinical trials [259,260]. Additionally, VLP-based vaccines are being explored for other diseases such as influenza, HIV, and malaria. By mimicking the structure of native viruses, VLPs can induce strong immune responses, making them a versatile platform for vaccine development [261,262,263,264].
3.5. Self-Assembling Peptide/Protein Nanovaccines
Self-assembling peptides and proteins can form diverse nanostructures, including nanofibers, NPs, and hydrogels. These structures offer unique advantages for vaccine delivery, such as controlled antigen release, enhanced immunogenicity, and targeted delivery to immune cells [265]. Recent advancements in peptide and protein engineering have enabled the development of self-assembling nanovaccines for various diseases. For example, ferritin-based NPs have been explored as a platform for delivering multiple antigens, including those targeting EBV, SARS-CoV-2, and cancer [266,267]. Additionally, peptide-based vaccines have been designed to induce specific immune responses against various pathogens [267,268].
4. Mechanism of Action of Nanovaccines
NPs can be functionalized as antigen carriers through encapsulation or surface conjugation. In encapsulation, antigens are enclosed within the NP matrix, providing a controlled release mechanism. In surface conjugation, antigens are attached to the NP surface, facilitating direct interaction with the immune system [59,269]. The mechanism of action of NPs is governed by their structure, size, surface charge, concentration, dosage, uptake, and processing by the immune system cells [270]. The size of NPs enhances tissue penetration and enables immune cell interaction and activation at the infected site [46]. They can protect antigens from premature enzymatic degradation and facilitate a sustained release, thereby ensuring prolonged antigen exposure and enhancing the immune response [55,271]. NPs can mimic the invasion process of pathogens, enhancing the immune response by presenting antigens more naturally and effectively [87]. NPs can also be functionalized with ligands that attach to certain receptors on antigen-presenting cells, including dendritic cells or macrophages. This targeted approach increases the uptake of antigens by the key immune cells. Once inside the APCs, the NPs degrade, releasing the antigens that are processed and presented on the surface of APCs by major histocompatibility complex molecules [272]. Potentially, the immune response to nanovaccines involves antibodies generated for both the NP itself and the incorporated antigen [273].
Adjuvants can be defined as substances added to vaccines to enhance the immune response, improve antigen presentation, activate the immune cells, and prolong antigen exposure. NPs can act as effective adjuvants by gradually releasing antigens and being engineered to specifically reach antigen-presenting cells. This targeted delivery optimizes immunization strategies, allowing effective immune activation with lower antigen doses [227,274].
Various types of NPs, including gold, dendrimers, carbon, polymers and liposomes, can induce cytokine and antibody responses. LNPs are particularly effective in encapsulating mRNA and delivering antigens to immune cells, facilitating CD8+ T cell activation. These LNPs have demonstrated success in clinical applications, such as in mRNA vaccines for SARS-CoV-2, where they enable efficient delivery of spike proteins to antigen-presenting cells, thereby triggering a strong immune response [189,275]. Additionally, NPs allow for the delivery of multiple antigens within a single vaccine. For example, a single NP could encapsulate spike proteins from various coronaviruses, potentially offering broader protection against related pathogens [276].
5. Nanovaccines for Food Safety
Foodborne and waterborne bacterial pathogens continue to pose significant global health challenges, causing substantial morbidity and mortality worldwide. Recent advances in NP-based vaccines have also emerged to form a promising strategy to combat these pathogens through immunization.
Recent studies have demonstrated significant progress in developing nanovaccines against Salmonella spp. Chitosan NPs conjugated with Salmonella outer membrane proteins have been shown to induce robust immune responses in broilers, protecting against lethal challenges. The nanoformulation showed superior stability when compared with conventional vaccines and also increased mRNA expression of toll-like receptors [277]. Additionally, oral vaccine administration (S. Enteritidis immunogenic outer membrane proteins and flagellin entrapped in mannose chitosan NPs) elicited cross-protective mucosal immune responses against S. Typhimurium colonization in broilers [278]
Innovative approaches using gold NPs conjugated with E. coli O157:H7 antigens have shown promising results. Recent studies have shown that vaccination with gold NPs conjugated to E. coli O157:H7 antigens elicits strong systemic and mucosal immune responses, leading to significant protection against E. coli O157:H7 colonization [279]. Furthermore, nanoformulations, combining antibiotics, NPs, and polymeric carriers, can improve drug delivery and efficacy in the treatment of urinary tract infection (UTI) treatment, especially UTIs caused by E. coli. These nanoformulations can enhance drug solubility, target specific sites of infection, and control drug release, leading to improved therapeutic outcomes and reduced side effects. These advancements in nanotechnology hold the potential to revolutionize UTI treatment by providing more effective and targeted therapeutic options [280].
A novel lipid nanoparticle (NP)-based vaccine incorporating the cholera toxin B subunit has been developed to combat V. cholerae. This formulation has demonstrated enhanced mucosal immunity compared to traditional oral cholera vaccines. Additionally, it has shown improved stability under gastric conditions and increased bioavailability, making it a promising alternative for cholera prevention [281]. Complementary research has shown that nanoparticle-based vaccines combining V. cholerae outer membrane vesicles induced long-lasting immunity [282].
Engineered polymer-based NPs incorporating flagellin and outer membrane proteins of Campylobacter jejuni have shown a significant reduction in bacterial colonization, achieving protection in poultry models [109]. Additionally, hybrid NPs combining chitosan and alginate have shown enhanced mucosal adherence and improved immune responses against C. jejuni [283,284].
Advanced NP formulations targeting L. monocytogenes have emerged as potential preventive strategies, where developed NPs conjugated with listeriolysin O have demonstrated enhanced cellular immunity and effective cross-presentation to CD8+ T-cells [285]. In addition, NPs loaded with multiple listeria antigens achieved broad-spectrum protection against different serotypes [285].
Innovative approaches in Shigella nanovaccine development have shown significant progress using NPs encapsulating Shigella invasion plasmid antigens, demonstrating robust protection against multiple Shigella species [286]. Furthermore, success has been reported with pH-responsive NPs that specifically release antigens in the intestinal environment, enhancing the efficiency of immune response induction [287].
6. Nanoparticle-Based Vaccines in Veterinary Medicine
The advancements in nanotechnology have revolutionized the field of vaccination and drug delivery, significantly enhancing the diagnosis, prevention, and treatment of both infectious and non-infectious diseases in humans and animals [269,288]. NPs, when used in vaccines, enable targeted delivery, enhance immune response, and potentially offer prolonged immunity, making them particularly advantageous in veterinary medicine applications. By reducing the reliance on antimicrobials, these nanovaccines play an essential role in promoting animal welfare and addressing antimicrobial resistance, a growing concern in veterinary and public health [289,290]. In veterinary medicine, nanoparticle-based vaccines have demonstrated effectiveness in conferring specific and durable immunity against a range of clinically significant bacterial pathogens, including Salmonella spp., E. coli, Staphylococcus spp., Helicobacter spp., Mycobacterium spp., Clostridium spp., and Pseudomonas spp. For example, studies have demonstrated that nanoformulations can enhance antigen stability and allow for controlled release, which can improve vaccine efficacy and immune memory [288,289,290]. This technology is also being explored to develop novel vaccine platforms that may improve immune responses against difficult-to-treat pathogens and reduce disease burden in livestock industries [290].
Secreting membrane vesicles are nanostructures that can stimulate T-cell responses against different antibiotic-resistant bacteria such as S. aureus and avian pathogenic E. coli. These extracellular vesicles produced by resistant bacterial strain surfaces and coated with indocyanine-loaded magnetic silica NPs could significantly reduce surface infection, systemic invasiveness, and complications of S. aureus in experimental models [97]. The outer membrane vesicles of avian pathogenic E. coli serotype O2 have experimentally promoted strong specific and non-specific protective immune responses against homologous infection with E. coli in broilers, which was achieved via the reduction of bacterial loads, reduction of proinflammatory cytokine production and activation of T-cell responses [98].
Carbon nanotubes and poly-anhydrous NPs have been tested against anaplasmosis or inactivated M. paratuberculosis in murine experimental models. The carbon nanotubes absorbed and displayed the A. marginale MSP1 protein on both their outer and inner surfaces. They then traversed biological membranes without compromising their integrity. In contrast, the polyanhydrides encapsulated whole-cell lysates and M. paratuberculosis culture filtrates through nanoprecipitation. [99].
Furthermore, nanovaccines based on the oligo-polysaccharide antigen and poly (lactic-co-glycolic acid) NPs have shown promise against B. melitensis through efficient engulfment by macrophages and other phagocytic cells [117]. Polyanhydride NPs encapsulating pathogenic fusion proteins co-adjuvanted with noncanonical CDGs are among the latest innovations that rapidly induce specific protective immune responses against highly virulent B. anthracis and Y. pestis [111]. F. columnare, which is a highly contagious bacteria affecting tilapia, can be controlled and suppressed by the immersion of mucoadhesive polymer chitosan-complexed nanovaccine, which modulates the mucosal immune response of tilapia against columnaris disease [103]. Avian pathogenic E. coli, which is a highly infectious pathogen impacting poultry production worldwide, has been minimized in chickens by using chitosan-based nanovaccines. These chitosan nanovaccines can encapsulate and load the outer membrane protein and flagellar antigen, resulting in improved vaccine efficacy and protection against avian pathogenic E. coli [108].
Additionally, chitosan nanovaccines containing S. Enteritidis that are surface-coated with F-protein and O-F antigen have successfully reduced the load of S. Enteritidis in chicken and pig intestines by stimulating specific memory B and T cell responses [109,110,291]. Moreover, nanoparticle-based vaccines have been used for the prevention and treatment of different parasitic infections and infestations such as malaria, leishmaniasis, toxoplasmosis, schistosomiasis, anaplasmosis, theileriosis, trypanosomiasis, and coccidiosis. They have also been applied to parasitic vectors like fleas, mosquitoes, and ticks [147,292]. Inoculation of nanovaccines in experimental animals against T. cruzi has been developed and optimized for both the protection and control of parasite replication and dissemination [112]. Moreover, new nanovaccine platforms, such as antigen-absorbing silica vesicles, have been evaluated against tick-borne diseases in laboratory models and have shown very promising results [113]. Another novel nano vaccine has shown synergistic efficacy and balanced immune response against T. parva [81]. Furthermore, precisely engineered nanovaccines have provided stable, long-lasting immunological and less toxigenic responses against highly pathogenic and mutated viruses, including avian influenza, RSV, and RVF virus [104,147,293,294].
7. Current Research on Nanoparticle-Based Vaccines
The use of NPs in vaccines can improve immunogenicity and offer a promising platform for future vaccine development. Researchers are utilizing microfluidic platforms to evaluate the performance of NPs in preclinical studies. These platforms can mimic in vivo conditions, allowing a more accurate assessment of NPs’ efficacy and safety [295]. In addition, membrane-based nanovaccines are under investigation, with several candidates in preclinical and clinical phases for use as effective cancer nanovaccines to inhibit tumor growth and metastasis [296,297,298]. NPs are being explored to improve the delivery and efficacy of cancer treatments. For instance, in lung cancer therapy, NPs can deliver drugs directly to tumor cells, enhancing the precision and effectiveness of the treatment while minimizing side effects [299]. Different types of NPs, such as polymer NPs, liposomes, quantum dots, dendrimers, and gold NPs, are being studied for their unique properties and applications in targeting tumor tissues [299,300,301]. Animal models remain the appropriate tool in demonstrating the efficacy of nanoparticle-based vaccines. Rats, guinea pigs, mice, sheep, canines, rabbits, swine, and non-human primates like African green monkeys and Asian macaques are among the most commonly used animal models. Small animal models offer advantages in handling, housing, and maintenance costs; however, selecting a suitable animal model is crucial for translating experimental findings to clinical applications and overcoming developmental barriers. For example, mice are more resistant to classic TB disease which minimizes their utilization for evaluating TB vaccines. The encapsulation of antigens in suitable animal models using microparticles/NPs enhances the immune responses, resulting in better immunity and broader protection compared with conventional vaccines [302]. Self-assembled peptide nanovaccines (SAPNs) can act as innovative nanovaccines that enhance antigen-presenting cell uptake, with B-cell activation and lymph node trafficking, resulting in a robust immune response and protection against various animal infectious diseases [303]. Additionally, VLPs have shown promise in vaccine development and have effectively protected animal models against SARS-CoV-2 infection [304].
8. Hurdles for Nanoparticle-Based Vaccines
Regulatory approval pathways for NPs can be extremely complex due to their unique features and potential applications across various fields, including medicine, cosmetics, and food packaging. The U.S. Food and Drug Administration (FDA) regulates NPs under existing statutory authorities, tailored to the specific product type (e.g., drugs, biologics, devices). For nanomedicine, the FDA requires thorough evaluations of safety, quality, and efficacy and often involves laboratory and clinical studies due to their novel properties. The regulatory bodies perform a risk-benefit analysis tailored to the intended use of the nanoparticle product with continuous monitoring of nanoparticle products post-approval to ensure ongoing safety and effectiveness [305,306,307]. Regarding NP-based vaccines, key considerations include validation methods for antigen selection, formulation stability, antigen release rates, the characterization of carrier pharmacokinetics and biodistribution, and rigorous clinical trials and safety assessments that include Phase I, II, and III trials which assess safety, efficacy, and immunogenicity [308,309,310].
Several critical considerations and challenges pertain to NPs, including safety concerns related to adverse reactions, as NPs may accumulate in tissues and organs causing harm. Some NPs may trigger allergic reactions, inflammations, and oxidative stress and there is potential for NPs to induce DNA damage, which could result in mutations and genotoxicity [311]. Regulatory bodies like the FDA, European Medicines Agency (EMA), and National Medical Products Administration (NMPA) have set guidelines and standards to ensure the safety and efficacy of these nanoparticle-based vaccines. Another challenge is the cold chain requirements of LNPs, as they are sensitive to temperature fluctuations and thus require stringent cold chain maintenance during storage and distribution [152]. Global harmonization is another challenge, ensuring consistent standards and social considerations across different countries. The U.S. FDA has set specific guidelines, including preclinical studies, clinical trials, and post-marketing surveillance to ensure the quality, safety, and efficacy of nanoparticle-based vaccines. The European Medicines Agency (EMA) also has stringent regulations focusing on safety, efficacy, and quality control for the approval of NP-based vaccines. In China, the NMPA focuses on clinical trial data and post-marketing monitoring for the regulation and approval of NP-based vaccines. Furthermore, regarding social considerations, there may be different levels of vaccine hesitancy due to mistrust or due to misinformation from the healthcare system, because some communities may have philosophical or religious objections to certain vaccines, and also because there may be disparities in healthcare access and distribution, particularly in low-income countries. Global organizations, like the World Health Organization (WHO), are working to harmonize vaccine standards and guidelines to ensure consistency and safety across countries [312]. Ongoing efforts to overcome cultural barriers and educate and engage communities about the importance of vaccination and increase acceptance. The complexity of nanoparticle design is another challenge and requires careful manufacturing to achieve an ideal product with the appropriate pharmacological, biological, and physicochemical characteristics [149]. The ability to overcome biological barriers to deliver therapeutics effectively to the target sites is a further challenge. There are additional challenges for mRNA-based nanovaccines, which include selecting the appropriate target antigen, synthesizing, and purifying mRNA, and ensuring effective encapsulation of mRNA within lipid NPs. There are several manufacturing challenges for NPs, including top-down manufacturing approaches for breaking down bulk materials into NPs. These techniques, such as milling, lithography, and laser ablation, can be energy-intensive and may lead to issues like contamination and non-uniform particle sizes [313]. In contrast, bottom-up, such as chemical vapor deposition, sol-gel processes, and self-assembly, offer greater control over particle size and composition [313].
There are also scalability challenges for NPs, including the need for uniformity and to achieve consistent size, shape, and distribution of NPs on a large scale, which can affect the performance and safety of the final product [313]. Even small amounts of impurities can significantly alter the properties of NPs, making it crucial to maintain purity and prevent contamination during large-scale production [314]. Additionally, scaling up nanoparticle production can be expensive due to the need for specialized equipment and materials.
Current research focuses on optimizing the manufacturing processes for nanovaccines to enhance their scalability, effectiveness, and cost-efficiency. mRNA vaccines have gained attention due to their rapid design, potent immune responses, and safety. Additionally, they can be designed within days, allowing fast production and scalability [315]. LNPs acting as effective delivery platforms for mRNA vaccines can enhance immunogenicity, target delivery, and improve stability [274]. Interestingly, another platform, called the Silicon Scalable Lipid NP Generation platform, has been developed with a scalable and efficient solution for LNP production and for addressing the challenges exposed during the COVID-19 pandemic [93].
Different measures can help in achieving high-quality and consistent NPs, which are essential for their reliable performance in various applications. Advanced techniques like electron microscopy (scanning, and transmission), and dynamic light scattering are used to analyze the size, shape, and distribution of NPs. Developing standardized protocols for synthesis and characterization helps in maintaining consistency across different batches. Implementing strict control over the production process, including temperature, pH, and reactant concentrations, ensures reproducibility. Regular testing and validation of NPs against predefined criteria help in identifying and mitigating any deviations. Using automated systems for production and quality control can reduce human errors and improve consistency. Adhering to regulatory standards and guidelines ensures that the NPs meet safety and efficacy requirements. Furthermore, rigorous quality control and adherence to regulatory guidelines are essential for successful nanoparticle-based vaccine safety and efficacy. The US Pharmacopeia has developed analytical methods by which to assess attributes such as identity verification, impurity control, and content measurement for mRNA vaccine and these quality standards contribute to enhanced regulatory confidence [316,317,318]. Preclinical assessment of nanovaccines involves measuring physical, chemical, and stability properties, as well as assessing immunogenicity and toxicity in vitro and in vivo, which are essential to ensure comprehensive characterization throughout vaccine development [319].
9. Innovations in NP Development
9.1. Nanocages, Dendrimers, and Other Novel Structures
Various innovative structures, including nanocages and dendrimers, have emerged in biomedical research and vaccine development. Nanocages are nanoscale, hollow structures with porous walls. Nanocages offer unique properties, including porosity, high loading capacity, controlled release mechanisms, and customizable surface properties. These characteristics make nanocages highly effective for delivering therapeutic and biological molecules. Additionally, nanocages are not recognized as exogenous particles and can be biologically or chemically engineered. Nanocages can be classified into two groups: organic nanocages (e.g., protein nanocages, DNA nanocages, etc.) and inorganic nanocages (e.g., gold nanocages, silica nanocages, carbon-based nanocages, etc.) [320]. Organic nanocages, particularly protein nanocages derived from endogenous proteins, exhibit minimal interactions with living cells. In contrast, inorganic nanocages offer high antigen-loading capacity, controllable drug loading and release kinetics, flexible surface chemical modification, and in vivo safety. They can also incorporate luminescent, radioactive, or magnetic reporter molecules for tracing purposes.
Dendrimers are highly branched nanosized polymers with a central core and multiple layers of branching units. Their characteristics, such as chemical stability, polyvalency, electrostatic interactions, self-assembling, solubility, and low cytotoxicity, make them suitable candidates for functionalization with antigens, adjuvants, and other nucleic acid-based vaccines [321,322]. This technology facilitates a rapid response to vaccines, with broader efficacy and reduced vaccination frequency. Notably, dendrimer-RNA nanoparticles have demonstrated significant protective effects against lethal pathogens such as H1N1 influenza, Ebola, and T. gondii, with a single dose protecting challenged mouse models [94].
Outer membrane vesicles (OMVs) are spherical double-layered nanostructures (20 to 250 nm) derived from the surface of gram-negative bacteria. OMVs do not cause diseases because they mimic the structures found on the bacterial cell surface and cannot be replicated. They have a promising role in the creation of nanoparticle vaccines and can be engineered to display multiple antigens against different bacterial and viral infections and malignant tumors. OMVs can strongly stimulate the innate and adaptive immune responses [323]. However, there remain some challenges that are being addressed, including possible degradation by proteases, protein misfolding, and inefficient translocation of complex antigens [324].
Metal–organic frameworks (MOFs) are examples of NPs that can be used in nanovaccines to enhance the immune response to antigens. MOFs can encapsulate and deliver viral proteins, such as the SARS-CoV-2 spike protein. Once inside the cells, MOFs break down and activate the innate immune system through toll-like receptors that lead to increased production of cytokines and other inflammatory molecules [325,326].
Nanogels show great potential as novel antigen-delivery vehicles. They are sub-micron structures with a network of cross-linked polymer chains. These nanogels can encapsulate biomolecules and target specific immune cells [327,328]. They also can be designed to release antigens in a controlled manner to prolong the immune response. In addition, nanogels can be designed to display multiple antigens to protect against multiple infectious agents [329].
Combinational nanovaccines can combine multiple antigens in a single formulation to induce a broader and stronger immune response. Despite the potential benefits of combinational nanovaccines, several challenges should be considered, as the multiple antigens and adjuvants in the combined nanovaccines can interfere with each other, resulting in adverse effects, while their design can be complex and requiring increased testing and evaluation. The other challenge is regarding combinational nanovaccines’ safety. There are different examples of combinational nanovaccines in the preclinical and clinical phases, including an influenza vaccine that can protect against multiple strains of influenza viruses [330], a tuberculosis vaccine that targets different TB antigens [331], a cancer vaccine that combines multiple cancer antigens [332] and an HIV combined vaccine that targets multiple strains of HIV [333].
Most traditional vaccines are administered through injection, which could be painful for some individuals. Needle-free nano vaccine administration (oral delivery, inhalation delivery, and transdermal delivery) offers several advantages, including simple administration, proven safety, reduced pain, and robust immunogenicity. For oral nanovaccine delivery to be suitable for GIT diseases, such as cholera and rotavirus infections, NPs must be designed to withstand the acidic pH of the stomach and to release the antigen in the lumen of the gut to initiate the immune response. Transdermal nanovaccine delivery is another delivery approach through which nanoparticle-based vaccines can penetrate the skin and reach the underlying tissues’ immune cells. The inhalation delivery of nanovaccine also ensures that nanoparticle-based vaccines can reach the immune cells in the lung and induce the desirable immune response [334,335,336,337,338]. Overall, needle-free nanovaccine administration offer many advantages, including reducing the risk of blood-borne disease transmission; reducing pain, especially in individuals with needle phobia; eliminating the need for specific equipment and trained personnel due to the ease of administration; and enhancing immunogenicity due to direct delivery of the vaccine to the immune cells.
Interestingly, there have been several suggestions regarding the utilization of spores of Bacillus spp. as a mucosal vaccine delivery carrier. These spores have the benefits of dormancy and extreme resistance properties. Many antigens have been presented on these spores through recombinant and non-recombinant methods and have exhibited robust antigen-specific immune responses. Additionally, probiotic-based spores have shown promise in enhancing immune responses by presenting antigens on probiotic strains [339,340].
9.2. Using NPs with Other Advanced Technologies
Other important advances in gene therapy in which NPs play a crucial role include the advancement of cutting-edge technologies like Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/associated protein 9 (Cas9) and mRNA-based therapies. CRISPR/Cas9 technology is renowned for its precise gene-editing capabilities and is considered one of the most promising technologies for genome manipulation. However, CRISPR/Cas9 has low intracellular delivery efficiency, which severely affects its potency and clinical application, meaning that the development of an effective delivery system for CRISPR/Cas9 system is crucial. LNPs and other nanocarriers like liposomes, cationic polymers, and gold NPs, have emerged as efficient carriers for CRISPR/Cas9 components, overcoming challenges such as inadequate cellular entry, off-target effects, and nuclease degradation [341,342]. A notable advancement in tumor targeting involves the use of a multiplexed dendrimer with an LNP [95]. This system involves the co-delivery of Cas9 mRNA, focal adhesion kinase siRNA, and sgRNA (siFAK + CRISPR-LNPs) to enable tumor delivery and enhance gene-editing efficacy. The study demonstrated that gene editing was enhanced and increased by more than 10-fold in tumor spheroids, due to increased cellular uptake and NP penetration mediated by FAK-knockdown in tumor tissues.
9.3. Personalized Vaccines
Personalized nanovaccines represent an exciting frontier in vaccine development, tailored to the genetic variations, existing immunity, age, sex, and disease history of individuals. Unlike traditional vaccines, which have a one-size-fits-all approach (standard antigen formulation and dose), personalized nanovaccines are specifically designed for each host’s unique immune system. Researchers have been able to develop personalized vaccines after analyzing biomarkers that can predict the host immune response to vaccines. The main advantage of personalized vaccines lies in their ability to target the individual’s unique immune system, thereby maximizing efficacy and minimizing adverse reactions [343]. However, their widespread adoption faces challenges including the need for biomarkers development, high costs, and logistical barriers [181,276,344]. Recent examples of engineered personalized nanovaccines include personalized flu vaccines and cancer immunotherapy. In cancer immunotherapy, the tailored nanovaccines not only enhance antigen presentation but also effectively modulate immunosuppression within the tumor microenvironment. A notable example is the mRNA-based personalized cancer vaccine mRNA-4157, which targets twenty tumor-associated antigens specifically expressed by cancer cells, demonstrating the potential for both immunostimulatory and antitumor activities [345]. Fluoropolymer-based nanovaccines represent another class of personalized nanovaccines. When used in combination with immune checkpoint blockade therapy, these tailored nanovaccines have exhibited prevention of post-surgical metastasis and tumor recurrence in breast cancer models [96]. Additionally, neoantigen nanovaccines, which target unique tumor-specific antigens arising from mutations in cancer cells, have shown promise as personalized nanovaccines. For example, a study has demonstrated that delivering neoantigen peptides along with a sting agonist through a nanoparticle-based system significantly improved the immune response and survival rates in a melanoma mouse model [346]. Moreover, the influenza virus is characterized by its rapid mutation, requiring traditional influenza vaccines to be updated periodically according to the circulating strains. However, all individuals cannot equally respond and be protected using these influenza vaccines. These challenges could be controlled with the development of personalized influenza vaccines that optimize the immune response of each individual.
10. Artificial Intelligence in Nanoparticle-Based Vaccines
Artificial intelligence (AI) has emerged as a transformative force in the development of NP-based vaccines, revolutionizing multiple aspects of the development pipeline from initial design to clinical implementation. The integration of AI technologies, particularly machine learning, and deep learning approaches, has significantly accelerated the vaccine development process while improving efficiency and predictive accuracy [347]. These advanced computational tools have enabled researchers to optimize antigen design, predict immune responses, and enhance the overall efficacy of NP-based vaccine platforms.
In the field of antigen design and NPs optimization, AI algorithms have demonstrated remarkable capabilities in predicting optimal epitope sequences and enhancing antigen stability. These systems can analyze vast datasets of protein structures and immunological responses to identify the most promising vaccine candidates, significantly reducing the time and resources required for traditional experimental approaches [348]. AI-driven platforms have revolutionized the characterization of nanoparticle properties, enabling precise control over particle size, shape, and surface chemistry, which are crucial parameters for vaccine efficacy [349].
The application of machine learning in formulation development has particularly enhanced our ability to optimize nanoparticle composition and predict stability profiles. These AI systems can analyze complex relationships between formulation parameters and vaccine performance, leading to more stable and effective vaccine candidates [350]. Additionally, AI models have proven invaluable in predicting immunological responses and potential adverse reactions, allowing researchers to optimize vaccine formulations before clinical trials begin [351].
Looking toward the future, the integration of multiple AI technologies presents exciting opportunities for nanovaccine development. The combination of big data analytics with sophisticated AI models is expected to enable more personalized vaccine approaches and improve prediction accuracy. However, challenges remain, including the need for larger, more diverse datasets and the validation of AI predictions in biological systems. As these technologies continue to evolve, they are expected to play an increasingly crucial role in developing next-generation nanovaccines that are more effective, safer, and more readily available to global populations.
11. Conclusions
Nanoparticle-based vaccines hold immense promise for revolutionizing immunization strategies. These vaccine platforms offer several advantages over traditional approaches, including enhanced immunogenicity, targeted delivery, and sustained antigen release. The ability to incorporate multiple antigens and adjuvants into a single nanoparticle formulation further simplifies vaccine administration and improves efficacy. However, significant challenges remain in translating these promising technologies into practical applications. Overcoming manufacturing hurdles, ensuring long-term stability, and conducting rigorous clinical trials are essential to bringing NP-based vaccines to market. Addressing potential toxicity and immune side effects is crucial for ensuring their safety and efficacy. Researchers can develop highly effective and personalized vaccines by combining nanotechnology with advancements in artificial intelligence and bioengineering. This interdisciplinary approach will facilitate the creation of vaccines capable of combating emerging infectious diseases, reducing the burden of chronic illnesses, and improving global health outcomes.
Author Contributions
Conceptualization, supervision, and funding, Y.A.H.; data curation, M.S. and Y.A.H.; original draft preparation, M.S., A.E.-M., A.H.E., W.I.A.S. and Y.A.H.; Review and editing, M.S., A.E.-M., A.H.E., W.I.A.S. and Y.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Center of Biomedical Research Excellence (COBRE) for Translational Chemical Biology (CTCB, NIH P20 GM130456), and the National Center for Advancing Translational Sciences, National Institutes of Health (Grant number KL2TR001996).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data were included in the published paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hedman, H.D.; Krawczyk, E.; Helmy, Y.A.; Zhang, L.; Varga, C. Host Diversity and Potential Transmission Pathways of SARS-CoV-2 at the Human-Animal Interface. Pathogens 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D.; Alash’le, G.A. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Mehendale, R.; Joshi, M.; Patravale, V.B. Nanomedicines for treatment of viral diseases. Crit. Rev. ™ Ther. Drug Carr. Syst. 2013, 30, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; El-Metwally, M.; Saber, W.; Negm, S.; El-Kott, A.; Mazroua, Y.; Makhlouf, A.; Moustafa, M. Secondary antiviral metabolites from fungi with special reference to coronaviruses. Biocell 2022, 46, 1979–1988. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Lamichhane, B.; Habib, T.; Adams, A.; El-Sheikh Ali, H.; Slovis, N.M.; Troedsson, M.H.T.; Helmy, Y.A. Antimicrobial Resistance in Equines: A Growing Threat to Horse Health and Beyond—A Comprehensive Review. Antibiotics 2024, 13, 713. [Google Scholar] [CrossRef]
- Roope, L.S.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Mullins, L.P.; Mason, E.; Winter, K.; Sadarangani, M. Vaccination is an integral strategy to combat antimicrobial resistance. PLoS Pathog. 2023, 19, e1011379. [Google Scholar] [CrossRef]
- Fawzy, M.; Helmy, Y.A. The One Health Approach is Necessary for the Control of Rift Valley Fever Infections in Egypt: A Comprehensive Review. Viruses 2019, 11, 139. [Google Scholar] [CrossRef]
- Helmy, Y.A.; El-Adawy, H.; Abdelwhab, E.M. A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt. Pathogens 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against viral infectious diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Kassem, I.I.; Rajashekara, G. Immuno-modulatory effect of probiotic E. coli Nissle 1917 in polarized human colonic cells against Campylobacter jejuni infection. Gut Microbes 2021, 13, 1857514. [Google Scholar] [CrossRef] [PubMed]
- Zepp, F. Principles of vaccine design—Lessons from nature. Vaccine 2010, 28, C14–C24. [Google Scholar] [CrossRef] [PubMed]
- Kiboneka, A.N. Basic concepts in clinical immunology: A review. World J. Adv. Res. Rev. 2021, 12, 490–496. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, K.; Helmy, Y.A.; Yitbarek, A.; Hodgins, D.C.; Sharafeldin, T.A.; Selim, M.S.H. Advances in Poultry Vaccines: Leveraging Biotechnology for Improving Vaccine Development, Stability, and Delivery. Vaccines 2024, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Elaish, M.; Ngunjiri, J.M.; Ali, A.; Xia, M.; Ibrahim, M.; Jang, H.; Hiremath, J.; Dhakal, S.; Helmy, Y.A.; Jiang, X. Supplementation of inactivated influenza vaccine with norovirus P particle-M2e chimeric vaccine enhances protection against heterologous virus challenge in chickens. PLoS ONE 2017, 12, e0171174. [Google Scholar] [CrossRef] [PubMed]
- Murugan, B.; Sagadevan, S. Nano-Vaccines: Opportunities and Challenges in Biomaterial-Based Vaccine Delivery. Biomater. -Inspired Nanomed. Target. Ther. 2024, 101–116. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cui, C.; Wang, Y.; Sun, X.; Wang, S.; Yang, M.; Yu, Y.; Wang, L. CpG ODN as an adjuvant arouses the vigor of B cells by relieving the negative regulation of surface TLR9 to enhance the antibody response to vaccine. Appl. Microbiol. Biotechnol. 2021, 105, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An overview of the use of nanoparticles in vaccine development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine development throughout history. Cureus 2021, 13, e16635. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Taylor, M.W. Smallpox; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Davidson, T. Vaccines: History, Science, and Issues; Bloomsbury Publishing USA: New York, NY, USA, 2017. [Google Scholar]
- Mamelund, S.-E. Influenza, historical. Medicine 2008, 54, 361–371. [Google Scholar]
- Lombard, M.; Pastoret, P.-P.; Moulin, A. A brief history of vaccines and vaccination. Rev. Sci. Tech. Off. Int. Epizoot. 2007, 26, 29–48. [Google Scholar] [CrossRef]
- Meeusen, E.N.; Walker, J.; Peters, A.; Pastoret, P.-P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Karin, H.; Lisa, B.; Damer, P. Vaccines as alternatives to antibiotics for food producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 64. [Google Scholar]
- Palomino-Tapia, V. Autogenous Vaccines in the Poultry Industry: A Field Perspective. In Poultry Farming-New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Nagpal, G.; Usmani, S.S.; Raghava, G.P. A web resource for designing subunit vaccine against major pathogenic species of bacteria. Front. Immunol. 2018, 9, 2280. [Google Scholar] [CrossRef]
- Malik, H.; Khan, F.H.; Ahsan, H. Human papillomavirus: Current status and issues of vaccination. Arch. Virol. 2014, 159, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiong, Y.; Li, N.; Jian, C. Vaccine types. In Vaccines—The History and Future; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Liljeqvist, S.; Ståhl, S. Production of recombinant subunit vaccines: Protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999, 73, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Liu, Y.; Rigsby, P.; Sesardic, D. An improved method for development of toxoid vaccines and antitoxins. J. Immunol. Methods 2008, 337, 42–48. [Google Scholar] [CrossRef]
- Andey, T.; Soni, S.; Modi, S. Conventional vaccination methods: Inactivated and live attenuated vaccines. Adv. Vaccin. Technol. Infect. Chronic Dis. 2024, 37–50. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479, 379–392. [Google Scholar] [CrossRef]
- Lopez, S.M.; Sato, A.I.; Chatterjee, A. Vaccines: An overview. Viral Parasit. Bact. Fungal Infect. 2023, 56, 699–717. [Google Scholar]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Teulon, J.-M.; Godon, C.; Chantalat, L.; Moriscot, C.; Cambedouzou, J.; Odorico, M.; Ravaux, J.; Podor, R.; Gerdil, A.; Habert, A. On the operational aspects of measuring nanoparticle sizes. Nanomaterials 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, H.; Zhang, S.; Hu, J.; Zhang, L. Delivery of nanovaccine towards lymphoid organs: Recent strategies in enhancing cancer immunotherapy. J. Nanobiotechnol. 2021, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Elattar, K.M.; Al-Otibi, F.O.; El-Hersh, M.S.; Attia, A.A.; Eldadamony, N.M.; Elsayed, A.; Menaa, F.; Saber, W.I. Multifaceted chemical and bioactive features of Ag@ TiO2 and Ag@ SeO2 core/shell nanoparticles biosynthesized using Beta vulgaris L. extract. Heliyon 2024, 10, e28359. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Chen, J.-Y.; Chen, H.-W.; Hu, C.-M.J. Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostics 2017, 1, 244. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Liu, Y.; Chen, X.; Liu, Q.; Jia, J.; Yang, T.; Qiu, S.; Ma, G. Immune responses to vaccines involving a combined antigen–nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials 2014, 35, 6086–6097. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Y.; Xiang, Y.; Xu, F. Nanovaccines: Merits, and diverse roles in boosting antitumor immune responses. Hum. Vaccines Immunother. 2022, 18, 2119020. [Google Scholar] [CrossRef]
- Li, M.; Kaminskas, L.M.; Marasini, N. Recent advances in nano/microparticle-based oral vaccines. J. Pharm. Investig. 2021, 51, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- González-García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailänder, V.; Landfester, K.; Vasilev, K. Nanoparticles surface chemistry influence on protein corona composition and inflammatory responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-based vaccines: Opportunities and limitations. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. [Google Scholar]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Manju, K.; Raj, S.N.; Ranjini, H.; Nayaka, S.C.; Ashwini, P.; Satish, S.; Prasad, M.N.; Chouhan, R.S.; Baker, S. Nanovaccines to combat drug resistance: The next-generation immunisation. Future J. Pharm. Sci. 2023, 9, 64. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, Z.P. Nanoparticle-based nanomedicines to promote cancer immunotherapy: Recent advances and future directions. Small 2019, 15, 1900262. [Google Scholar] [CrossRef]
- Kumru, O.S.; Joshi, S.B.; Smith, D.E.; Middaugh, C.R.; Prusik, T.; Volkin, D.B. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals 2014, 42, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced nanobiomaterials: Vaccines, diagnosis and treatment of infectious diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Abusalah, M.A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; Sundriyal, A. Nanovaccines: A game changing approach in the fight against infectious diseases. Biomed. Pharmacother. 2023, 167, 115597. [Google Scholar]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and microparticles as potential oral vaccine carriers and adjuvants against infectious diseases. Front. Pharmacol. 2021, 12, 682286. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Rodriguez Pozo, A.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A. Vaccine inoculation route modulates early immunity and consequently antigen-specific immune response. Front. Immunol. 2021, 12, 645210. [Google Scholar] [CrossRef]
- Xie, S.; Pan, B.; Wang, M.; Zhu, L.; Wang, F.; Dong, Z.; Wang, X.; Zhou, W. Formulation, characterization and pharmacokinetics of praziquantel-loaded hydrogenated castor oil solid lipid nanoparticles. Nanomedicine 2010, 5, 693–701. [Google Scholar] [CrossRef]
- Heidari-Kharaji, M.; Taheri, T.; Doroud, D.; Habibzadeh, S.; Badirzadeh, A.; Rafati, S. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite Immunol. 2016, 38, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Kondel, R.; Shafiq, N.; Kaur, I.P.; Singh, M.P.; Pandey, A.K.; Ratho, R.K.; Malhotra, S. Effect of acyclovir solid lipid nanoparticles for the treatment of herpes simplex virus (HSV) infection in an animal model of HSV-1 infection. Pharm. Nanotechnol. 2019, 7, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.; Ryu, M.; Kim, S.; Joo, M.; Yeom, J.-H.; Kim, S.; Park, Y.; Lee, K.; Bae, J. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep. 2017, 7, 13572. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent gold nanoparticle–peptide conjugates for targeting intracellular bacterial infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Judy, B.M.; Qazi, O.; Blumentritt, C.A.; Brown, K.A.; Shaw, A.M.; Torres, A.G.; Titball, R.W. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 447–456. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, J.-A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Farfán-Castro, S.; García-Soto, M.J.; Betancourt-Mendiola, L.; Cervantes, J.; Segura, R.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and evaluation of gold nanoparticles conjugated with five antigenic peptides derived from the spike protein of SARS-CoV-2 for vaccine development. Front. Nanotechnol. 2024, 6, 1335346. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.; Alipour, M.; Azghani, A.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Theivendran, S.; Lazarev, S.; Yu, C. Mesoporous silica/organosilica nanoparticles for cancer immunotherapy. Exploration 2023, 3, 20220086. [Google Scholar] [CrossRef]
- Song, C.; Li, F.; Wang, S.; Wang, J.; Wei, W.; Ma, G. Recent advances in particulate adjuvants for cancer vaccination. Adv. Ther. 2020, 3, 1900115. [Google Scholar] [CrossRef]
- Madapong, A.; Petro-Turnquist, E.M.; Webby, R.J.; McCormick, A.A.; Weaver, E.A. Immunity and Protective Efficacy of a Plant-Based Tobacco Mosaic Virus-like Nanoparticle Vaccine against Influenza a Virus in Mice. Vaccines 2024, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Díaz, M.C.; Phan, S.; Elson, A.; Nuñez, I.; Wei, H.; Sakamoto, K.; He, B. Parainfluenza virus 5 (PIV5) amplifying virus-like particles expressing respiratory syncytial virus (RSV) antigens protect mice against RSV infection. Vaccine 2019, 37, 2925–2934. [Google Scholar] [CrossRef]
- Lacasta, A.; Mody, K.T.; De Goeyse, I.; Yu, C.; Zhang, J.; Nyagwange, J.; Mwalimu, S.; Awino, E.; Saya, R.; Njoroge, T. Synergistic effect of two nanotechnologies enhances the protective capacity of the Theileria parva sporozoite p67C antigen in cattle. J. Immunol. 2021, 206, 686–699. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2022, 12, 790121. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Michel, M.-L.; Tiollais, P. Hepatitis B vaccines: Protective efficacy and therapeutic potential. Pathol. Biol. 2010, 58, 288–295. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.N.; Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Current and future nanoparticle vaccines for COVID-19. EBioMedicine 2021, 74, 103699. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, J.; Lo, P.-C.; Lovell, J.F.; Jin, H.; Yang, K. Self-adjuvanting cancer nanovaccines. J. Nanobiotechnol. 2022, 20, 345. [Google Scholar] [CrossRef]
- Li, Y.; Su, T.; Zhang, Y.; Huang, X.; Li, J.; Li, C. Liposomal co-delivery of daptomycin and clarithromycin at an optimized ratio for treatment of methicillin-resistant Staphylococcus aureus infection. Drug Deliv. 2015, 22, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant–biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Hanson, M.C.; Abraham, W.; Crespo, M.P.; Chen, S.H.; Liu, H.; Szeto, G.L.; Kim, M.; Reinherz, E.L.; Irvine, D.J. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine 2015, 33, 861–868. [Google Scholar] [CrossRef]
- Smith, L.R.; Wloch, M.K.; Ye, M.; Reyes, L.R.; Boutsaboualoy, S.; Dunne, C.E.; Chaplin, J.A.; Rusalov, D.; Rolland, A.P.; Fisher, C.L. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010, 28, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Ma, G.; Xia, Y. Unlocking the therapeutic applicability of LNP-mRNA: Chemistry, formulation, and clinical strategies. Research 2024, 7, 0370. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Yu, X.; Wei, T.; Farbiak, L.; Johnson, L.T.; Taylor, A.M.; Xu, J.; Hong, Y.; Zhu, H. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 2022, 17, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Li, Z.; Wang, F.; Fan, X.; Zhou, X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics 2020, 10, 7131. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, H.; Wang, M.; Li, J.; Lin, H.; Liang, M.; Gao, Y.; Yang, M. An OMV-based nanovaccine confers safety and protection against pathogenic Escherichia coli via both humoral and predominantly Th1 immune responses in poultry. Nanomaterials 2020, 10, 2293. [Google Scholar] [CrossRef]
- Thukral, A.; Ross, K.; Hansen, C.; Phanse, Y.; Narasimhan, B.; Steinberg, H.; Talaat, A.M. A single dose polyanhydride-based nanovaccine against paratuberculosis infection. npj Vaccines 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Etewa, S.E.; El-Maaty, D.A.A.; Hamza, R.S.; Metwaly, A.S.; Sarhan, M.H.; Abdel-Rahman, S.A.; Fathy, G.M.; El-Shafey, M.A. Assessment of spiramycin-loaded chitosan nanoparticles treatment on acute and chronic toxoplasmosis in mice. J. Parasit. Dis. 2018, 42, 102–113. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Gao, C.; Wang, Y.; Cheng, X.; Yang, Y.; Gou, Q.; Lei, L.; Chen, Y.; Wang, X. Development of a chitosan--modified PLGA nanoparticle vaccine for protection against Escherichia coli K1 caused meningitis in mice. J. Nanobiotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef] [PubMed]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune response of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish. Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef]
- El-Sissi, A.F.; Mohamed, F.H.; Danial, N.M.; Gaballah, A.Q.; Ali, K.A. Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 2020, 10, 88. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Chen, G.; Shi, X.-m.; Gao, T.-t.; Li, W.; Zhao, Y.; Zhang, F.-q.; Wu, J.; Cui, X.; Wang, Y.-F. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS ONE 2012, 7, e53314. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Padhi, A.; Mukherjee, S.; Dash, D.P.; Kar, S.; Sonawane, A. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of γδ T cells in mice. Nanotechnology 2017, 28, 165101. [Google Scholar] [CrossRef]
- Mohammed, G.M.; ElZorkany, H.E.; Farroh, K.Y.; Abd El-Aziz, W.R.; Elshoky, H.A. Potential improvement of the immune response of chickens against E. coli vaccine by using two forms of chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 167, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Renukaradhya, G.J. Chitosan nanoparticle based mucosal vaccines delivered against infectious diseases of poultry and pigs. Front. Bioeng. Biotechnol. 2020, 8, 558349. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef]
- Kelly, S.M.; Larsen, K.R.; Darling, R.; Petersen, A.C.; Bellaire, B.H.; Wannemuehler, M.J.; Narasimhan, B. Single-dose combination nanovaccine induces both rapid and durable humoral immunity and toxin neutralizing antibody responses against Bacillus anthracis. Vaccine 2021, 39, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.H.; Lokugamage, N.; Garg, N.J. Experimental nanovaccine offers protection against repeat exposures to Trypanosoma cruzi through activation of polyfunctional T cell response. Front. Immunol. 2020, 11, 595039. [Google Scholar] [CrossRef]
- Mody, K.T.; Zhang, B.; Li, X.; Fletcher, N.L.; Akhter, D.T.; Jarrett, S.; Zhang, J.; Yu, C.; Thurecht, K.J.; Mahony, T.J. Characterization of the biodistribution of a silica vesicle nanovaccine carrying a Rhipicephalus (Boophilus) microplus protective antigen with in vivo live animal imaging. Front. Bioeng. Biotechnol. 2021, 8, 606652. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Bharath, L. Controlling of food borne pathogens by nanoparticles. Bioorganic Phase Nat. Food Overv. 2018, 15, 293–322. [Google Scholar]
- Vinayamohan, P.G.; Joseph, D.; Viju, L.S.; Venkitanarayanan, K. Efficacy of selenium for controlling infectious diseases. In Selenium and Human Health; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Maleki, M.; Salouti, M.; Shafiee Ardestani, M.; Talebzadeh, A. Preparation of a nanovaccine against Brucella melitensis M16 based on PLGA nanoparticles and oligopolysaccharide antigen. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4248–4256. [Google Scholar] [CrossRef]
- Lee, J.-A.; Jung, B.-G.; Kim, T.-H.; Kim, Y.-M.; Park, M.-H.; Hyun, P.-m.; Jeon, J.-w.; Park, J.-k.; Cho, C.-W.; Suh, G.-H. Poly d, l-lactide-co-glycolide (PLGA) nanoparticle-encapsulated honeybee (Apis melifera) venom promotes clearance of Salmonella enterica serovar Typhimurium infection in experimentally challenged pigs through the up-regulation of T helper type 1 specific immune responses. Vet. Immunol. Immunopathol. 2014, 161, 193–204. [Google Scholar] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef]
- Toti, U.S.; Guru, B.R.; Hali, M.; McPharlin, C.M.; Wykes, S.M.; Panyam, J.; Whittum-Hudson, J.A. Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials 2011, 32, 6606–6613. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, S.; Ganesh, N.; Bhattacharya, S.; Jain, N.K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target. 2009, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.S.; Fordoei, A.S.; Abdoli, A.; Ahangari Cohan, R.; Bahramali, G.; Sadat, S.M.; Siadat, S.D.; Moloudian, H.; Nassiri Koopaei, N.; Bolhasani, A. Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity. J. Mater. Sci. Mater. Med. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.Q.; Thanh, N.T.H.; Thuy, N.T.; Van Chung, P.; Hung, P.N.; Le, A.-T.; Hanh, N.T.H. Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villamil, J.I.; Tapia, D.; Torres, A.G. Development of a gold nanoparticle vaccine against enterohemorrhagic Escherichia coli O157: H7. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Tiwari, V.; Tiwari, M.; Solanki, V. Polyvinylpyrrolidone-capped silver nanoparticle inhibits infection of carbapenem-resistant strain of Acinetobacter baumannii in the human pulmonary epithelial cell. Front. Immunol. 2017, 8, 973. [Google Scholar] [CrossRef]
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Pangestika, R.; Ernawati, R. Antiviral activity effect of silver nanoparticles (Agnps) solution against the growth of infectious bursal disease virus on embryonated chicken eggs with Elisa test. KnE Life Sci. 2017, 3, 536–548. [Google Scholar] [CrossRef]
- Kuppan, G.; Sangeeta, K. Dose and Size-Dependent Antiviral Effects of Silver Nanoparticles on Feline Calicivirus, a Human Norovirus Surrogate. Foodborne Pathog Dis. 2016, 13, 239–244. [Google Scholar]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef]
- Folliero, V.; Zannella, C.; Chianese, A.; Stelitano, D.; Ambrosino, A.; De Filippis, A.; Galdiero, M.; Franci, G.; Galdiero, M. Application of dendrimers for treating parasitic diseases. Pharmaceutics 2021, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- El Bissati, K.; Zhou, Y.; Paulillo, S.M.; Raman, S.K.; Karch, C.P.; Roberts, C.W.; Lanar, D.E.; Reed, S.; Fox, C.; Carter, D. Protein nanovaccine confers robust immunity against Toxoplasma. npj Vaccines 2017, 2, 24. [Google Scholar] [CrossRef]
- Kwon, E.J.; Skalak, M.; Bertucci, A.; Braun, G.; Ricci, F.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Porous silicon nanoparticle delivery of tandem peptide anti-infectives for the treatment of Pseudomonas aeruginosa lung infections. Adv. Mater. 2017, 29, 1701527. [Google Scholar] [CrossRef]
- Gowri, M.; Latha, N.; Suganya, K.; Murugan, M.; Rajan, M. Calcium alginate nanoparticle crosslinked phosphorylated polyallylamine to the controlled release of clindamycin for osteomyelitis treatment. Drug Dev. Ind. Pharm. 2021, 47, 280–291. [Google Scholar] [CrossRef]
- Wang, L.; Xing, D.; Le Van, A.; Jerse, A.E.; Wang, S. Structure-based design of ferritin nanoparticle immunogens displaying antigenic loops of Neisseria gonorrhoeae. FEBS Open Bio 2017, 7, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kiderlen, A.F.; Petri, B.; Kayser, O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 597–603. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, Y.; Hua, L.; Liu, X.; Xiao, M.; Xu, T.; Zhu, B.; Cao, H. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3485–3491. [Google Scholar] [CrossRef]
- Thi, E.P.; Mire, C.E.; Lee, A.C.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362–365. [Google Scholar] [CrossRef]
- Lauster, D.; Glanz, M.; Bardua, M.; Ludwig, K.; Hellmund, M.; Hoffmann, U.; Hamann, A.; Böttcher, C.; Haag, R.; Hackenberger, C.P. Multivalent peptide–nanoparticle conjugates for influenza-virus inhibition. Angew. Chem. Int. Ed. 2017, 56, 5931–5936. [Google Scholar] [CrossRef]
- Rodrigues-Jesus, M.; Fotoran, W.L.; Cardoso, R.M.; Araki, K.; Wunderlich, G.; Ferreira, L.C. Nano-multilamellar lipid vesicles (NMVs) enhance protective antibody responses against Shiga toxin (Stx2a) produced by enterohemorrhagic Escherichia coli strains (EHEC). Braz. J. Microbiol. 2019, 50, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cavallaro, A.S.; Mody, K.T.; Zhang, J.; Deringer, J.R.; Brown, W.C.; Mahony, T.J.; Yu, C.; Mitter, N. Nanoparticle-based delivery of Anaplasma marginale membrane proteins; virb9-1 and virb10 produced in the Pichia pastoris expression system. Nanomaterials 2016, 6, 201. [Google Scholar] [CrossRef]
- Rungrojcharoenkit, K.; Sunintaboon, P.; Ellison, D.; Macareo, L.; Midoeng, P.; Chaisuwirat, P.; Fernandez, S.; Ubol, S. Development of an adjuvanted nanoparticle vaccine against influenza virus, an in vitro study. PLoS ONE 2020, 15, e0237218. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Lung, P.; Yang, J.; Li, Q. Nanoparticle formulated vaccines: Opportunities and challenges. Nanoscale 2020, 12, 5746–5763. [Google Scholar] [CrossRef]
- Verma, M.; Ozer, I.; Xie, W.; Gallagher, R.; Teixeira, A.; Choy, M. The landscape for lipid-nanoparticle-based genomic medicines. Nat. Rev. Drug Discov. 2023, 22, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xue, Y.; Álvarez-Benedicto, E.; Shi, Y.; Chen, W.; Koo, S.; Siegwart, D.J.; Dong, Y.; Tao, W. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Primers 2023, 3, 63. [Google Scholar] [CrossRef]
- Look, M.; Bandyopadhyay, A.; Blum, J.S.; Fahmy, T.M. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv. Drug Deliv. Rev. 2010, 62, 378–393. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J. Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef]
- Smith, J.F.; Brownlow, M.; Brown, M.; Kowalski, R.; Esser, M.T.; Ruiz, W.; Barr, E.; Brown, D.R.; Bryan, J.T. Antibodies from women immunized with Gardasil® cross-neutralize HPV 45 pseudovirions. Hum. Vaccines 2007, 3, 109–115. [Google Scholar] [CrossRef]
- Passero Jr, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer. Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Nordquist, L.T.; Shore, N.D.; Elist, J.J.; Oliver, J.C.; Gannon, W.; Shahlaee, A.H.; Fuller, S.A.; Ghanbari, H.A. Phase 1 open-label trial to evaluate the safety and immunogenicity of PAN-301-1, a novel nanoparticle therapeutic vaccine, in patients with biochemically relapsed prostate cancer. J. Clin. Oncol. 2018, 29 (Suppl. S15), e15166. [Google Scholar] [CrossRef]
- Lee, D.-H.; Choi, S.; Park, Y.; Jin, H.-s. Mucin1 and Mucin16: Therapeutic targets for cancer therapy. Pharmaceuticals 2021, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Cech, P.G.; Aebi, T.; Abdallah, M.S.; Mpina, M.; Machunda, E.B.; Westerfeld, N.; Stoffel, S.A.; Zurbriggen, R.; Pluschke, G.; Tanner, M. Virosome-formulated Plasmodium falciparum AMA-1 & CSP derived peptides as malaria vaccine: Randomized phase 1b trial in semi-immune adults & children. PLoS ONE 2011, 6, e22273. [Google Scholar]
- Porras, C.; Sampson, J.N.; Herrero, R.; Gail, M.H.; Cortés, B.; Hildesheim, A.; Cyr, J.; Romero, B.; Schiller, J.T.; Montero, C. Rationale and design of a double-blind randomized non-inferiority clinical trial to evaluate one or two doses of vaccine against human papillomavirus including an epidemiologic survey to estimate vaccine efficacy: The Costa Rica ESCUDDO trial. Vaccine 2022, 40, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory syncytial virus vaccines: A review of the candidates and the approved vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bhangde, S.; Lodaya, R.N.; Amiji, M.M. Nanoscale Vaccines for Influenza. In Nanomedicines for the Prevention and Treatment of Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 331–347. [Google Scholar]
- Low, J.G.; De Alwis, R.; Chen, S.; Kalimuddin, S.; Leong, Y.S.; Mah, T.K.L.; Yuen, N.; Tan, H.C.; Zhang, S.L.; Sim, J.X. A phase I/II randomized, double-blinded, placebo-controlled trial of a self-amplifying COVID-19 mRNA vaccine. npj Vaccines 2022, 7, 161. [Google Scholar] [CrossRef]
- Al, L.I.R.; Sönmezer, M.Ç.; Ünal, S. RNA-Based COVID-19 vaccine candidates with clinical phase trials in progress. Turk. J. Med. Sci. 2021, 51, 3246–3252. [Google Scholar]
- Kalnin, K.V.; Plitnik, T.; Kishko, M.; Zhang, J.; Zhang, D.; Beauvais, A.; Anosova, N.G.; Tibbitts, T.; DiNapoli, J.; Ulinski, G. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 2021, 6, 61. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Alameh, M.-G.; Tharakhet, K.; Kaewpang, P.; Yostrerat, N.; Pitakpolrat, P.; Buranapraditkun, S.; Manopwisedjaroen, S.; Thitithanyanont, A. Immunogenicity and protective efficacy of SARS-CoV-2 mRNA vaccine encoding secreted non-stabilized spike in female mice. Nat. Commun. 2023, 14, 2309. [Google Scholar] [CrossRef]
- World Health Organization. Emergency use designation of COVID-19 candidate vaccines: Ethical considerations for current and future COVID-19 placebo-controlled vaccine trials and trial unblinding: Policy brief, 18 December 2020. In Emergency Use Designation of COVID-19 Candidate Vaccines: Ethical Considerations for Current and Future COVID-19 Placebo-Controlled Vaccine Trials and Trial Unblinding: Policy Brief, 18 December 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Beloki, U.; Salaberry, L.; Smerdou, C. Self-Amplifying RNA: A Second Revolution of mRNA Vaccines against COVID-19. Vaccines 2024, 12, 318. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. npj Vaccines 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Ho Um, S.; Khant, H. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Li, A.V.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 2012, 109, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Huang, B.; Irvine, D.J. Engineering nano- and microparticles to tune immunity. Adv. Mater. 2012, 24, 3724–3746. [Google Scholar] [CrossRef]
- Chong, P.; Huang, J.-H.; Leng, C.-H.; Liu, S.-J.; Chen, H.-W. Recombinant lipoproteins as novel vaccines with intrinsic adjuvant. Adv. Protein Chem. Struct. Biol. 2015, 99, 55–74. [Google Scholar]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.-F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and art of developing lipid nanoparticles for biologics delivery: Focus on development and scale-up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, Y.; Wang, Y.; Yin, Q. Emerging peptide-based nanovaccines: From design synthesis to defense against cancer and infection. Biomed. Pharmacother. 2023, 158, 114117. [Google Scholar] [CrossRef]
- Abdul Ghaffar, K.; Kumar Giddam, A.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Tretiakova, D.; Vodovozova, E. Liposomes as adjuvants and vaccine delivery systems. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef] [PubMed]
- Yanasarn, N.; Sloat, B.R.; Cui, Z. Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol. Pharm. 2011, 8, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Shetye, L.; Sherlekar, A.; Mendhulkar, V. Liposome-Based Drug Delivery—A New Therapeutic Paradigm. In Advanced Drug Delivery: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–48. [Google Scholar]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Pensado, A.; Seijo, B.; Sanchez, A. Current strategies for DNA therapy based on lipid nanocarriers. Expert Opin. Drug Deliv. 2014, 11, 1721–1731. [Google Scholar] [CrossRef]
- Peres, L.B.; Peres, L.B.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A recent update: Solid lipid nanoparticles for effective drug delivery. Adv. Pharm. Bull. 2022, 12, 17. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Santos, P.; Almeida, F. Exosome-based vaccines: History, current state, and clinical trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Mahdipour, M.; Nourazarian, A.; Habibollahi, P.; Sokullu, E.; Avci, Ç.B.; Rahbarghazi, R. Application of exosomes for the alleviation of COVID-19-related pathologies. Cell Biochem. Funct. 2022, 40, 430–438. [Google Scholar] [CrossRef]
- Wallis, J.; Shenton, D.; Carlisle, R. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019, 196, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Glück, R.; Metcalfe, I. New technology platforms in the development of vaccines for the future. Vaccine 2002, 20, B10–B16. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Tang, J.-l.; Sun, J.; He, Z.-G. Self-emulsifying drug delivery systems: Strategy for improving oral delivery of poorly soluble drugs. Curr. Drug Ther. 2007, 2, 85–93. [Google Scholar] [CrossRef]
- Akagi, T.; Baba, M.; Akashi, M. Biodegradable nanoparticles as vaccine adjuvants and delivery systems: Regulation of immune responses by nanoparticle-based vaccine. Polym. Nanomed. 2012, 247, 31–64. [Google Scholar]
- Liu, B.; Wu, Z.; Liu, T.; Qian, R.; Wu, T.; Liu, Q.; Shen, A. Polymeric nanoparticles engineered as a vaccine adjuvant-delivery system. In Immunization-Vaccine Adjuvant Delivery System and Strategies; IntechOpen: London, UK, 2018. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Veiga, F.; Ribeiro, A.J. Preparation of drug-loaded polymeric nanoparticles. In Nanomedicine in Cancer; Jenny Stanford Publishing: Oxon, UK, 2017; pp. 171–214. [Google Scholar]
- Maji, I.; Mahajan, S.; Sriram, A.; Mehra, N.K.; Singh, P.K. Polymeric nanomaterials: Fundamentals and therapeutic applications. In Nanomaterial-Based Drug Delivery Systems: Therapeutic and Theranostic Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 33–64. [Google Scholar]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnology 2020, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Stern, T.; González, M.F.; Epstein, J. Biodegradable poly(ethylene oxide)/poly(ϵ-caprolactone) multiblock copolymers. J. Biomed. Mater. Res. 2002, 59, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-loaded mixed micelles and polymersomes composed of poly (caprolactone)-poly(ethylene glycol)(PEG-PCL) and poly (lactic acid)-poly (ethylene glycol)(PEG-PLA): Preparation and in-vitro characterization. Iran. J. Pharm. Res. IJPR 2019, 18, 142. [Google Scholar]
- Tian, J.-H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; van Meijgaarden, K.E.; Franken, K.L.; Junginger, H.E.; Borchard, G.; Ottenhoff, T.H.; Geluk, A. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A* 0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 2004, 22, 1609–1615. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Rezigue, M. Lipid and Polymeric Nanoparticles: Drug Delivery Applications; Springer: Cham, Switzerland, 2020; pp. 167–230. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Elzoghby, A.; M Abd-Elwakil, M.; Abd-Elsalam, K.; T Elsayed, M.; Hashem, Y.; Mohamed, O. Natural polymeric nanoparticles for brain-targeting: Implications on drug and gene delivery. Curr. Pharm. Des. 2016, 22, 3305–3323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, H.; Ma, L.; Shi, Y.; Ji, M.; Sun, X.; Ma, D.; Zhou, W.; Huang, T.; Zhang, D. The quest for nanoparticle-powered vaccines in cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Vasudevan, S.S.; Kandrikar, T.Y.; Sayyed, A.A.; Sarker, P.; Nasir, N.S.; Venugopalan, S.; Mariajohn, R.; Chavda, V.P.; Gondaliya, P. Nanoparticle-based vaccines and future vaccine technologies. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Elsevier: Amsterdam, The Netherlands, 2024; pp. 477–495. [Google Scholar]
- Cable, J.; Graham, B.S.; Koup, R.A.; Seder, R.A.; Karikó, K.; Pardi, N.; Barouch, D.H.; Sharma, B.; Rauch, S.; Nachbagauer, R. Progress in vaccine development for infectious diseases—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2023, 1524, 65–86. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Gnanaraj, M.; Sisubalan, N.; Jebastin, T.; Vijayan, A.; Muneeshwaran, T.; Manikandan, R. Gold Nanoparticles as Antibacterial and Antiviral Agents: Biomedical Applications and Theranostic Potential. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 19–45. [Google Scholar]
- Chaudhary, K.; Masram, D.T. Biological activities of nanoparticles and mechanism of action. In Model Organisms to Study Biological Activities and Toxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2020; pp. 19–34. [Google Scholar]
- Chernykh, I.; Kopanitsa, M.; Shchul’kin, A.; Yakusheva, E.; Frolova, M. Gold nanoparticles as potential antitumor agents. Pharm. Chem. J. 2021, 55, 934–941. [Google Scholar] [CrossRef]
- He, J.-s.; Liu, S.-j.; Zhang, Y.-r.; Chu, X.-d.; Lin, Z.-b.; Zhao, Z.; Qiu, S.-h.; Guo, Y.-g.; Ding, H.; Pan, Y.-l. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021, 12, 687399. [Google Scholar] [CrossRef] [PubMed]
- Farfán-Castro, S.; García-Soto, M.J.; Aguilar-Aguilar, A.; González-Ortega, O.; Rosales-Mendoza, S. Application of gold nanoparticles in vaccine development. In Gold Nanoparticles for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 445–493. [Google Scholar]
- Xu, L.; Wang, X.; Wang, W.; Sun, M.; Choi, W.J.; Kim, J.-Y.; Hao, C.; Li, S.; Qu, A.; Lu, M. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef]
- Tapia, D.; Sanchez-Villamil, J.I.; Torres, A.G. Multicomponent gold nano-glycoconjugate as a highly immunogenic and protective platform against Burkholderia mallei. npj Vaccines 2020, 5, 82. [Google Scholar] [CrossRef]
- Gao, L.; Song, Y.; Zhong, J.; Lin, X.; Zhou, S.-F.; Zhan, G. Biocompatible 2D Cu-TCPP nanosheets derived from Cu2O nanocubes as multifunctional nanoplatforms for combined anticancer therapy. ACS Biomater. Sci. Eng. 2022, 8, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2018, 16, 1–23. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021, 21, 627–630. [Google Scholar] [CrossRef]
- Nandanwar, R.; Singh, P.; Haque, F.Z. Synthesis and properties of silica nanoparticles by sol-gel method for the application in green chemistry. Mater. Sci. Res. India 2013, 10, 85–92. [Google Scholar]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Machado, Y.; Feinle, A.; Thalhamer, J.; Hüsing, N.; Weiss, R. Potential of nanoparticles for allergen-specific immunotherapy–use of silica nanoparticles as vaccination platform. Expert Opin. Drug Deliv. 2016, 13, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Seré, S.; Vounckx, U.; Seo, J.W.; Lenaerts, I.; Van Gool, S.; Locquet, J.-P. Proof of concept study: Mesoporous silica nanoparticles, from synthesis to active specific immunotherapy. Front. Nanotechnol. 2020, 2, 584233. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sayes, C.M. A toxicological profile of silica nanoparticles. Toxicol. Res. 2022, 11, 565–582. [Google Scholar] [CrossRef]
- Pallavi, P.; Harini, K.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; Gowtham, P.; Girigoswami, K.; Shakeel, F.; Girigoswami, A. From synthetic route of silica nanoparticles to theranostic applications. Processes 2022, 10, 2595. [Google Scholar] [CrossRef]
- Xiao, D.; Qi, H.; Teng, Y.; Pierre, D.; Kutoka, P.T.; Liu, D. Advances and challenges of fluorescent nanomaterials for synthesis and biomedical applications. Nanoscale Res. Lett. 2021, 16, 1–23. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 1–27. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Yang, X.; Lu, X.; Xu, X.-N.; Zhang, J.; Chen, R. Potentiating the Immune Responses of HBsAg-VLP Vaccine Using a Polyphosphoester-Based Cationic Polymer Adjuvant. ACS Appl. Mater. Interfaces 2023, 15, 48871–48881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-B.; Quan, F.-S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Patil, S. A review of virus-like particle-based SARS-CoV-2 vaccines in clinical trial phases. Iran. J. Pharm. Res. IJPR 2021, 13, 2901–2905. [Google Scholar]
- Aboshi, M.; Matsuda, K.; Kawakami, D.; Kono, K.; Kazami, Y.; Sekida, T.; Komori, M.; Morey, A.L.; Suga, S.; Smith, J.F. Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine. iScience 2024, 27, 108964. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Vandoolaeghe, P.; Schuerman, L. The RTS, S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Expert Rev. Vaccines 2016, 15, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Syomin, B.; Ilyin, Y. Virus-like particles as an instrument of vaccine production. Mol. Biol. 2019, 53, 323–334. [Google Scholar] [CrossRef]
- Erdmann, N.B.; Williams, W.B.; Walsh, S.R.; Grunenberg, N.; Edlefsen, P.T.; Goepfert, P.A.; Cain, D.W.; Cohen, K.W.; Maenza, J.; Mayer, K.H. A HIV-1 Gp41 peptide-liposome vaccine elicits neutralizing epitope-targeted antibody responses in healthy individuals. medRxiv 2024. [Google Scholar] [CrossRef]
- Zeigler, D.F.; Gage, E.; Roque, R.; Clegg, C.H. Epitope targeting with self-assembled peptide vaccines. NPJ Vaccines 2019, 4, 30. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Longping, V.T.; Shehata, L.; O’Connor, M.A.; Chen, C. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Josheghani, S.; Bakhshi, B.; Najar-peerayeh, S. The influence of nanoparticle on vaccine responses against bacterial infection. J. Nanotechnol. 2022, 2022, 6856982. [Google Scholar] [CrossRef]
- Chang, H.C.; Zou, Z.Z.; Wang, Q.H.; Li, J.; Jin, H.; Yin, Q.X.; Xing, D. Targeting and specific activation of antigen--presenting cells by endogenous antigen-loaded nanoparticles elicits tumor-specific immunity. Adv. Sci. 2020, 7, 1900069. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, O.; Bertero, A.; Gagliardi, M.; Bardi, G. Biomedical nanoparticles: Overview of their surface immune-compatibility. Coatings 2014, 4, 139–159. [Google Scholar] [CrossRef]
- Zaman, M.; Good, M.F.; Toth, I. Nanovaccines and their mode of action. Methods 2013, 60, 226–231. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.; Wang, K.; Liang, L.; Zhang, H. An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer. Vaccines 2024, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-modified chitosan-nanoparticle-based Salmonella subunit oralvaccine-induced immune response and efficacy in a challenge trial in broilers. Vaccines 2020, 8, 299. [Google Scholar] [CrossRef]
- Dolatyabi, S.; Renu, S.; Schrock, J.; Renukaradhya, G.J. Chitosan-nanoparticle-based oral Salmonella enteritidis subunit vaccine elicits cross-protection against Salmonella typhimurium in broilers. Poult. Sci. 2024, 103, 103569. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D. Evaluation of a Gold Nanoparticle Platform as Highly Immunogenic and Protective Therapy Against Burkholderia Mallei, B. pseudomallei, and Enterohemorrhagic Escherichia coli O157: H7. Ph.D. Published ETD Collection 2022. Available online: https://hdl.handle.net/2152.3/11436 (accessed on 13 January 2025).
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary tract infections caused by uropathogenic Escherichia coli strains—New strategies for an old pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Wang, L.; Lin, J.; Liu, Y. Advances of Nanotechnology Toward Vaccine Development Against Animal Infectious Diseases. Adv. Funct. Mater. 2023, 33, 2305061. [Google Scholar] [CrossRef]
- Mat Rani, N.N.I.; Alzubaidi, Z.M.; Butt, A.M.; Mohammad Faizal, N.D.F.; Sekar, M.; Azhari, H.; Mohd Amin, M.C.I. Outer membrane vesicles as biomimetic vaccine carriers against infections and cancers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1784. [Google Scholar] [CrossRef] [PubMed]
- Mawad, A.; Helmy, Y.A.; Shalkami, A.-G.; Kathayat, D.; Rajashekara, G.E. coli Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 10675–10690. [Google Scholar] [CrossRef]
- Helmy Yosra, A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens. Infect. Immun. 2022, 90, e0033722. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical experience and recent advances in the development of listeria-based tumor immunotherapies. Front. Immunol. 2021, 12, 642316. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Rajeev, A.; Chattopadhyay, I. Application of antimicrobial peptides as next-generation therapeutics in the biomedical world. Biotechnol. Genet. Eng. Rev. 2023, 40, 2458–2496. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Zhang, L.; Cheng, Y.; Chen, Z.; Zhang, Y.; Zheng, X.; Lv, Y.; Wang, S.; Li, Z. pH-responsive CuS/DSF/EL/PVP nanoplatform alleviates inflammatory bowel disease in mice via regulating gut immunity and microbiota. Acta Biomater. 2024, 178, 265–286. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.K.; Yasaswini, D.; Reddy, P.P.R.; Kumar, D.S.; Elghandour, M.M.; Salem, A. Nanotechnology in Veterinary Sector: Current Applications, Limitations and Future Perspective. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1541–1567. [Google Scholar]
- Celis-Giraldo, C.T.; López-Abán, J.; Muro, A.; Patarroyo, M.A.; Manzano-Román, R. Nanovaccines against animal pathogens: The latest findings. Vaccines 2021, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, Y.; Zhao, S.; Tang, J.; Li, H.; Xing, Y.; Qu, G.; Li, X.; Dai, J.; Zhu, Y. PAMAM-Lys, a novel vaccine delivery vector, enhances the protective effects of the SjC23 DNA vaccine against Schistosoma japonicum infection. PLoS ONE 2014, 9, e86578. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Liu, Y.V.; Mu, H.; Taylor, J.K.; Massare, M.; Flyer, D.C.; Glenn, G.M.; Smith, G.E.; Frieman, M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014, 32, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Long, Q.; Xu, Y.; Xing, J. Evaluating nanoparticles in preclinical research using microfluidic systems. Micromachines 2019, 10, 414. [Google Scholar] [CrossRef]
- Desai, N.; Chavda, V.; Singh, T.R.R.; Thorat, N.D.; Vora, L.K. Cancer nanovaccines: Nanomaterials and clinical perspectives. Small 2024, 20, 2401631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, Y.; Ma, P.; Wang, S.; Nie, G.; Li, N. Membrane-based cancer nanovaccines: The time is now. QJM Int. J. Med. 2023, 116, 621–624. [Google Scholar] [CrossRef]
- Sharma, H.; Thakur, S. 2 Membrane-Based Nano. In Nanoparticles in Cancer Therapy: Innovations and Clinical Applications; CRC Press: Boca Raton, FL, USA, 2024; p. 19. [Google Scholar]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, J.; Rajendran, V.; Ramalingam, B. Nanovaccine: A Modern Approach to Vaccinology. Nanotechnol. Med. 2021, 4, 57–74. [Google Scholar]
- Sun, M.; Pratama, A.C.; Qiu, H.; Liu, Z.; He, F. Toward innovative veterinary nanoparticle vaccines. Anim. Dis. 2024, 4, 14. [Google Scholar] [CrossRef]
- Geng, Q.; Tai, W.; Baxter, V.K.; Shi, J.; Wan, Y.; Zhang, X.; Montgomery, S.A.; Taft-Benz, S.A.; Anderson, E.J.; Knight, A.C. Novel virus-like nanoparticle vaccine effectively protects animal model from SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009897. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.N.; Knight, K. FDA Regulation of Nanotechnology; Beveridge and Diamond, PG: Washington, DC, USA, 2012. [Google Scholar]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Kumar, P.; Chaurasiya, A.; Kenwat, R.; Katke, S.; Paliwal, S.R. Development of nanomedicines and nano-similars: Recent advances in regulatory landscape. Curr. Pharm. Des. 2022, 28, 165–177. [Google Scholar] [CrossRef]
- Souto, E.B.; Blanco-Llamero, C.; Krambeck, K.; Kiran, N.S.; Yashaswini, C.; Postwala, H.; Severino, P.; Priefer, R.; Prajapati, B.G.; Maheshwari, R. Regulatory Insights into Nanomedicine and Gene Vaccine Innovation: Safety Assessment, Challenges, and Regulatory Perspectives. Acta Biomater. 2024, 180, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.R.; Bezbaruah, R.; Patel, V.; Satasia, R.; Bhattacharjee, B.; Mazumder, B. Regulatory Pathways for Nanocarrier Vaccine. In Nanocarrier Vaccines: Biopharmaceutics-Based Fast Track Development; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 465–485. [Google Scholar]
- Florindo, H.; Lopes, J.; Silva, L.; Corvo, M.; Martins, M.; Gaspar, R. Regulatory development of nanotechnology-based vaccines. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–410. [Google Scholar]
- Araste, F.; Bakker, A.D.; Zandieh-Doulabi, B. Potential and risks of nanotechnology applications in COVID-19-related strategies for pandemic control. J. Nanoparticle Res. 2023, 25, 229. [Google Scholar] [CrossRef]
- World Health Organization. State of the World’s Vaccines and Immunization; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Fernandes, C.; Jathar, M.; Sawant, B.K.S.; Warde, T. Scale-up of nanoparticle manufacturing process. In Pharmaceutical Process Engineering and Scale-Up Principles; Springer: Berlin/Heidelberg, Germany, 2023; pp. 173–203. [Google Scholar]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Borrajo, M.L.; Lou, G.; Anthiya, S.; Lapuhs, P.; Álvarez, D.M.; Tobío, A.; Loza, M.I.; Vidal, A.; Alonso, M.J. Nanoemulsions and nanocapsules as carriers for the development of intranasal mRNA vaccines. In Drug Delivery and Translational Research; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–16. [Google Scholar]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef]
- Whitley, J.; Zwolinski, C.; Denis, C.; Maughan, M.; Hayles, L.; Clarke, D.; Snare, M.; Liao, H.; Chiou, S.; Marmura, T. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl. Res. 2022, 242, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.; Azevedo, A.M.; Marques, M.P. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- George, E.; Goswami, A.; Lodhiya, T.; Padwal, P.; Iyer, S.; Gauttam, I.; Sethi, L.; Jeyasankar, S.; Sharma, P.R.; Dravid, A.A. Immunomodulatory effect of mycobacterial outer membrane vesicles coated nanoparticles. Biomater. Adv. 2022, 139, 213003. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Wang, Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, Y.; Bai, M.; Gu, H.; Li, X. Metal–organic frameworks as a therapeutic strategy for lung diseases. J. Mater. Chem. B 2022, 10, 5666–5695. [Google Scholar] [CrossRef]
- Tousian, B.; Khosravi, A.R.; Ghasemi, M.H.; Kadkhodaie, M. Biomimetic Functionalized Metal Organic Frameworks as Multifunctional Agents: Paving the Way for Cancer Vaccine Advances. Mater. Today Bio 2024, 27, 101134. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S. Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Fries, L.; Wu, Y.; Agrawal, S.; Cho, I.; Thomas, D.N.; Spindler, M.; Lindner, E.; Hahn, T.; Plested, J. Improved titers against influenza drift variants with a nanoparticle vaccine. N. Engl. J. Med. 2018, 378, 2346–2348. [Google Scholar] [CrossRef]
- Brazzoli, M.; Piccioli, D.; Marchetti, F. Challenges in development of vaccines directed toward antimicrobial resistant bacterial species. Hum. Vaccines Immunother. 2023, 19, 2228669. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A. mRNA vaccines and clinical research in Africa-From hope to reality. Int. J. Surg. 2022, 105, 106833. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and using the appropriate influenza vaccine for each individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Q.; Wei, J.; Liu, B. Personalized cancer neoantigen vaccines come of age. Theranostics 2018, 8, 4238. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, W.; Xu, X. Oral vaccination with liposome-encapsulated recombinant fusion peptide of urease B epitope and cholera toxin B subunit affords prophylactic and therapeutic effects against H. pylori infection in BALB/c mice. Vaccine 2007, 25, 7664–7673. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bowersock, T.; Griebel, P.; Kidane, A.; Babiuk, L.; Sanchez, M.; Attah-Poku, S.; Kaushik, R.; Mutwiri, G. Mucosal immune responses following oral immunization with rotavirus antigens encapsulated in alginate microspheres. J. Control. Release 2002, 85, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, transfersomes and niosomes: Production methods and their applications in the vaccinal field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef]
- Saggese, A.; Baccigalupi, L.; Donadio, G.; Ricca, E.; Isticato, R. The bacterial spore as a mucosal vaccine delivery system. Int. J. Mol. Sci. 2023, 24, 10880. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Chin, J.S.; Chooi, W.H.; Wang, H.; Ong, W.; Leong, K.W.; Chew, S.Y. Scaffold-mediated non-viral delivery platform for CRISPR/Cas9-based genome editing. Acta Biomater. 2019, 90, 60–70. [Google Scholar] [CrossRef]
- Hii, A.R.K.; Qi, X.; Wu, Z. Advanced strategies for CRISPR/Cas9 delivery and applications in gene editing, therapy, and cancer detection using nanoparticles and nanocarriers. J. Mater. Chem. B 2024, 12, 1467–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Peng, Y.; Du, Y.; Yang, Z.; Qi, X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control. Release 2023, 353, 423–433. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Smith, C.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z.; Hyun, H. Neoantigen nanovaccine improves personalized cancer immunotherapy. Cancer Res. 2020, 80, 2866. [Google Scholar] [CrossRef]
- Feng, R.; Yu, F.; Xu, J.; Hu, X. Knowledge gaps in immune response and immunotherapy involving nanomaterials: Databases and artificial intelligence for material design. Biomaterials 2021, 266, 120469. [Google Scholar] [CrossRef]
- Zohuri, B.; Behgounia, F. Application of artificial intelligence driving nano-based drug delivery system. In A Handbook of Artificial Intelligence in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 145–212. [Google Scholar]
- Hasanzadeh, A.; Hamblin, M.R.; Kiani, J.; Noori, H.; Hardie, J.M.; Karimi, M.; Shafiee, H. Could artificial intelligence revolutionize the development of nanovectors for gene therapy and mRNA vaccines? Nano Today 2022, 47, 101665. [Google Scholar] [CrossRef]
- Hamilton, S.; Kingston, B.R. Applying artificial intelligence and computational modeling to nanomedicine. Curr. Opin. Biotechnol. 2024, 85, 103043. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, L.; Zhu, M.; Zhao, Y. The Future of Nanomedicine. In Nanomedicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).