Revolutionizing Nanovaccines: A New Era of Immunization
Abstract
1. Introduction
2. Conventional vs. Nanoparticle-Based Vaccines
2.1. Conventional Vaccines
2.1.1. Inactivated Vaccines
2.1.2. Live Vaccines
2.1.3. Subunit Vaccines
2.1.4. Toxoid Vaccines
2.2. Nanoparticle-Based Vaccines
3. Types of NPs Used in Vaccines
3.1. Lipid-Based NPs
3.1.1. Interbilayer-Crosslinked Multilamellar Vesicles (ICMVs)
3.1.2. Lipoproteins
3.1.3. Liposomes
3.1.4. Solid Lipid Nanoparticles (SLNs)
3.1.5. Exosomes
3.1.6. Virosomes
3.1.7. Emulsions
3.2. Polymeric NPs
3.3. Inorganic NPs
3.4. Virus-like Particles (VLPs)
3.5. Self-Assembling Peptide/Protein Nanovaccines
4. Mechanism of Action of Nanovaccines
5. Nanovaccines for Food Safety
6. Nanoparticle-Based Vaccines in Veterinary Medicine
7. Current Research on Nanoparticle-Based Vaccines
8. Hurdles for Nanoparticle-Based Vaccines
9. Innovations in NP Development
9.1. Nanocages, Dendrimers, and Other Novel Structures
9.2. Using NPs with Other Advanced Technologies
9.3. Personalized Vaccines
10. Artificial Intelligence in Nanoparticle-Based Vaccines
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hedman, H.D.; Krawczyk, E.; Helmy, Y.A.; Zhang, L.; Varga, C. Host Diversity and Potential Transmission Pathways of SARS-CoV-2 at the Human-Animal Interface. Pathogens 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D.; Alash’le, G.A. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Mehendale, R.; Joshi, M.; Patravale, V.B. Nanomedicines for treatment of viral diseases. Crit. Rev. ™ Ther. Drug Carr. Syst. 2013, 30, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; El-Metwally, M.; Saber, W.; Negm, S.; El-Kott, A.; Mazroua, Y.; Makhlouf, A.; Moustafa, M. Secondary antiviral metabolites from fungi with special reference to coronaviruses. Biocell 2022, 46, 1979–1988. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Lamichhane, B.; Habib, T.; Adams, A.; El-Sheikh Ali, H.; Slovis, N.M.; Troedsson, M.H.T.; Helmy, Y.A. Antimicrobial Resistance in Equines: A Growing Threat to Horse Health and Beyond—A Comprehensive Review. Antibiotics 2024, 13, 713. [Google Scholar] [CrossRef]
- Roope, L.S.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Mullins, L.P.; Mason, E.; Winter, K.; Sadarangani, M. Vaccination is an integral strategy to combat antimicrobial resistance. PLoS Pathog. 2023, 19, e1011379. [Google Scholar] [CrossRef]
- Fawzy, M.; Helmy, Y.A. The One Health Approach is Necessary for the Control of Rift Valley Fever Infections in Egypt: A Comprehensive Review. Viruses 2019, 11, 139. [Google Scholar] [CrossRef]
- Helmy, Y.A.; El-Adawy, H.; Abdelwhab, E.M. A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt. Pathogens 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against viral infectious diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Kassem, I.I.; Rajashekara, G. Immuno-modulatory effect of probiotic E. coli Nissle 1917 in polarized human colonic cells against Campylobacter jejuni infection. Gut Microbes 2021, 13, 1857514. [Google Scholar] [CrossRef] [PubMed]
- Zepp, F. Principles of vaccine design—Lessons from nature. Vaccine 2010, 28, C14–C24. [Google Scholar] [CrossRef] [PubMed]
- Kiboneka, A.N. Basic concepts in clinical immunology: A review. World J. Adv. Res. Rev. 2021, 12, 490–496. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, K.; Helmy, Y.A.; Yitbarek, A.; Hodgins, D.C.; Sharafeldin, T.A.; Selim, M.S.H. Advances in Poultry Vaccines: Leveraging Biotechnology for Improving Vaccine Development, Stability, and Delivery. Vaccines 2024, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Elaish, M.; Ngunjiri, J.M.; Ali, A.; Xia, M.; Ibrahim, M.; Jang, H.; Hiremath, J.; Dhakal, S.; Helmy, Y.A.; Jiang, X. Supplementation of inactivated influenza vaccine with norovirus P particle-M2e chimeric vaccine enhances protection against heterologous virus challenge in chickens. PLoS ONE 2017, 12, e0171174. [Google Scholar] [CrossRef] [PubMed]
- Murugan, B.; Sagadevan, S. Nano-Vaccines: Opportunities and Challenges in Biomaterial-Based Vaccine Delivery. Biomater. -Inspired Nanomed. Target. Ther. 2024, 101–116. [Google Scholar] [CrossRef]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cui, C.; Wang, Y.; Sun, X.; Wang, S.; Yang, M.; Yu, Y.; Wang, L. CpG ODN as an adjuvant arouses the vigor of B cells by relieving the negative regulation of surface TLR9 to enhance the antibody response to vaccine. Appl. Microbiol. Biotechnol. 2021, 105, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An overview of the use of nanoparticles in vaccine development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine development throughout history. Cureus 2021, 13, e16635. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Taylor, M.W. Smallpox; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Davidson, T. Vaccines: History, Science, and Issues; Bloomsbury Publishing USA: New York, NY, USA, 2017. [Google Scholar]
- Mamelund, S.-E. Influenza, historical. Medicine 2008, 54, 361–371. [Google Scholar]
- Lombard, M.; Pastoret, P.-P.; Moulin, A. A brief history of vaccines and vaccination. Rev. Sci. Tech. Off. Int. Epizoot. 2007, 26, 29–48. [Google Scholar] [CrossRef]
- Meeusen, E.N.; Walker, J.; Peters, A.; Pastoret, P.-P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Karin, H.; Lisa, B.; Damer, P. Vaccines as alternatives to antibiotics for food producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 64. [Google Scholar]
- Palomino-Tapia, V. Autogenous Vaccines in the Poultry Industry: A Field Perspective. In Poultry Farming-New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Nagpal, G.; Usmani, S.S.; Raghava, G.P. A web resource for designing subunit vaccine against major pathogenic species of bacteria. Front. Immunol. 2018, 9, 2280. [Google Scholar] [CrossRef]
- Malik, H.; Khan, F.H.; Ahsan, H. Human papillomavirus: Current status and issues of vaccination. Arch. Virol. 2014, 159, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiong, Y.; Li, N.; Jian, C. Vaccine types. In Vaccines—The History and Future; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Liljeqvist, S.; Ståhl, S. Production of recombinant subunit vaccines: Protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999, 73, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Liu, Y.; Rigsby, P.; Sesardic, D. An improved method for development of toxoid vaccines and antitoxins. J. Immunol. Methods 2008, 337, 42–48. [Google Scholar] [CrossRef]
- Andey, T.; Soni, S.; Modi, S. Conventional vaccination methods: Inactivated and live attenuated vaccines. Adv. Vaccin. Technol. Infect. Chronic Dis. 2024, 37–50. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479, 379–392. [Google Scholar] [CrossRef]
- Lopez, S.M.; Sato, A.I.; Chatterjee, A. Vaccines: An overview. Viral Parasit. Bact. Fungal Infect. 2023, 56, 699–717. [Google Scholar]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Teulon, J.-M.; Godon, C.; Chantalat, L.; Moriscot, C.; Cambedouzou, J.; Odorico, M.; Ravaux, J.; Podor, R.; Gerdil, A.; Habert, A. On the operational aspects of measuring nanoparticle sizes. Nanomaterials 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, H.; Zhang, S.; Hu, J.; Zhang, L. Delivery of nanovaccine towards lymphoid organs: Recent strategies in enhancing cancer immunotherapy. J. Nanobiotechnol. 2021, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Elattar, K.M.; Al-Otibi, F.O.; El-Hersh, M.S.; Attia, A.A.; Eldadamony, N.M.; Elsayed, A.; Menaa, F.; Saber, W.I. Multifaceted chemical and bioactive features of Ag@ TiO2 and Ag@ SeO2 core/shell nanoparticles biosynthesized using Beta vulgaris L. extract. Heliyon 2024, 10, e28359. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Chen, J.-Y.; Chen, H.-W.; Hu, C.-M.J. Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostics 2017, 1, 244. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Liu, Y.; Chen, X.; Liu, Q.; Jia, J.; Yang, T.; Qiu, S.; Ma, G. Immune responses to vaccines involving a combined antigen–nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials 2014, 35, 6086–6097. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Y.; Xiang, Y.; Xu, F. Nanovaccines: Merits, and diverse roles in boosting antitumor immune responses. Hum. Vaccines Immunother. 2022, 18, 2119020. [Google Scholar] [CrossRef]
- Li, M.; Kaminskas, L.M.; Marasini, N. Recent advances in nano/microparticle-based oral vaccines. J. Pharm. Investig. 2021, 51, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- González-García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailänder, V.; Landfester, K.; Vasilev, K. Nanoparticles surface chemistry influence on protein corona composition and inflammatory responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef]
- Diaz-Arévalo, D.; Zeng, M. Nanoparticle-based vaccines: Opportunities and limitations. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–150. [Google Scholar]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Manju, K.; Raj, S.N.; Ranjini, H.; Nayaka, S.C.; Ashwini, P.; Satish, S.; Prasad, M.N.; Chouhan, R.S.; Baker, S. Nanovaccines to combat drug resistance: The next-generation immunisation. Future J. Pharm. Sci. 2023, 9, 64. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, Z.P. Nanoparticle-based nanomedicines to promote cancer immunotherapy: Recent advances and future directions. Small 2019, 15, 1900262. [Google Scholar] [CrossRef]
- Kumru, O.S.; Joshi, S.B.; Smith, D.E.; Middaugh, C.R.; Prusik, T.; Volkin, D.B. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals 2014, 42, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced nanobiomaterials: Vaccines, diagnosis and treatment of infectious diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Abusalah, M.A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; Sundriyal, A. Nanovaccines: A game changing approach in the fight against infectious diseases. Biomed. Pharmacother. 2023, 167, 115597. [Google Scholar]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and microparticles as potential oral vaccine carriers and adjuvants against infectious diseases. Front. Pharmacol. 2021, 12, 682286. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Rodriguez Pozo, A.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A. Vaccine inoculation route modulates early immunity and consequently antigen-specific immune response. Front. Immunol. 2021, 12, 645210. [Google Scholar] [CrossRef]
- Xie, S.; Pan, B.; Wang, M.; Zhu, L.; Wang, F.; Dong, Z.; Wang, X.; Zhou, W. Formulation, characterization and pharmacokinetics of praziquantel-loaded hydrogenated castor oil solid lipid nanoparticles. Nanomedicine 2010, 5, 693–701. [Google Scholar] [CrossRef]
- Heidari-Kharaji, M.; Taheri, T.; Doroud, D.; Habibzadeh, S.; Badirzadeh, A.; Rafati, S. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite Immunol. 2016, 38, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Kondel, R.; Shafiq, N.; Kaur, I.P.; Singh, M.P.; Pandey, A.K.; Ratho, R.K.; Malhotra, S. Effect of acyclovir solid lipid nanoparticles for the treatment of herpes simplex virus (HSV) infection in an animal model of HSV-1 infection. Pharm. Nanotechnol. 2019, 7, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.; Ryu, M.; Kim, S.; Joo, M.; Yeom, J.-H.; Kim, S.; Park, Y.; Lee, K.; Bae, J. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep. 2017, 7, 13572. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent gold nanoparticle–peptide conjugates for targeting intracellular bacterial infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Judy, B.M.; Qazi, O.; Blumentritt, C.A.; Brown, K.A.; Shaw, A.M.; Torres, A.G.; Titball, R.W. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 447–456. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, J.-A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Farfán-Castro, S.; García-Soto, M.J.; Betancourt-Mendiola, L.; Cervantes, J.; Segura, R.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and evaluation of gold nanoparticles conjugated with five antigenic peptides derived from the spike protein of SARS-CoV-2 for vaccine development. Front. Nanotechnol. 2024, 6, 1335346. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.; Alipour, M.; Azghani, A.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Theivendran, S.; Lazarev, S.; Yu, C. Mesoporous silica/organosilica nanoparticles for cancer immunotherapy. Exploration 2023, 3, 20220086. [Google Scholar] [CrossRef]
- Song, C.; Li, F.; Wang, S.; Wang, J.; Wei, W.; Ma, G. Recent advances in particulate adjuvants for cancer vaccination. Adv. Ther. 2020, 3, 1900115. [Google Scholar] [CrossRef]
- Madapong, A.; Petro-Turnquist, E.M.; Webby, R.J.; McCormick, A.A.; Weaver, E.A. Immunity and Protective Efficacy of a Plant-Based Tobacco Mosaic Virus-like Nanoparticle Vaccine against Influenza a Virus in Mice. Vaccines 2024, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Díaz, M.C.; Phan, S.; Elson, A.; Nuñez, I.; Wei, H.; Sakamoto, K.; He, B. Parainfluenza virus 5 (PIV5) amplifying virus-like particles expressing respiratory syncytial virus (RSV) antigens protect mice against RSV infection. Vaccine 2019, 37, 2925–2934. [Google Scholar] [CrossRef]
- Lacasta, A.; Mody, K.T.; De Goeyse, I.; Yu, C.; Zhang, J.; Nyagwange, J.; Mwalimu, S.; Awino, E.; Saya, R.; Njoroge, T. Synergistic effect of two nanotechnologies enhances the protective capacity of the Theileria parva sporozoite p67C antigen in cattle. J. Immunol. 2021, 206, 686–699. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2022, 12, 790121. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Michel, M.-L.; Tiollais, P. Hepatitis B vaccines: Protective efficacy and therapeutic potential. Pathol. Biol. 2010, 58, 288–295. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.N.; Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Current and future nanoparticle vaccines for COVID-19. EBioMedicine 2021, 74, 103699. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, J.; Lo, P.-C.; Lovell, J.F.; Jin, H.; Yang, K. Self-adjuvanting cancer nanovaccines. J. Nanobiotechnol. 2022, 20, 345. [Google Scholar] [CrossRef]
- Li, Y.; Su, T.; Zhang, Y.; Huang, X.; Li, J.; Li, C. Liposomal co-delivery of daptomycin and clarithromycin at an optimized ratio for treatment of methicillin-resistant Staphylococcus aureus infection. Drug Deliv. 2015, 22, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant–biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef]
- Hanson, M.C.; Abraham, W.; Crespo, M.P.; Chen, S.H.; Liu, H.; Szeto, G.L.; Kim, M.; Reinherz, E.L.; Irvine, D.J. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine 2015, 33, 861–868. [Google Scholar] [CrossRef]
- Smith, L.R.; Wloch, M.K.; Ye, M.; Reyes, L.R.; Boutsaboualoy, S.; Dunne, C.E.; Chaplin, J.A.; Rusalov, D.; Rolland, A.P.; Fisher, C.L. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 2010, 28, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Ma, G.; Xia, Y. Unlocking the therapeutic applicability of LNP-mRNA: Chemistry, formulation, and clinical strategies. Research 2024, 7, 0370. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Yu, X.; Wei, T.; Farbiak, L.; Johnson, L.T.; Taylor, A.M.; Xu, J.; Hong, Y.; Zhu, H. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 2022, 17, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Li, Z.; Wang, F.; Fan, X.; Zhou, X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics 2020, 10, 7131. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, H.; Wang, M.; Li, J.; Lin, H.; Liang, M.; Gao, Y.; Yang, M. An OMV-based nanovaccine confers safety and protection against pathogenic Escherichia coli via both humoral and predominantly Th1 immune responses in poultry. Nanomaterials 2020, 10, 2293. [Google Scholar] [CrossRef]
- Thukral, A.; Ross, K.; Hansen, C.; Phanse, Y.; Narasimhan, B.; Steinberg, H.; Talaat, A.M. A single dose polyanhydride-based nanovaccine against paratuberculosis infection. npj Vaccines 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Etewa, S.E.; El-Maaty, D.A.A.; Hamza, R.S.; Metwaly, A.S.; Sarhan, M.H.; Abdel-Rahman, S.A.; Fathy, G.M.; El-Shafey, M.A. Assessment of spiramycin-loaded chitosan nanoparticles treatment on acute and chronic toxoplasmosis in mice. J. Parasit. Dis. 2018, 42, 102–113. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Gao, C.; Wang, Y.; Cheng, X.; Yang, Y.; Gou, Q.; Lei, L.; Chen, Y.; Wang, X. Development of a chitosan--modified PLGA nanoparticle vaccine for protection against Escherichia coli K1 caused meningitis in mice. J. Nanobiotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef] [PubMed]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune response of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish. Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef]
- El-Sissi, A.F.; Mohamed, F.H.; Danial, N.M.; Gaballah, A.Q.; Ali, K.A. Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 2020, 10, 88. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Chen, G.; Shi, X.-m.; Gao, T.-t.; Li, W.; Zhao, Y.; Zhang, F.-q.; Wu, J.; Cui, X.; Wang, Y.-F. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS ONE 2012, 7, e53314. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Padhi, A.; Mukherjee, S.; Dash, D.P.; Kar, S.; Sonawane, A. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of γδ T cells in mice. Nanotechnology 2017, 28, 165101. [Google Scholar] [CrossRef]
- Mohammed, G.M.; ElZorkany, H.E.; Farroh, K.Y.; Abd El-Aziz, W.R.; Elshoky, H.A. Potential improvement of the immune response of chickens against E. coli vaccine by using two forms of chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 167, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Renukaradhya, G.J. Chitosan nanoparticle based mucosal vaccines delivered against infectious diseases of poultry and pigs. Front. Bioeng. Biotechnol. 2020, 8, 558349. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef]
- Kelly, S.M.; Larsen, K.R.; Darling, R.; Petersen, A.C.; Bellaire, B.H.; Wannemuehler, M.J.; Narasimhan, B. Single-dose combination nanovaccine induces both rapid and durable humoral immunity and toxin neutralizing antibody responses against Bacillus anthracis. Vaccine 2021, 39, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.H.; Lokugamage, N.; Garg, N.J. Experimental nanovaccine offers protection against repeat exposures to Trypanosoma cruzi through activation of polyfunctional T cell response. Front. Immunol. 2020, 11, 595039. [Google Scholar] [CrossRef]
- Mody, K.T.; Zhang, B.; Li, X.; Fletcher, N.L.; Akhter, D.T.; Jarrett, S.; Zhang, J.; Yu, C.; Thurecht, K.J.; Mahony, T.J. Characterization of the biodistribution of a silica vesicle nanovaccine carrying a Rhipicephalus (Boophilus) microplus protective antigen with in vivo live animal imaging. Front. Bioeng. Biotechnol. 2021, 8, 606652. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Bharath, L. Controlling of food borne pathogens by nanoparticles. Bioorganic Phase Nat. Food Overv. 2018, 15, 293–322. [Google Scholar]
- Vinayamohan, P.G.; Joseph, D.; Viju, L.S.; Venkitanarayanan, K. Efficacy of selenium for controlling infectious diseases. In Selenium and Human Health; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Maleki, M.; Salouti, M.; Shafiee Ardestani, M.; Talebzadeh, A. Preparation of a nanovaccine against Brucella melitensis M16 based on PLGA nanoparticles and oligopolysaccharide antigen. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4248–4256. [Google Scholar] [CrossRef]
- Lee, J.-A.; Jung, B.-G.; Kim, T.-H.; Kim, Y.-M.; Park, M.-H.; Hyun, P.-m.; Jeon, J.-w.; Park, J.-k.; Cho, C.-W.; Suh, G.-H. Poly d, l-lactide-co-glycolide (PLGA) nanoparticle-encapsulated honeybee (Apis melifera) venom promotes clearance of Salmonella enterica serovar Typhimurium infection in experimentally challenged pigs through the up-regulation of T helper type 1 specific immune responses. Vet. Immunol. Immunopathol. 2014, 161, 193–204. [Google Scholar] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef]
- Toti, U.S.; Guru, B.R.; Hali, M.; McPharlin, C.M.; Wykes, S.M.; Panyam, J.; Whittum-Hudson, J.A. Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials 2011, 32, 6606–6613. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, S.; Ganesh, N.; Bhattacharya, S.; Jain, N.K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target. 2009, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.S.; Fordoei, A.S.; Abdoli, A.; Ahangari Cohan, R.; Bahramali, G.; Sadat, S.M.; Siadat, S.D.; Moloudian, H.; Nassiri Koopaei, N.; Bolhasani, A. Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity. J. Mater. Sci. Mater. Med. 2015, 26, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.Q.; Thanh, N.T.H.; Thuy, N.T.; Van Chung, P.; Hung, P.N.; Le, A.-T.; Hanh, N.T.H. Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villamil, J.I.; Tapia, D.; Torres, A.G. Development of a gold nanoparticle vaccine against enterohemorrhagic Escherichia coli O157: H7. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjugate Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef]
- Tiwari, V.; Tiwari, M.; Solanki, V. Polyvinylpyrrolidone-capped silver nanoparticle inhibits infection of carbapenem-resistant strain of Acinetobacter baumannii in the human pulmonary epithelial cell. Front. Immunol. 2017, 8, 973. [Google Scholar] [CrossRef]
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Pangestika, R.; Ernawati, R. Antiviral activity effect of silver nanoparticles (Agnps) solution against the growth of infectious bursal disease virus on embryonated chicken eggs with Elisa test. KnE Life Sci. 2017, 3, 536–548. [Google Scholar] [CrossRef]
- Kuppan, G.; Sangeeta, K. Dose and Size-Dependent Antiviral Effects of Silver Nanoparticles on Feline Calicivirus, a Human Norovirus Surrogate. Foodborne Pathog Dis. 2016, 13, 239–244. [Google Scholar]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef]
- Folliero, V.; Zannella, C.; Chianese, A.; Stelitano, D.; Ambrosino, A.; De Filippis, A.; Galdiero, M.; Franci, G.; Galdiero, M. Application of dendrimers for treating parasitic diseases. Pharmaceutics 2021, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- El Bissati, K.; Zhou, Y.; Paulillo, S.M.; Raman, S.K.; Karch, C.P.; Roberts, C.W.; Lanar, D.E.; Reed, S.; Fox, C.; Carter, D. Protein nanovaccine confers robust immunity against Toxoplasma. npj Vaccines 2017, 2, 24. [Google Scholar] [CrossRef]
- Kwon, E.J.; Skalak, M.; Bertucci, A.; Braun, G.; Ricci, F.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Porous silicon nanoparticle delivery of tandem peptide anti-infectives for the treatment of Pseudomonas aeruginosa lung infections. Adv. Mater. 2017, 29, 1701527. [Google Scholar] [CrossRef]
- Gowri, M.; Latha, N.; Suganya, K.; Murugan, M.; Rajan, M. Calcium alginate nanoparticle crosslinked phosphorylated polyallylamine to the controlled release of clindamycin for osteomyelitis treatment. Drug Dev. Ind. Pharm. 2021, 47, 280–291. [Google Scholar] [CrossRef]
- Wang, L.; Xing, D.; Le Van, A.; Jerse, A.E.; Wang, S. Structure-based design of ferritin nanoparticle immunogens displaying antigenic loops of Neisseria gonorrhoeae. FEBS Open Bio 2017, 7, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kiderlen, A.F.; Petri, B.; Kayser, O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 597–603. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, Y.; Hua, L.; Liu, X.; Xiao, M.; Xu, T.; Zhu, B.; Cao, H. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3485–3491. [Google Scholar] [CrossRef]
- Thi, E.P.; Mire, C.E.; Lee, A.C.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362–365. [Google Scholar] [CrossRef]
- Lauster, D.; Glanz, M.; Bardua, M.; Ludwig, K.; Hellmund, M.; Hoffmann, U.; Hamann, A.; Böttcher, C.; Haag, R.; Hackenberger, C.P. Multivalent peptide–nanoparticle conjugates for influenza-virus inhibition. Angew. Chem. Int. Ed. 2017, 56, 5931–5936. [Google Scholar] [CrossRef]
- Rodrigues-Jesus, M.; Fotoran, W.L.; Cardoso, R.M.; Araki, K.; Wunderlich, G.; Ferreira, L.C. Nano-multilamellar lipid vesicles (NMVs) enhance protective antibody responses against Shiga toxin (Stx2a) produced by enterohemorrhagic Escherichia coli strains (EHEC). Braz. J. Microbiol. 2019, 50, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cavallaro, A.S.; Mody, K.T.; Zhang, J.; Deringer, J.R.; Brown, W.C.; Mahony, T.J.; Yu, C.; Mitter, N. Nanoparticle-based delivery of Anaplasma marginale membrane proteins; virb9-1 and virb10 produced in the Pichia pastoris expression system. Nanomaterials 2016, 6, 201. [Google Scholar] [CrossRef]
- Rungrojcharoenkit, K.; Sunintaboon, P.; Ellison, D.; Macareo, L.; Midoeng, P.; Chaisuwirat, P.; Fernandez, S.; Ubol, S. Development of an adjuvanted nanoparticle vaccine against influenza virus, an in vitro study. PLoS ONE 2020, 15, e0237218. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Lung, P.; Yang, J.; Li, Q. Nanoparticle formulated vaccines: Opportunities and challenges. Nanoscale 2020, 12, 5746–5763. [Google Scholar] [CrossRef]
- Verma, M.; Ozer, I.; Xie, W.; Gallagher, R.; Teixeira, A.; Choy, M. The landscape for lipid-nanoparticle-based genomic medicines. Nat. Rev. Drug Discov. 2023, 22, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xue, Y.; Álvarez-Benedicto, E.; Shi, Y.; Chen, W.; Koo, S.; Siegwart, D.J.; Dong, Y.; Tao, W. Nanotechnology-based mRNA vaccines. Nat. Rev. Methods Primers 2023, 3, 63. [Google Scholar] [CrossRef]
- Look, M.; Bandyopadhyay, A.; Blum, J.S.; Fahmy, T.M. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv. Drug Deliv. Rev. 2010, 62, 378–393. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J. Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef]
- Smith, J.F.; Brownlow, M.; Brown, M.; Kowalski, R.; Esser, M.T.; Ruiz, W.; Barr, E.; Brown, D.R.; Bryan, J.T. Antibodies from women immunized with Gardasil® cross-neutralize HPV 45 pseudovirions. Hum. Vaccines 2007, 3, 109–115. [Google Scholar] [CrossRef]
- Passero Jr, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer. Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Nordquist, L.T.; Shore, N.D.; Elist, J.J.; Oliver, J.C.; Gannon, W.; Shahlaee, A.H.; Fuller, S.A.; Ghanbari, H.A. Phase 1 open-label trial to evaluate the safety and immunogenicity of PAN-301-1, a novel nanoparticle therapeutic vaccine, in patients with biochemically relapsed prostate cancer. J. Clin. Oncol. 2018, 29 (Suppl. S15), e15166. [Google Scholar] [CrossRef]
- Lee, D.-H.; Choi, S.; Park, Y.; Jin, H.-s. Mucin1 and Mucin16: Therapeutic targets for cancer therapy. Pharmaceuticals 2021, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Cech, P.G.; Aebi, T.; Abdallah, M.S.; Mpina, M.; Machunda, E.B.; Westerfeld, N.; Stoffel, S.A.; Zurbriggen, R.; Pluschke, G.; Tanner, M. Virosome-formulated Plasmodium falciparum AMA-1 & CSP derived peptides as malaria vaccine: Randomized phase 1b trial in semi-immune adults & children. PLoS ONE 2011, 6, e22273. [Google Scholar]
- Porras, C.; Sampson, J.N.; Herrero, R.; Gail, M.H.; Cortés, B.; Hildesheim, A.; Cyr, J.; Romero, B.; Schiller, J.T.; Montero, C. Rationale and design of a double-blind randomized non-inferiority clinical trial to evaluate one or two doses of vaccine against human papillomavirus including an epidemiologic survey to estimate vaccine efficacy: The Costa Rica ESCUDDO trial. Vaccine 2022, 40, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory syncytial virus vaccines: A review of the candidates and the approved vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bhangde, S.; Lodaya, R.N.; Amiji, M.M. Nanoscale Vaccines for Influenza. In Nanomedicines for the Prevention and Treatment of Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 331–347. [Google Scholar]
- Low, J.G.; De Alwis, R.; Chen, S.; Kalimuddin, S.; Leong, Y.S.; Mah, T.K.L.; Yuen, N.; Tan, H.C.; Zhang, S.L.; Sim, J.X. A phase I/II randomized, double-blinded, placebo-controlled trial of a self-amplifying COVID-19 mRNA vaccine. npj Vaccines 2022, 7, 161. [Google Scholar] [CrossRef]
- Al, L.I.R.; Sönmezer, M.Ç.; Ünal, S. RNA-Based COVID-19 vaccine candidates with clinical phase trials in progress. Turk. J. Med. Sci. 2021, 51, 3246–3252. [Google Scholar]
- Kalnin, K.V.; Plitnik, T.; Kishko, M.; Zhang, J.; Zhang, D.; Beauvais, A.; Anosova, N.G.; Tibbitts, T.; DiNapoli, J.; Ulinski, G. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 2021, 6, 61. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Alameh, M.-G.; Tharakhet, K.; Kaewpang, P.; Yostrerat, N.; Pitakpolrat, P.; Buranapraditkun, S.; Manopwisedjaroen, S.; Thitithanyanont, A. Immunogenicity and protective efficacy of SARS-CoV-2 mRNA vaccine encoding secreted non-stabilized spike in female mice. Nat. Commun. 2023, 14, 2309. [Google Scholar] [CrossRef]
- World Health Organization. Emergency use designation of COVID-19 candidate vaccines: Ethical considerations for current and future COVID-19 placebo-controlled vaccine trials and trial unblinding: Policy brief, 18 December 2020. In Emergency Use Designation of COVID-19 Candidate Vaccines: Ethical Considerations for Current and Future COVID-19 Placebo-Controlled Vaccine Trials and Trial Unblinding: Policy Brief, 18 December 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Beloki, U.; Salaberry, L.; Smerdou, C. Self-Amplifying RNA: A Second Revolution of mRNA Vaccines against COVID-19. Vaccines 2024, 12, 318. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. npj Vaccines 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Ho Um, S.; Khant, H. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Li, A.V.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 2012, 109, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Huang, B.; Irvine, D.J. Engineering nano- and microparticles to tune immunity. Adv. Mater. 2012, 24, 3724–3746. [Google Scholar] [CrossRef]
- Chong, P.; Huang, J.-H.; Leng, C.-H.; Liu, S.-J.; Chen, H.-W. Recombinant lipoproteins as novel vaccines with intrinsic adjuvant. Adv. Protein Chem. Struct. Biol. 2015, 99, 55–74. [Google Scholar]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.-F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and art of developing lipid nanoparticles for biologics delivery: Focus on development and scale-up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, Y.; Wang, Y.; Yin, Q. Emerging peptide-based nanovaccines: From design synthesis to defense against cancer and infection. Biomed. Pharmacother. 2023, 158, 114117. [Google Scholar] [CrossRef]
- Abdul Ghaffar, K.; Kumar Giddam, A.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Tretiakova, D.; Vodovozova, E. Liposomes as adjuvants and vaccine delivery systems. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef] [PubMed]
- Yanasarn, N.; Sloat, B.R.; Cui, Z. Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol. Pharm. 2011, 8, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Shetye, L.; Sherlekar, A.; Mendhulkar, V. Liposome-Based Drug Delivery—A New Therapeutic Paradigm. In Advanced Drug Delivery: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–48. [Google Scholar]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Pensado, A.; Seijo, B.; Sanchez, A. Current strategies for DNA therapy based on lipid nanocarriers. Expert Opin. Drug Deliv. 2014, 11, 1721–1731. [Google Scholar] [CrossRef]
- Peres, L.B.; Peres, L.B.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A recent update: Solid lipid nanoparticles for effective drug delivery. Adv. Pharm. Bull. 2022, 12, 17. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Santos, P.; Almeida, F. Exosome-based vaccines: History, current state, and clinical trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Mahdipour, M.; Nourazarian, A.; Habibollahi, P.; Sokullu, E.; Avci, Ç.B.; Rahbarghazi, R. Application of exosomes for the alleviation of COVID-19-related pathologies. Cell Biochem. Funct. 2022, 40, 430–438. [Google Scholar] [CrossRef]
- Wallis, J.; Shenton, D.; Carlisle, R. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019, 196, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Glück, R.; Metcalfe, I. New technology platforms in the development of vaccines for the future. Vaccine 2002, 20, B10–B16. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Tang, J.-l.; Sun, J.; He, Z.-G. Self-emulsifying drug delivery systems: Strategy for improving oral delivery of poorly soluble drugs. Curr. Drug Ther. 2007, 2, 85–93. [Google Scholar] [CrossRef]
- Akagi, T.; Baba, M.; Akashi, M. Biodegradable nanoparticles as vaccine adjuvants and delivery systems: Regulation of immune responses by nanoparticle-based vaccine. Polym. Nanomed. 2012, 247, 31–64. [Google Scholar]
- Liu, B.; Wu, Z.; Liu, T.; Qian, R.; Wu, T.; Liu, Q.; Shen, A. Polymeric nanoparticles engineered as a vaccine adjuvant-delivery system. In Immunization-Vaccine Adjuvant Delivery System and Strategies; IntechOpen: London, UK, 2018. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Veiga, F.; Ribeiro, A.J. Preparation of drug-loaded polymeric nanoparticles. In Nanomedicine in Cancer; Jenny Stanford Publishing: Oxon, UK, 2017; pp. 171–214. [Google Scholar]
- Maji, I.; Mahajan, S.; Sriram, A.; Mehra, N.K.; Singh, P.K. Polymeric nanomaterials: Fundamentals and therapeutic applications. In Nanomaterial-Based Drug Delivery Systems: Therapeutic and Theranostic Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 33–64. [Google Scholar]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnology 2020, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Stern, T.; González, M.F.; Epstein, J. Biodegradable poly(ethylene oxide)/poly(ϵ-caprolactone) multiblock copolymers. J. Biomed. Mater. Res. 2002, 59, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-loaded mixed micelles and polymersomes composed of poly (caprolactone)-poly(ethylene glycol)(PEG-PCL) and poly (lactic acid)-poly (ethylene glycol)(PEG-PLA): Preparation and in-vitro characterization. Iran. J. Pharm. Res. IJPR 2019, 18, 142. [Google Scholar]
- Tian, J.-H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; van Meijgaarden, K.E.; Franken, K.L.; Junginger, H.E.; Borchard, G.; Ottenhoff, T.H.; Geluk, A. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A* 0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 2004, 22, 1609–1615. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Rezigue, M. Lipid and Polymeric Nanoparticles: Drug Delivery Applications; Springer: Cham, Switzerland, 2020; pp. 167–230. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Elzoghby, A.; M Abd-Elwakil, M.; Abd-Elsalam, K.; T Elsayed, M.; Hashem, Y.; Mohamed, O. Natural polymeric nanoparticles for brain-targeting: Implications on drug and gene delivery. Curr. Pharm. Des. 2016, 22, 3305–3323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, H.; Ma, L.; Shi, Y.; Ji, M.; Sun, X.; Ma, D.; Zhou, W.; Huang, T.; Zhang, D. The quest for nanoparticle-powered vaccines in cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Vasudevan, S.S.; Kandrikar, T.Y.; Sayyed, A.A.; Sarker, P.; Nasir, N.S.; Venugopalan, S.; Mariajohn, R.; Chavda, V.P.; Gondaliya, P. Nanoparticle-based vaccines and future vaccine technologies. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Elsevier: Amsterdam, The Netherlands, 2024; pp. 477–495. [Google Scholar]
- Cable, J.; Graham, B.S.; Koup, R.A.; Seder, R.A.; Karikó, K.; Pardi, N.; Barouch, D.H.; Sharma, B.; Rauch, S.; Nachbagauer, R. Progress in vaccine development for infectious diseases—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2023, 1524, 65–86. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Gnanaraj, M.; Sisubalan, N.; Jebastin, T.; Vijayan, A.; Muneeshwaran, T.; Manikandan, R. Gold Nanoparticles as Antibacterial and Antiviral Agents: Biomedical Applications and Theranostic Potential. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 19–45. [Google Scholar]
- Chaudhary, K.; Masram, D.T. Biological activities of nanoparticles and mechanism of action. In Model Organisms to Study Biological Activities and Toxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2020; pp. 19–34. [Google Scholar]
- Chernykh, I.; Kopanitsa, M.; Shchul’kin, A.; Yakusheva, E.; Frolova, M. Gold nanoparticles as potential antitumor agents. Pharm. Chem. J. 2021, 55, 934–941. [Google Scholar] [CrossRef]
- He, J.-s.; Liu, S.-j.; Zhang, Y.-r.; Chu, X.-d.; Lin, Z.-b.; Zhao, Z.; Qiu, S.-h.; Guo, Y.-g.; Ding, H.; Pan, Y.-l. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021, 12, 687399. [Google Scholar] [CrossRef] [PubMed]
- Farfán-Castro, S.; García-Soto, M.J.; Aguilar-Aguilar, A.; González-Ortega, O.; Rosales-Mendoza, S. Application of gold nanoparticles in vaccine development. In Gold Nanoparticles for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 445–493. [Google Scholar]
- Xu, L.; Wang, X.; Wang, W.; Sun, M.; Choi, W.J.; Kim, J.-Y.; Hao, C.; Li, S.; Qu, A.; Lu, M. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef]
- Tapia, D.; Sanchez-Villamil, J.I.; Torres, A.G. Multicomponent gold nano-glycoconjugate as a highly immunogenic and protective platform against Burkholderia mallei. npj Vaccines 2020, 5, 82. [Google Scholar] [CrossRef]
- Gao, L.; Song, Y.; Zhong, J.; Lin, X.; Zhou, S.-F.; Zhan, G. Biocompatible 2D Cu-TCPP nanosheets derived from Cu2O nanocubes as multifunctional nanoplatforms for combined anticancer therapy. ACS Biomater. Sci. Eng. 2022, 8, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2018, 16, 1–23. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021, 21, 627–630. [Google Scholar] [CrossRef]
- Nandanwar, R.; Singh, P.; Haque, F.Z. Synthesis and properties of silica nanoparticles by sol-gel method for the application in green chemistry. Mater. Sci. Res. India 2013, 10, 85–92. [Google Scholar]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Machado, Y.; Feinle, A.; Thalhamer, J.; Hüsing, N.; Weiss, R. Potential of nanoparticles for allergen-specific immunotherapy–use of silica nanoparticles as vaccination platform. Expert Opin. Drug Deliv. 2016, 13, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Seré, S.; Vounckx, U.; Seo, J.W.; Lenaerts, I.; Van Gool, S.; Locquet, J.-P. Proof of concept study: Mesoporous silica nanoparticles, from synthesis to active specific immunotherapy. Front. Nanotechnol. 2020, 2, 584233. [Google Scholar] [CrossRef]
- Liu, J.Y.; Sayes, C.M. A toxicological profile of silica nanoparticles. Toxicol. Res. 2022, 11, 565–582. [Google Scholar] [CrossRef]
- Pallavi, P.; Harini, K.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; Gowtham, P.; Girigoswami, K.; Shakeel, F.; Girigoswami, A. From synthetic route of silica nanoparticles to theranostic applications. Processes 2022, 10, 2595. [Google Scholar] [CrossRef]
- Xiao, D.; Qi, H.; Teng, Y.; Pierre, D.; Kutoka, P.T.; Liu, D. Advances and challenges of fluorescent nanomaterials for synthesis and biomedical applications. Nanoscale Res. Lett. 2021, 16, 1–23. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 1–27. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Yang, X.; Lu, X.; Xu, X.-N.; Zhang, J.; Chen, R. Potentiating the Immune Responses of HBsAg-VLP Vaccine Using a Polyphosphoester-Based Cationic Polymer Adjuvant. ACS Appl. Mater. Interfaces 2023, 15, 48871–48881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-B.; Quan, F.-S. Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Patil, S. A review of virus-like particle-based SARS-CoV-2 vaccines in clinical trial phases. Iran. J. Pharm. Res. IJPR 2021, 13, 2901–2905. [Google Scholar]
- Aboshi, M.; Matsuda, K.; Kawakami, D.; Kono, K.; Kazami, Y.; Sekida, T.; Komori, M.; Morey, A.L.; Suga, S.; Smith, J.F. Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine. iScience 2024, 27, 108964. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Vandoolaeghe, P.; Schuerman, L. The RTS, S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Expert Rev. Vaccines 2016, 15, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Syomin, B.; Ilyin, Y. Virus-like particles as an instrument of vaccine production. Mol. Biol. 2019, 53, 323–334. [Google Scholar] [CrossRef]
- Erdmann, N.B.; Williams, W.B.; Walsh, S.R.; Grunenberg, N.; Edlefsen, P.T.; Goepfert, P.A.; Cain, D.W.; Cohen, K.W.; Maenza, J.; Mayer, K.H. A HIV-1 Gp41 peptide-liposome vaccine elicits neutralizing epitope-targeted antibody responses in healthy individuals. medRxiv 2024. [Google Scholar] [CrossRef]
- Zeigler, D.F.; Gage, E.; Roque, R.; Clegg, C.H. Epitope targeting with self-assembled peptide vaccines. NPJ Vaccines 2019, 4, 30. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Longping, V.T.; Shehata, L.; O’Connor, M.A.; Chen, C. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Josheghani, S.; Bakhshi, B.; Najar-peerayeh, S. The influence of nanoparticle on vaccine responses against bacterial infection. J. Nanotechnol. 2022, 2022, 6856982. [Google Scholar] [CrossRef]
- Chang, H.C.; Zou, Z.Z.; Wang, Q.H.; Li, J.; Jin, H.; Yin, Q.X.; Xing, D. Targeting and specific activation of antigen--presenting cells by endogenous antigen-loaded nanoparticles elicits tumor-specific immunity. Adv. Sci. 2020, 7, 1900069. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, O.; Bertero, A.; Gagliardi, M.; Bardi, G. Biomedical nanoparticles: Overview of their surface immune-compatibility. Coatings 2014, 4, 139–159. [Google Scholar] [CrossRef]
- Zaman, M.; Good, M.F.; Toth, I. Nanovaccines and their mode of action. Methods 2013, 60, 226–231. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.; Wang, K.; Liang, L.; Zhang, H. An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer. Vaccines 2024, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-modified chitosan-nanoparticle-based Salmonella subunit oralvaccine-induced immune response and efficacy in a challenge trial in broilers. Vaccines 2020, 8, 299. [Google Scholar] [CrossRef]
- Dolatyabi, S.; Renu, S.; Schrock, J.; Renukaradhya, G.J. Chitosan-nanoparticle-based oral Salmonella enteritidis subunit vaccine elicits cross-protection against Salmonella typhimurium in broilers. Poult. Sci. 2024, 103, 103569. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D. Evaluation of a Gold Nanoparticle Platform as Highly Immunogenic and Protective Therapy Against Burkholderia Mallei, B. pseudomallei, and Enterohemorrhagic Escherichia coli O157: H7. Ph.D. Published ETD Collection 2022. Available online: https://hdl.handle.net/2152.3/11436 (accessed on 13 January 2025).
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary tract infections caused by uropathogenic Escherichia coli strains—New strategies for an old pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Wang, L.; Lin, J.; Liu, Y. Advances of Nanotechnology Toward Vaccine Development Against Animal Infectious Diseases. Adv. Funct. Mater. 2023, 33, 2305061. [Google Scholar] [CrossRef]
- Mat Rani, N.N.I.; Alzubaidi, Z.M.; Butt, A.M.; Mohammad Faizal, N.D.F.; Sekar, M.; Azhari, H.; Mohd Amin, M.C.I. Outer membrane vesicles as biomimetic vaccine carriers against infections and cancers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1784. [Google Scholar] [CrossRef] [PubMed]
- Mawad, A.; Helmy, Y.A.; Shalkami, A.-G.; Kathayat, D.; Rajashekara, G.E. coli Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 10675–10690. [Google Scholar] [CrossRef]
- Helmy Yosra, A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens. Infect. Immun. 2022, 90, e0033722. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, M.; Paterson, Y.; Wood, L.M. Clinical experience and recent advances in the development of listeria-based tumor immunotherapies. Front. Immunol. 2021, 12, 642316. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Rajeev, A.; Chattopadhyay, I. Application of antimicrobial peptides as next-generation therapeutics in the biomedical world. Biotechnol. Genet. Eng. Rev. 2023, 40, 2458–2496. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Zhang, L.; Cheng, Y.; Chen, Z.; Zhang, Y.; Zheng, X.; Lv, Y.; Wang, S.; Li, Z. pH-responsive CuS/DSF/EL/PVP nanoplatform alleviates inflammatory bowel disease in mice via regulating gut immunity and microbiota. Acta Biomater. 2024, 178, 265–286. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.K.; Yasaswini, D.; Reddy, P.P.R.; Kumar, D.S.; Elghandour, M.M.; Salem, A. Nanotechnology in Veterinary Sector: Current Applications, Limitations and Future Perspective. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1541–1567. [Google Scholar]
- Celis-Giraldo, C.T.; López-Abán, J.; Muro, A.; Patarroyo, M.A.; Manzano-Román, R. Nanovaccines against animal pathogens: The latest findings. Vaccines 2021, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, Y.; Zhao, S.; Tang, J.; Li, H.; Xing, Y.; Qu, G.; Li, X.; Dai, J.; Zhu, Y. PAMAM-Lys, a novel vaccine delivery vector, enhances the protective effects of the SjC23 DNA vaccine against Schistosoma japonicum infection. PLoS ONE 2014, 9, e86578. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.M.; Liu, Y.V.; Mu, H.; Taylor, J.K.; Massare, M.; Flyer, D.C.; Glenn, G.M.; Smith, G.E.; Frieman, M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014, 32, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-based vaccines against respiratory viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Long, Q.; Xu, Y.; Xing, J. Evaluating nanoparticles in preclinical research using microfluidic systems. Micromachines 2019, 10, 414. [Google Scholar] [CrossRef]
- Desai, N.; Chavda, V.; Singh, T.R.R.; Thorat, N.D.; Vora, L.K. Cancer nanovaccines: Nanomaterials and clinical perspectives. Small 2024, 20, 2401631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, Y.; Ma, P.; Wang, S.; Nie, G.; Li, N. Membrane-based cancer nanovaccines: The time is now. QJM Int. J. Med. 2023, 116, 621–624. [Google Scholar] [CrossRef]
- Sharma, H.; Thakur, S. 2 Membrane-Based Nano. In Nanoparticles in Cancer Therapy: Innovations and Clinical Applications; CRC Press: Boca Raton, FL, USA, 2024; p. 19. [Google Scholar]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, J.; Rajendran, V.; Ramalingam, B. Nanovaccine: A Modern Approach to Vaccinology. Nanotechnol. Med. 2021, 4, 57–74. [Google Scholar]
- Sun, M.; Pratama, A.C.; Qiu, H.; Liu, Z.; He, F. Toward innovative veterinary nanoparticle vaccines. Anim. Dis. 2024, 4, 14. [Google Scholar] [CrossRef]
- Geng, Q.; Tai, W.; Baxter, V.K.; Shi, J.; Wan, Y.; Zhang, X.; Montgomery, S.A.; Taft-Benz, S.A.; Anderson, E.J.; Knight, A.C. Novel virus-like nanoparticle vaccine effectively protects animal model from SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009897. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.N.; Knight, K. FDA Regulation of Nanotechnology; Beveridge and Diamond, PG: Washington, DC, USA, 2012. [Google Scholar]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Kumar, P.; Chaurasiya, A.; Kenwat, R.; Katke, S.; Paliwal, S.R. Development of nanomedicines and nano-similars: Recent advances in regulatory landscape. Curr. Pharm. Des. 2022, 28, 165–177. [Google Scholar] [CrossRef]
- Souto, E.B.; Blanco-Llamero, C.; Krambeck, K.; Kiran, N.S.; Yashaswini, C.; Postwala, H.; Severino, P.; Priefer, R.; Prajapati, B.G.; Maheshwari, R. Regulatory Insights into Nanomedicine and Gene Vaccine Innovation: Safety Assessment, Challenges, and Regulatory Perspectives. Acta Biomater. 2024, 180, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.R.; Bezbaruah, R.; Patel, V.; Satasia, R.; Bhattacharjee, B.; Mazumder, B. Regulatory Pathways for Nanocarrier Vaccine. In Nanocarrier Vaccines: Biopharmaceutics-Based Fast Track Development; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 465–485. [Google Scholar]
- Florindo, H.; Lopes, J.; Silva, L.; Corvo, M.; Martins, M.; Gaspar, R. Regulatory development of nanotechnology-based vaccines. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–410. [Google Scholar]
- Araste, F.; Bakker, A.D.; Zandieh-Doulabi, B. Potential and risks of nanotechnology applications in COVID-19-related strategies for pandemic control. J. Nanoparticle Res. 2023, 25, 229. [Google Scholar] [CrossRef]
- World Health Organization. State of the World’s Vaccines and Immunization; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Fernandes, C.; Jathar, M.; Sawant, B.K.S.; Warde, T. Scale-up of nanoparticle manufacturing process. In Pharmaceutical Process Engineering and Scale-Up Principles; Springer: Berlin/Heidelberg, Germany, 2023; pp. 173–203. [Google Scholar]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Borrajo, M.L.; Lou, G.; Anthiya, S.; Lapuhs, P.; Álvarez, D.M.; Tobío, A.; Loza, M.I.; Vidal, A.; Alonso, M.J. Nanoemulsions and nanocapsules as carriers for the development of intranasal mRNA vaccines. In Drug Delivery and Translational Research; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–16. [Google Scholar]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef]
- Whitley, J.; Zwolinski, C.; Denis, C.; Maughan, M.; Hayles, L.; Clarke, D.; Snare, M.; Liao, H.; Chiou, S.; Marmura, T. Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials. Transl. Res. 2022, 242, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.; Azevedo, A.M.; Marques, M.P. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- George, E.; Goswami, A.; Lodhiya, T.; Padwal, P.; Iyer, S.; Gauttam, I.; Sethi, L.; Jeyasankar, S.; Sharma, P.R.; Dravid, A.A. Immunomodulatory effect of mycobacterial outer membrane vesicles coated nanoparticles. Biomater. Adv. 2022, 139, 213003. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Wang, Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1523. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, Y.; Bai, M.; Gu, H.; Li, X. Metal–organic frameworks as a therapeutic strategy for lung diseases. J. Mater. Chem. B 2022, 10, 5666–5695. [Google Scholar] [CrossRef]
- Tousian, B.; Khosravi, A.R.; Ghasemi, M.H.; Kadkhodaie, M. Biomimetic Functionalized Metal Organic Frameworks as Multifunctional Agents: Paving the Way for Cancer Vaccine Advances. Mater. Today Bio 2024, 27, 101134. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S. Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Fries, L.; Wu, Y.; Agrawal, S.; Cho, I.; Thomas, D.N.; Spindler, M.; Lindner, E.; Hahn, T.; Plested, J. Improved titers against influenza drift variants with a nanoparticle vaccine. N. Engl. J. Med. 2018, 378, 2346–2348. [Google Scholar] [CrossRef]
- Brazzoli, M.; Piccioli, D.; Marchetti, F. Challenges in development of vaccines directed toward antimicrobial resistant bacterial species. Hum. Vaccines Immunother. 2023, 19, 2228669. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A. mRNA vaccines and clinical research in Africa-From hope to reality. Int. J. Surg. 2022, 105, 106833. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and using the appropriate influenza vaccine for each individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Q.; Wei, J.; Liu, B. Personalized cancer neoantigen vaccines come of age. Theranostics 2018, 8, 4238. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, W.; Xu, X. Oral vaccination with liposome-encapsulated recombinant fusion peptide of urease B epitope and cholera toxin B subunit affords prophylactic and therapeutic effects against H. pylori infection in BALB/c mice. Vaccine 2007, 25, 7664–7673. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bowersock, T.; Griebel, P.; Kidane, A.; Babiuk, L.; Sanchez, M.; Attah-Poku, S.; Kaushik, R.; Mutwiri, G. Mucosal immune responses following oral immunization with rotavirus antigens encapsulated in alginate microspheres. J. Control. Release 2002, 85, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, transfersomes and niosomes: Production methods and their applications in the vaccinal field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef]
- Saggese, A.; Baccigalupi, L.; Donadio, G.; Ricca, E.; Isticato, R. The bacterial spore as a mucosal vaccine delivery system. Int. J. Mol. Sci. 2023, 24, 10880. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Chin, J.S.; Chooi, W.H.; Wang, H.; Ong, W.; Leong, K.W.; Chew, S.Y. Scaffold-mediated non-viral delivery platform for CRISPR/Cas9-based genome editing. Acta Biomater. 2019, 90, 60–70. [Google Scholar] [CrossRef]
- Hii, A.R.K.; Qi, X.; Wu, Z. Advanced strategies for CRISPR/Cas9 delivery and applications in gene editing, therapy, and cancer detection using nanoparticles and nanocarriers. J. Mater. Chem. B 2024, 12, 1467–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Peng, Y.; Du, Y.; Yang, Z.; Qi, X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. J. Control. Release 2023, 353, 423–433. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Smith, C.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z.; Hyun, H. Neoantigen nanovaccine improves personalized cancer immunotherapy. Cancer Res. 2020, 80, 2866. [Google Scholar] [CrossRef]
- Feng, R.; Yu, F.; Xu, J.; Hu, X. Knowledge gaps in immune response and immunotherapy involving nanomaterials: Databases and artificial intelligence for material design. Biomaterials 2021, 266, 120469. [Google Scholar] [CrossRef]
- Zohuri, B.; Behgounia, F. Application of artificial intelligence driving nano-based drug delivery system. In A Handbook of Artificial Intelligence in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 145–212. [Google Scholar]
- Hasanzadeh, A.; Hamblin, M.R.; Kiani, J.; Noori, H.; Hardie, J.M.; Karimi, M.; Shafiee, H. Could artificial intelligence revolutionize the development of nanovectors for gene therapy and mRNA vaccines? Nano Today 2022, 47, 101665. [Google Scholar] [CrossRef]
- Hamilton, S.; Kingston, B.R. Applying artificial intelligence and computational modeling to nanomedicine. Curr. Opin. Biotechnol. 2024, 85, 103043. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, L.; Zhu, M.; Zhao, Y. The Future of Nanomedicine. In Nanomedicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28. [Google Scholar]
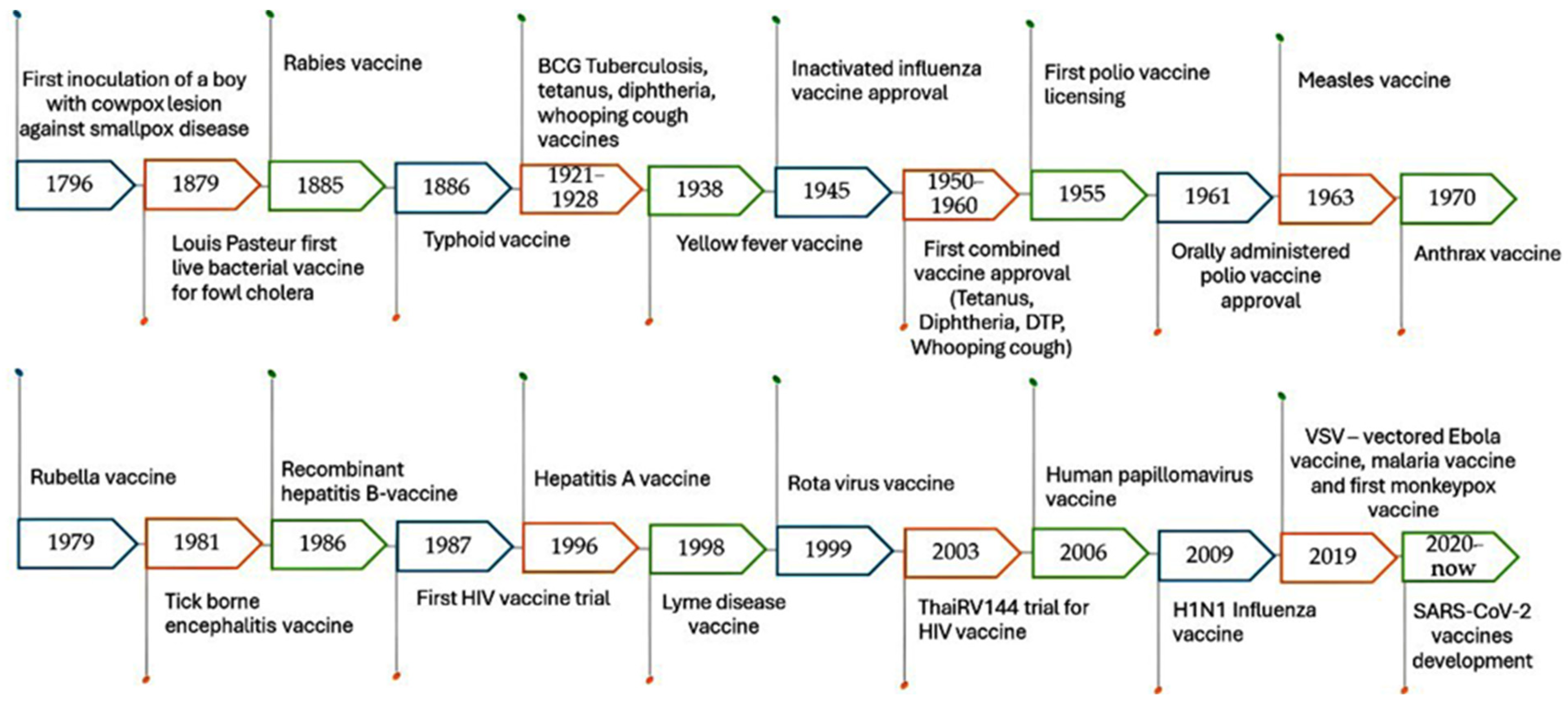

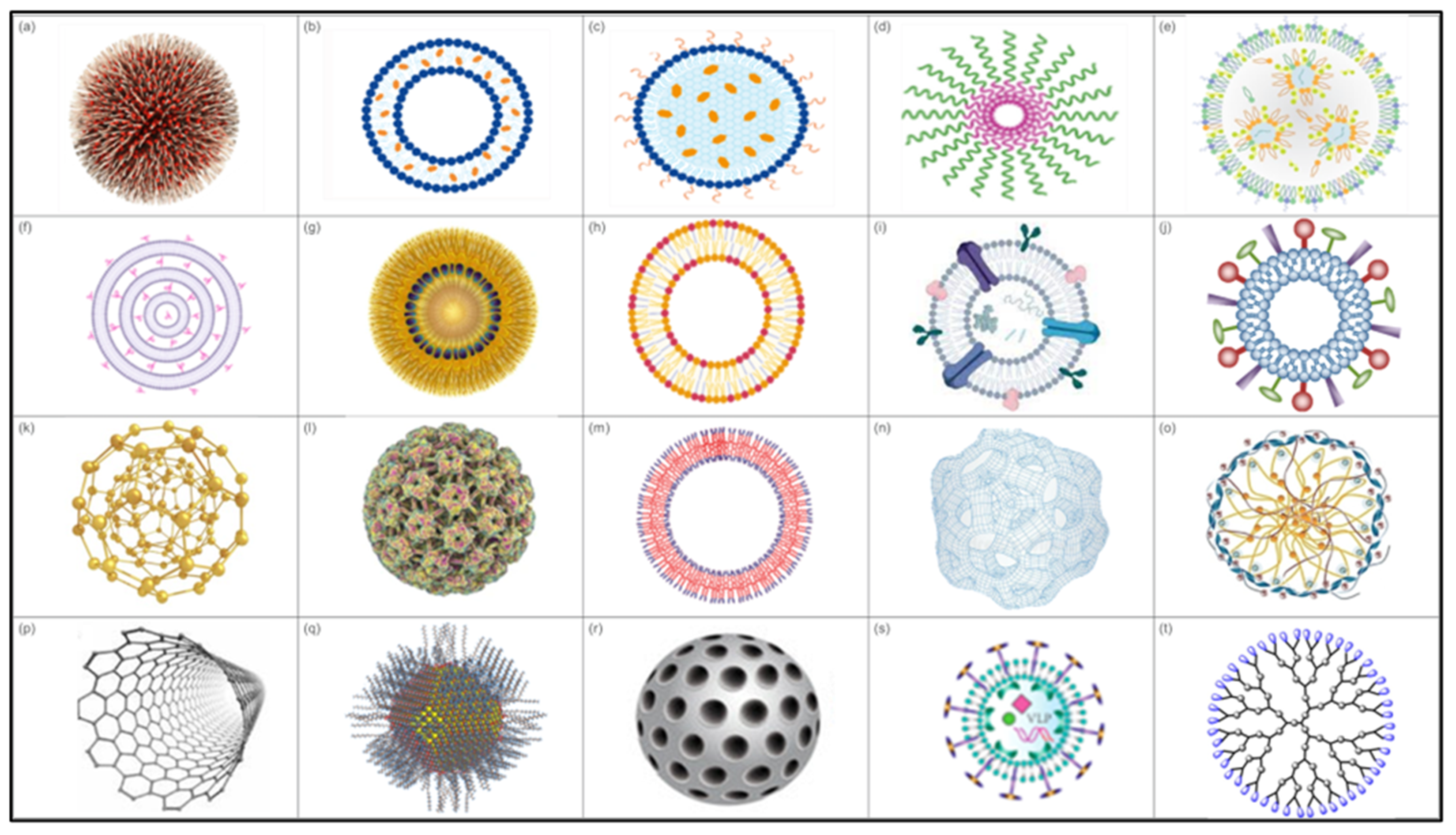
| Criterion | Conventional Vaccines | Nanoparticle-Based Vaccines |
|---|---|---|
| Types | Live attenuated, inactivated, subunit and toxoid vaccines | Protein NPs, lipid NPs, polymer-based NPs, inorganic NPs, and virus-like particles |
| Advantages | Proven track record, strong and long-lasting immunity | Can carry multiple antigens, enhanced stability, targeted delivery, potentially stronger response |
| Mechanism | Uses attenuated/killed pathogens or pathogen components to stimulate immune response | Delivers antigens in a targeted manner for better recognition and immune response |
| Immune response | Immunogenicity can vary and often requires adjuvants to elicit a robust response | Designed for a more specific, potent immune response with built-in adjuvants |
| Antigen delivery | Direct administration, often less targeted | Targeted delivery of specific cells or tissues |
| Antigen stability | Require strict temperature control and maintenance | Nanocarriers extend the shelf life of antigens and protect them from degradation |
| Production Complexity | Long-established manufacturing processes | Advanced bioengineering techniques, more complex production |
| Stability | Generally unstable, requires cold chain storage | Often more stable, some formulations may require less stringent storage conditions |
| Customization | Limited adaptability to rapidly mutated pathogens | Highly customizable for emerging pathogens |
| Dosage | Multiple administrations may be required for optimal immunogenicity, depending on the vaccine and target population | Targeted delivery has minimized the administration of multiple doses |
| Safety | Almost safe with few risks of reversion to virulent form regarding live attenuated vaccines | Potential toxicity of some NPs |
| Examples | Polio, hepatitis B, IB, NDV, tetanus, fowl pox | Novavax (protein NPs) and COVID-19 mRNA vaccines (using lipid NPs) |
| Nanoparticle Type | Targeted Infectious Pathogen/Disease | Pathogen Classification | Reference |
|---|---|---|---|
| Lipid-based NPs | SARS-CoV-2, herpes simplex virus, leishmania and Schistosoma | Virus/parasite | [66,67,68,69] |
| Gold NPs (AuNPs) | SARS-CoV-2, Hepatitis C Virus, Foot and Mouth Disease (FMD), Clostridia tetani, Burkholderia mallei, Salmonella Typhi and Vibrio vulnificus | Virus/bacteria | [70,71,72,73,74,75,76] |
| Mesoporous Silica NPs | Tumor cells | Tumor cells | [77,78] |
| Virus-like particles | Hepatitis B virus, malaria, Theileria parva, Respiratory Syncytial virus (RSV), influenza, circoviruses and SARS-CoV-2 | virus/parasite | [79,80,81,82,83,84] |
| Protein-based NPs | SARS-CoV-2 | Virus | [85,86] |
| Self-adjuvanting polyguanidine nanovaccines | Tumor cells | Tumor cells | [87] |
| Liposomes | Influenza virus, hepatitis A, Human immunosuppressive Virus (HIV), Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus | Virus/bacteria | [76,88,89,90,91,92] |
| Silicon scalable lipid NPs | SARS-CoV-2 | Virus | [93] |
| Dendrimer-RNA NPs | H1N1 influenza, Ebola, and Toxoplasma gondii | Virus /parasite | [94] |
| Multiplexed dendrimer with lipid-based NPs | Tumor cells | Tumor cells | [95] |
| Fluoropolymer-based nanovaccines | Tumor cells | Tumor cells | [96] |
| Extracellular vesicle-coated multi-antigenic nanovaccines | Multidrug resistant S. aureus | Bacteria | [97] |
| OMV-based nanovaccines | Escherichia coli serotype O2 | Bacteria | [98] |
| Carbon nanotubes and poly-anhydrous NPs | Anaplasma marginale and Mycobacterium paratuberculosis | Protozoa/bacteria | [99] |
| Chitosan | Salmonella Enteritidis, Streptococcus, avian pathogenic E. coli, NDV, tuberculosis, Flavobacterium columnare, S. aureus, E. coli K1 and Toxoplasma | Bacteria/virus/parasite | [100,101,102,103,104,105,106,107,108,109,110] |
| Polyanhydride NPs | Bacillus anthracis and Yersinia pestis | Bacteria | [111] |
| Nanoplasmids | Trypanosoma cruzi | Parasite | [112] |
| Antigen-absorbing silica vesicles | Tick-borne diseases | Tick-borne diseases | [113] |
| Zinc oxide NPs | E. coli, Salmonella spp. and H1N1 | Bacteria/Virus | [114,115] |
| Selenium NPs | S. aureus and E. coli | Bacteria | [116] |
| Poly (lactic acid-coglycolic acid) polymeric NPs | C. trachomatis, C. pneumoniae, Helicobacter pylori, Listeria monocytogenes, Salmonella Typhimurium and Brucella melitensis | Bacteria | [117,118,119,120,121] |
| Chitosan and glutamic acid polymeric NPs | H. pylori | Bacteria | [122] |
| Silver NPs (AgNPs) | H3N2, feline calicivirus, infectious bursal disease virus (IBDV), respiratory syncytial virus (RSV), Rift Valley Fever virus (RVF), HIV, adenovirus, poliovirus, norovirus, Acinetobacter baumannii, feline coronavirus, E. coli, S. aureus and P. aeruginosa | Virus/bacteria | [123,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| Dendrimers | Schistosoma | Parasite | [137] |
| Self-assembling polypeptide NPs | Toxoplasmosis | Parasite | [138] |
| Porous silicon NPs | P. aeruginosa | Bacteria | [139] |
| Calcium-alginate NPs | Methicillin-resistant S. aureus | Bacteria | [140] |
| Ferritin NPs | Neisseria gonorrhoeae | Bacteria | [141] |
| Nanosuspensions | Cryptosporidium parvum | Parasite | [142] |
| Selenium nanoparticle functionalized with oseltamivir | Enterovirus | Virus | [143] |
| LNP- siVP35-3 | EBOV | Virus | [144] |
| Multivalent peptide–polymer NPs | Influenza | Virus | [145] |
| Nano-vesicles | A. marginale and shiga toxin by E. coli | Parasite/bacteria | [146,147] |
| TMC NPs | Influenza | Virus | [148] |
| Trade Name | Composition | Indication | Ref. |
|---|---|---|---|
| Vaxfectin® | Liposomal vaccine | Herpes simplex virus type 2 and influenza virus | [91] |
| Epaxal® | Liposomal vaccine | Hepatitis A infection | [92,159] |
| Doxil® and Abraxane® | Liposomal doxorubicin and albumin-bound paclitaxel | Approved by the FDA and are used in treating Various cancers | [160] |
| Shingrix® | Recombinant VZV glycoprotein E on liposomes carrier | Approved by FDA against herpes virus | [161] |
| Onivyde® | Liposomal irinotecan | Pancreatic cancer | [162] |
| W_ova1 | Liposome-formulated mRNAs | Ovarian cancer | [163] |
| DPX-0907 | Liposomes containing a polynucleotide adjuvant | Ovarian, breast, and prostate cancer | [164] |
| Lipovaxin-MM | Tumor antigen-containing multicomponent liposomes with the DC-targeting molecule DMS-5000 | Metastatic melanoma | [165] |
| Vyxeos® | Liposomal cytarabine and daunorubicin | Acute myeloid leukemia | [166,167] |
| VaxiSome™ | Liposomal adjuvant | Influenza | [168] |
| MPER-656 | HIV-1 gp41 membrane-proximal external region (MPER) on liposome carrier | HIV | [169] |
| LNP CL-0059/CL-0137 | mRNA LNP | RSV | [170] |
| BNT165b1 | mRNA LNP | Malaria | [171] |
| MRT5413 | LNP-formulated, modified mRNA | Influenza | [172] |
| ARCT-021, MRT5500, Pfizer-BioNTech and Moderna COVID-19, ChulaCov19, mRNA-1273, CVnCoV, LNP-nCoV-saRNA | mRNA LNP | COVID-19 | [68,173,174,175,176,177,178,179,180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, M.; El-Moghazy, A.; Elgohary, A.H.; Saber, W.I.A.; Helmy, Y.A. Revolutionizing Nanovaccines: A New Era of Immunization. Vaccines 2025, 13, 126. https://doi.org/10.3390/vaccines13020126
Saleh M, El-Moghazy A, Elgohary AH, Saber WIA, Helmy YA. Revolutionizing Nanovaccines: A New Era of Immunization. Vaccines. 2025; 13(2):126. https://doi.org/10.3390/vaccines13020126
Chicago/Turabian StyleSaleh, Mohammed, Ahmed El-Moghazy, Adel H. Elgohary, WesamEldin I. A. Saber, and Yosra A. Helmy. 2025. "Revolutionizing Nanovaccines: A New Era of Immunization" Vaccines 13, no. 2: 126. https://doi.org/10.3390/vaccines13020126
APA StyleSaleh, M., El-Moghazy, A., Elgohary, A. H., Saber, W. I. A., & Helmy, Y. A. (2025). Revolutionizing Nanovaccines: A New Era of Immunization. Vaccines, 13(2), 126. https://doi.org/10.3390/vaccines13020126








