Booster Vaccination Against Invasive Pneumococcal Disease and Hepatitis B in Previously Vaccinated Solid Organ Transplant Recipients Without Seroprotection
Abstract
1. Introduction
2. Materials & Methods
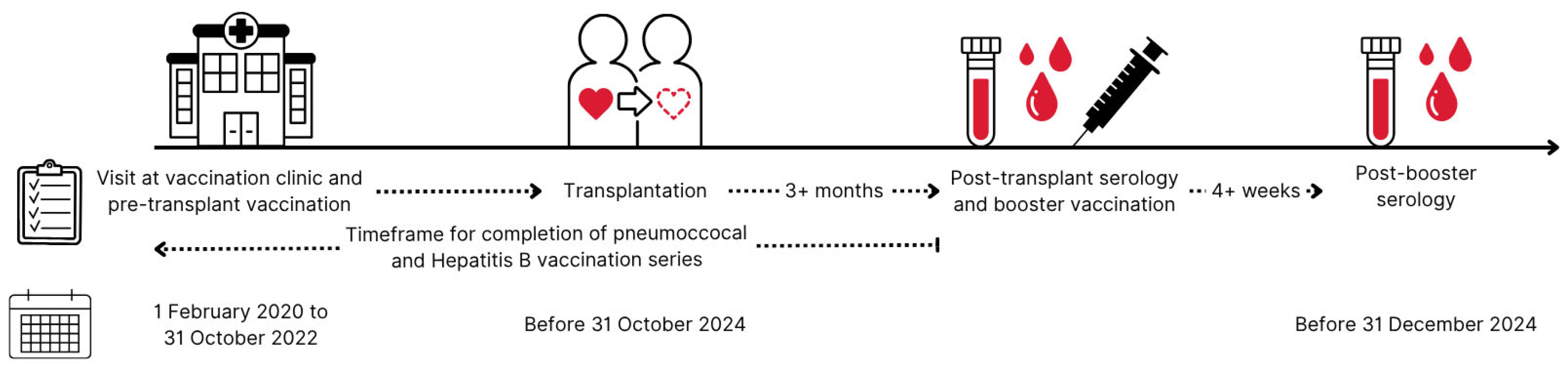
2.1. Study Overview
2.2. Booster Vaccination Strategy
2.3. Serology
2.4. Definitions
2.5. Statistical Analysis
3. Results
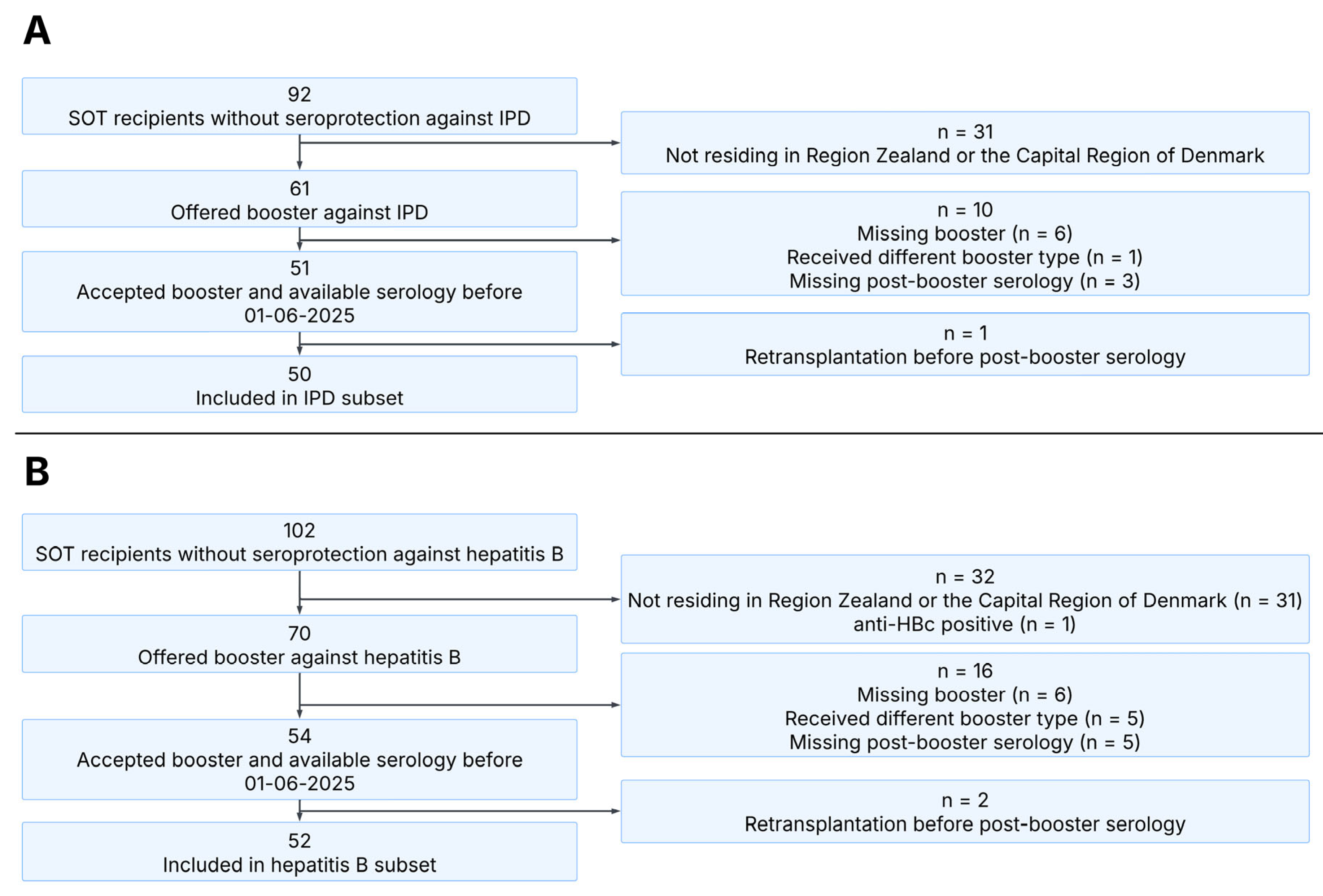
3.1. Cohort Characteristics
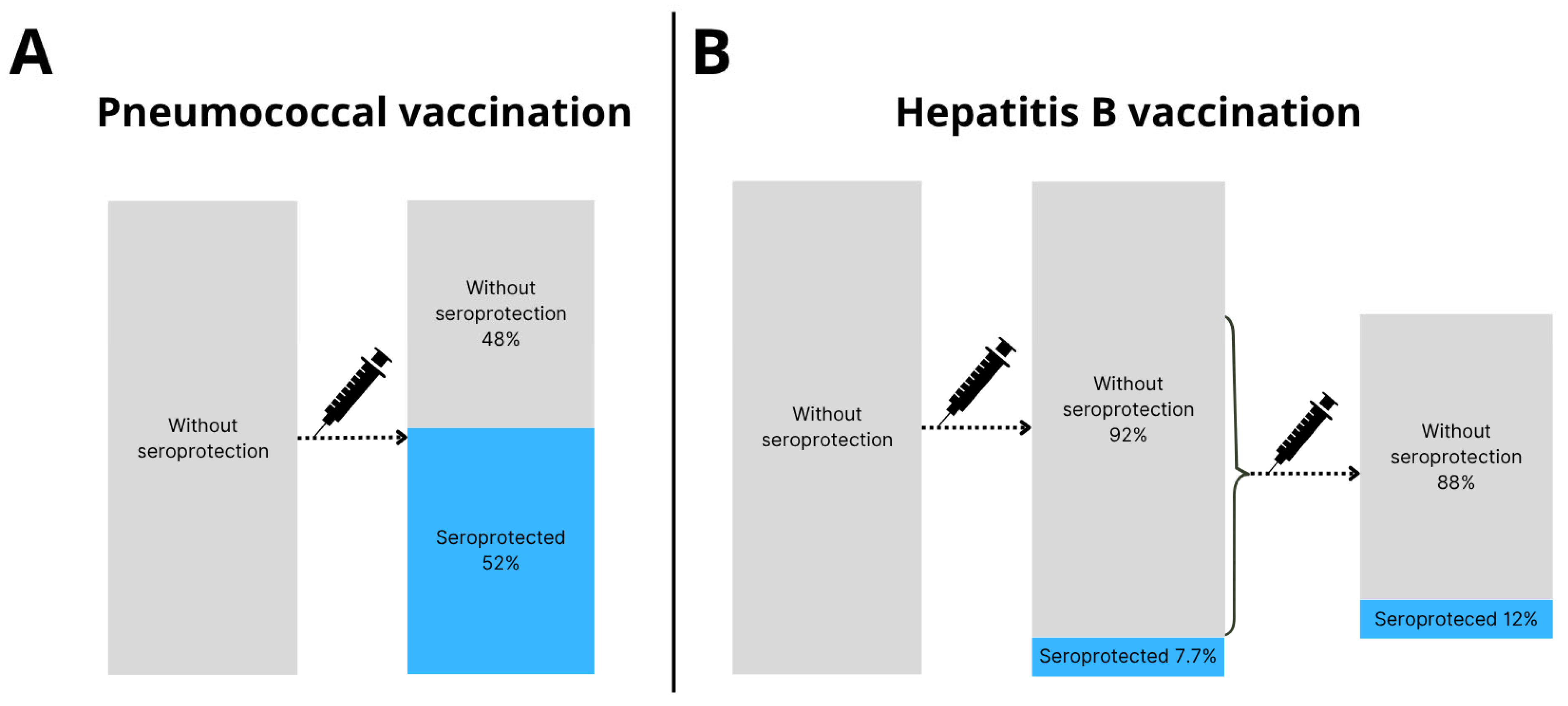
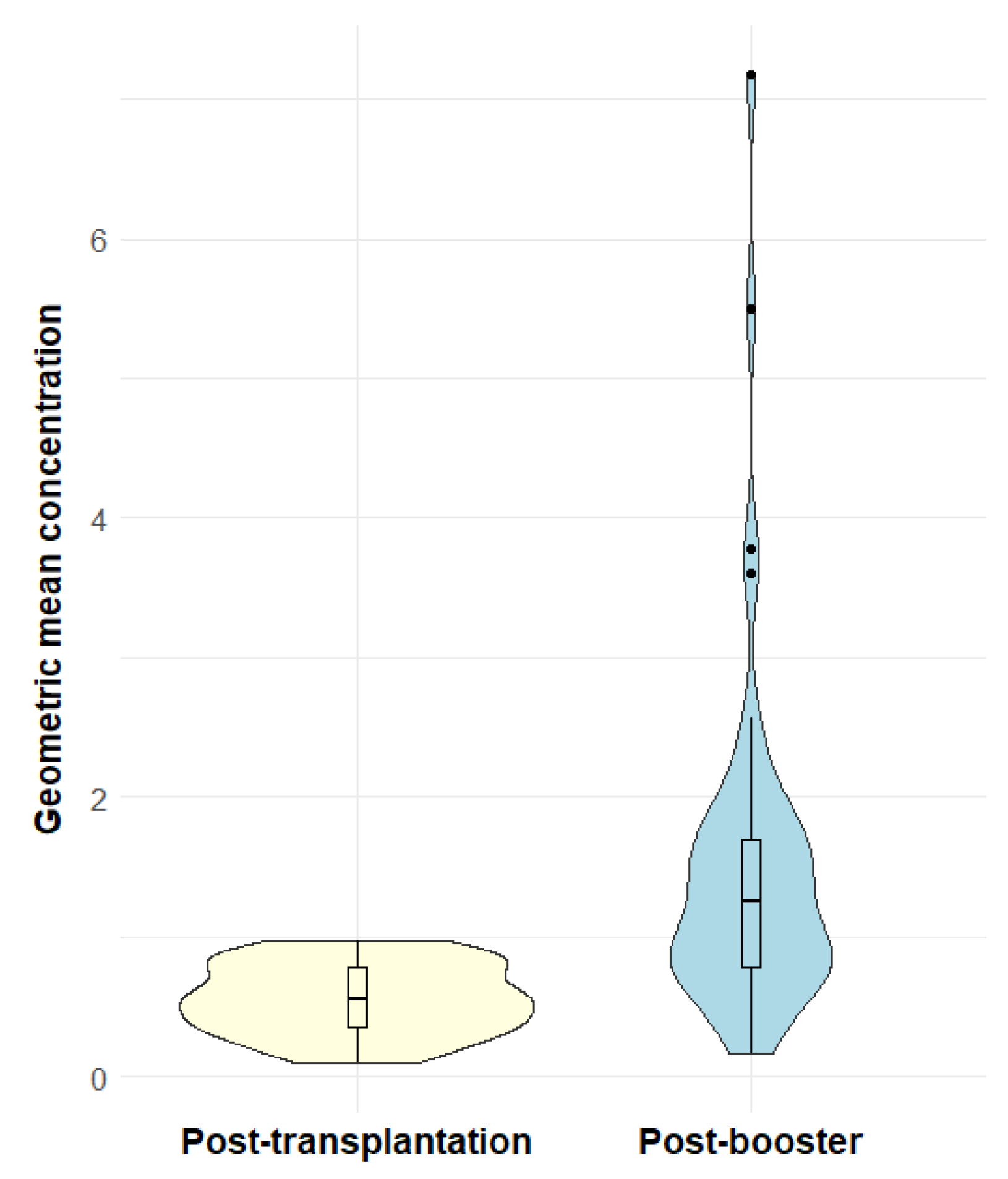
3.2. Invasive Pneumococcal Disease
3.3. Hepatitis B
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid Organ Transplantation in the 21st Century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef]
- Pagalilauan, G.L.; Limaye, A.P. Infections in Transplant Patients. Med. Clin. N. Am. 2013, 97, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Humar, A.; Plevneshi, A.; Green, K.; Prasad, G.V.R.; Siegal, D.; McGeer, A. Invasive Pneumococcal Disease in Solid Organ Transplant Recipients—10-Year Prospective Population Surveillance. Am. J. Transplant. 2007, 7, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Dendle, C.; Stuart, R.L.; Mulley, W.R.; Holdsworth, S.R. Pneumococcal Vaccination in Adult Solid Organ Transplant Recipients: A Review of Current Evidence. Vaccine 2018, 36, 6253–6261. [Google Scholar] [CrossRef] [PubMed]
- Skalski, M.; Patkowski, W.; Grąt, M.; Zieniewicz, K.; Wróblewski, T.; Hołówko, W.; Krawczyk, M. Utilization of Donors with Hepatitis B Core Antibodies in Liver Transplantation. Ann. Transpl. 2015, 20, 667–675. [Google Scholar] [CrossRef]
- Segovia, R.; Sánchez-Fueyo, A.; Rimola, A.; Grande, L.; Bruguera, M.; Costa, J.; Soguero, C.; Uriz, J. Evidence of Serious Graft Damage Induced by de Novo Hepatitis B Virus Infection after Liver Transplantation. Liver Transplant. 2001, 7, 106–112. [Google Scholar] [CrossRef]
- Walti, L.N.; Mugglin, C.; Mombelli, M.; Manuel, O.; Hirsch, H.H.; Khanna, N.; Mueller, N.J.; Berger, C.; Boggian, K.; Garzoni, C.; et al. Vaccine-Preventable Infections Among Solid Organ Transplant Recipients in Switzerland. JAMA Netw. Open 2023, 6, e2310687. [Google Scholar] [CrossRef]
- Moberley, S.; Holden, J.; Tatham, D.P.; Andrews, R.M. Vaccines for Preventing Pneumococcal Infection in Adults. Cochrane Database Syst. Rev. 2013, 2013, CD000422. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Martins, R.M.; Bensabath, G.; Arraes, L.C.; Oliveira, M.d.L.A.; Miguel, J.C.; Barbosa, G.G.; Camacho, L.A.B. Multicenter Study on the Immunogenicity and Safety of Two Recombinant Vaccines against Hepatitis B. Mem. Inst. Oswaldo Cruz. 2004, 99, 865–871. [Google Scholar] [CrossRef][Green Version]
- Bakker, M.; Bunge, E.M.; Marano, C.; de Ridder, M.; De Moerlooze, L. Immunogenicity, Effectiveness and Safety of Combined Hepatitis A and B Vaccine: A Systematic Literature Review. Expert Rev. Vaccines 2016, 15, 829–851. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Larsen, C.S.; Ekenberg, C.; Carstens, J.; Rezahosseini, O.; Poulsen, S.D.; Jensen-Fangel, S.; Harboe, Z.B. Guidelines for Vaccination Af Voksne i Forbindelse Med Organtransplantation. Available online: https://organtransplantation.dk/wp-content/uploads/2023/12/Guidelines-for-vaccination-af-voksne-kandidater-og-recipienter-til-solid-organtransplantation_maj2022.pdf (accessed on 15 December 2025).
- Eckerle, I.; Rosenberger, K.D.; Zwahlen, M.; Junghanss, T. Serologic Vaccination Response after Solid Organ Transplantation: A Systematic Review. PLoS ONE 2013, 8, e56974. [Google Scholar] [CrossRef] [PubMed]
- See, K.C. Vaccination for the Prevention of Infection among Immunocompromised Patients: A Concise Review of Recent Systematic Reviews. Vaccines 2022, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Smallwood, G.; Halcomb, J.; Fried, M.W.; Boyer, T.D. Is Vaccination against Hepatitis B Infection Indicated in Patients Waiting for or after Orthotopic Liver Transplantation? Liver Transplant. Surg. 1998, 4, 128–132. [Google Scholar] [CrossRef]
- Kumar, D.; Rotstein, C.; Miyata, G.; Arlen, D.; Humar, A. Randomized, Double-Blind, Controlled Trial of Pneumococcal Vaccination in Renal Transplant Recipients. J. Infect. Dis. 2003, 187, 1639–1645. [Google Scholar] [CrossRef]
- Hamm, S.R.; Møller, D.L.; Pérez-Alós, L.; Hansen, C.B.; Pries-Heje, M.M.; Heftdal, L.D.; Hasselbalch, R.B.; Fogh, K.; Madsen, J.R.; Almagro Armenteros, J.J.; et al. Decline in Antibody Concentration 6 Months After Two Doses of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients and Healthy Controls. Front. Immunol. 2022, 13, 832501. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D. Vaccination of Solid Organ Transplant Candidates and Recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Hornung, L.B.; Hamm, S.R.; Hald, A.; Harboe, Z.B.; Lundbo, L.F.; Wareham, N.E.; Heftdal, L.D.; Ekenberg, C.; Bjerrum, S.; Holler, J.G.; et al. Post-Transplantation Seroprotection Rates in Liver, Lung, and Heart Transplant Recipients Vaccinated Pre-Transplantation against Hepatitis B Virus and Invasive Pneumococcal Disease. Vaccines 2024, 12, 1092. [Google Scholar] [CrossRef]
- Sticchi, L.; Iavarone, I.G.; Durando, P.; Di Biagio, A.; Schiavetti, I.; Murgia, F.; Icardi, G. The Role of Hepatitis B Vaccine Challenge Dose in Patients with Underlying Health Conditions. Hum. Vaccines Immunother. 2021, 17, 575–579. [Google Scholar] [CrossRef]
- Chiu, C.; Sampathkumar, P.; Brumble, L.M.; Vikram, H.R.; Watt, K.D.; Beam, E. Posttransplant HBV Vaccine Compliance, Seroprotection, and Kinetics of Hepatitis B Surface Antibody in Thoracic Organ Transplant Recipients. Transpl. Infect. Dis. 2025, 27, e70015. [Google Scholar] [CrossRef]
- Lindemann, M.; Zaslavskaya, M.; Fiedler, M.; Wilde, B.; Heinemann, F.M.; Heinold, A.; Horn, P.A.; Witzke, O. Humoral and Cellular Responses to a Single Dose of Fendrix in Renal Transplant Recipients with Non-response to Previous Hepatitis B Vaccination. Scand. J. Immunol. 2017, 85, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Harboe, Z.B.; Hald, A.; Ekenberg, C.; Ete Wareham, N.; Fogt Lundbo, L.; Holler, J.G.; Qvist, T.; Rask Hamm, S.; Bjerrum, S.; Rezahosseini, O.; et al. Implementation of a Vaccination Clinic for Adult Solid Organ Transplant Candidates: A Single-Center Experience. Vaccine 2023, 41, 6637–6644. [Google Scholar] [CrossRef] [PubMed]
- Grove Krause, T.; Jakobsen, S.; Haarh, M.; Mølbak, K. The Danish Vaccination Register. Eurosurveillance 2012, 17, 20155. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-Specific Effectiveness and Correlates of Protection for the 13-Valent Pneumococcal Conjugate Vaccine: A Postlicensure Indirect Cohort Study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- World Health Organization. Correlates of Vaccine-Induced Protection: Methods and Implications; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Lal, G.; Balmer, P.; Stanford, E.; Martin, S.; Warrington, R.; Borrow, R. Development and Validation of a Nonaplex Assay for the Simultaneous Quantitation of Antibodies to Nine Streptococcus Pneumoniae Serotypes. J. Immunol. Methods 2005, 296, 135–147. [Google Scholar] [CrossRef]
- Cruse, J.M.; Lewis, R.E.; Wang, H.; Schreuder, G.M.T.; Marsh, S.G.E.; Kennedy, L.J. Antigens, Immunogens, Vaccines, and Immunization. In Immunology Guidebook; Elsevier: Amsterdam, The Netherlands, 2004; pp. 17–45. [Google Scholar]
- Tönshoff, B. Immunosuppressants in Organ Transplantation. Handb. Exp. Pharmacol. 2019, 261, 441–469. [Google Scholar]
- Fakhrmousavi, S.A.A.; Hadadi, A.; Hosseini, S.H.; Rahbar, M.; Hamidian, R.; Ramezani, A.; Pourmand, G.; Razeghi, E. Immunogenicity of Four Doses of Double-Strength Intramuscular Hepatitis B. Iran J. Pathol. 2016, 11, 127–132. [Google Scholar]
- Simons, B.C.; Spradling, P.R.; Bruden, D.J.T.; Zanis, C.; Case, S.; Choromanski, T.L.; Apodaca, M.; Brogdon, H.D.; Dwyer, G.; Snowball, M.; et al. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-Lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J. Infect. Dis. 2016, 214, 273–280. [Google Scholar] [CrossRef]
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef]
- Friedrich, P.; Sattler, A.; Müller, K.; Nienen, M.; Reinke, P.; Babel, N. Comparing Humoral and Cellular Immune Response Against HBV Vaccine in Kidney Transplant Patients. Am. J. Transpl. 2015, 15, 3157–3165. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Xie, S.; Gong, M.; Yan, R.; Zheng, A.; Xu, Y.; Wu, H.; Wang, Z. The Dynamics and Association of B and T Cell Receptor Repertoires upon Antibody Response to Hepatitis B Vaccination in Healthy Adults. Hum. Vaccines Immunother. 2021, 17, 3203–3213. [Google Scholar] [CrossRef]
- Tsuda, K.; Yamanaka, K.; Kitagawa, H.; Akeda, T.; Naka, M.; Niwa, K.; Nakanishi, T.; Kakeda, M.; Gabazza, E.C.; Mizutani, H. Calcineurin Inhibitors Suppress Cytokine Production from Memory T Cells and Differentiation of Naïve T Cells into Cytokine-Producing Mature T Cells. PLoS ONE 2012, 7, e31465. [Google Scholar] [CrossRef]
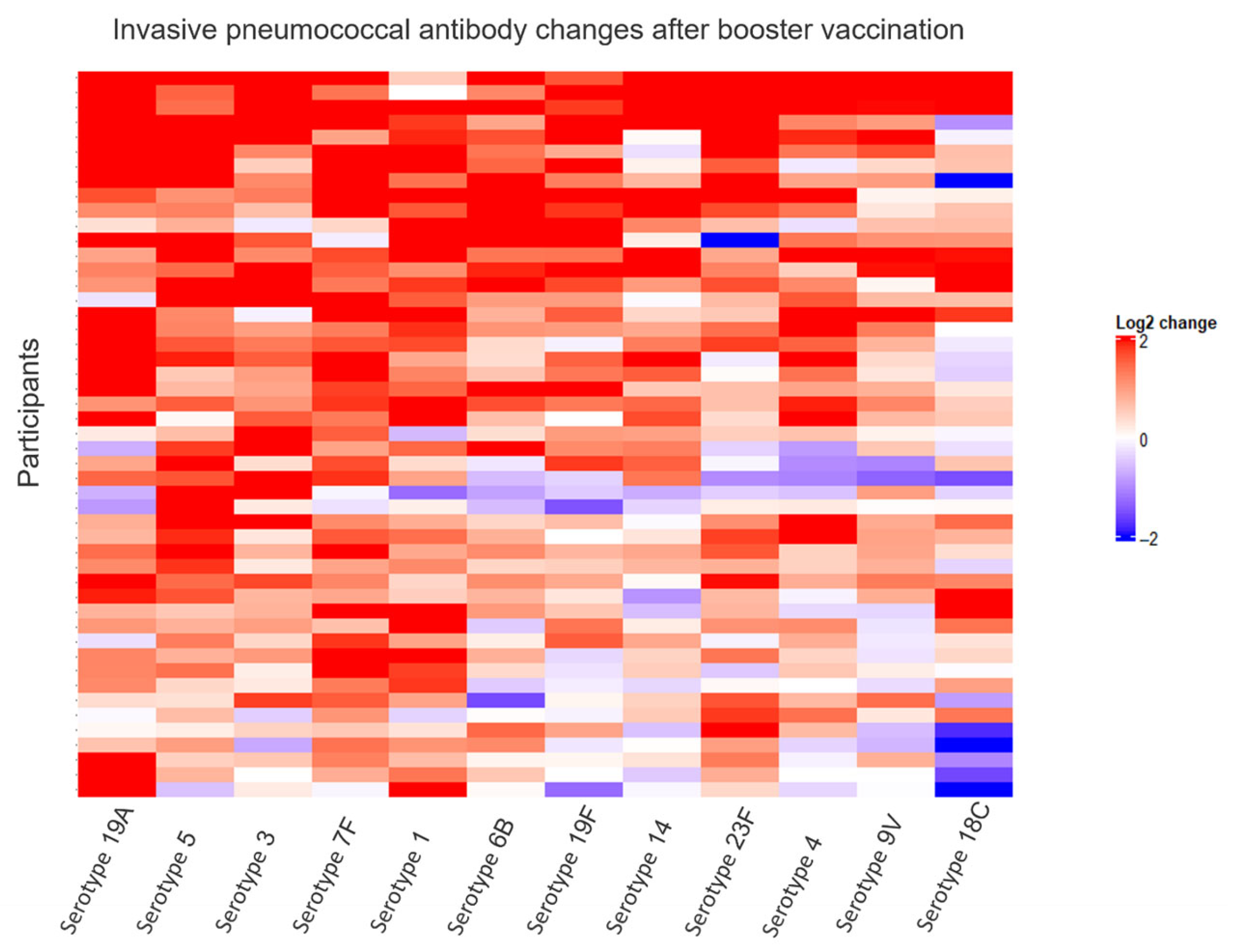
| Invasive Pneumococcal Disease Subset (n = 50) | Hepatitis B Subset (n = 52) | |
|---|---|---|
| General | ||
| Age (years), median (IQR) | 55 (47–61) | 59 (46–63) |
| Male sex, n (%) | 31 (61%) | 26 (50%) |
| Organ transplant type, n (%) | ||
| Liver | 29 (58%) | 30 (58%) |
| Lung | 10 (20%) | 15 (29%) |
| Heart | 6 (12%) | 3 (5.8%) |
| Kidney | 3 (6.0%) | 1 (1.9%) |
| Dual organ transplantation | 2 (4.0%) | 3 (5.8%) |
| Re-transplantation, n (%) | 3 (6.0%) | 5 (9.6%) |
| Vaccination Information | ||
| Vaccination completed after transplantation, n (%) | 14 (28%) | 34 (65%) |
| Seroprotection at time of pre-transplantation vaccination, n (%) | 9 (18%) | 1 (1.9%) |
| Time from transplantation to booster (years), median (IQR) | 2.24 (1.31–2.81) | 1.59 (1.12–2.29) |
| Time between vaccination completion to booster (years), median (IQR) | 2.58 (2.01–3.59) | 1.36 (1.04–2.06) |
| Time from booster to post-booster serology (months), median (IQR) | 4.92 (4.58–7.87) | 5.21 (4.29–7.31) |
| Comorbidity | ||
| Diabetes, n (%) | 11 (22%) | 7 (13%) |
| Non-BCC cancer, n (%) | 3 (6.0%) | 5 (9.6%) |
| Dialysis, n (%) | 0 (0%) | 0 (0%) |
| Immunosuppressive Therapy | ||
| Corticosteroids, n (%) | 35 (70%) | 38 (73%) |
| Calcineurin inhibitors, n (%) | ||
| Tacrolimus | 43 (86%) | 42 (81%) |
| Ciclosporin | 6 (12%) | 9 (17%) |
| None | 1 (2.0%) | 7 (13%) |
| Antimetabolites, n (%) | ||
| MMF | 43 (86%) | 42 (81%) |
| Azathioprine | 2 (4.0%) | 3 (5.8%) |
| None | 5 (10%) | 7 (13%) |
| mTOR inhibitors, n (%) | 4 (8.0%) | 4 (7.7%) |
| Clinical Characteristics at Time of Pneumococcal Booster Vaccine | Seroprotected (n = 26) | Not Seroprotected (n = 24) | p |
|---|---|---|---|
| General | |||
| Age (years), median (IQR) | 54 (50–59) | 58 (47–64) | 0.482 |
| Male sex, n (%) | 19 (73%) | 12 (50%) | 0.165 |
| Organ transplant type, n (%) | 0.829 | ||
| Liver | 16 (62%) | 13 (54%) | |
| Lung | 6 (23%) | 4 (17%) | |
| Heart | 3 (12%) | 3 (13%) | |
| Kidney | 1 (3.8%) | 2 (8.3%) | |
| Dual organ transplantation | 0 (0%) | 2 (8.3%) | |
| Re-transplantation, n (%) | 2 (7.7%) | 1 (4.2%) | >0.99 |
| Vaccination Information | |||
| Vaccination completed after transplantation, n (%) | 7 (27%) | 7 (29%) | >0.99 |
| Seroprotection at time of pre-transplantation vaccination, n (%) | 8 (31%) | 1 (4.2%) | 0.024 |
| Time from transplantation to booster (years), median (IQR) | 2.21 (1.51–2.96) | 2.27 (1.29–2.67) | 0.763 |
| Time between vaccination completion to booster (years), median (IQR) | 2.58 (1.93–3.68) | 2.72 (2.35–3.44) | 0.962 |
| Time from booster to post-booster serology (months), median (IQR) | 5.04 (4.60–8.19) | 4.83 (4.50–7.15) | 0.442 |
| Comorbidity | |||
| Diabetes, n (%) | 5 (19%) | 6 (25%) | 0.738 |
| Non-BCC cancer, n (%) | 1 (3.8%) | 2 (8.3%) | 0.602 |
| Dialysis, n (%) | 0 (0%) | 0 (0%) | >0.99 |
| Immunosuppressive Therapy | |||
| Corticosteroids, n (%) | 17 (65%) | 18 (75%) | 0.545 |
| Calcineurin inhibitors, n (%) | 0.827 | ||
| Tacrolimus | 23 (88%) | 20 (83%) | |
| Ciclosporin | 3 (12%) | 3 (13%) | |
| None | 0 (0%) | 1 (4.2%) | |
| Antimetabolites, n (%) | 0.409 | ||
| MMF | 23 (88%) | 20 (83%) | |
| Azathioprine | 0 (0%) | 2 (8.3%) | |
| None | 3 (12%) | 2 (8.3%) | |
| mTOR inhibitors, n (%) | 2 (7.7%) | 2 (8.3%) | >0.99 |
| Unadjusted Model | Adjusted Model * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | |
| Age (per year of age) | 1.01 | 0.96; 1.06 | 0.690 | |||
| Vaccine series completed post-transplantation | 1.12 | 0.32; 3.91 | 0.860 | |||
| Male sex | 0.37 | 0.11; 1.17 | 0.097 | 0.36 | 0.11; 1.16 | 0.095 |
| Seroprotection at time of pre-transplantation vaccination | 0.10 | 0.01; 0.60 | 0.036 | 0.07 | 0.003; 0.49 | 0.022 |
| Time between transplantation and booster (per year) | 0.96 | 0.53; 1.75 | 0.898 | 0.92 | 0.46; 1.83 | 0.818 |
| Time between booster and blood sample (per month) | 0.97 | 0.89; 1.04 | 0.431 | 0.97 | 0.88; 1.04 | 0.433 |
| Diabetes | 1.40 | 0.36; 5.61 | 0.624 | 1.32 | 0.33; 5.53 | 0.692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejerøe-Olsen, J.; Suarez-Zdunek, M.A.; Helbo, T.; Hornung, L.B.; Jørgensen, C.S.; Rossing, K.; Perch, M.; Rasmussen, A.; Hamm, S.R.; Nielsen, S.D. Booster Vaccination Against Invasive Pneumococcal Disease and Hepatitis B in Previously Vaccinated Solid Organ Transplant Recipients Without Seroprotection. Vaccines 2025, 13, 1253. https://doi.org/10.3390/vaccines13121253
Sejerøe-Olsen J, Suarez-Zdunek MA, Helbo T, Hornung LB, Jørgensen CS, Rossing K, Perch M, Rasmussen A, Hamm SR, Nielsen SD. Booster Vaccination Against Invasive Pneumococcal Disease and Hepatitis B in Previously Vaccinated Solid Organ Transplant Recipients Without Seroprotection. Vaccines. 2025; 13(12):1253. https://doi.org/10.3390/vaccines13121253
Chicago/Turabian StyleSejerøe-Olsen, Julie, Moises Alberto Suarez-Zdunek, Thomas Helbo, Lise Bank Hornung, Charlotte Sværke Jørgensen, Kasper Rossing, Michael Perch, Allan Rasmussen, Sebastian Rask Hamm, and Susanne Dam Nielsen. 2025. "Booster Vaccination Against Invasive Pneumococcal Disease and Hepatitis B in Previously Vaccinated Solid Organ Transplant Recipients Without Seroprotection" Vaccines 13, no. 12: 1253. https://doi.org/10.3390/vaccines13121253
APA StyleSejerøe-Olsen, J., Suarez-Zdunek, M. A., Helbo, T., Hornung, L. B., Jørgensen, C. S., Rossing, K., Perch, M., Rasmussen, A., Hamm, S. R., & Nielsen, S. D. (2025). Booster Vaccination Against Invasive Pneumococcal Disease and Hepatitis B in Previously Vaccinated Solid Organ Transplant Recipients Without Seroprotection. Vaccines, 13(12), 1253. https://doi.org/10.3390/vaccines13121253






