Vaccinations Included in the National Immunization Calendar as a Tool to Tackle Antimicrobial Resistance: Current Evidence for Selected Pathogens in Italy
Abstract
1. Introduction
1.1. The General Frame of Antimicrobial Resistance
1.2. The Italian Landscape
1.3. The Role of Vaccines in Combating AMR
2. Methods
Search Strategy
3. Results
3.1. Influenza Virus
3.2. Pneumococcus
3.3. Varicella
3.4. Respiratory Syncytial Virus (RSV)
3.5. Rotavirus Vaccine
3.6. Meningococcus B (MenB)
3.7. Meningococcus ACWY (MenACWY)
3.8. Pertussis
3.9. Measles
3.10. Herpes Zoster
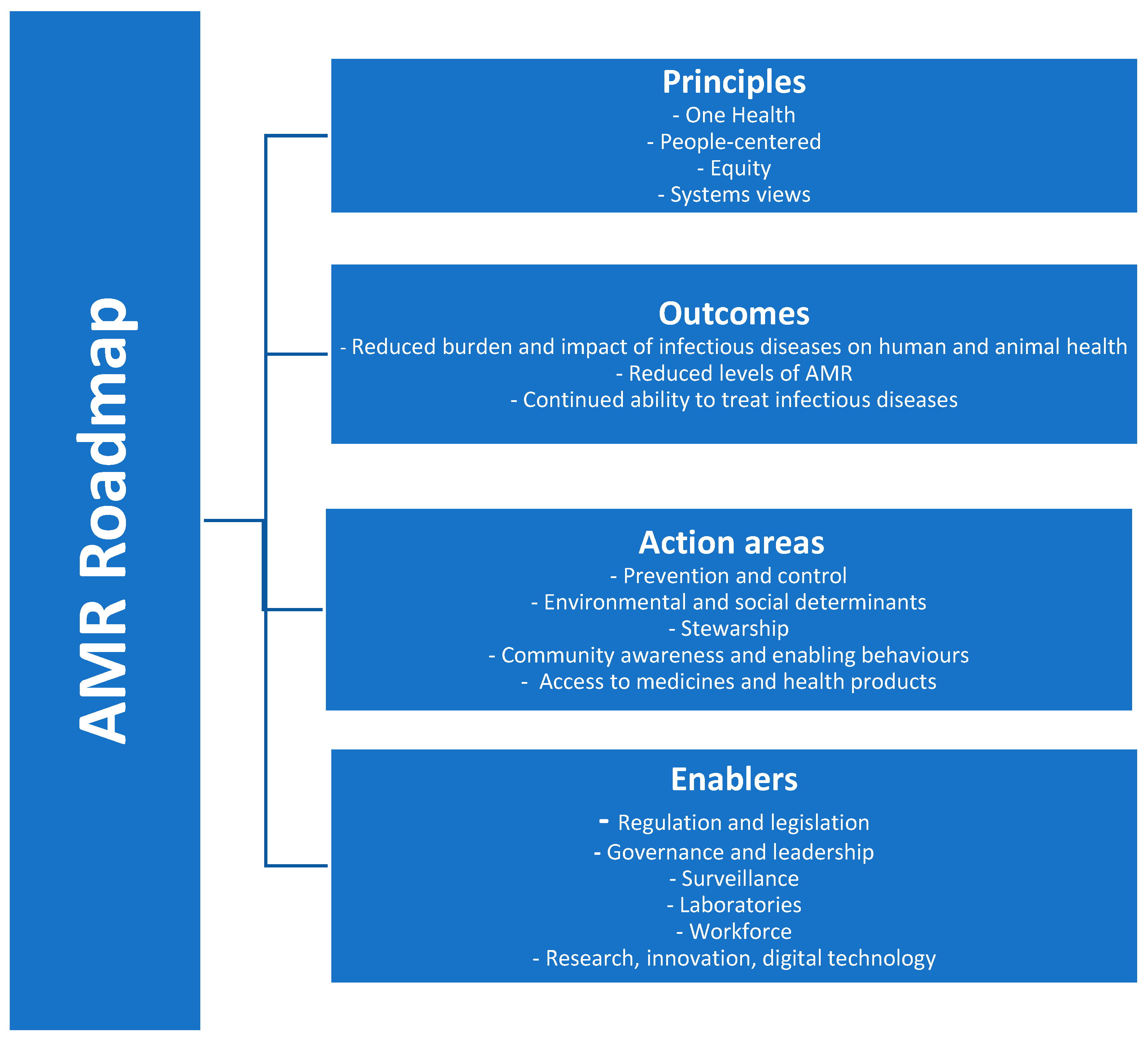
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Antimicrobial Resistance. Key Facts. 21 November 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 25 September 2025).
- Vitiello, A.; Sabbatucci, M.; Boccellino, M.; Ponzo, A.; Langella, R.; Zovi, A. Therapeutic and Unconventional Strategies to Contrast Antimicrobial Resistance: A Literature Review. Discov. Med. 2023, 35, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2 of 2): Final Report; HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.; World Bank Group: Washington, DC, USA, 2017; Available online: http://documents.worldbank.org/curated/en/323311493396993758 (accessed on 25 September 2025).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Research Agenda for Antimicrobial Resistance in Human Health. Policy Brief. June 2023. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/who-global-research-agenda-for-amr-in-human-health---policy-brief.pdf?sfvrsn=f86aa073_4&download=true (accessed on 25 September 2025).
- ECDC 2024: European Antibiotic Awareness Day 2024: Key messages. Available online: https://antibiotic.ecdc.europa.eu/en/european-antibiotic-awareness-day-2024-key-messages (accessed on 25 September 2025).
- WHO Antibiotics Most Responsible for Drug Resistance Are Overused—WHO Report. 29 April 2025. Available online: https://www.who.int/news/item/29-04-2025-antibiotics-most-responsible-for-drug-resistance-are-overused---who-report (accessed on 25 September 2025).
- WHO. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 25 September 2025).
- WHO Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 25 September 2025).
- Vekemans, J.; Hasso-Agopsowicz, M.; Kang, G.; Hausdorff, W.P.; Fiore, A.; Tayler, E.; Klemm, E.J.; Laxminarayan, R.; Srikantiah, P.; Friede, M.; et al. Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: A World Health Organization Action Framework. Clin. Infect. Dis. 2021, 73, e1011–e1017. [Google Scholar] [CrossRef] [PubMed]
- WHO. People-Centered Approach to Addressing Antimicrobial Resistance in Human Health: WHO Core Package of Interventions to Support National Action Plans; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-008249-6. [Google Scholar]
- FAO; UNEP; WHO; WOAH. One Health Joint Plan of Action (2022–2026). Working Together for the Health of Humans, Animals, Plants and the Environment. Rome. 2022. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/fc522db2-9619-4f70-b6ba-64177f4865e6/content (accessed on 25 September 2025).
- Sabbatucci, M.; Ashiru-Oredope, D.; Barbier, L.; Bohin, E.; Bou-Antoun, S.; Brown, C.; Clarici, A.; Fuentes, C.; Goto, T.; Maraglino, F.; et al. Tracking progress on antimicrobial resistance by the quadripartite country self-assessment survey (TrACSS) in G7 countries, 2017–2023: Opportunities and gaps. Pharmacol. Res. 2024, 204, 107188. [Google Scholar] [CrossRef]
- WHO European Region. Roadmap on Antimicrobial Resistance for the WHO European Region 2023–2030. Working Document. Available online: https://iris.who.int/bitstream/handle/10665/372503/73wd07e-AMR-Roadmap-230574.pdf?sequence=5 (accessed on 25 September 2025).
- AIFA Osservatorio Nazionale sull’impiego dei Medicinali. L’uso degli antibiotici in Italia. Rapporto Nazionale 2023; Agenzia Italiana del Farmaco: Roma, Italy, 2025.
- Iacchini, S.; Boros, S.; Pezzotti, P.; Errico, G.; Del Grosso, M.; Camilli, R.; Giufrè, M.; Pantosti, A.; Maraglino, F.; Palamara, A.T.; et al. AR-ISS: Sorveglianza nazionale dell’Antibiotico-Resistenza. Dati 2023, Rapporti ISS Sorveglianza RIS-5/2024; Istituto Superiore di Sanità: Roma, Italy, 2024. [Google Scholar]
- Ministero della Salute. Piano Nazionale di Contrasto all’Antibiotico-Resistenza (PNCAR) 2022–2025. 2023. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3294_allegato.pdf (accessed on 25 September 2025).
- Ministero della Salute. Intesa ai Sensi Dell’articolo, 8.; Comma, 6.; Della Legge 5 Giugno, 2.0.0.3.; n, 1.3.1.; trai l Governo le Regioni e le Province Autonome di Trento e di Bolzano sul Documento Recante <<Piano Nazionale di Prevenzione Vaccinale (PNPV) 2023–2025>> e sul Documento Recante <<Calendario Nazionale Vaccinale>> (Rep atti n 193/CSRdel 2 agosto 2023) (23A04685), (.G.U. Serie Generale, n. 194 del 21 Agosto 2023). Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=95963&completo=true (accessed on 25 September 2025).
- Task Force, A.M.R. Raccomandazioni per una strategia efficace contro la resistenza antimicrobica. Dalla prevenzione vaccinale allo sviluppo e utilizzo dei nuovi antibiotici. Rivista SIMG 2022, 29, 22–25. Available online: https://www.pacinimedicina.it/wp-content/uploads/06_Raccomandazioni.pdf (accessed on 25 September 2025).
- Costanzo, V.; Roviello, G.N. The Potential Role of Vaccines in Preventing Antimicrobial Resistance (AMR): An Update and Future Perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef]
- Iwu-Jaja, C.; Gahimbare, L.; Mazingisa, A.V.; Fuller, W.; Mazengiya, D.Y.; Okeibunor, J.; Olu, O.O.; Katoto, P.M.C.; Yahaya, A.A.; Nyarko, K.; et al. Mapping the role of vaccines in combating AMR in the WHO African region: A scoping review and implications for research and policy. BMC Infect. Dis. 2025, 25, 702. [Google Scholar] [CrossRef]
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2023, 4, e113–e125. [Google Scholar] [CrossRef]
- WHO. Estimating the Impact of Vaccines in Reducing Antimicrobial Resistance and Antibiotic Use: Technical Report; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/379116/9789240098787-eng.pdf?sequence=1 (accessed on 25 September 2025).
- Brazzoli, M.; Piccioli, D.; Marchetti, F. Challenges in development of vaccines directed toward antimicrobial resistant bacterial species. Hum. Vaccin. Immunother. 2023, 19, 2228669. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Impalli, I.; Rangarajan, R.; Cohn, J.; Ramjeet, K.; Trainor, B.W.; Strathdee, S.; Sumpradit, N.; Berman, D.; Wertheim, H.; et al. Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. Lancet 2024, 403, 2534–2550. [Google Scholar] [CrossRef]
- Yemeke, T.; Chen, H.H.; Ozawa, S. Economic and cost-effectiveness aspects of vaccines in combating antibiotic resistance. Hum. Vaccin. Immunother. 2023, 19, 2215149. [Google Scholar] [CrossRef]
- Calendario Vaccinale per la Vita, 5 Edizione 2025. Available online: https://www.igienistionline.it/docs/2024/19cdv.pdf (accessed on 25 September 2025).
- Tessmer, A.; Welte, T.; Schmidt-Ott, R.; Eberle, S.; Barten, G.; Suttorp, N.; Schaberg, T. CAPNETZStudy Group Influenza vaccination is associated with reduced severity of community-acquired pneumonia. Eur. Respir. J. 2011, 38, 147–153. [Google Scholar] [CrossRef]
- Loeb, M.; Russell, M.L.; Moss, L.; Fonseca, K.; Fox, J.; Earn, D.J.D.; Aoki, F.; Horsman, G.; Van Caeseele, P.; Chokani, K.; et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: A randomized trial. JAMA 2010, 303, 943–950. [Google Scholar] [CrossRef]
- Wang, B.; Russell, M.L.; Moss, L.; Fonseca, K.; Earn, D.J.D.; Aoki, F.; Horsman, G.; Van Caeseele, P.; Chokani, K.; Vooght, M.; et al. Effect of Influenza Vaccination of Children on Infection Rate in Hutterite Communities: Follow-Up Study of a Randomized Trial. PLoS ONE 2016, 11, e0167281. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Vinci, R.; Agodi, A. The Inverse Relationship between Influenza Vaccination and Antimicrobial Resistance: An Ecological Analysis of Italian Data. Vaccines 2022, 10, 554. [Google Scholar] [CrossRef]
- Buckley, B.S.; Henschke, N.; Bergman, N.; Skidmore, B.; Klemm, E.J.; Villanueva, G.; Garritty, C.; Paul, M. Impact of vaccination on antibiotic usage: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- van Heuvel, L.; Paget, J.; Dückers, M.; Caini, S. The impact of influenza and pneumococcal vaccination on antibiotic use: An updated systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Sikuler-Cohen, M.; Zamir, O.; Janco, J.; Givon-Lavi, N.; Fraser, D. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr. Infect. Dis. J. 2001, 20, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.H.; Lynfield, R.; Schaffner, W.; Craig, A.S.; Hadler, J.; Reingold, A.; Thomas, A.R.; Harrison, L.H.; Bennett, N.M.; Farley, M.M.; et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 2006, 354, 1455–1463. [Google Scholar] [CrossRef]
- Kim, C.; Holm, M.; Frost, I.; Hasso-Agopsowicz, M.; Abbas, K. Global and regional burden of attributable and associated bacterial antimicrobial resistance avertable by vaccination: Modelling study. BMJ Glob. Health 2023, 8, e011341. [Google Scholar] [CrossRef]
- Bernal, J.L.; Hobbelen, P.; Amirthalingam, G. Burden of varicella complications in secondary care, England, 2004 to 2017. Eurosurveillance 2019, 24, 1900233. [Google Scholar] [CrossRef]
- Vandenhaute, J.; Tsakeu, E.; Chevalier, P.; Pawaskar, M.; Benčina, G.; Vertriest, J. Assessing the use of antibiotics and the burden of varicella in Belgium using a retrospective GP database analysis. BMC Infect. Dis. 2021, 21, 1150. [Google Scholar] [CrossRef]
- Pawaskar, M.; Fergie, J.; Harley, C.; Samant, S.; Veeranki, P.; Diaz, O.; Conway, J.H. Impact of universal varicella vaccination on the use and cost of antibiotics and antivirals for varicella management in the United States. PLoS ONE 2015, 17, e0269916. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Cocchio, S.; Furlan, P.; Scamarcia, P.; Cantarutti, L.; Giaquinto, C.; Baldo, V. A population database analysis to describe the residual burden of varicella in Italy-a high vaccination coverage area-from 2004 to 2022. Front. Public Health 2025, 3, 1412620. [Google Scholar] [CrossRef]
- Feikin, D.; Karron, R.A.; Saha, S.K.; Sparrow, E.; Srikantiah, P.; Weinberger, D.M.; Zar, H.J. The full value of immunisation against respiratory syncytial virus for infants younger than 1 year: Effects beyond prevention of acute respiratory illness. Rev. Lancet Infect. Dis. 2024, 24, e318–e327. [Google Scholar] [CrossRef]
- Miller, L.; Beaney, T.; Hope, R.; Cunningham, M.; Robotham, J.V.; Pouwels, K.B.; Costelloe, C.E. General practice antibiotic prescriptions attributable to respiratory syncytial virus by age and antibiotic class: An ecological analysis of the English population. J. Antimicrob. Chemother. 2025, 80, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Puggina, A.; Dovizio, M.; Domnich, A.; Marijam, A.; Veronesi, C.; Rizzo, C.; Vicentini, M.; Degli Esposti, L.; Calabrò, G.E.; Fonseca, M.J. Healthcare Resource Utilization and Economic Outcomes of RSV-Hospitalized Patients Aged ≥ 60 Years: A Retrospective Cohort Study. Diseases 2025, 13, 68. [Google Scholar] [CrossRef]
- Puggina, A.; Fonseca, M.J.; Nugnes, M.; Domnich, A.; Veronesi, C.; Rizzo, C.; Vicentini, M.; Degli Esposti, L.; Calabrò, G.E.; Marchetti, F. Pattern of antibiotic prescriptions in adult patients hospitalized with respiratory syncytial virus in Italy. In Proceedings of the 58th National Congress of the Italian Society of Public Health and Preventive Medicine, Bologna, Italy, 22–25 October 2025. Abstract code SIT22612-93. [Google Scholar]
- Puggina, A.; Rumi, F.; Zarkadoulas, E.; Marijam, A.; Calabró, G.E. The Potential Public Health Impact of the Adjuvanted Respiratory Syncytial Virus Prefusion F Protein Vaccine Among Older Adults in Italy. Vaccines 2025, 13, 212. [Google Scholar] [CrossRef]
- Hall, E.; Tippett, A.; Fridkin, S.; Anderson, E.J.; Lopman, B.; Benkeser, D.; Baker, J.M. Association Between Rotavirus Vaccination and Antibiotic Prescribing Among Commercially Insured US Children, 2007–2018. Open Forum Infect. Dis. 2022, 9, ofac276. [Google Scholar] [CrossRef] [PubMed]
- Isonne, C.; Petrone, D.; Del Manso, M.; Iera, J.; Caramia, A.; Bandini, L.; Fadda, G.; Grossi, A.; Baccolini, V.; Costantino, C.; et al. The Impact of Rotavirus Vaccination on Discharges for Pediatric Gastroenteritis in Italy: An Eleven Year (2009–2019) Nationwide Analysis. Vaccines 2023, 11, 1037. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Andrews, N.; Parikh, S.R.; Campbell, H.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N. Engl. J. Med. 2020, 382, 309–317. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.; Shumate, A.M.; Johnsen, P.; MacNeil, J.R.; Patel, M.; Bhavsar, T.; Cohn, A.C.; Dinitz-Sklar, J.; Duffy, J.; Finnie, J.; et al. First Use of a Serogroup B Meningococcal Vaccine in the US in Response to a University Outbreak. Pediatrics 2015, 135, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Sorveglianza Nazionale Delle Malattie Batteriche Invasive. Dati 2021–2023; Rapporti ISS Sorveglianza RIS-2/2024; Istituto Superiore di Sanità: Rome, Italy, 2021. [Google Scholar]
- Lodi, L.; Barbati, F.; Amicizia, D.; Baldo, V.; Barbui, A.M.; Bondi, A.; Costantino, C.; Da Dalt, L.; Ferrara, L.; Fortunato, F.; et al. Four-Component Recombinant Protein-Based Vaccine Effectiveness Against Serogroup B Meningococcal Disease in Italy. JAMA Netw. Open. 2023, 6, e2329678. [Google Scholar] [CrossRef]
- Abara, W.E.; Kirkcaldy, R.D.; Bernstein, K.T.; Galloway, E.; Learner, E.R. Effectiveness of MenB-4C Vaccine Against Gonorrhea: A Systematic Review and Meta-analysis. J. Infect. Dis. 2025, 231, 61–70. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. A Guide to the Meningococcal B Vaccine for Protection Against Gonorrhoea. Guidance Updated 28 July 2025. Available online: https://www.gov.uk/government/publications/meningococcal-b-menb-vaccination-against-gonorrhoea-guide/a-guide-to-the-meningococcal-b-vaccine-for-protection-against-gonorrhoea (accessed on 25 September 2025).
- Istituto Superiore di Sanità. Notiziario ISS; Istituto Superiore di Sanità: Rome, Italy, 2025; Volume 38, Numero 7-8 Luglio-Agosto. [Google Scholar]
- European Centre for Disease Prevention and Control. Gonococcal Antimicrobial Susceptibility Surveillance in the European Union/European Economic Area, 2022; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- McMillan, M.; Chandrakumar, A.; Wang, H.L.R.; Clarke, M.; Sullivan, T.R.; Andrews, R.M.; Ramsay, M.; Marshall, H.S. Effectiveness of Meningococcal Vaccines at Reducing Invasive Meningococcal Disease and Pharyngeal Neisseria meningitidis Carriage: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2021, 73, e609–e619. [Google Scholar] [CrossRef]
- Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Impact of an adolescent meningococcal ACWY immunisation programme to control a national outbreak of group W meningococcal disease in England: A national surveillance and modelling study. Lancet Child Adolesc. Health 2022, 6, 96–105. [Google Scholar] [CrossRef]
- Pertussis Annual Epidemiological Report for 2022, E.-C.D.C. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/PERT_AER_2022_Report.pdf (accessed on 25 September 2025).
- World Health Organization. Pertussis Vaccines: WHO Position Paper—August 2015 Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2015; Volume 90, p. 35. [Google Scholar]
- Tozzi, A.; Croci, I.; Gesualdo, F.; Perno, C.F.; Linardos, G.; Villani, A.; Russo, L.; Campagna, I.; Ferro, D.; Pandolfi, E. Effect of Early Administration of Clarithromycin or Azithromycin on Symptoms of Pertussis in Infants. Antibiotics 2025, 14, 279. [Google Scholar] [CrossRef]
- Gabutti, G. Available evidence and potential for vaccines for reduction in antibiotic prescriptions. Hum. Vaccin. Immunother. 2022, 18, 2151291. [Google Scholar] [CrossRef]
- Bagordo, F.; Grassi, T.; Savio, M.; Rota, M.C.; Baldovin, T.; Vicentini, C.; Napolitano, F.; Trombetta, C.M.; Gabutti, G. Seroepidemiological Study Group Assessment of Pertussis Underreporting in Italy. J. Clin. Med. 2023, 12, 1732. [Google Scholar] [CrossRef]
- Morbillo e Rosolia News, Rapporto n.37, Gennaio 2018. Istituto Superiore di Sanità. 2018. Available online: www.epicentro.iss.it/morbillo/bollettino/RM_News_2018_37%20def.pdf (accessed on 25 September 2025).
- Filia, A.; Del Manso, M.; Petrone, D.; Magurano, F.; Gioacchini, S.; Pezzotti, P.; Palamara, A.T.; Bella, A. Surge in Measles Cases in Italy from August 2023 to January 2025: Characteristics of Cases and Public Health Relevance. Vaccines 2025, 13, 663. [Google Scholar] [CrossRef]
- Giannelos, N.; Curran, D.; Nguyen, C.; Kagia, C.; Vroom, N.; Vroling, N. The Incidence of Herpes Zoster Complications: A Systematic Literature Review. Infect. Dis. Ther. 2024, 13, 1461–1486. [Google Scholar] [CrossRef]
- Rapporto Epidemiologico RespiVirNet Rapporto, N. 25 del 5 Maggio 2025. Available online: https://respivirnet.iss.it/pagine/rapportoInflunet.aspx (accessed on 25 September 2025).
- Dougan, G.; Hugo-Webb, E. Impact of vaccines on antimicrobial resistance. J. Med. Microbiol. 2025, 74, 002050. [Google Scholar] [CrossRef]
- Smith, G.C.S.; Pell, J.P. Parachute use to prevent death and major trauma related to gravitational challenge: Systematic review of randomised controlled trials. BMJ 2003, 327, 1459–1461. [Google Scholar] [CrossRef]
- Yeh, R.W.; Valsdottir, L.R.; Yeh, M.W.; Shen, C.; Kramer, D.B.; Strom, J.B.; Secemsky, E.A.; Healy, J.L.; Domeier, R.M.; Kazi, D.S.; et al. Parachute use to prevent death and major trauma when jumping from aircraft: Randomized controlled trial. BMJ 2018, 363, k5094. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Cambieri, P.; Baldanti, F.; Andreoni, M.; Perrone, F.; Pedrazzol, P.; Silvestris, N. How Should We Manage the Impact of Antimicrobial Resistance in Patients With Cancer? An Oncological and Infectious Disease Specialist Point of View. JCO Oncol. Pract. 2025, 21, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Prato, R.; Viale, P. Survey among Italian experts on existing vaccines’ role in limiting antibiotic resistance. Hum. Vaccin. Immunother. 2021, 17, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
| Documented | Postulated |
|---|---|
| Pneumococcus | Pertussis |
| Influenza | Meningococcus B |
| Rotavirus | Meningococcus ACWY |
| Varicella | Measles |
| Respiratory Syncytial Virus | Herpes Zoster |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, G.C.; Castiglia, P.G.; Lombardi, A.; Marchetti, F.; Gabutti, G. Vaccinations Included in the National Immunization Calendar as a Tool to Tackle Antimicrobial Resistance: Current Evidence for Selected Pathogens in Italy. Vaccines 2025, 13, 1141. https://doi.org/10.3390/vaccines13111141
Marchetti GC, Castiglia PG, Lombardi A, Marchetti F, Gabutti G. Vaccinations Included in the National Immunization Calendar as a Tool to Tackle Antimicrobial Resistance: Current Evidence for Selected Pathogens in Italy. Vaccines. 2025; 13(11):1141. https://doi.org/10.3390/vaccines13111141
Chicago/Turabian StyleMarchetti, Giulia Carla, Paolo Giuseppino Castiglia, Andrea Lombardi, Federico Marchetti, and Giovanni Gabutti. 2025. "Vaccinations Included in the National Immunization Calendar as a Tool to Tackle Antimicrobial Resistance: Current Evidence for Selected Pathogens in Italy" Vaccines 13, no. 11: 1141. https://doi.org/10.3390/vaccines13111141
APA StyleMarchetti, G. C., Castiglia, P. G., Lombardi, A., Marchetti, F., & Gabutti, G. (2025). Vaccinations Included in the National Immunization Calendar as a Tool to Tackle Antimicrobial Resistance: Current Evidence for Selected Pathogens in Italy. Vaccines, 13(11), 1141. https://doi.org/10.3390/vaccines13111141








