The Central Importance of Vaccines to Mitigate the Threat of Antibiotic-Resistant Bacterial Pathogens
Abstract
1. Introduction
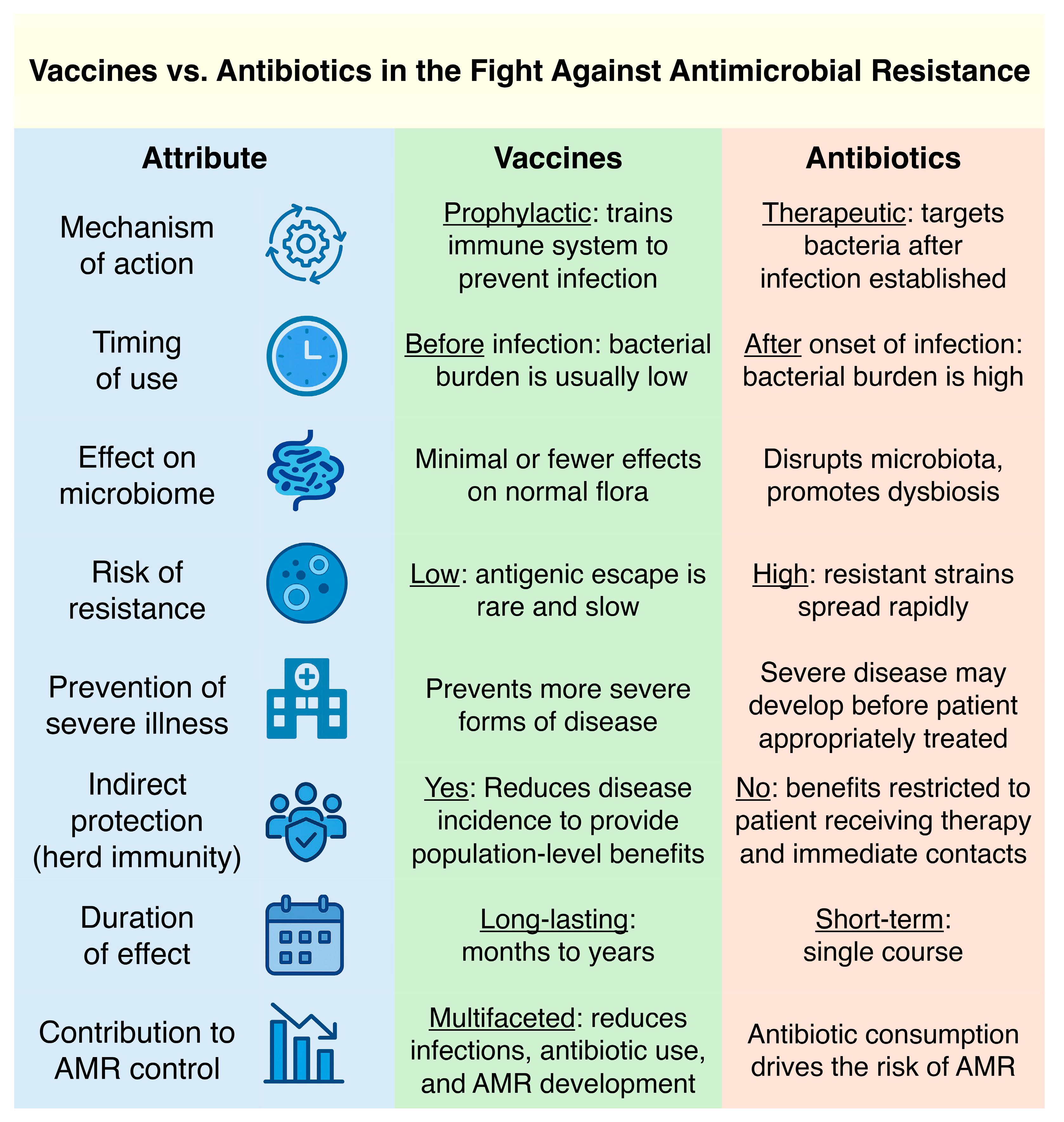
2. Advantages of Vaccines over Antibiotics in Confronting AMR
3. Lessons from Successful Vaccination Campaigns Against AMR
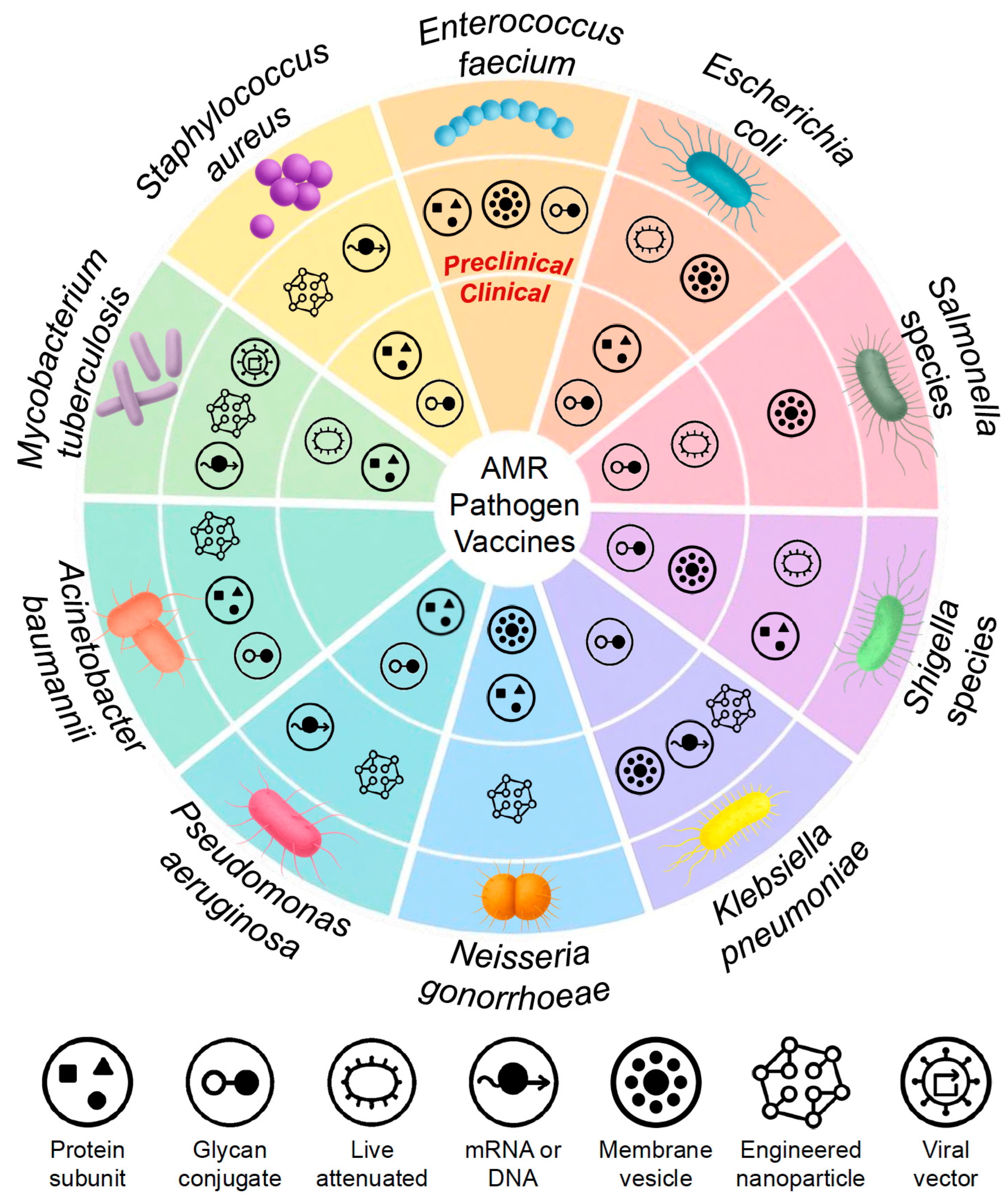
4. Pathogen-Specific Vaccine Strategies to Combat AMR
4.1. Mycobacterium tuberculosis
4.2. Staphylococcus aureus
4.3. Enterococcus spp.
4.4. Escherichia coli
4.5. Salmonella spp.
4.6. Shigella spp.
4.7. Klebsiella pneumoniae
4.8. Neisseria gonorrhoeae
4.9. Pseudomonas aeruginosa
4.10. Acinetobacter baumannii
4.11. Clostridiodes difficile
5. Addressing AMR Through Vaccines: Strategic Imperatives and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Buchy, P.; Ascioglu, S.; Buisson, Y.; Datta, S.; Nissen, M.; Tambyah, P.A.; Vong, S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2020, 90, 188–196. [Google Scholar] [CrossRef]
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines against antimicrobial resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A One Health perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. WHO’s top health threats for 2019. JAMA 2019, 321, 1041. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust & HM Government: London, UK, 2016.
- Zurawski, D.V.; McLendon, M.K. Monoclonal antibodies as an antibacterial approach against bacterial pathogens. Antibiotics 2020, 9, 155. [Google Scholar] [CrossRef]
- Motley, M.P.; Banerjee, K.; Fries, B.C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 2019, 32, 210–216. [Google Scholar] [CrossRef]
- Chan, B.K.; Stanley, G.L.; Kortright, K.E.; Vill, A.C.; Modak, M.; Ott, I.M.; Sun, Y.; Würstle, S.; Grun, C.N.; Kazmierczak, B.I.; et al. Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis. Nat. Med. 2025, 31, 1494–1501. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Barrios Steed, D.; Koundakjian, D.; Harris, A.D.; Rosato, A.E.; Konstantinidis, K.T.; Woodworth, M.H. Leveraging strain competition to address antimicrobial resistance with microbiota therapies. Gut Microbes 2025, 17, 2488046. [Google Scholar] [CrossRef]
- Abavisani, M.; Khoshrou, A.; Eshaghian, S.; Karav, S.; Sahebkar, A. Overcoming antibiotic resistance: The potential and pitfalls of drug repurposing. J. Drug Target. 2025, 33, 341–367. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Burnham, C.-A.D.; Leeds, J.; Nordmann, P.; O’Grady, J.; Patel, J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 2017, 15, 697–703. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [PubMed]
- Nazir, J.; Manzoor, T.; Saleem, A.; Gani, U.; Bhat, S.S.; Khan, S.; Haq, Z.; Jha, P.; Ahmad, S.M. Combatting Salmonella: A focus on antimicrobial resistance and the need for effective vaccination. BMC Infect. Dis. 2025, 25, 84. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of vaccines; health, economic and social perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine technologies and platforms for infectious diseases: Current progress, challenges, and opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Mullins, L.P.; Mason, E.; Winter, K.; Sadarangani, M. Vaccination is an integral strategy to combat antimicrobial resistance. PLoS Pathog. 2023, 19, e1011379. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Gruber, W.C.; Simon, R.; Wassil, J.; Anderson, A.S. The impact of human vaccines on bacterial antimicrobial resistance: A review. Environ. Chem. Lett. 2021, 19, 4031–4062. [Google Scholar] [CrossRef]
- Thänert, R.; Sawhney, S.S.; Schwartz, D.J.; Dantas, G. The resistance within: Antibiotic disruption of the gut microbiome and resistome dynamics in infancy. Cell Host Microbe 2022, 30, 675–683. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Boston, R.H.; Guan, R.; Kalmar, L.; Beier, S.; Horner, E.C.; Beristain-Covarrubias, N.; Yam-Puc, J.C.; Pereyra Gerber, P.; Faria, L.; Kuroshchenkova, A.; et al. Stability of gut microbiome after COVID-19 vaccination in healthy and immuno-compromised individuals. Life Sci. Alliance 2024, 7, e202302529. [Google Scholar] [CrossRef]
- Panwar, R.B.; Sequeira, R.P.; Clarke, T.B. Microbiota-mediated protection against antibiotic-resistant pathogens. Genes Immun. 2021, 22, 255–267. [Google Scholar] [CrossRef]
- La Guidara, C.; Adamo, R.; Sala, C.; Micoli, F. Vaccines and monoclonal antibodies as alternative strategies to antibiotics to fight antimicrobial resistance. Int. J. Mol. Sci. 2024, 25, 5487. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Read, A.F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. Biol. Sci. 2017, 284, 20162562. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Siber, G.R. How can vaccines contribute to solving the antimicrobial resistance problem? mBio 2016, 7, e00428-16. [Google Scholar] [CrossRef] [PubMed]
- Gilsdorf, J.R. Hib vaccines: Their impact on Haemophilus influenzae type b disease. J. Infect. Dis. 2021, 224, S321–S330. [Google Scholar] [CrossRef]
- Slack, M.P.E.; Cripps, A.W.; Grimwood, K.; Mackenzie, G.A.; Ulanova, M. Invasive Haemophilus influenzae infections after 3 decades of Hib protein conjugate vaccine use. Clin. Microbiol. Rev. 2021, 34, e00028-21. [Google Scholar] [CrossRef]
- Kyaw, M.H.; Lynfield, R.; Schaffner, W.; Craig, A.S.; Hadler, J.; Reingold, A.; Thomas, A.R.; Harrison, L.H.; Bennett, N.M.; Farley, M.M.; et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 2006, 354, 1455–1463. [Google Scholar] [CrossRef]
- Schroeder, M.R.; Chancey, S.T.; Thomas, S.; Kuo, W.-H.; Satola, S.W.; Farley, M.M.; Stephens, D.S. A population-based assessment of the impact of 7- and 13-valent pneumococcal conjugate vaccines on macrolide-resistant invasive pneumococcal disease: Emergence and decline of Streptococcus pneumoniae serotype 19A (CC320) with dual macrolide resistance mechanisms. Clin. Infect. Dis. 2017, 65, 990–998. [Google Scholar] [CrossRef]
- Rodgers, G.L.; Whitney, C.G.; Klugman, K.P. Triumph of pneumococcal conjugate vaccines: Overcoming a common foe. J. Infect. Dis. 2021, 224, S352–S359. [Google Scholar] [CrossRef] [PubMed]
- van Heuvel, L.; Paget, J.; Dückers, M.; Caini, S. The impact of influenza and pneumococcal vaccination on antibiotic use: An updated systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 70. [Google Scholar] [CrossRef]
- Kwong, J.C.; Maaten, S.; Upshur, R.E.G.; Patrick, D.M.; Marra, F. The effect of universal influenza immunization on antibiotic prescriptions: An ecological study. Clin. Infect. Dis. 2009, 49, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Schueller, E.; Tseng, K.K.; Morgan, D.J.; Laxminarayan, R.; Nandi, A. The impact of influenza vaccination on antibiotic use in the United States, 2010–2017. Open Forum Infect. Dis. 2020, 7, ofaa223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; ISBN 9789240083851. [Google Scholar]
- Bu, Q.; Qiang, R.; Fang, L.; Peng, X.; Zhang, H.; Cheng, H. Global trends in the incidence rates of MDR and XDR tuberculosis: Findings from the Global Burden of Disease Study 2019. Front. Pharmacol. 2023, 14, 1156249. [Google Scholar] [CrossRef]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial resistance in Mycobacterium tuberculosis: Mechanistic and evolutionary perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Ellner, J.J.; Hirsch, C.S.; Whalen, C.C. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin. Infect. Dis. 2000, 30 (Suppl. 3), S279–S282. [Google Scholar] [CrossRef]
- Nemes, E.; Fiore-Gartland, A.; Boggiano, C.; Coccia, M.; D’Souza, P.; Gilbert, P.; Ginsberg, A.; Hyrien, O.; Laddy, D.; Makar, K.; et al. The quest for vaccine-induced immune correlates of protection against tuberculosis. Vaccine Insights 2022, 1, 165–181. [Google Scholar] [CrossRef]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A functional role for antibodies in tuberculosis. Cell 2016, 167, 433–443.e14. [Google Scholar] [CrossRef]
- Young, D. Animal models of tuberculosis. Eur. J. Immunol. 2009, 39, 2011–2014. [Google Scholar] [CrossRef]
- Williams, A.; Orme, I.M. Animal models of tuberculosis: An overview. Microbiol. Spectr. 2016, 4, TBTB2-0004-2015. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef]
- Nuttall, J.J.C.; Eley, B.S. BCG vaccination in HIV-infected children. Tuberc. Res. Treat. 2011, 2011, 712736. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H.E. The recombinant Bacille Calmette-Guérin vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Cotton, M.F.; Madhi, S.A.; Luabeya, A.K.; Tameris, M.; Hesseling, A.C.; Shenje, J.; Schoeman, E.; Hatherill, M.; Desai, S.; Kapse, D.; et al. Safety and immunogenicity of VPM1002 versus BCG in South African newborn babies: A randomised, phase 2 non-inferiority double-blind controlled trial. Lancet Infect. Dis. 2022, 22, 1472–1483. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.; Gullick, J.; Clark, S.; Gleeson, F.; McIntyre, A.; Arlehamn, C.L.; Sette, A.; et al. MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies. NPJ Vaccines 2021, 6, 4. [Google Scholar] [CrossRef]
- Aguilo, N.; Uranga, S.; Marinova, D.; Monzon, M.; Badiola, J.; Martin, C. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis 2016, 96, 71–74. [Google Scholar] [CrossRef]
- Martín, C.; Marinova, D.; Aguiló, N.; Gonzalo-Asensio, J. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine 2021, 39, 7277–7285. [Google Scholar] [CrossRef]
- Scriba, T.J.; Netea, M.G.; Ginsberg, A.M. Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin. Immunol. 2020, 50, 101431. [Google Scholar] [CrossRef]
- Garcia-Basteiro, A.L.; White, R.G.; Tait, D.; Schmidt, A.C.; Rangaka, M.X.; Quaife, M.; Nemes, E.; Mogg, R.; Hill, P.C.; Harris, R.C.; et al. End-point definition and trial design to advance tuberculosis vaccine development. Eur. Respir. Rev. 2022, 31, 220044. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.W.; Choi, H.-G.; Kim, K.S.; Park, S.A.; Kim, H.-J.; Shin, S.J. BCG-booster vaccination with HSP90-ESAT-6-HspX-RipA multivalent subunit vaccine confers durable protection against hypervirulent Mtb in mice. NPJ Vaccines 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Li, X.; Wei, J.; Du, W.; Zhao, A.; Xu, M. RNA vaccines: The dawn of a new age for tuberculosis? Hum. Vaccines Immunother. 2025, 21, 2469333. [Google Scholar] [CrossRef] [PubMed]
- Szachniewicz, M.M.; van den Eeden, S.J.F.; van Meijgaarden, K.E.; Franken, K.L.M.C.; van Veen, S.; Geluk, A.; Bouwstra, J.A.; Ottenhoff, T.H.M. Intradermal versus subcutaneous immunization: Effects of administration route using a lipid-PLGA hybrid nanoparticle tuberculosis vaccine. Eur. J. Pharm. Sci. 2025, 205, 106995. [Google Scholar] [CrossRef]
- Sefat, K.M.S.R.; Kumar, M.; Kehl, S.; Kulkarni, R.; Leekha, A.; Paniagua, M.-M.; Ackart, D.F.; Jones, N.; Spencer, C.; Podell, B.K.; et al. An intranasal nanoparticle vaccine elicits protective immunity against Mycobacterium tuberculosis. Vaccine 2024, 42, 125909. [Google Scholar] [CrossRef]
- Tafaghodi, M.; Khademi, F.; Shiehzadeh, F.; Firouzi, Z. Polymer-based nanoparticles as delivery systems for treatment and vaccination of tuberculosis. In Nanotechnology Based Approaches for Tuberculosis Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–142. ISBN 9780128198117. [Google Scholar]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Audran, R.; Karoui, O.; Donnet, L.; Soumas, V.; Fares, F.; Lovis, A.; Noirez, L.; Cavassini, M.; Fayet-Mello, A.; Satti, I.; et al. Randomised, double-blind, controlled phase 1 trial of the candidate tuberculosis vaccine ChAdOx1-85A delivered by aerosol versus intramuscular route. J. Infect. 2024, 89, 106205. [Google Scholar] [CrossRef]
- Cáceres, G.; Calderon, R.; Ugarte-Gil, C. Tuberculosis and comorbidities: Treatment challenges in patients with comorbid diabetes mellitus and depression. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095831. [Google Scholar] [CrossRef]
- Falugi, F.; Kim, H.K.; Missiakas, D.M.; Schneewind, O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 2013, 4, e00575-13. [Google Scholar] [CrossRef]
- Shi, M.; Willing, S.E.; Kim, H.K.; Schneewind, O.; Missiakas, D. Peptidoglycan contribution to the B cell superantigen activity of staphylococcal protein A. mBio 2021, 12, e00039-21. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Caldera, J.R.; Hajam, I.A.; Chiang, A.W.T.; Tsai, C.-H.; Li, H.; Díez, M.L.; Gonzalez, C.; Trieu, D.; Martins, G.A.; et al. Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine. Cell Host Microbe 2022, 30, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Hajam, I.A.; Tsai, C.-M.; Gonzalez, C.; Caldera, J.R.; Lázaro Díez, M.; Du, X.; Aralar, A.; Lin, B.; Duong, W.; Liu, G.Y. Pathobiont-induced suppressive immune imprints thwart T cell vaccine responses. Nat. Commun. 2024, 15, 10335. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.; Allen, K.B.; Moreira, E.D.; Moustafa, M.; Isgro, F.; Boucher, H.W.; Corey, G.R.; Carmeli, Y.; Betts, R.; Hartzel, J.S.; et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: A randomized trial. JAMA 2013, 309, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.; Matalon, A.; Buerkert, J.; Taylor, K.; Damaso, S.; Boutriau, D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum. Vaccines Immunother. 2015, 11, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, H.; Baber, J.; Begier, E.; Noriega, D.C.; Konishi, H.; Yato, Y.; Wang, M.Y.; Le Huec, J.C.; Patel, V.; Varga, P.; et al. Efficacy of a 4-antigen Staphylococcus aureus vaccine in spinal surgery: The STaphylococcus aureus suRgical Inpatient Vaccine Efficacy (STRIVE) randomized clinical trial. Clin. Infect. Dis. 2023, 77, 312–320. [Google Scholar] [CrossRef]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef]
- Tam, K.; Lacey, K.A.; Devlin, J.C.; Coffre, M.; Sommerfield, A.; Chan, R.; O’Malley, A.; Koralov, S.B.; Loke, P.; Ng, J.; et al. Targeting leukocidin-mediated immune evasion protects mice from Staphylococcus aureus bacteremia. J. Exp. Med. 2020, 217, e20190541. [Google Scholar] [CrossRef]
- Tran, V.G.; Venkatasubramaniam, A.; Adhikari, R.P.; Krishnan, S.; Wang, X.; Le, V.T.M.; Le, H.N.; Vu, T.T.T.; Schneider-Smith, E.; Aman, M.J.; et al. Efficacy of active immunization with attenuated α-hemolysin and Panton-Valentine leukocidin in a rabbit model of Staphylococcus aureus necrotizing pneumonia. J. Infect. Dis. 2020, 221, 267–275. [Google Scholar] [CrossRef]
- Roetzer, A.; Stich, N.; Model, N.; Schwameis, M.; Firbas, C.; Jilma, B.; Eibl, M.M. High titer persistent neutralizing antibodies induced by TSST-1 variant vaccine against toxic shock cytokine storm. Toxins 2020, 12, 640. [Google Scholar] [CrossRef]
- Schlievert, P.M. Staphylococcal enterotoxin B and C mutants and vaccine toxoids. Microbiol. Spectr. 2023, 11, e0444622. [Google Scholar] [CrossRef]
- Schoergenhofer, C.; Gelbenegger, G.; Hasanacevic, D.; Schöner, L.; Steiner, M.M.; Firbas, C.; Buchtele, N.; Derhaschnig, U.; Tanzmann, A.; Model, N.; et al. A randomized, double-blind study on the safety and immunogenicity of rTSST-1 variant vaccine: Phase 2 results. EClinicalMedicine 2024, 67, 102404. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Gong, M.-Q.; Zhang, H.-J.; Peng, A.-Q.; Xie, Z.; Sun, D.; Liu, L.; Zhou, S.-Q.; Chen, H.; Yang, X.-F.; et al. The safety and immunogenicity of a recombinant five-antigen Staphylococcus aureus vaccine among patients undergoing elective surgery for closed fractures: A randomized, double-blind, placebo-controlled, multicenter phase 2 clinical trial. Vaccine 2023, 41, 5562–5571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Zeng, H.; Li, J.-X.; Wang, B.; Meng, F.-Y.; Yang, F.; Gu, J.; Liang, H.-Y.; Hu, Y.-M.; Liu, P.; et al. Evaluation of a recombinant five-antigen Staphylococcus aureus vaccine: The randomized, single-centre phase 1a/1b clinical trials. Vaccine 2022, 40, 3216–3227. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus vaccine research and development: The past, present and future, including novel therapeutic strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef]
- Millar, E.V.; Bennett, J.W.; Barin, B.; Carey, P.M.; Law, N.N.; English, C.E.; Schwartz, M.M.; Cochrane, T.; Ellis, M.W.; Tribble, D.R.; et al. Safety, immunogenicity, and efficacy of NDV-3A against Staphylococcus aureus colonization: A phase 2 vaccine trial among US Army infantry trainees. Vaccine 2021, 39, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef]
- Wang, F.; Fang, R.H.; Luk, B.T.; Hu, C.-M.J.; Thamphiwatana, S.; Dehaini, D.; Angsantikul, P.; Kroll, A.V.; Pang, Z.; Gao, W.; et al. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv. Funct. Mater. 2016, 26, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Xu, C.; Zhang, C.; Tan, A.; Lu, D.; Luo, P.; Cheng, P.; Zhang, W.; Bai, L.; Yu, C.; et al. mRNA-based platform for preventing and treating Staphylococcus aureus by targeted staphylococcal enterotoxin B. Front. Immunol. 2024, 15, 1490044. [Google Scholar] [CrossRef]
- Marchitto, M.C.; Dillen, C.A.; Liu, H.; Miller, R.J.; Archer, N.K.; Ortines, R.V.; Alphonse, M.P.; Marusina, A.I.; Merleev, A.A.; Wang, Y.; et al. Clonal Vγ6⁺Vδ4⁺ T cells promote IL-17-mediated immunity against Staphylococcus aureus skin infection. Proc. Natl. Acad. Sci. USA 2019, 116, 10917–10926. [Google Scholar] [CrossRef]
- Brown, A.F.; Murphy, A.G.; Lalor, S.J.; Leech, J.M.; O’Keeffe, K.M.; Mac Aogáin, M.; O’Halloran, D.P.; Lacey, K.A.; Tavakol, M.; Hearnden, C.H.; et al. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 2015, 11, e1005226. [Google Scholar] [CrossRef]
- Park, B.; Liu, G.Y. Staphylococcus aureus and hyper-IgE syndrome. Int. J. Mol. Sci. 2020, 21, 9152. [Google Scholar] [CrossRef]
- Mancini, F.; Monaci, E.; Lofano, G.; Torre, A.; Bacconi, M.; Tavarini, S.; Sammicheli, C.; Arcidiacono, L.; Galletti, B.; Laera, D.; et al. One dose of Staphylococcus aureus 4C-Staph vaccine formulated with a novel TLR7-dependent adjuvant rapidly protects mice through antibodies, effector CD4⁺ T cells, and IL-17A. PLoS ONE 2016, 11, e0147767. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; McCarthy, K.N.; Claxton, T.J.; Carlile, S.R.; O’Brien, E.C.; Vozza, E.G.; Mills, K.H.; McLoughlin, R.M. IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection. JCI Insight 2024, 9, e178216. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, M.-H.; Jeon, J.; Kim, O.Y.; Choi, Y.; Seo, J.; Hong, S.-W.; Lee, W.-H.; Jeon, S.G.; Gho, Y.S.; et al. Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS ONE 2015, 10, e0136021. [Google Scholar] [CrossRef]
- Ferraro, A.; Buonocore, S.M.; Auquier, P.; Nicolas, I.; Wallemacq, H.; Boutriau, D.; van der Most, R.G. Role and plasticity of Th1 and Th17 responses in immunity to Staphylococcus aureus. Hum. Vaccines Immunother. 2019, 15, 2980–2992. [Google Scholar] [CrossRef]
- Wei, Y.; Palacios Araya, D.; Palmer, K.L. Enterococcus faecium: Evolution, adaptation, pathogenesis and emerging therapeutics. Nat. Rev. Microbiol. 2024, 22, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Cairns, K.A.; Udy, A.A.; Peel, T.N.; Abbott, I.J.; Dooley, M.J.; Peleg, A.Y. Therapeutics for vancomycin-resistant enterococcal bloodstream infections. Clin. Microbiol. Rev. 2023, 36, e0005922. [Google Scholar] [CrossRef] [PubMed]
- Kalfopoulou, E.; Huebner, J. Advances and prospects in vaccine development against enterococci. Cells 2020, 9, 2397. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.M.; Romero-Saavedra, F.; Laverde, D.; Johannessen, M.; Hübner, J.; Hegstad, K. Enterococcal membrane vesicles as vaccine candidates. Int. J. Mol. Sci. 2023, 24, 16051. [Google Scholar] [CrossRef]
- Romero-Saavedra, F.; Laverde, D.; Kalfopoulou, E.; Martini, C.; Torelli, R.; Martinez-Matamoros, D.; Sanguinetti, M.; Huebner, J. Conjugation of different immunogenic enterococcal vaccine target antigens leads to extended strain coverage. J. Infect. Dis. 2019, 220, 1589–1598. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary tract infections caused by uropathogenic Escherichia coli: Mechanisms of infection and treatment options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Bonten, M.; Johnson, J.R.; van den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; de Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli bacteremia: A systematic literature review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef]
- Hoffman, A.; Satyavolu, S.; Muhanna, D.; Malay, S.; Raffay, T.; Windau, A.; Ransom, E.M.; Mukherjee, D. Predictors of mortality and severe illness from Escherichia coli sepsis in neonates. J. Perinatol. 2024, 44, 1816–1821. [Google Scholar] [CrossRef]
- Doua, J.; Rodríguez-Baño, J.; Froget, R.; Puranam, P.; Go, O.; Geurtsen, J.; van Rooij, S.; Vilken, T.; Minoru, I.; Yasumori, I.; et al. Clinical presentation and antimicrobial resistance of invasive Escherichia coli disease in hospitalized older adults: A prospective multinational observational study. Infection 2024, 52, 1073–1085. [Google Scholar] [CrossRef]
- Fierro, C.A.; Sarnecki, M.; Doua, J.; Spiessens, B.; Go, O.; Davies, T.A.; van den Dobbelsteen, G.; Poolman, J.; Abbanat, D.; Haazen, W. Safety, reactogenicity, immunogenicity, and dose selection of 10-valent extraintestinal pathogenic Escherichia coli bioconjugate vaccine (VAC52416) in adults aged 60–85 years in a randomized, multicenter, interventional, first-in-human, phase 1/2a study. Open Forum Infect. Dis. 2023, 10, ofad417. [Google Scholar] [CrossRef]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997, 276, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Chorro, L.; Ciolino, T.; Torres, C.L.; Illenberger, A.; Aglione, J.; Corts, P.; Lypowy, J.; Ponce, C.; La Porte, A.; Burt, D.; et al. A cynomolgus monkey E. coli urinary tract infection model confirms efficacy of new FimH vaccine candidates. Infect. Immun. 2024, 92, e0016924. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, G.R.; Hughey, H.; Rosenberger, L.; Martin, S.M.; Shapiro, A.M.; D’Antonio, E.; Krejci, K.G.; Shore, N.; Peterson, J.; Lukes, A.S.; et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: Results from a first-in-human phase 1 study. Hum. Vaccines Immunother. 2021, 17, 1262–1270. [Google Scholar] [CrossRef]
- Nickel, J.C.; Doiron, R.C. An effective sublingual vaccine, MV140, safely reduces risk of recurrent urinary tract infection in women. Pathogens 2023, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Foley, S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018, 121, 289–292. [Google Scholar] [CrossRef]
- Carrión-López, P.; Martínez-Ruiz, J.; Giménez-Bachs, J.M.; Fernández-Anguita, P.J.; Díaz de Mera-Sánchez Migallón, I.; Legido-Gómez, O.; Rico-Marco, S.; Lorenzo-Sánchez, M.V.; Salinas-Sánchez, A.S. Cost-effectiveness of a sublingual bacterial vaccine for the prophylaxis of recurrent urinary tract infections. Urol. Int. 2022, 106, 730–736. [Google Scholar] [CrossRef]
- Xing, Y.; Clark, J.R.; Chang, J.D.; Chirman, D.M.; Green, S.; Zulk, J.J.; Jelinski, J.; Patras, K.A.; Maresso, A.W. Broad protective vaccination against systemic Escherichia coli with autotransporter antigens. PLoS Pathog. 2023, 19, e1011082. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.-M.J.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Sereme, Y.; Schrimp, C.; Faury, H.; Agapoff, M.; Lefebvre-Wloszczowski, E.; Chang Marchand, Y.; Ageron-Ardila, E.; Panafieu, E.; Blec, F.; Coureuil, M.; et al. A live attenuated vaccine to prevent severe neonatal Escherichia coli K1 infections. Nat. Commun. 2024, 15, 3021. [Google Scholar] [CrossRef]
- Svennerholm, A.-M.; Lundgren, A. Developments in oral enterotoxigenic Escherichia coli vaccines. Curr. Opin. Immunol. 2023, 84, 102372. [Google Scholar] [CrossRef]
- Gutiérrez, R.L.; Riddle, M.S.; Porter, C.K.; Maciel, M., Jr.; Poole, S.T.; Laird, R.M.; Lane, M.; Turiansky, G.W.; Jarell, A.; Savarino, S.J. A first in human clinical trial assessing the safety and immunogenicity of two intradermally delivered enterotoxigenic Escherichia coli CFA/I fimbrial tip adhesin antigens with and without heat-labile enterotoxin with mutation LT(R192G). Microorganisms 2023, 11, 2689. [Google Scholar] [CrossRef] [PubMed]
- Sukwa, N.; Mubanga, C.; Hatyoka, L.M.; Chilyabanyama, O.N.; Chibuye, M.; Mundia, S.; Munyinda, M.; Kamuti, E.; Siyambango, M.; Badiozzaman, S.; et al. Safety, tolerability, and immunogenicity of an oral inactivated ETEC vaccine (ETVAX®) with dmLT adjuvant in healthy adults and children in Zambia: An age descending randomised, placebo-controlled trial. Vaccine 2023, 41, 6884–6894. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Riekkinen, M.; Jokiranta, T.S.; Pakkanen, S.H.; Pietilä, J.-P.; Patjas, A.; Eriksson, M.; Khawaja, T.; Klemets, P.; Marttinen, K.; et al. Safety and immunogenicity of ETVAX®, an oral inactivated vaccine against enterotoxigenic Escherichia coli diarrhoea: A double-blinded, randomized, placebo-controlled trial amongst Finnish travellers to Benin, West Africa. J. Travel Med. 2023, 30, taad045. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.R.; Baker, S.; Marks, F.; Alsan, M.; Garrett, D.; Gellin, B.G.; Saha, S.K.; Qamar, F.N.; Yousafzai, M.T.; Bogoch, I.I.; et al. Typhoid conjugate vaccines: A new tool in the fight against antimicrobial resistance. Lancet Infect. Dis. 2019, 19, e26–e30. [Google Scholar] [CrossRef]
- Qamar, F.N.; Yousafzai, M.T.; Dehraj, I.F.; Shakoor, S.; Irfan, S.; Hotwani, A.; Hunzai, M.J.; Thobani, R.S.; Rahman, N.; Mehmood, J.; et al. Antimicrobial resistance in typhoidal Salmonella: Surveillance for Enteric Fever in Asia Project, 2016–2019. Clin. Infect. Dis. 2020, 71, S276–S284. [Google Scholar] [CrossRef]
- Hoffman, S.A.; LeBoa, C.; Date, K.; Haldar, P.; Harvey, P.; Shimpi, R.; An, Q.; Zhang, C.; Jayaprasad, N.; Horng, L.; et al. Programmatic effectiveness of a pediatric typhoid conjugate vaccine campaign in Navi Mumbai, India. Clin. Infect. Dis. 2023, 77, 138–144. [Google Scholar] [CrossRef]
- Birkhold, M.; Mwisongo, A.; Pollard, A.J.; Neuzil, K.M. Typhoid conjugate vaccines: Advancing the research and public health agendas. J. Infect. Dis. 2021, 224, S781–S787. [Google Scholar] [CrossRef]
- Shakya, M.; Colin-Jones, R.; Theiss-Nyland, K.; Voysey, M.; Pant, D.; Smith, N.; Liu, X.; Tonks, S.; Mazur, O.; Farooq, Y.G.; et al. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N. Engl. J. Med. 2019, 381, 2209–2218. [Google Scholar] [CrossRef]
- Luthra, K.; Watts, E.; Debellut, F.; Pecenka, C.; Bar-Zeev, N.; Constenla, D. A review of the economic evidence of typhoid fever and typhoid vaccines. Clin. Infect. Dis. 2019, 68, S83–S95. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Stanaway, J.; Grow, S.; Vannice, K.; Steele, A.D. Salmonella combination vaccines: Moving beyond typhoid. Open Forum Infect. Dis. 2023, 10, S58–S66. [Google Scholar] [CrossRef]
- Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of broad immunity against invasive Salmonella disease by a quadrivalent combination Salmonella MAPS vaccine targeting Salmonella enterica serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.Y.; Lin, F.Y.; Hó, V.A.; Thuy, N.T.; Van Bay, P.; Thanh, T.C.; Khiem, H.B.; Trach, D.D.; Karpas, A.B.; Li, J.; et al. Phase 1 and phase 2 studies of Salmonella enterica serovar Paratyphi A O-specific polysaccharide-tetanus toxoid conjugates in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 2000, 68, 1529–1534. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Gavini, M.; Lanzilao, L.; Medaglini, D.; Saul, A.; Martin, L.B. O:2-CRM197 conjugates against Salmonella Paratyphi A. PLoS ONE 2012, 7, e47039. [Google Scholar] [CrossRef]
- Wahid, R.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Cell mediated immune responses elicited in volunteers following immunization with candidate live oral Salmonella enterica serovar Paratyphi A attenuated vaccine strain CVD 1902. Clin. Immunol. 2019, 201, 61–69. [Google Scholar] [CrossRef]
- McCann, N.; Emary, K.; Singh, N.; Mclean, F.; Camara, S.; Jones, E.; Kim, Y.C.; Liu, X.; Greenland, M.; Conlin, K.; et al. Accelerating clinical development of a live attenuated vaccine against Salmonella Paratyphi A (VASP): Study protocol for an observer-participant-blind randomised control trial of a novel oral vaccine using a human challenge model of Salmonella Paratyphi A infection in healthy adult volunteers. BMJ Open 2023, 13, e068966. [Google Scholar] [CrossRef] [PubMed]
- Soulier, A.; Prevosto, C.; Chol, M.; Deban, L.; Cranenburgh, R.M. Engineering a novel bivalent oral vaccine against enteric fever. Int. J. Mol. Sci. 2021, 22, 3287. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Potey, A.V.; Bharati, S.; Kunhihitlu, A.; Narasimha, B.; Yallapa, S.; Dharmadhikari, A.; Gavade, V.; Kamat, C.D.; Mallya, A.; et al. The safety and immunogenicity of a bivalent conjugate vaccine against Salmonella enterica Typhi and Paratyphi A in healthy Indian adults: A phase 1, randomised, active-controlled, double-blind trial. Lancet 2024, 403, 1554–1562. [Google Scholar] [CrossRef]
- Pinto, M.; Durante, S.; Carducci, M.; Massai, L.; Alfini, R.; Mylona, E.; Karkey, A.; Baker, S.; Micoli, F.; Giannelli, C.; et al. The Salmonella Paratyphi A O-antigen glycoconjugate vaccine is able to induce antibodies with bactericidal activity against a panel of clinical isolates. Vaccines 2025, 13, 122. [Google Scholar] [CrossRef]
- Alfini, R.; Carducci, M.; Massai, L.; De Simone, D.; Mariti, M.; Rossi, O.; Rondini, S.; Micoli, F.; Giannelli, C. Design of a glycoconjugate vaccine against Salmonella Paratyphi A. Vaccines 2024, 12, 1272. [Google Scholar] [CrossRef]
- Fiorino, F.; Pettini, E.; Koeberling, O.; Ciabattini, A.; Pozzi, G.; Martin, L.B.; Medaglini, D. Long-term anti-bacterial immunity against systemic infection by Salmonella enterica serovar Typhimurium elicited by a GMMA-based vaccine. Vaccines 2021, 9, 495. [Google Scholar] [CrossRef]
- Fiorino, F.; Rondini, S.; Micoli, F.; Lanzilao, L.; Alfini, R.; Mancini, F.; MacLennan, C.A.; Medaglini, D. Immunogenicity of a bivalent adjuvanted glycoconjugate vaccine against Salmonella Typhimurium and Salmonella Enteritidis. Front. Immunol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Hanumunthadu, B.; Kanji, N.; Owino, N.; Ferreira da Silva, C.; Robinson, H.; White, R.; Ferruzzi, P.; Nakakana, U.; Canals, R.; Pollard, A.J.; et al. Salmonella Vaccine Study in Oxford (SALVO) trial: Protocol for an observer-participant blind randomised placebo-controlled trial of the iNTS-GMMA vaccine within a European cohort. BMJ Open 2023, 13, e072938. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.N.; Hilbi, H. Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 2008, 21, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Satija, K.; Anjankar, V.P. Molecular characterization of multidrug-resistant Shigella flexneri. Cureus 2024, 16, e53276. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Steele, A.D. Frontiers in Shigella vaccine development. Vaccines 2022, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Fang, W.; Li, H.; Chen, J.; Hu, X.; Wang, B.; Feng, Z.; Shi, H.; He, Y.; Huang, D.; et al. Safety and immunogenicity of a Shigella bivalent conjugate vaccine (ZF0901) in 3-month- to 5-year-old children in China. Vaccines 2021, 10, 33. [Google Scholar] [CrossRef]
- Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a four-component GMMA-based vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Rossi, O.; Citiulo, F.; Giannelli, C.; Cappelletti, E.; Gasperini, G.; Mancini, F.; Acquaviva, A.; Raso, M.M.; Sollai, L.; Alfini, R.; et al. A next-generation GMMA-based vaccine candidate to fight shigellosis. NPJ Vaccines 2023, 8, 130. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Conti, V.; Ferruzzi, P.; Ndiaye, A.G.W.; Parker, S.; McNeal, M.M.; Dickey, M.; Granada, J.P.; Cilio, G.L.; De Ryck, I.; et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: Results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine 2021, 39, 101076. [Google Scholar] [CrossRef]
- Randall, A.Z.; Conti, V.; Nakakana, U.; Liang, X.; Teng, A.A.; Di Pasquale, A.L.; Kapulu, M.; Frenck, R., Jr.; Launay, O.; Ferruzzi, P.; et al. Protein-specific immune response elicited by the Shigella sonnei 1790GAHB GMMA-based candidate vaccine in adults with varying exposure to Shigella. mSphere 2025, 10, e0105724. [Google Scholar] [CrossRef]
- Martin, P.; Alaimo, C. The ongoing journey of a Shigella bioconjugate vaccine. Vaccines 2022, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.M.; Ranallo, R.T. Live-attenuated Shigella vaccines. Expert Rev. Vaccines 2006, 5, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Upadhyay, I.; Seo, H.; Vakamalla, S.S.R.; Madhwal, A.; Sack, D.A.; Zhang, W. Immunogenicity and preclinical efficacy characterization of ShecVax, a combined vaccine against Shigella and enterotoxigenic Escherichia coli. Infect. Immun. 2025, 93, e00004-25. [Google Scholar] [CrossRef] [PubMed]
- Boerth, E.M.; Gong, J.; Roffler, B.; Hancock, Z.; Berger, L.; Song, B.; Malley, S.F.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Evaluation of a quadrivalent Shigella flexneri serotype 2a, 3a, 6, and Shigella sonnei O-specific polysaccharide and IpaB MAPS vaccine. Vaccines 2024, 12, 1091. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Milton, R.; Gillespie, D.; Dyer, C.; Taiyari, K.; Carvalho, M.J.; Thomson, K.; Sands, K.; Portal, E.A.R.; Hood, K.; Ferreira, A.; et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: An international multisite prospective observational study. Lancet Glob. Health 2022, 10, e661–e672. [Google Scholar] [CrossRef]
- Mendes, G.; Santos, M.L.; Ramalho, J.F.; Duarte, A.; Caneiras, C. Virulence factors in carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1325077. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Judd, L.M.; Holt, K.E.; Wyres, K.L. Kaptive 2.0: Updated capsule and lipopolysaccharide locus typing for the Klebsiella pneumoniae species complex. Microb. Genom. 2022, 8, 000800. [Google Scholar] [CrossRef]
- Feldman, M.F.; Mayer Bridwell, A.E.; Scott, N.E.; Vinogradov, E.; McKee, S.R.; Chavez, S.M.; Twentyman, J.; Stallings, C.L.; Rosen, D.A.; Harding, C.M. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 18655–18663. [Google Scholar] [CrossRef]
- Lin, T.-L.; Yang, F.-L.; Ren, C.-T.; Pan, Y.-J.; Liao, K.-S.; Tu, I.-F.; Chang, Y.-P.; Cheng, Y.-Y.; Wu, C.-Y.; Wu, S.-H.; et al. Development of Klebsiella pneumoniae capsule polysaccharide-conjugated vaccine candidates using phage depolymerases. Front. Immunol. 2022, 13, 843183. [Google Scholar] [CrossRef]
- Choi, M.; Hegerle, N.; Nkeze, J.; Sen, S.; Jamindar, S.; Nasrin, S.; Permala-Booth, J.; Sinclair, J.; Tapia, M.D.; Tennant, S.M.; et al. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front. Microbiol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Cuscino, N.; Fatima, A.; Di Pilato, V.; Bulati, M.; Alfano, C.; Monaca, E.; Di Mento, G.; Di Carlo, D.; Cardinale, F.; Monaco, F.; et al. Computational design and characterization of a multiepitope vaccine against carbapenemase-producing Klebsiella pneumoniae strains, derived from antigens identified through reverse vaccinology. Comput. Struct. Biotechnol. J. 2022, 20, 4446–4463. [Google Scholar] [CrossRef]
- Hegerle, N.; Choi, M.; Sinclair, J.; Amin, M.N.; Ollivault-Shiflett, M.; Curtis, B.; Laufer, R.S.; Shridhar, S.; Brammer, J.; Toapanta, F.R.; et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0203143. [Google Scholar] [CrossRef]
- Wantuch, P.L.; Knoot, C.J.; Robinson, L.S.; Vinogradov, E.; Scott, N.E.; Harding, C.M.; Rosen, D.A. Heptavalent O-antigen bioconjugate vaccine exhibiting differential functional antibody responses against diverse Klebsiella pneumoniae isolates. J. Infect. Dis. 2024, 230, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Dentovskaya, S.V.; Vagaiskaya, A.S.; Trunyakova, A.S.; Kartseva, A.S.; Ivashchenko, T.A.; Gerasimov, V.N.; Platonov, M.E.; Firstova, V.V.; Anisimov, A.P. Genetically engineered bacterial ghosts as vaccine candidates against Klebsiella pneumoniae infection. Vaccines 2025, 13, 59. [Google Scholar] [CrossRef]
- Huang, T.; Che, S.; Lv, Z.; Hao, D.; Wang, R.; Yi, Q.; Mei, L.; Yuan, Y.; Zou, H.; Guo, Y.; et al. mRNA-LNP vaccines combined with tPA signal sequence elicit strong protective immunity against Klebsiella pneumoniae. mSphere 2025, 10, e00775-24. [Google Scholar] [CrossRef]
- Shahbazi, S.; Habibi, M.; Badmasti, F.; Sabzi, S.; Farokhi, M.; Asadi Karam, M.R. Design and fabrication of a vaccine candidate based on rOmpA from Klebsiella pneumoniae encapsulated in silk fibroin-sodium alginate nanoparticles against pneumonia infection. Int. Immunopharmacol. 2023, 125, 111171. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Galas, M.; et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: A retrospective observational study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Sabbatucci, M.; Zovi, A. Antimicrobial resistance in gonorrhea. Microb. Drug Resist. 2024, 30, 297–303. [Google Scholar] [CrossRef]
- Russell, M.W.; Jerse, A.E.; Gray-Owen, S.D. Progress toward a gonococcal vaccine: The way forward. Front. Immunol. 2019, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Ruiz García, Y.; Marrazzo, J.; Martinón-Torres, F.; Workowski, K.; Giordano, G.; Pizza, M.; Sohn, W.-Y. Urgent need to understand and prevent gonococcal infection: From the laboratory to real-world context. J. Infect. Dis. 2024, 230, e758–e767. [Google Scholar] [CrossRef]
- Wang, B.; Mohammed, H.; Andraweera, P.; McMillan, M.; Marshall, H. Vaccine effectiveness and impact of meningococcal vaccines against gonococcal infections: A systematic review and meta-analysis. J. Infect. 2024, 89, 106225. [Google Scholar] [CrossRef]
- Paynter, J.; Goodyear-Smith, F.; Morgan, J.; Saxton, P.; Black, S.; Petousis-Harris, H. Effectiveness of a group B outer membrane vesicle meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: A retrospective cohort study. Vaccines 2019, 7, 5. [Google Scholar] [CrossRef]
- Azze, R.F.O. A meningococcal B vaccine induces cross-protection against gonorrhea. Clin. Exp. Vaccine Res. 2019, 8, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Micoli, F.; Necchi, F.; Pizza, M.; Berlanda Scorza, F.; Rossi, O. GMMA-based vaccines: The known and the unknown. Front. Immunol. 2021, 12, 715393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B. GSK’s gonorrhea vaccine receives fast-track designation to expedite clinical trials. Nat. Med. 2023, 29, 2146–2147. [Google Scholar] [CrossRef]
- Bagwe, P.; Bajaj, L.; Menon, I.; Braz Gomes, K.; Kale, A.; Patil, S.; Vijayanand, S.; Gala, R.; D’Souza, M.J.; Zughaier, S.M. Gonococcal microparticle vaccine in dissolving microneedles induced immunity and enhanced bacterial clearance in infected mice. Int. J. Pharm. 2023, 642, 123182. [Google Scholar] [CrossRef]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel whole-cell inactivated Neisseria gonorrhoeae microparticles as vaccine formulation in microneedle-based transdermal immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef]
- Schwartz, B.; Klamer, K.; Zimmerman, J.; Kale-Pradhan, P.B.; Bhargava, A. Multidrug resistant Pseudomonas aeruginosa in clinical settings: A review of resistance mechanisms and treatment strategies. Pathogens 2024, 13, 975. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO bacterial priority pathogens list 2024: A prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect. Dis. 2025; online before print. [Google Scholar]
- Asamenew, T.; Worku, S.; Motbainor, H.; Mekonnen, D.; Deribe, A. Antimicrobial resistance profile of Pseudomonas aeruginosa from different clinical samples in Debre Tabor Comprehensive Specialized Hospital, Northwest Ethiopia. Ethiop. J. Health Sci. 2023, 33, 423–432. [Google Scholar] [CrossRef]
- Santamarina-Fernández, R.; Fuentes-Valverde, V.; Silva-Rodríguez, A.; García, P.; Moscoso, M.; Bou, G. Pseudomonas aeruginosa vaccine development: Lessons, challenges, and future innovations. Int. J. Mol. Sci. 2025, 26, 2012. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Wurm, R.; Depuydt, P.; Spapen, H.; Lorente, J.A.; Staudinger, T.; Creteur, J.; Zauner, C.; Meier-Hellmann, A.; Eller, P.; et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients—A randomized clinical trial. Crit. Care 2020, 24, 74. [Google Scholar] [CrossRef]
- Askarian, F.; Tsai, C.-M.; Cordara, G.; Zurich, R.H.; Bjånes, E.; Golten, O.; Vinther Sørensen, H.; Kousha, A.; Meier, A.; Chikwati, E.; et al. Immunization with lytic polysaccharide monooxygenase CbpD induces protective immunity against Pseudomonas aeruginosa pneumonia. Proc. Natl. Acad. Sci. USA 2023, 120, e2301538120. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Morici, L.A. Vaccination to prevent Pseudomonas aeruginosa bloodstream infections. Front. Microbiol. 2022, 13, 870104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Liang, Y.; Zhang, L.; Fan, J.; Yang, Y. A semisynthetic oligomannuronic acid-based glycoconjugate vaccine against Pseudomonas aeruginosa. ACS Cent. Sci. 2024, 10, 1515–1523. [Google Scholar] [CrossRef]
- Zhu, F.; Qin, R.; Ma, S.; Zhou, Z.; Tan, C.; Yang, H.; Zhang, P.; Xu, Y.; Luo, Y.; Chen, J.; et al. Designing a multi-epitope vaccine against Pseudomonas aeruginosa via integrating reverse vaccinology with immunoinformatics approaches. Sci. Rep. 2025, 15, 10425. [Google Scholar] [CrossRef]
- Ma, C.; Ma, X.; Jiang, B.; Pan, H.; Liao, X.; Zhang, L.; Li, W.; Luo, Y.; Shen, Z.; Cheng, X.; et al. A novel inactivated whole-cell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal Transduct. Target. Ther. 2021, 6, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Wan, C.; Wei, J.; Gao, C.; Zhang, Y.; Zeng, H.; Peng, L.; Luo, P.; Lu, D.; et al. Development of a chimeric vaccine against Pseudomonas aeruginosa based on the Th17-stimulating epitopes of PcrV and AmpC. Front. Immunol. 2020, 11, 601601. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Rcheulishvili, N.; Papukashvili, D.; Xie, F.; Zhao, J.; Hu, X.; Yu, K.; Yang, N.; Pan, X.; et al. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa. NPJ Vaccines 2023, 8, 76. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Y.; Yang, L.; Yang, Y.Y.; Yuan, P.; Chen, X.; Tian, G.-B.; Ding, X. A multiantigenic antibacterial nanovaccine utilizing hybrid membrane vesicles for combating Pseudomonas aeruginosa infections. J. Extracell. Vesicles 2024, 13, e12524. [Google Scholar] [CrossRef]
- Bjånes, E.; Krishnan, N.; Koh, T.; Ngo, A.T.; Cole, J.; Olson, J.; Cornax, I.; Chen, C.-H.; Chavarria, N.; Dahesh, S.; et al. STING-adjuvanted outer membrane vesicle nanoparticle vaccine against Pseudomonas aeruginosa. JCI Insight 2025, in press. [Google Scholar] [CrossRef]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-H.; Sung, W.-C.; Lien, S.-P.; Chen, Y.-Z.; Lo, A.F.-Y.; Huang, J.-H.; Kuo, S.-C.; Chong, P. Identification of novel vaccine candidates against Acinetobacter baumannii using reverse vaccinology. Hum. Vaccines Immunother. 2015, 11, 1065–1073. [Google Scholar] [CrossRef]
- Shahid, F.; Zaheer, T.; Ashraf, S.T.; Shehroz, M.; Anwer, F.; Naz, A.; Ali, A. Chimeric vaccine designs against Acinetobacter baumannii using pan genome and reverse vaccinology approaches. Sci. Rep. 2021, 11, 13213. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.; Mahapatra, S.R.; Singh, P.K.; Prabhuswamimath, S.C.; Misra, N.; Suar, M. Designing of multi-epitope peptide vaccine against Acinetobacter baumannii through combined immunoinformatics and protein interaction-based approaches. Immunol. Res. 2023, 71, 639–662. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, F.; Zhang, P.; Xu, Y.; Zhou, Z.; Yang, H.; Tan, C.; Chen, J.; Pan, P. Development of a novel multi-epitope subunit mRNA vaccine candidate to combat Acinetobacter baumannii. Sci. Rep. 2025, 15, 1410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, C.; Liu, Z.; Sun, P.; Hua, X.; Feng, E.; Yu, Y.; Wu, J.; Zhu, L.; Wang, H. Safety and immunogenicity of a new glycoengineered vaccine against Acinetobacter baumannii in mice. Microb. Biotechnol. 2022, 15, 703–716. [Google Scholar] [CrossRef]
- Hagag, Y.A.; Said, H.S.; Kenawy, H.I.; Hassan, R. A novel pentavalent vaccine candidate completely protects against Acinetobacter baumannii in a mouse model of peritonitis. Appl. Microbiol. Biotechnol. 2022, 106, 8151–8167. [Google Scholar] [CrossRef] [PubMed]
- Behrouz, B.; Rasooli, I.; Badmasti, F. Inserting Omp22 into the flagellin protein, replacing its hypervariable region, results in stronger protection against lethal Acinetobacter baumannii infection. Sci. Rep. 2024, 14, 27646. [Google Scholar] [CrossRef] [PubMed]
- Dollery, S.J.; Zurawski, D.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Tobin, J.K.; Wiggins, T.J.; Bushnell, R.V.; MacLeod, D.A.; Alamneh, Y.A.; Abu-Taleb, R.; et al. Radiation-inactivated Acinetobacter baumannii vaccine candidates. Vaccines 2021, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Bjånes, E.; Zhou, J.; Qayum, T.; Krishnan, N.; Zurich, R.H.; Menon, N.D.; Hoffman, A.; Fang, R.H.; Zhang, L.; Nizet, V. Outer membrane vesicle-coated nanoparticle vaccine protects against Acinetobacter baumannii pneumonia and sepsis. Adv. Nanobiomed. Res. 2023, 3, 2200130. [Google Scholar] [CrossRef]
- Pumiglia, L.; Wilson, L.; Rashidi, L. Clostridioides difficile colitis. Surg. Clin. 2024, 104, 545–556. [Google Scholar] [CrossRef]
- Aguilar-Zamora, E.; Weimer, B.C.; Torres, R.C.; Gómez-Delgado, A.; Ortiz-Olvera, N.; Aparicio-Ozores, G.; Barbero-Becerra, V.J.; Torres, J.; Camorlinga-Ponce, M. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in hospitalized patients from Mexico. Front. Microbiol. 2021, 12, 787451. [Google Scholar] [CrossRef]
- Remich, S.; Kitchin, N.; Peterson, J.; Li, P.; Pride, M.W.; Brock, L.; Anderson, A.S.; Gruber, W.C.; Jansen, K.U.; Lockhart, S.P.; et al. A phase 2 extension study evaluating the immunogenicity, safety, and tolerability of 3 or 4 doses of a Clostridioides difficile vaccine in healthy US adults aged 65 to 85 years. J. Infect. Dis. 2024, 229, 367–375. [Google Scholar] [CrossRef]
- Razim, A.; Górska, S.; Gamian, A. Non-toxin-based Clostridioides difficile vaccination approaches. Pathogens 2023, 12, 235. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.; Tian, S. Against Clostridioides difficile infection: An update on vaccine development. Toxins 2025, 17, 222. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Semon, A.; Bayard, N.U.; Pan, Y.-G.; Dwivedi, G.; Knox, J.; Glover, R.C.; Rangel, P.C.; Tanes, C.; Bittinger, K.; et al. A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection. Science 2024, 386, 69–75. [Google Scholar] [CrossRef]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.; Zellweger, R.M.; Poudyal, N.; Smith, K.H.T.; Joh, H.S.; Marks, F. Measuring the link between vaccines and antimicrobial resistance in low resource settings—Limitations and opportunities in direct and indirect assessments and implications for impact studies. Front. Trop. Dis. 2022, 3, 805833. [Google Scholar] [CrossRef]
- Breeze, P.R.; Squires, H.; Ennis, K.; Meier, P.; Hayes, K.; Lomax, N.; Shiell, A.; Kee, F.; de Vocht, F.; O’Flaherty, M.; et al. Guidance on the use of complex systems models for economic evaluations of public health interventions. Health Econ. 2023, 32, 1603–1625. [Google Scholar] [CrossRef]
- Moore, M.R.; Gertz, R.E., Jr.; Woodbury, R.L.; Barkocy-Gallagher, G.A.; Schaffner, W.; Lexau, C.; Gershman, K.; Reingold, A.; Farley, M.; Harrison, L.H.; et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 2008, 197, 1016–1027. [Google Scholar] [CrossRef]
- Olarte, L.; Kaplan, S.L.; Barson, W.J.; Romero, J.R.; Lin, P.L.; Tan, T.Q.; Hoffman, J.A.; Bradley, J.S.; Givner, L.B.; Mason, E.O.; et al. Emergence of multidrug-resistant pneumococcal serotype 35B among children in the United States. J. Clin. Microbiol. 2017, 55, 724–734. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Weinberger, R.; van der Linden, M.; Imöhl, M.; von Kries, R. Vaccine effectiveness of PCV13 in a 3+1 vaccination schedule. Vaccine 2016, 34, 2062–2065. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.L.; Childs, L.; Ouattara, M.; Akhter, F.; Britton, A.; Pilishvili, T.; Kobayashi, M. Systematic review and meta-analysis of the efficacy and effectiveness of pneumococcal vaccines in adults. Pathogens 2023, 12, 732. [Google Scholar] [CrossRef]
- Jesudason, T. Impact of vaccines in reducing antimicrobial resistance. Lancet Microbe 2025, 6, 101040. [Google Scholar] [CrossRef]
- Kim, C.; Holm, M.; Frost, I.; Hasso-Agopsowicz, M.; Abbas, K. Global and regional burden of attributable and associated bacterial antimicrobial resistance avertable by vaccination: Modelling study. BMJ Glob. Health 2023, 8, e011341. [Google Scholar] [CrossRef]
- Lu, E.Y.; Chen, H.-H.; Zhao, H.; Ozawa, S. Health and economic impact of the pneumococcal conjugate vaccine in hindering antimicrobial resistance in China. Proc. Natl. Acad. Sci. USA 2021, 118, e2004933118. [Google Scholar] [CrossRef] [PubMed]
- Wazed, S. 50th anniversary of Expanded Programme on Immunization: Shaping the next 50 years in the WHO South-East Asia region. Indian J. Med. Res. 2024, 160, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; McKee, M.; Howard, N. The role of global health partnerships in vaccine equity: A scoping review. PLoS Glob. Public Health 2024, 4, e0002834. [Google Scholar] [CrossRef]
- Halabi, S.; Gostin, L.O.; Aneja, K.; Nardi, F.; Gottschalk, K.; Monahan, J. The Coalition for Epidemic Preparedness Innovations (CEPI) and the partnerships of equitable vaccine access. J. Law Med. Ethics 2023, 51, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, E. Exploring future pandemic preparedness through the development of preventive vaccine platforms and the key roles of international organizations in a global health crisis. Vaccines 2025, 13, 56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.A.; Nizet, V. The Central Importance of Vaccines to Mitigate the Threat of Antibiotic-Resistant Bacterial Pathogens. Vaccines 2025, 13, 893. https://doi.org/10.3390/vaccines13090893
Zhang JA, Nizet V. The Central Importance of Vaccines to Mitigate the Threat of Antibiotic-Resistant Bacterial Pathogens. Vaccines. 2025; 13(9):893. https://doi.org/10.3390/vaccines13090893
Chicago/Turabian StyleZhang, Jiaqi Amber, and Victor Nizet. 2025. "The Central Importance of Vaccines to Mitigate the Threat of Antibiotic-Resistant Bacterial Pathogens" Vaccines 13, no. 9: 893. https://doi.org/10.3390/vaccines13090893
APA StyleZhang, J. A., & Nizet, V. (2025). The Central Importance of Vaccines to Mitigate the Threat of Antibiotic-Resistant Bacterial Pathogens. Vaccines, 13(9), 893. https://doi.org/10.3390/vaccines13090893





