A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Population, Design, and Period
2.2. Sample Collection
2.3. Plasmodium falciparum Infection Diagnosis
2.4. Multiplex Luminex Assay for Antibody Quantification
Antigen Panel
2.5. Antibody Detection Immunoassay
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
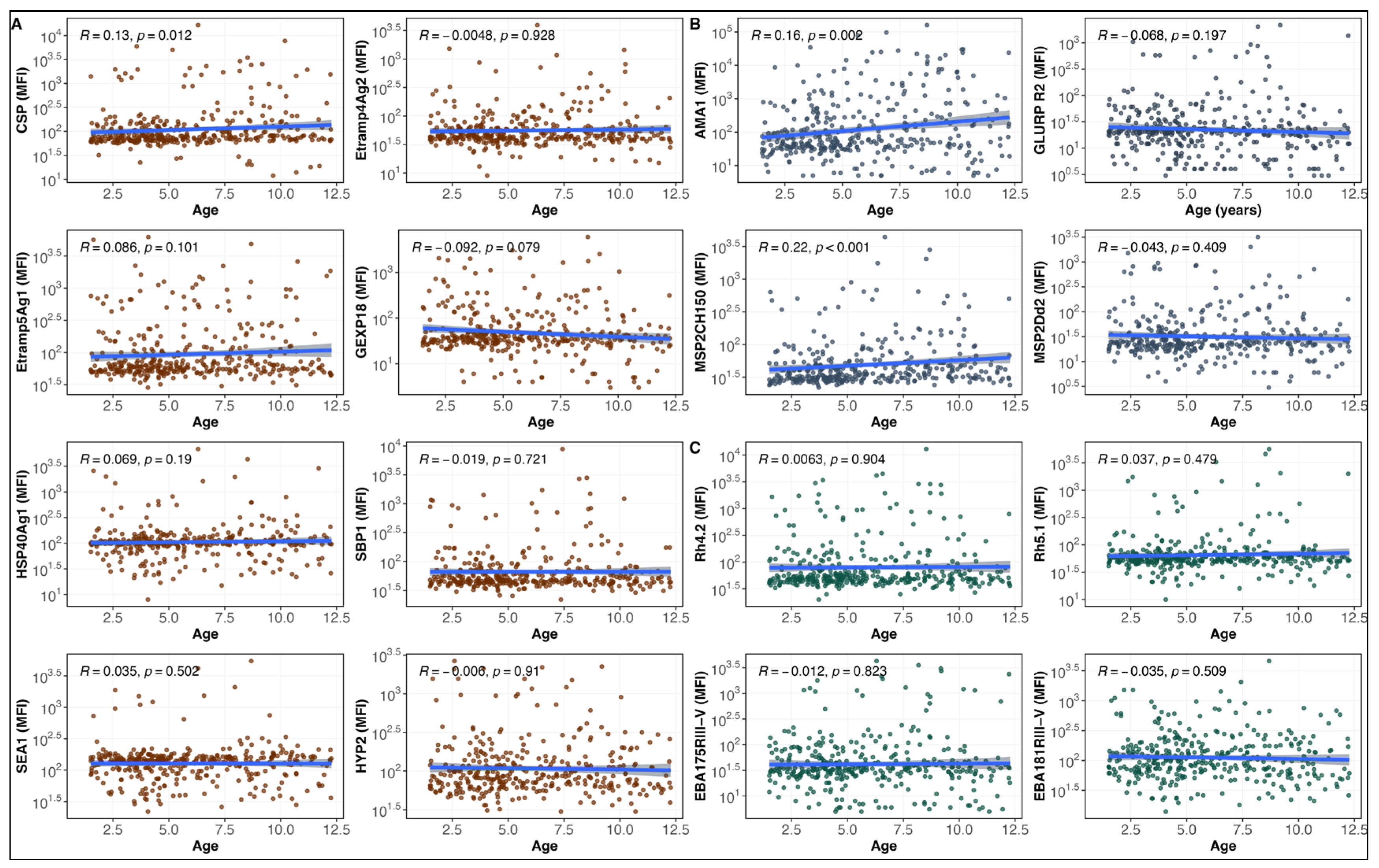
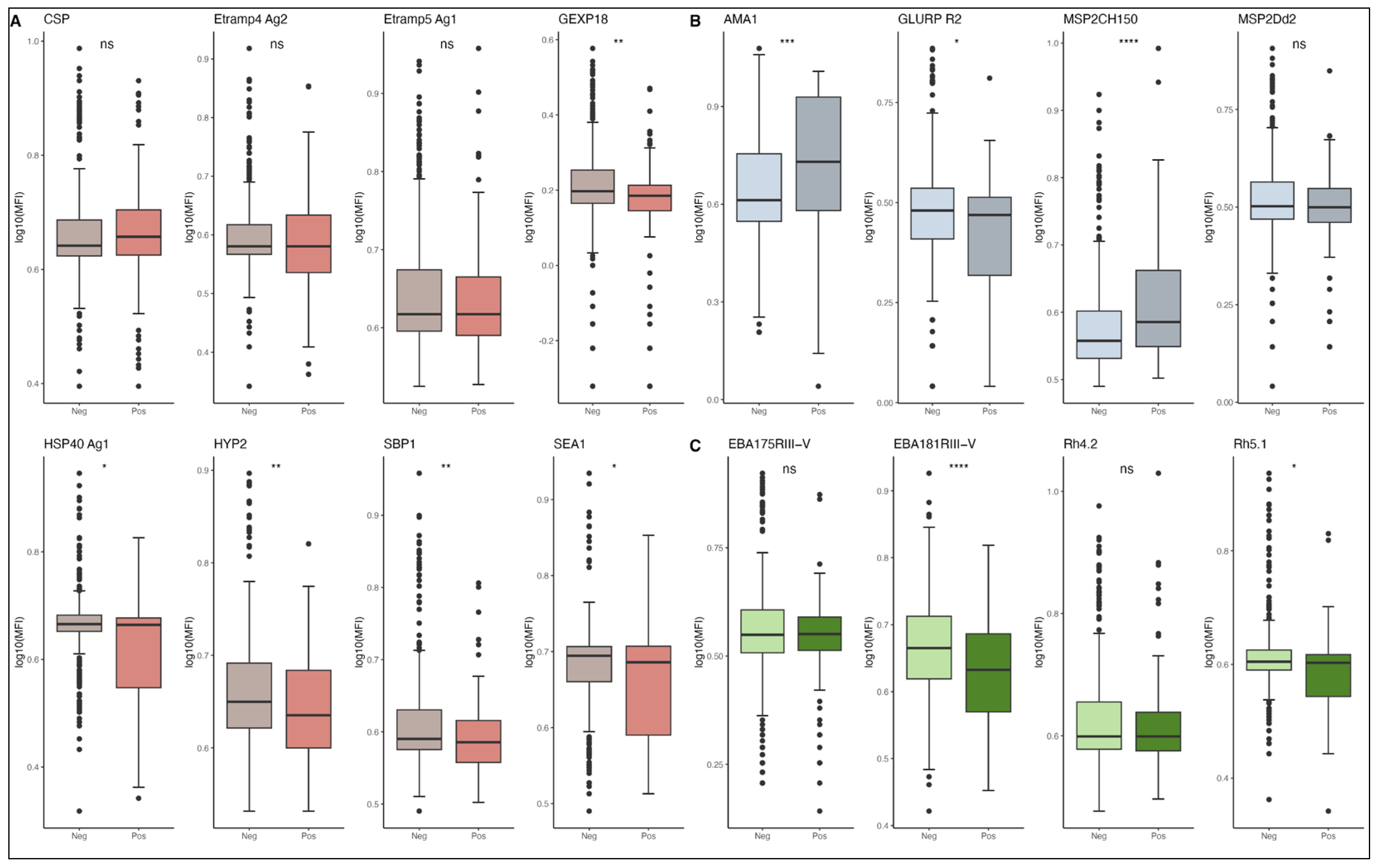
3.2. IgG Antibody Responses to Malaria Antigens and Age
3.3. Antibody Levels and Risk of P. falciparum Malaria Episodes
3.4. Predictive Modeling of Antibody Responses for Clinical Protection Against Malaria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| EBA175 | Erythrocyte binding antigen 175, region III-V |
| Etramp4 Ag2 | Early transcribed membrane protein 4, antigen 2 |
| Etramp5 Ag1 | Early transcribed membrane protein 5, antigen 1 |
| GEXP18 | Gametocyte-exported protein 18 |
| GLURP R2 | Glutamate-rich protein R2 region |
| HSP40 Ag1 | Heat shock protein 40 |
| Hyp2 | Exported putative protein |
| IgG | immunoglobulin G |
| LLINs | Long-lasting insecticide-treated nets |
| MFI | Mean fluorescence intensity |
| MSP2 CH150/9 | Merozoite surface protein 2 |
| nPCR | nested polymerase chain reaction |
| P. falciparum | Plasmodium falciparum |
| PfAMA1 | Apical membrane antigen 1, N terminal region |
| PfCSP | Circumsporozoite protein NANP repeat |
| Rh4.2 | Reticulocyte binding homolog 4 |
| RH5.1 | Reticulocyte binding protein homolog 5 |
| SBP1 | Skeleton binding protein 1 |
| SEA1 | Schizont egress antigen 1 |
| SMC | Seasonal malaria chemoprevention |
| SOP | Standard operating procedure |
| WHO | World Health Organization |
References
- World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 20 May 2025).
- Yazdani, S.S.; Mukherjee, P.; Chauhan, V.S.; Chitnis, C.E. Immune Responses to Asexual Blood-Stages of Malaria Parasites. Curr. Mol. Med. 2006, 6, 187–203. [Google Scholar] [CrossRef]
- Tijani, M.K.; Lugaajju, A.; Persson, K.E.M. Naturally Acquired Antibodies against Plasmodium Falciparum: Friend or Foe? Pathogens 2021, 10, 832. [Google Scholar] [CrossRef]
- Barua, P.; Beeson, J.G.; Maleta, K.; Ashorn, P.; Rogerson, S.J. The Impact of Early Life Exposure to Plasmodium Falciparum on the Development of Naturally Acquired Immunity to Malaria in Young Malawian Children. Malar. J. 2019, 18, 11. [Google Scholar] [CrossRef]
- Addy, J.W.G.; Bediako, Y.; Ndungu, F.M.; Valetta, J.J.; Reid, A.J.; Mwacharo, J.; Ngoi, J.M.; Wambua, J.; Otieno, E.; Musyoki, J.; et al. 10-Year Longitudinal Study of Malaria in Children: Insights into Acquisition and Maintenance of Naturally Acquired Immunity. Wellcome Open Res. 2021, 6, 79. [Google Scholar] [CrossRef]
- King, L.D.W.; Pulido, D.; Barrett, J.R.; Davies, H.; Quinkert, D.; Lias, A.M.; Silk, S.E.; Pattinson, D.J.; Diouf, A.; Williams, B.G.; et al. Preclinical Development of a Stabilized RH5 Virus-like Particle Vaccine That Induces Improved Antimalarial Antibodies. Cell Rep. Med. 2024, 5, 101654. [Google Scholar] [CrossRef] [PubMed]
- Nguetse, C.N.; Ojo, J.A.; Nchotebah, C.; Ikegbunam, M.N.; Meyer, C.G.; Thomas, B.N.; Velavan, T.P.; Ojurongbe, O. Genetic Diversity of the Plasmodium Falciparum Glutamate-Rich Protein R2 Region Before and Twelve Years after Introduction of Artemisinin Combination Therapies among Febrile Children in Nigeria. Am. J. Trop. Med. Hyg. 2018, 98, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, C.E.; Mukherjee, P.; Mehta, S.; Yazdani, S.S.; Dhawan, S.; Shakri, A.R.; Bhardwaj, R.; Gupta, P.K.; Hans, D.; Mazumdar, S.; et al. Correction: Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium Falciparum Malaria Based on MSP1 and EBA175. PLoS ONE 2015, 10, e0137816. [Google Scholar] [CrossRef]
- Takashima, E.; Otsuki, H.; Morita, M.; Ito, D.; Nagaoka, H.; Yuguchi, T.; Hassan, I.; Tsuboi, T. The Need for Novel Asexual Blood-Stage Malaria Vaccine Candidates for Plasmodium falciparum. Biomolecules 2024, 14, 100. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Marjason, J.; Elliott, S.; Fahey, P.; Bang, G.; Malkin, E.; Tierney, E.; Aked-Hurditch, H.; Adda, C.; Cross, N.; et al. A Phase 1 Trial of MSP2-C1, a Blood-Stage Malaria Vaccine Containing 2 Isoforms of MSP2 Formulated with Montanide® ISA 720. PLoS ONE 2011, 6, e24413. [Google Scholar] [CrossRef]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Laurens, M.B.; Kone, A.K.; Guindo, A.B.; Traore, K.; Sissoko, M.; Diallo, D.A.; Diarra, I.; et al. Safety and Immunogenicity of an AMA1 Malaria Vaccine in Malian Children: Results of a Phase 1 Randomized Controlled Trial. PLoS ONE 2010, 5, e9041. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Lopez, F.R.; Natama, H.M.; Weston, S.; et al. Safety and Efficacy of Malaria Vaccine Candidate R21/Matrix-M in African Children: A Multicentre, Double-Blind, Randomised, Phase 3 Trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Merle, C.S.; Group, R.-S. working Implementation Strategies for the Introduction of the RTS,S/AS01 (RTS,S) Malaria Vaccine in Countries with Areas of Highly Seasonal Transmission: Workshop Meeting Report. Malar. J. 2023, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Tuju, J.; Kamuyu, G.; Murungi, L.M.; Osier, F.H.A. Vaccine Candidate Discovery for the next Generation of Malaria Vaccines. Immunology 2017, 152, 195. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, D.R.; Gómez, M.; Camargo, A.; Cuy-Chaparro, L.; Molina-Franky, J.; Reyes, C.; Patarroyo, M.A.; Patarroyo, M.E. Plasmodium Falciparum Blood Stage Antimalarial Vaccines: An Analysis of Ongoing Clinical Trials and New Perspectives Related to Synthetic Vaccines. Front. Microbiol. 2019, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ciubotariu, I.I.; Broyles, B.K.; Xie, S.; Thimmapuram, J.; Mwenda, M.C.; Mambwe, B.; Mulube, C.; Matoba, J.; Schue, J.L.; Moss, W.J.; et al. Diversity and Selection Analyses Identify Transmission-Blocking Antigens as the Optimal Vaccine Candidates in Plasmodium Falciparum. eBioMedicine 2024, 106, 105227. [Google Scholar] [CrossRef]
- Wu, L.; Mwesigwa, J.; Affara, M.; Bah, M.; Correa, S.; Hall, T.; Singh, S.K.; Beeson, J.G.; Tetteh, K.K.A.; Kleinschmidt, I.; et al. Antibody Responses to a Suite of Novel Serological Markers for Malaria Surveillance Demonstrate Strong Correlation with Clinical and Parasitological Infection across Seasons and Transmission Settings in The Gambia. BMC Med. 2020, 18, 304. [Google Scholar] [CrossRef]
- Wu, L.; Hall, T.; Ssewanyana, I.; Oulton, T.; Patterson, C.; Vasileva, H.; Singh, S.; Affara, M.; Mwesigwa, J.; Correa, S.; et al. Optimisation and standardisation of a multiplex immunoassay of diverse Plasmodium falciparum antigens to assess changes in malaria transmission using sero-epidemiology. Wellcome Open Res. 2020, 4, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perrin, A.J.; Bisson, C.; Faull, P.A.; Renshaw, M.J.; Lees, R.A.; Fleck, R.A.; Saibil, H.R.; Snijders, A.P.; Baker, D.A.; Blackman, M.J. Malaria Parasite Schizont Egress Antigen-1 Plays an Essential Role in Nuclear Segregation during Schizogony. mBio 2021, 12, e03377-20. [Google Scholar] [CrossRef]
- Roper, B.; Kannan, D.; Mathews, E.S.; John, A.R.O. Essential Role for HSP40 in Asexual Replication and Thermotolerance of Malaria Parasites. bioRxiv 2024. [Google Scholar] [CrossRef]
- Direction Régionale Sanitaire Des Cascades. Available online: https://www.sante.gov.bf/detail-structure?tx_news_pi1%5Baction%5D=detail&tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Bnews%5D=51&cHash=2b6881ec7031613785256d89cbb00fd4 (accessed on 27 February 2025).
- Tiono, A.B.; Kangoye, D.T.; Rehman, A.M.; Kargougou, D.G.; Kaboré, Y.; Diarra, A.; Ouedraogo, E.; Nébié, I.; Ouédraogo, A.; Okech, B.; et al. Malaria Incidence in Children in South-West Burkina Faso: Comparison of Active and Passive Case Detection Methods. PLoS ONE 2014, 9, e86936. [Google Scholar] [CrossRef]
- Kirakoya-Samadoulougou, F.; De Brouwere, V.; Fokam, A.F.; Ouédraogo, M.; Yé, Y. Assessing the Effect of Seasonal Malaria Chemoprevention on Malaria Burden among Children under 5 Years in Burkina Faso. Malar. J. 2022, 21, 143. [Google Scholar] [CrossRef]
- Druetz, T.; Corneau-Tremblay, N.; Millogo, T.; Kouanda, S.; Ly, A.; Bicaba, A.; Haddad, S. Impact Evaluation of Seasonal Malaria Chemoprevention under Routine Program Implementation: A Quasi-Experimental Study in Burkina Faso. Am. J. Trop. Med. Hyg. 2018, 98, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, H.; Shahrakipour, A.; Mehravaran, A.; Rahmati-Balaghaleh, M.; Zarean, M.; Etemadi, S.; Shahraki, M.; Solgi, R. Evaluation of Multiplex/Nested Polymerase Chain Reaction and Loop-Mediated Isothermal Amplification for Malaria Diagnosis in Southeastern Iran. Am. J. Trop. Med. Hyg. 2022, 106, 841–845. [Google Scholar] [CrossRef]
- Xu, Y.; Goodacre, R. On Splitting Training and Validation Set: A Comparative Study of Cross-Validation, Bootstrap and Systematic Sampling for Estimating the Generalization Performance of Supervised Learning. J. Anal. Test. 2018, 2, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, L.; Drew, D.R.; Dent, A.E.; Kazura, J.W.; Beeson, J.G. Antibody Targets and Properties for Complement-Fixation Against the Circumsporozoite Protein in Malaria Immunity. Front. Immunol. 2021, 12, 775659. [Google Scholar] [CrossRef] [PubMed]
- Mugyenyi, C.K.; Elliott, S.R.; McCallum, F.J.; Anders, R.F.; Marsh, K.; Beeson, J.G. Antibodies to Polymorphic Invasion-Inhibitory and Non-Inhibitory Epitopes of Plasmodium falciparum Apical Membrane Antigen 1 in Human Malaria. PLoS ONE 2013, 8, e68304. [Google Scholar] [CrossRef]
- Mugo, R.M.; Mwai, K.; Mwacharo, J.; Shee, F.M.; Musyoki, J.N.; Wambua, J.; Otieno, E.; Bejon, P.; Ndungu, F.M. Seven-Year Kinetics of RTS, S/AS01-Induced Anti-CSP Antibodies in Young Kenyan Children. Malar. J. 2021, 20, 452. [Google Scholar] [CrossRef]
- Chan, J.-A.; Loughland, J.R.; de la Parte, L.; Okano, S.; Ssewanyana, I.; Nalubega, M.; Nankya, F.; Musinguzi, K.; Rek, J.; Arinaitwe, E.; et al. Age-Dependent Changes in Circulating Tfh Cells Influence Development of Functional Malaria Antibodies in Children. Nat. Commun. 2022, 13, 4159. [Google Scholar] [CrossRef]
- Proietti, C.; Verra, F.; Bretscher, M.T.; Stone, W.; Kanoi, B.N.; Balikagala, B.; Egwang, T.G.; Corran, P.; Ronca, R.; Arcà, B.; et al. Influence of Infection on Malaria-Specific Antibody Dynamics in a Cohort Exposed to Intense Malaria Transmission in Northern Uganda. Parasite Immunol. 2013, 35, 164–173. [Google Scholar] [CrossRef]
- Somé, A.F.; Bazié, T.; Zongo, I.; Yerbanga, R.S.; Nikiéma, F.; Neya, C.; Taho, L.K.; Ouédraogo, J.-B. Plasmodium Falciparum Msp1 and Msp2 Genetic Diversity and Allele Frequencies in Parasites Isolated from Symptomatic Malaria Patients in Bobo-Dioulasso, Burkina Faso. Parasit. Vectors 2018, 11, 323. [Google Scholar] [CrossRef]
- Bailey, J.A.; Berry, A.A.; Travassos, M.A.; Ouattara, A.; Boudova, S.; Dotsey, E.Y.; Pike, A.; Jacob, C.G.; Adams, M.; Tan, J.C.; et al. Microarray Analyses Reveal Strain-Specific Antibody Responses to Plasmodium falciparum Apical Membrane Antigen 1 Variants Following Natural Infection and Vaccination. Sci. Rep. 2020, 10, 3952. [Google Scholar] [CrossRef]
- Dodoo, D.; Atuguba, F.; Bosomprah, S.; Ansah, N.A.; Ansah, P.; Lamptey, H.; Egyir, B.; Oduro, A.R.; Gyan, B.; Hodgson, A.; et al. Antibody Levels to Multiple Malaria Vaccine Candidate Antigens in Relation to Clinical Malaria Episodes in Children in the Kasena-Nankana District of Northern Ghana. Malar. J. 2011, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Mathews, E.S.; Jezewski, A.J.; Odom John, A.R. Protein Prenylation and Hsp40 in Thermotolerance of Plasmodium falciparum Malaria Parasites. mBio 2021, 12, e00760-21. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.G.; King, L.D.W.; Pulido, D.; Quinkert, D.; Lias, A.M.; Silk, S.E.; Ragotte, R.J.; Davies, H.; Barrett, J.R.; McHugh, K.; et al. Development of an Improved Blood-Stage Malaria Vaccine Targeting the Essential RH5-CyRPA-RIPR Invasion Complex. Nat. Commun. 2024, 15, 4857. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Thompson, J.K.; Mackwell, K.L.; Browne, C.D.; Seager, B.A.; Ngo, A.; Lowes, K.N.; Silk, S.E.; Pulido, D.; King, L.D.W.; et al. RH5.1-CyRPA-Ripr Antigen Combination Vaccine Shows Little Improvement over RH5.1 in a Preclinical Setting. Front. Cell Infect. Microbiol. 2022, 12, 1049065. [Google Scholar] [CrossRef]
- Adu, B.; Cherif, M.K.; Bosomprah, S.; Diarra, A.; Arthur, F.K.N.; Dickson, E.K.; Corradin, G.; Cavanagh, D.R.; Theisen, M.; Sirima, S.B.; et al. Antibody Levels against GLURP R2, MSP1 Block 2 Hybrid and AS202.11 and the Risk of Malaria in Children Living in Hyperendemic (Burkina Faso) and Hypo-Endemic (Ghana) Areas. Malar. J. 2016, 15, 123. [Google Scholar] [CrossRef]
- van den Hoogen, L.L.; Walk, J.; Oulton, T.; Reuling, I.J.; Reiling, L.; Beeson, J.G.; Coppel, R.L.; Singh, S.K.; Draper, S.J.; Bousema, T.; et al. Antibody Responses to Antigenic Targets of Recent Exposure Are Associated with Low-Density Parasitemia in Controlled Human Plasmodium falciparum Infections. Front. Microbiol. 2019, 9, 3300. [Google Scholar] [CrossRef]
- Wu, L.; Hsiang, M.S.; Prach, L.M.; Schrubbe, L.; Ntuku, H.; Dufour, M.-S.K.; Whittemore, B.; Scott, V.; Yala, J.; Roberts, K.W.; et al. Serological Evaluation of the Effectiveness of Reactive Focal Mass Drug Administration and Reactive Vector Control to Reduce Malaria Transmission in Zambezi Region, Namibia: Results from a Secondary Analysis of a Cluster Randomised Trial. eClinicalMedicine 2022, 44, 101272. [Google Scholar] [CrossRef]
- Marchioni, J.; Seeger, A.; Barrett, J.R.; McHugh, K.; Kain, J.; MacGill, R.; Georgiou, G.; Draper, S.J.; Lavinder, J.J.; Ippolito, G.C. Characterizing the Plasma IgG Antibody Repertoire of Malaria-Naïve United Kingdom Adults Vaccinated with Novel Blood Stage Vaccine RH5.1/AS01 B. J. Immunol. 2023, 210, 141.18. [Google Scholar] [CrossRef]
- Macalinao, M.L.M.; Fornace, K.M.; Reyes, R.A.; Hall, T.; Bareng, A.P.N.; Adams, J.H.; Huon, C.; Chitnis, C.E.; Luchavez, J.S.; Tetteh, K.K.A.; et al. Analytical Approaches for Antimalarial Antibody Responses to Confirm Historical and Recent Malaria Transmission: An Example from the Philippines. Lancet Reg. Health West. Pac. 2023, 37, 100792. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Ogada, E.; Drakeley, C.; Marsh, K.; Bejon, P. Estimating Individual Exposure to Malaria Using Local Prevalence of Malaria Infection in the Field. PLoS ONE 2012, 7, e32929. [Google Scholar] [CrossRef]
- Dutta, S.; Dlugosz, L.S.; Drew, D.R.; Ge, X.; Ababacar, D.; Rovira, Y.I.; Moch, J.K.; Shi, M.; Long, C.A.; Foley, M.; et al. Overcoming Antigenic Diversity by Enhancing the Immunogenicity of Conserved Epitopes on the Malaria Vaccine Candidate Apical Membrane Antigen-1. PLoS Pathog. 2013, 9, e1003840. [Google Scholar] [CrossRef]
- Osier, F.H.A.; Weedall, G.D.; Verra, F.; Murungi, L.; Tetteh, K.K.A.; Bull, P.; Faber, B.W.; Remarque, E.; Thomas, A.; Marsh, K.; et al. Allelic Diversity and Naturally Acquired Allele-Specific Antibody Responses to Plasmodium falciparum Apical Membrane Antigen 1 in Kenya. Infect. Immun. 2010, 78, 4625–4633. [Google Scholar] [CrossRef]

| Parameters | Total | 1.5–5 Years | 5–12 Years | p-Value 2 |
|---|---|---|---|---|
| N = 474 1 | N = 188 1 | N = 286 1 | ||
| Gender (n, %) | 0.8 | |||
| M | 233 (49%) | 94 (50%) | 139 (49%) | |
| F | 241 (51%) | 94 (50%) | 147 (51%) | |
| Age (mean, range) | 6.42 (1.51–12.27) | 3.40 (1.51–4.98) | 8.40 (5.06–12.27) | <0.001 |
| Baseline asexual Pf infection (microscopy), (n, %) | 109 (23%) | 15 (8.0%) | 94 (33%) | <0.001 |
| Baseline asexual Pf parasite density (/µL of blood) * | 743.35 (492.47–1122.03) | 1350.34 (496.6–3675.79) | 675.81 (431.41–1058.66) | <0.001 |
| Pf infection by PCR (n, %) | 147 (31%) | 33 (18%) | 114 (40%) | <0.001 |
| Malaria Primary Case Definition | Malaria Secondary Case Definition | |||||
|---|---|---|---|---|---|---|
| Antigen | Crude IRR (95% CI) | IRR Adjusted for Age (95% CI) | p-Value for Adjusted IRR | Crude IRR (95% CI) | IRR Adjusted for Age (95% CI) | p-Value for Adjusted IRR |
| AMA1 | 1.32 (0.92–1.92) | 1.36 (0.94–2.00) | 0.06 | 1.27 (0.96–1.68) | 1.28 (0.97–1.71) | 0.06 |
| CSP | 0.84 (0.39–1.79) | 0.84 (0.39–1.78) | 0.66 | 0.72 (0.38–1.34) | 0.72 (0.38–1.34) | 0.31 |
| EBA175 RIII-V | 1.21 (0.55–2.73) | 1.23 (0.56–2.78) | 0.52 | 0.94 (0.49–1.77) | 0.94 (0.49–1.78) | 0.84 |
| EBA181 RIII-V | 0.67 (0.30–1.46) | 0.65 (0.29–1.43) | 0.34 | 0.62 (0.33–1.17) | 0.61 (0.32–1.16) | 0.17 |
| Etramp4 Ag2 | 0.50 (0.15–1.52) | 0.49 (0.15–1.48) | 0.23 | 0.65 (0.27–1.46) | 0.64 (0.27–1.45) | 0.32 |
| Etramp5 Ag1 | 0.57 (0.22–1.35) | 0.56 (0.22–1.34) | 0.20 | 0.53 (0.25–1.07) | 0.53 (0.25–1.06) | 0.08 |
| GEXP18 | 1.04 (0.50–2.16) | 1.03 (0.50–2.14) | 0.90 | 0.88 (0.48–1.59) | 0.88 (0.48–1.58) | 0.64 |
| GLURP R2 | 0.69 (0.29–1.61) | 0.70 (0.29–1.61) | 0.35 | 0.52 (0.26–1.01) | 0.52 (0.25–1.00) | 0.04 |
| HSP40 Ag1 | 0.65 (0.26–1.55) | 0.65 (0.26–1.54) | 0.38 | 0.49 (0.23–1.01) | 0.49 (0.22–1.00) | 0.07 |
| HYP2 | 0.86 (0.34–2.14) | 0.85 (0.34–2.13) | 0.76 | 0.71 (0.32–1.52) | 0.70 (0.32–1.51) | 0.40 |
| MSP2 CH150 | 1.10 (0.43–2.93) | 1.14 (0.44–3.06) | 0.75 | 0.98 (0.45–2.12) | 0.99 (0.45–2.14) | 0.98 |
| MSP2 Dd2 | 1.01 (0.40–2.54) | 1.01 (0.41–2.55) | 0.96 | 0.86 (0.41–1.79) | 0.86 (0.41–1.78) | 0.63 |
| Rh4.2 | 0.84 (0.37–1.89) | 0.85 (0.37–1.90) | 0.67 | 0.68 (0.34–1.31) | 0.68 (0.34–1.32) | 0.24 |
| Rh5.1 | 0.61 (0.21–1.57) | 0.62 (0.22–1.61) | 0.40 | 0.40 (0.16–0.93) | 0.40 (0.16–0.93) | 0.07 |
| SBP1 | 0.58 (0.18–1.72) | 0.57 (0.18–1.70) | 0.32 | 0.54 (0.21–1.27) | 0.54 (0.21–1.26) | 0.17 |
| SEA1 | 0.43 (0.14–1.21) | 0.43 (0.14–1.21) | 0.14 | 0.40 (0.17–0.92) | 0.40 (0.16–0.92) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezinmegnon, S.; Nébié, I.; Marlais, T.; Ouattara, D.; Diarra, A.; Patterson, C.; Tetteh, K.; Ouédraogo, A.; Drakeley, C.; Tiono, A.B.; et al. A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso. Vaccines 2025, 13, 1091. https://doi.org/10.3390/vaccines13111091
Ezinmegnon S, Nébié I, Marlais T, Ouattara D, Diarra A, Patterson C, Tetteh K, Ouédraogo A, Drakeley C, Tiono AB, et al. A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso. Vaccines. 2025; 13(11):1091. https://doi.org/10.3390/vaccines13111091
Chicago/Turabian StyleEzinmegnon, Sem, Issa Nébié, Tegwen Marlais, Daouda Ouattara, Amidou Diarra, Catriona Patterson, Kevin Tetteh, Alphonse Ouédraogo, Chris Drakeley, Alfred B. Tiono, and et al. 2025. "A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso" Vaccines 13, no. 11: 1091. https://doi.org/10.3390/vaccines13111091
APA StyleEzinmegnon, S., Nébié, I., Marlais, T., Ouattara, D., Diarra, A., Patterson, C., Tetteh, K., Ouédraogo, A., Drakeley, C., Tiono, A. B., & Sirima, S. B. (2025). A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso. Vaccines, 13(11), 1091. https://doi.org/10.3390/vaccines13111091






