Dual-Function Adjuvant Cyclosporin A: Enhancing RSV-Specific Humoral Immunity via Treg-Driven B-Cell Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Virus, and Infection
2.2. Vaccine Preparations and Immunization
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Viral Neutralizing Antibody Assay
2.5. Pulmonary Lymph Nodes and Spleen Isolation and Flow Cytometry
2.6. Treg Depletion
2.7. Treg, Tcon, and B-Cell Isolation and Co-Culture Assay
2.8. IL-10 and CD40L Blockade In Vitro and In Vivo
2.9. Statistical Analysis
3. Results
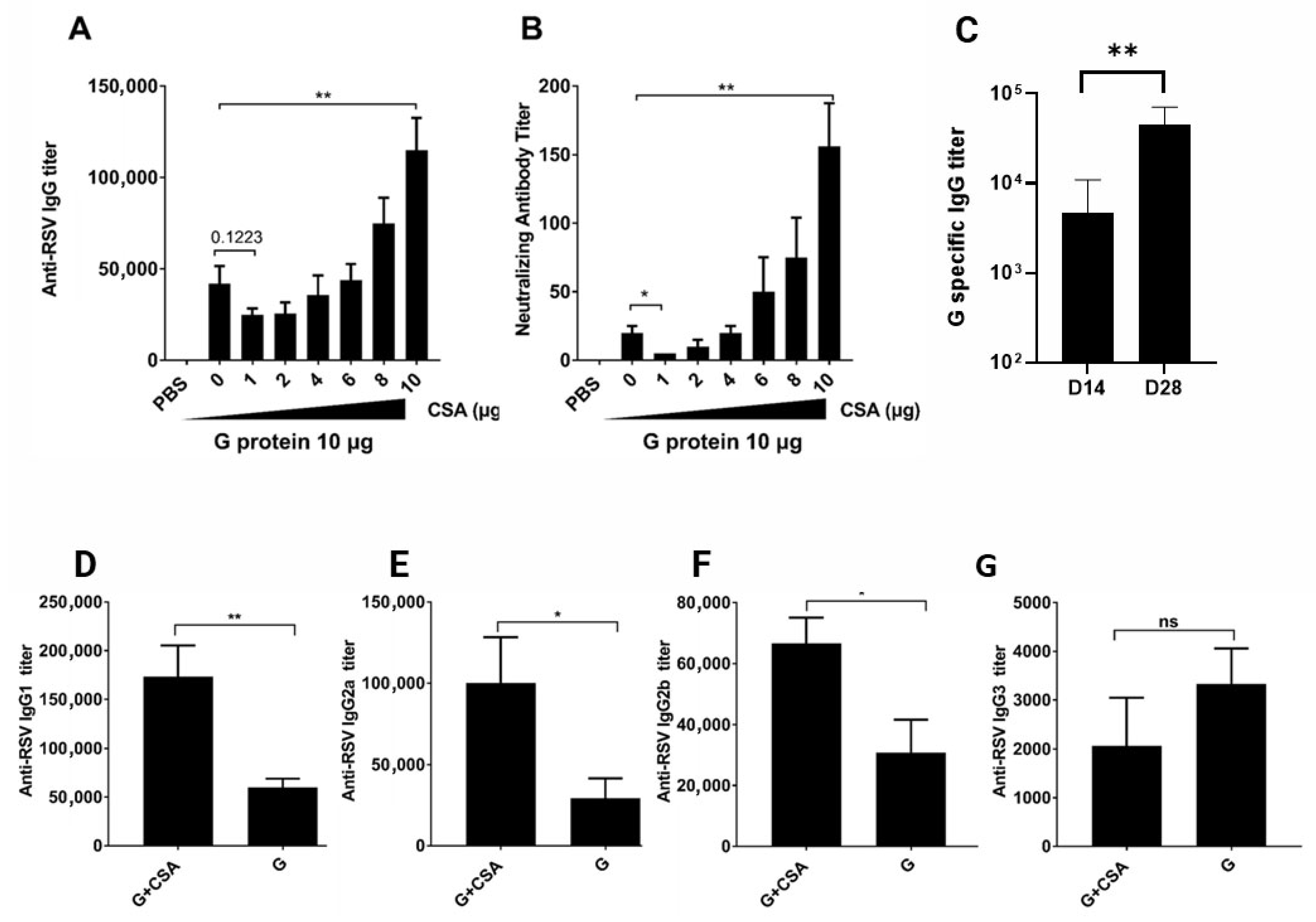
3.1. G+CsA Immunization Promotes RSV-Specific IgG Production and Isotype Switching
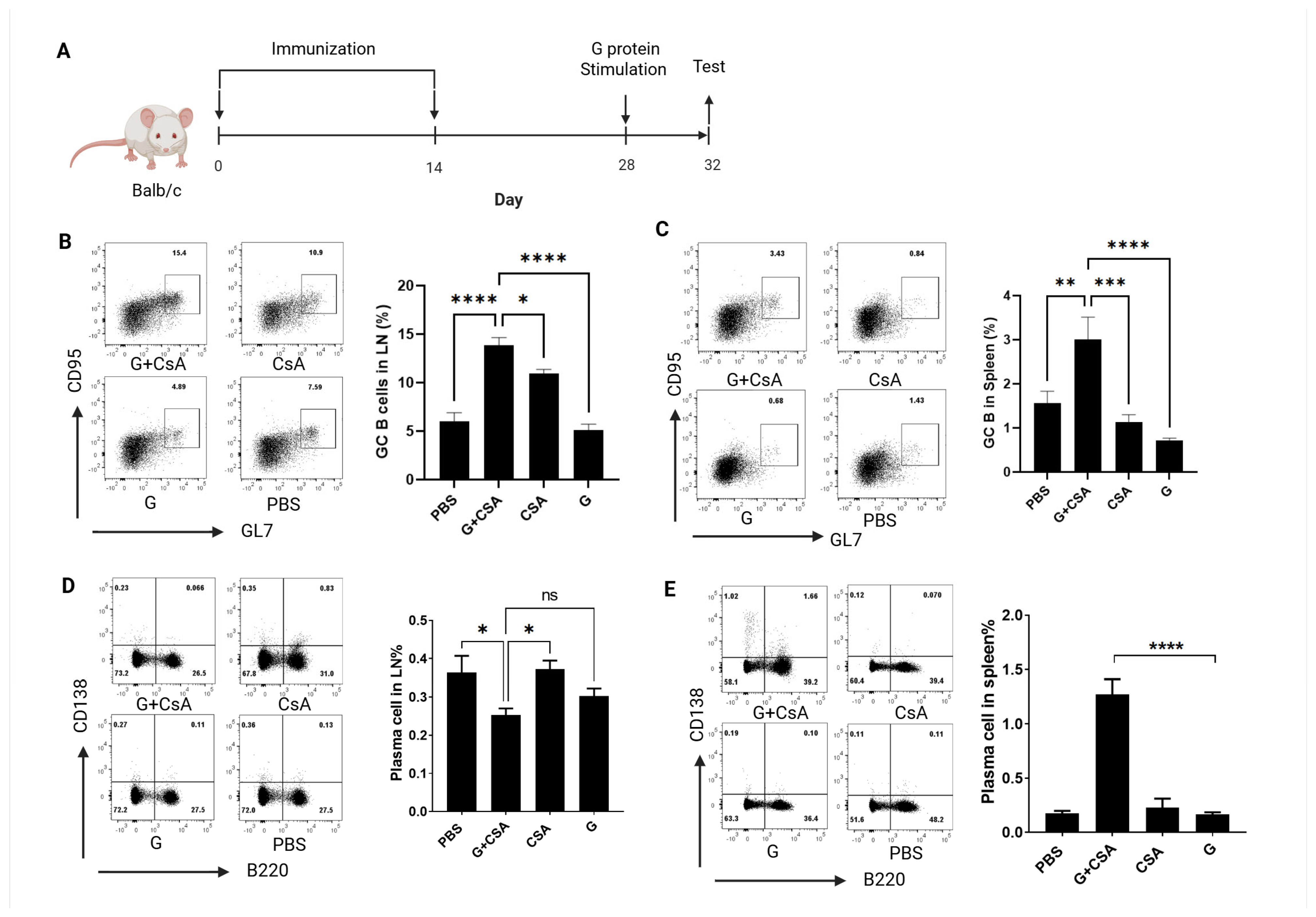
3.2. Effects of G+CsA Immunization on B-Cell Differentiation
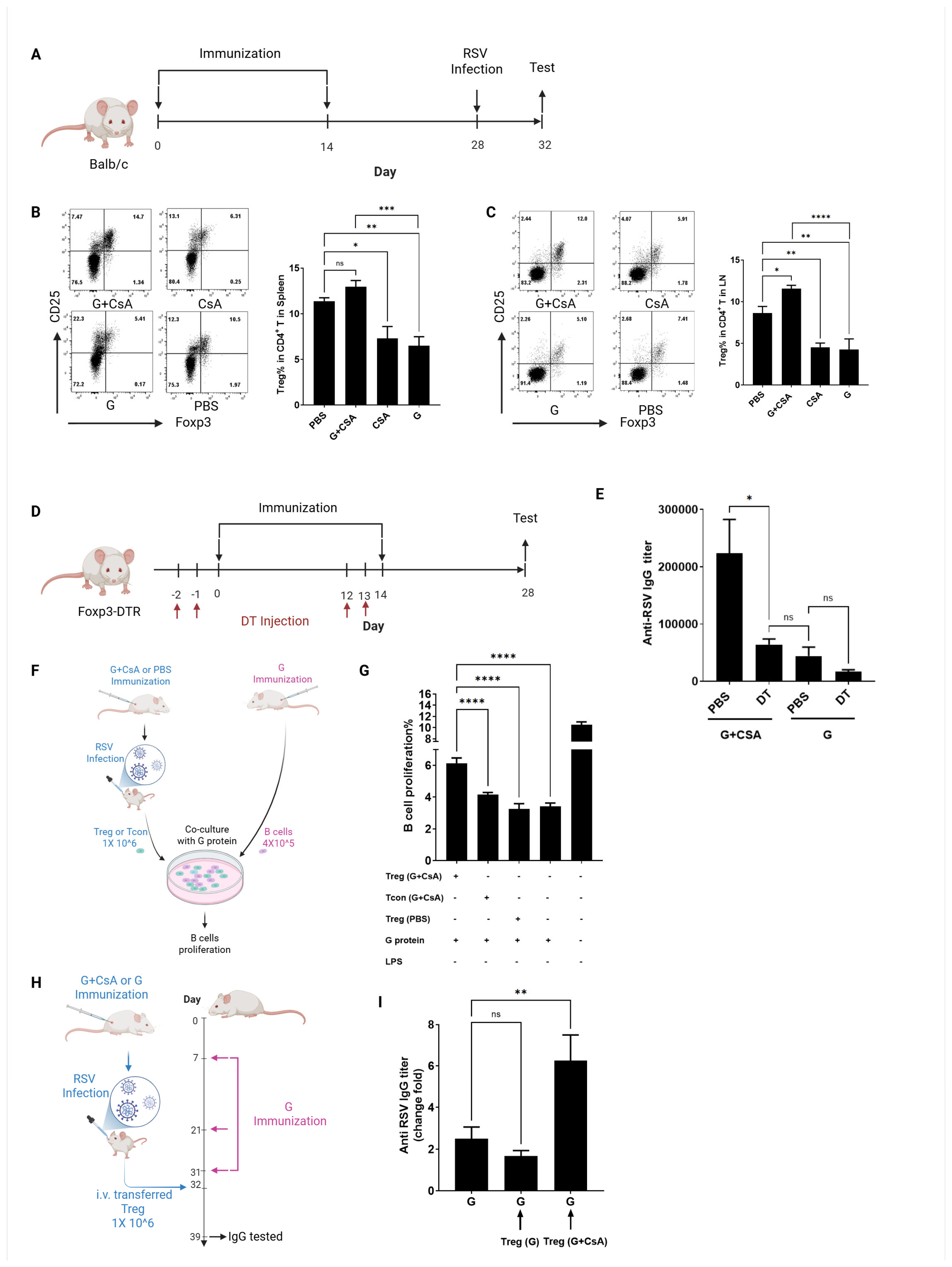
3.3. Treg Cells Are Critical for B-Cell Activation Post-G+CsA Immunization
3.4. Treg Cell-Derived CD40L and IL-10 in Drives B-Cell Differentiation into Plasma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMorrow, M.L.; Moline, H.L.; Toepfer, A.P.; Halasa, N.B.; Schuster, J.E.; Staat, M.A.; Williams, J.V.; Klein, E.J.; Weinberg, G.A.; Clopper, B.R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations in Children <5 Years: 2016–2022. Pediatrics 2024, 154, e2023065623. [Google Scholar] [CrossRef]
- Peng, R.; Chen, C.; Chen, Q.; Zhang, Y.; Huang, R.; Zhang, Y.; Li, J. Global progress in clinical research on human respiratory syncytial virus vaccines. Front. Microbiol. 2024, 15, 1457703. [Google Scholar] [CrossRef]
- Rainisch, G.; Adhikari, B.; Meltzer, M.I.; Langley, G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine 2020, 38, 251–257. [Google Scholar] [CrossRef]
- Bowser, D.M.; Rowlands, K.R.; Hariharan, D.; Gervasio, R.M.; Buckley, L.; Halasa-Rappel, Y.; Glaser, E.L.; Nelson, C.B.; Shepard, D.S. Cost of Respiratory Syncytial Virus Infections in US Infants: Systematic Literature Review and Analysis. J. Infect. Dis. 2022, 226 (Suppl. S2), S225–S235. [Google Scholar] [CrossRef]
- Matias, G.; Taylor, R.; Haguinet, F.; Schuck-Paim, C.; Lustig, R.; Shinde, V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health 2017, 17, 271. [Google Scholar] [CrossRef]
- Kulkarni, H.; Smith, C.M.; Lee Ddo, H.; Hirst, R.A.; Easton, A.J.; O’Callaghan, C. Evidence of Respiratory Syncytial Virus Spread by Aerosol. Time to Revisit Infection Control Strategies? Am. J. Respir. Crit. Care Med. 2016, 194, 308–316. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, e984–e991. [Google Scholar] [CrossRef]
- Weinberg, G.A. Respiratory syncytial virus mortality among young children. Lancet Glob. Health 2017, 5, e951–e952. [Google Scholar] [CrossRef]
- Piedimonte, G.; Perez, M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef]
- Sande, C.J. The long-term efficacy of a respiratory syncytial virus vaccine for older adults. Lancet Infect. Dis. 2024, 24, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Yoshizaki, K.; Watabe, S.; Ishige, M.; Hinoki, A.; Kondo, T.; Taguchi, T.; Hasegawa, H.; Hatata, T.; Tanuma, N.; et al. Safety, efficacy and pharmacokinetics of palivizumab in off-label neonates, infants, and young children at risk for serious respiratory syncytial virus infection: A multicenter phase II clinical trial. Lancet Reg. Health West. Pac. 2023, 39, 100847. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E.; Cutland, C.L., Jr.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef]
- Scotta, M.C.; Stein, R.T. Current strategies and perspectives for active and passive immunization against Respiratory Syncytial Virus in childhood. J. Pediatr. 2023, 99 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef]
- Madhi Shabir, A.; Polack Fernando, P.; Piedra Pedro, A.; Munoz Flor, M.; Trenholme Adrian, A.; Simões Eric, A.F.; Swamy Geeta, K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Wesley, C.; Winckworth, L.C. Respiratory syncytial virus vaccination in pregnancy is not effective enough at reducing infant infections. Arch. Dis. Child. Educ. Pract. Ed. 2022, 107, 389. [Google Scholar] [CrossRef]
- Knudson, C.J.; Hartwig, S.M.; Meyerholz, D.K.; Varga, S.M. RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets. PLOS Pathog. 2015, 11, e1004757. [Google Scholar] [CrossRef]
- Bigay, J.; Le Grand, R.; Martinon, F.; Maisonnasse, P. Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Front. Microbiol. 2022, 13, 932408. [Google Scholar] [CrossRef]
- Acosta Patricio, L.; Caballero Mauricio, T.; Polack Fernando, P. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin. Vaccine Immunol. 2016, 23, 189–195. [Google Scholar] [CrossRef]
- Christiaansen, A.F.; Knudson, C.J.; Weiss, K.A.; Varga, S.M. The CD4 T cell response to respiratory syncytial virus infection. Immunol. Res. 2014, 59, 109–117. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Dyer, K.D.; Domachowske, J.B. Respiratory viruses and eosinophils: Exploring the connections. Antivir. Res. 2009, 83, 1–9. [Google Scholar] [CrossRef]
- Habibi, M.S.; Jozwik, A.; Makris, S.; Dunning, J.; Paras, A.; DeVincenzo, J.P.; de Haan, C.A.M.; Wrammert, J.; Openshaw, P.J.M.; Chiu, C. Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am. J. Respir. Crit. Care Med. 2015, 191, 1040–1049. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor. Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef]
- Jordan, E.; Jenkins, V.; Silbernagl, G.; Chávez, M.P.V.; Schmidt, D.; Schnorfeil, F.; Schultz, S.; Chen, L.; Salgado, F.; Jacquet, J.-M.; et al. A multivalent RSV vaccine based on the modified vaccinia Ankara vector shows moderate protection against disease caused by RSV in older adults in a phase 3 clinical study. Vaccine 2024, 42, 126427. [Google Scholar] [CrossRef]
- Loebbermann, J.; Durant, L.; Thornton, H.; Johansson, C.; Openshaw, P.J. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.B.; Meyerholz, D.K.; Varga, S.M. Foxp3+ CD4 Regulatory T Cells Limit Pulmonary Immunopathology by Modulating the CD8 T Cell Response during Respiratory Syncytial Virus Infection. J. Immunol. 2010, 185, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Ochoa, J.L.; Kazemian, M.; Afzali, B. The role of transcription factors in shaping regulatory T cell identity. Nat. Rev. Immunol. 2023, 23, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Lu, Y.; Amezquita, R.A.; Weinstein, J.S.; Vander Heiden, J.A.; Gupta, N.T.; Kleinstein, S.H.; Kaech, S.M.; Craft, J. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci. Immunol. 2017, 2, eaan4767. [Google Scholar] [CrossRef]
- León, B.; Bradley, J.E.; Lund, F.E.; Randall, T.D.; Ballesteros-Tato, A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat. Commun. 2014, 5, 3495. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Su, C.; Li, C.; Zhou, X.; Zhao, W.; Zhong, Y.; He, Z.; Peng, H.; Dong, A.; et al. Neonatal priming and infancy boosting with a novel respiratory syncytial virus vaccine induces protective immune responses without concomitant respiratory disease upon RSV challenge. Hum. Vaccines Immunother. 2020, 16, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, X.; Zhong, Y.; Li, C.; Dong, A.; He, Z.; Zhang, S.; Wang, B. A Recombinant G Protein Plus Cyclosporine A-Based Respiratory Syncytial Virus Vaccine Elicits Humoral and Regulatory T Cell Responses against Infection without Vaccine-Enhanced Disease. J. Immunol. 2016, 196, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xu, L.; Wang, B.; Chen, A.; Zheng, G. Cutting edge: Immunosuppressant as adjuvant for tolerogenic immunization. J. Immunol. 2008, 180, 5172–5176. [Google Scholar] [CrossRef]
- Fedechkin, S.O.; George, N.L.; Wolff, J.T.; Kauvar, L.M.; DuBois, R.M. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci. Immunol. 2018, 3, eaar3534. [Google Scholar] [CrossRef]
- Caidi, H.; Miao, C.; Thornburg, N.J.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antivir. Res. 2018, 154, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruckwardt Tracy, J.; Chen, M.; Johnson Teresa, R.; Graham Barney, S. Characterization of Respiratory Syncytial Virus M- and M2-Specific CD4 T Cells in a Murine Model. J. Virol. 2009, 83, 4934–4941. [Google Scholar] [CrossRef][Green Version]
- Miroux, C.; Moralès, O.; Carpentier, A.; Dharancy, S.; Conti, F.; Boleslowski, E.; Podevin, P.; Auriault, C.; Pancré, V.; Delhem, N. Inhibitory Effects of Cyclosporine on Human Regulatory T Cells In Vitro. Transplant. Proc. 2009, 41, 3371–3374. [Google Scholar] [CrossRef]
- Nowosad, C.R.; Spillane, K.M.; Tolar, P. Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat. Immunol. 2016, 17, 870–877. [Google Scholar] [CrossRef]
- Bian, L.; Zheng, Y.; Guo, X.; Li, D.; Zhou, J.; Jing, L.; Chen, Y.; Lu, J.; Zhang, K.; Jiang, C.; et al. Intramuscular Inoculation of AS02-Adjuvanted Respiratory Syncytial Virus (RSV) F Subunit Vaccine Shows Better Efficiency and Safety Than Subcutaneous Inoculation in BALB/c Mice. Front. Immunol. 2022, 13, 938598. [Google Scholar] [CrossRef]
- Su, C.; Zhong, Y.; Zhao, G.; Hou, J.; Zhang, S.; Wang, B. RSV pre-fusion F protein enhances the G protein antibody and anti-infectious responses. npj Vaccines 2022, 7, 168. [Google Scholar] [CrossRef]
- Ersching, J.; Efeyan, A.; Mesin, L.; Jacobsen, J.T.; Pasqual, G.; Grabiner, B.C.; Dominguez-Sola, D.; Sabatini, D.M.; Victora, G.D. Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 2017, 46, 1045–1058.e6. [Google Scholar] [CrossRef]
- McCarron, M.J.; Park, P.W.; Fooksman, D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood 2017, 129, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Bonaparte, K.L.; Nason, M.C.; Graham, B.S. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 2009, 83, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Mangodt, T.C.; Van Herck, M.A.; Nullens, S.; Ramet, J.; De Dooy, J.J.; Jorens, P.G.; De Winter, B.Y. The role of Th17 and Treg responses in the pathogenesis of RSV infection. Pediatr. Res. 2015, 78, 483–491. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwenhuijze, A.; Liston, A. The Molecular Control of Regulatory T Cell Induction. Prog. Mol. Biol. Transl. Sci. 2015, 136, 69–97. [Google Scholar] [CrossRef]
- Connors, M.; Kulkarni, A.B.; Firestone, C.Y.; Holmes, K.L.; Morse, H.C., 3rd; Sotnikov, A.V.; Murphy, B.R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J. Virol. 1992, 66, 7444–7451. [Google Scholar] [CrossRef]
- Cassese, G.; Arce, S.; Hauser, A.E.; Lehnert, K.; Moewes, B.; Mostarac, M.; Muehlinghaus, G.; Szyska, M.; Radbruch, A.; Manz, R.A. Plasma Cell Survival Is Mediated by Synergistic Effects of Cytokines and Adhesion-Dependent Signals. J. Immunol. 2003, 171, 1684–1690. [Google Scholar] [CrossRef]
- Bryant, V.L.; Ma, C.S.; Avery, D.T.; Li, Y.; Good, K.L.; Corcoran, L.M.; de Waal Malefyt, R.; Tangye, S.G. Cytokine-Mediated Regulation of Human B Cell Differentiation into Ig-Secreting Cells: Predominant Role of IL-21 Produced by CXCR5+ T Follicular Helper Cells. J. Immunol. 2007, 179, 8180–8190. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Tangye, S.G.; Tarlinton, D.M. Memory B cells: Effectors of long-lived immune responses. Eur. J. Immunol. 2009, 39, 2065–2075. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Cyster, J.G. Transcriptional regulation of memory B cell differentiation. Nat. Rev. Immunol. 2021, 21, 209–220. [Google Scholar] [CrossRef]
- Fooksman, D.R.; Jing, Z.; Park, R. New insights into the ontogeny, diversity, maturation and survival of long-lived plasma cells. Nat. Rev. Immunol. 2024, 24, 461–470. [Google Scholar] [CrossRef]
- Smit, J.J.; Rudd, B.D.; Lukacs, N.W. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2006, 203, 1153–1159. [Google Scholar] [CrossRef]
- Han, R.; Wang, T.; Cheng, X.; Bing, J.; Li, J.; Deng, Y.; Shan, X.; Zhang, X.; Wang, D.; Sun, S.; et al. Immune Responses and Protection Profiles in Mice Induced by Subunit Vaccine Candidates Based on the Extracellular Domain Antigen of Respiratory Syncytial Virus G Protein Combined with Different Adjuvants. Vaccines 2024, 12, 686. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhong, Y.; Zhang, S.; Su, C.; Zhao, G.; Wang, B. Dual-Function Adjuvant Cyclosporin A: Enhancing RSV-Specific Humoral Immunity via Treg-Driven B-Cell Activation. Vaccines 2025, 13, 997. https://doi.org/10.3390/vaccines13100997
Li C, Zhong Y, Zhang S, Su C, Zhao G, Wang B. Dual-Function Adjuvant Cyclosporin A: Enhancing RSV-Specific Humoral Immunity via Treg-Driven B-Cell Activation. Vaccines. 2025; 13(10):997. https://doi.org/10.3390/vaccines13100997
Chicago/Turabian StyleLi, Chaofan, Yiwei Zhong, Shuren Zhang, Caixia Su, Gan Zhao, and Bin Wang. 2025. "Dual-Function Adjuvant Cyclosporin A: Enhancing RSV-Specific Humoral Immunity via Treg-Driven B-Cell Activation" Vaccines 13, no. 10: 997. https://doi.org/10.3390/vaccines13100997
APA StyleLi, C., Zhong, Y., Zhang, S., Su, C., Zhao, G., & Wang, B. (2025). Dual-Function Adjuvant Cyclosporin A: Enhancing RSV-Specific Humoral Immunity via Treg-Driven B-Cell Activation. Vaccines, 13(10), 997. https://doi.org/10.3390/vaccines13100997





