1. Introduction
Poliomyelitis is an acute infectious disease caused by one of the three poliovirus serotypes (types 1, 2, or 3). Prior to the introduction of vaccination, nearly all children contracted poliovirus, and approximately 1 in 200 infections resulted in irreversible paralysis. However, the implementation of widespread immunization programs has led to significant progress in controlling the disease [
1]. Nevertheless, the emergence of virulent circulating vaccine-derived polioviruses (cVDPVs) from oral Sabin vaccines remains a concern, as they can cause vaccine-associated paralytic poliomyelitis in a small proportion of recipients [
2,
3]. This highlights the need for improved methods to assess vaccine quality [
4].
Immunologically, poliovirus particles of types 2 and 3 are classified into two native forms: D-antigen, representing infectious, mature virions, and H-antigen (is also called C-antigen), representing non-infectious, immature particles [
5]. D-antigen particles are composed of 60 copies of four capsid proteins: VP1, VP2, VP3, and VP4 (with VP4 located on the inner surface of the capsid) [
6]. VP2 and VP4 result from the cleavage of the precursor protein VP0 [
6,
7,
8]. In contrast, H-antigen particles lack genomic RNA and consist of VP0, VP1, and VP3 [
9]. Only D-antigen particles are thought to be capable of inducing neutralizing antibodies, making their quantification essential for evaluating the immunogenic potency of poliovirus vaccines [
10,
11].
However, while D-antigen particles are critical for generating neutralizing antibodies, H-antigen particles can still play a role in modulating immune responses [
5,
12,
13].
Also D-antigen can be converted to “imitative” form of H-antigen by various environmental stress conditions, including high temperatures [
14,
15]. For example in the article Rezapkin G, etc., D-antigen was heat for 1 h at 56 °C to form H-antigen [
16].
Among the available methods for quantifying D-antigen units in poliovirus serotypes 1, 2, and 3, the sandwich enzyme-linked immunosorbent assay (ELISA) is the most widely employed due to its sensitivity and adaptability [
17,
18]. In such assays, the specificity of antibodies is critical, as cross-reactivity with H-antigen can compromise the accuracy of results [
16,
19].
It is noteworthy that many laboratories rely on monoclonal antibodies in D-antigen ELISA [
20,
21]. However, targeting a single epitope may not adequately reflect the overall antigenic activity of the vaccine. Although numerous neutralizing epitopes have been identified using monoclonal antibodies, there is still limited knowledge about specific epitopes that confer protective immunity. While combining multiple monoclonal antibodies may offer broader epitope coverage, it also increases assay complexity and cost [
16,
22].
To address these limitations, we utilized polyclonal antibodies raised against attenuated Sabin strains types 1, 2, and 3, enabling recognition of a broad spectrum of viral epitopes. Previous studies have demonstrated that inactivated poliovirus particles can undergo epitope modification or partial destruction, which may reduce the binding efficiency of monoclonal antibodies—further supporting the use of polyclonal antibodies [
23]. This reaffirms the advantage of using polyclonal antibodies.
The selection of attenuated Sabin strains was motivated by the WHO’s recommendation to transition IPV production from wild-type to Sabin strains for enhanced biosafety [
24,
25]. Additionally, antigenic differences between wild and attenuated strains may influence antibody binding and vaccine performance [
26].
In this study, we adapted our poliovirus purification method for isolating purified D- and H-antigens of Sabin strain polioviruses types 1, 2, and 3. These antigens were subsequently used to immunize rabbits, resulting in the production of specific polyclonal antibodies. The resulting antibodies are suitable for ELISA-based quantification of both D- and H-antigens in vaccine products and stability testing.
2. Materials and Methods
2.1. Purification of D- and H-Antigens of Poliovirus Strain Sabin Types 1, 2 and 3
FSUE “Institute of Poliomyelitis and Viral Encephalitis named after M.P. Chumakov” in October 1999 received 15 ampoules of the WHO Vero RCB 10–87 of the Vero cell line. Directly from the cell suspension of vial No.0519 of this bank (without making the seed bank of the Manufacturer’s cell line), a working bank of the Vero cell line (Vero WCB) was prepared at passage 139. In this study, the Vero WHO RCB 10–87 cell line (passages 141–149) was used for poliovirus production. Poliovirus strains Sabin type 1 (LSc 2ab), type 2 (P712 Ch 2ab), and type 3 (Leon 12a1b) were used for the antigen production. Vero cells (inoculation densities 0.2 ± 0.05 × 106 cells/mL) were cultivated in single-use bioreactors (Cytiva, Marlborough, MA, USA and Sartorius Stedim, Aubagne, France) in EMEM (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis, Moscow, Russia) with fetal bovine serum (FBS, 5%, Gibco, Thermo Fisher Scientific, Waltham, MA, USA, cat. #10091148) on microcarriers Cytodex 1 (Cytiva, Marlborough, MA, USA) with concentration 3 g/L). To ensure optimal culture conditions, dissolved oxygen (DO), temperature, and pH were maintained at 70%, 37 °C, and 7.2, respectively. After reaching confluency, the Vero cell monolayer was washed with Hank’s buffer solution. Then, buffer solution was changed to M199 (FSASI “Chumakov FSC R&D IBP RAS”, Moscow, Russia). The cells were infected with 0.01–0.1 TCID50/cell MOI. Vero cells were incubated with the virus at 34 °C until complete monolayer degeneration was observed.
Virus particles produced in Vero cells were used as antigens for immunization. To obtain crude virus preparations, culture supernatants were cleared of cell debris by centrifugation at 17.050 rcf for 30 min at 4 °C using an Optima XPN-100 ultracentrifuge (Beckman Coulter, Brea, CA, USA). The clarified supernatant was then subjected to ultracentrifugation through a 3 mL 30% sucrose cushion (30% sucrose [cat. #S0389, Sigma-Aldrich, St. Louis, MO, USA], 1 M NaCl [cat. #S9888, Sigma-Aldrich, St. Louis, MO], and 20 mM Trizma, pH 7.5 [cat. #T7818, Sigma-Aldrich, St. Louis, MO]) at 106.559 rcf for 4 h at 4 °C. The resulting pellet was resuspended in 3 mL of 20 mM PBS (pH 7.2, FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)).
To remove residual lipids, 1.5 volumes of trichlorotrifluoroethane (CFC-113, cat. #48411, Sigma-Aldrich, St. Louis, MO) were added to the virus suspension, followed by vigorous mixing for 2–3 min on a vortex. The mixture was centrifuged at 986 rcf for 5 min at 4 °C, and the upper aqueous phase containing the virus was collected (Eppendorf Research, London, UK, 5810R).
Further purification was achieved by ultracentrifugation in a cesium chloride density gradient. Specifically, 3.265 mL of virus suspension was mixed with 1.735 mL of saturated cesium chloride solution (cat. #15554, Serva, Heidelberg, Germany; density 1.34 g/cc) and centrifuged at 181.799 rcf for 16–18 h at 4 °C (Optima XPN-100, Beckman Coulter). This procedure resulted in the formation of two distinct visible bands: the lower band corresponding to D-antigen and the upper band to H-antigen. Fractions of 200–300 μL were collected in wells of a 96-well plate after piercing the bottom of the tube with a needle from a disposable syringe. To identify protein-rich fractions, 3 µL of each collected fraction was applied to a nitrocellulose membrane and stained with Ponceau S solution. Ponceau S solution was poured onto the membrane for 30–40 s and the membrane was washed with water. The fractions with the highest protein content were pooled.
Desalting of antigen-containing fractions was performed by gel filtration chromatography on Bio-Gel P-2 columns (cat. #1504118, Bio-Rad, Hercules, CA, USA) using 20 mM PBS (pH 7.0) as the mobile phase.
As a result, five antigen preparations were obtained: D-antigen from Sabin type 1; D- and H-antigens from Sabin types 2 and 3. These were designated as Sabin 1, Sabin 2D, Sabin 2H, Sabin 3D, and Sabin 3H, respectively.
2.2. Transmission Electron Microscopy
Antigen-containing fractions were adsorbed onto glow discharged Carbon/Formvar-coated copper grids (Formvar/Carbon 200 Mesh, 3–4 nm carbon, FCF200-CU-SB, Electron Microscopy Sciences, Hatfield, PA, USA) for 5 min. The grids were then washed with 1 M EDTA (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)), followed by staining with 2% uranyl acetate for 2 min (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)), air-dried and examined in a transmission electron microscope JEM-100C (Jeol, Tokyo, Japan), magnification: X30000. The size of the obtained viral particles was determined using BioVision 4.0 (West Medica, Wiener Neudorf, Austria).
2.3. SDS-PAGE Under Denaturing Conditions
Poliovirus antigen preparations were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 20% polyacrylamide gels under denaturing conditions, in accordance with OFS.1.2.1.0023.15 Polyacrylamide Gel Electrophoresis, State Pharmacopoeia of the Russian Federation XIV, Volume 1. Electrophoresis was performed using a Mini-PROTEAN Tetra Cell system (Bio-Rad, USA). Protein bands were visualized by staining with Coomassie Brilliant Blue.
2.4. Lowry Assay
Protein concentrations in antigen and antibody samples were determined using the Lowry assay, in accordance with OFS.1.2.3.0012.15 Determination of Protein, State Pharmacopoeia of the Russian Federation XIV, Volume 1. Optical density at 750 nm (OD750) was measured using a Multiskan FC microplate spectrophotometer (ThermoFisher, Waltham, MA, USA). Bovine serum albumin (BSA; FSBI “SCEEMP” of the Ministry of Health of Russia) was used as a standard and serially diluted to final concentrations ranging from 200 to 10 µg/mL. A standard calibration curve was constructed in Microsoft Excel by plotting the average blank-corrected absorbance values at 750 nm against BSA concentrations (µg/mL), and was subsequently used to calculate the protein concentrations of the test samples.
2.5. Preparation of Rabbit Polyclonal Serum Against D- and H-Antigens of Poliovirus Strain Sabin Types 1, 2 and 3 and IgG Purification
Five rabbits of the Soviet Chinchilla breed were immunized twice with the obtained antigens Sabin 1, Sabin 2D, Sabin 2H, Sabin 3D, Sabin 3H, respectively (concentration range 170–560 µg/mL). During primary and second immunization, pure antigen was injected subcutaneously in a volume of 1 mL mixed with 300 μL complete Freund’s adjuvant (Calbiochem Research Biochemicals, Merck KGaA, Darmstadt, Hesse, Germany). A 21-day interval was maintained between immunizations. Following each immunization, control serum samples were collected no earlier than 14 days post-immunization, after which a neutralization assay was conducted. Upon reaching satisfactory titers of neutralizing antibodies (not less than 1:1024 for the D-antigen), a total blood sampling was carried out. IgG (against antigens Sabin 1, Sabin 2D, Sabin 2H, Sabin 3D, Sabin 3H) were isolated and purified from each serum sample by affinity chromatography on HiTrap columns filled with Protein G HP (cat #17-0405-01, Cytiva). Antibodies were eluted with 0.1 M Glycine-HCl buffer pH 2.7 (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)) and pH immediately was adjusted to pH 7 by adding 1 M Tris-HCl pH 9 (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)).
2.6. Neutralization Assay
The identification of titers of neutralizing antibodies was performed using a neutralization assay according to the standard WHO protocol [
27].
2.7. Biotin-Conjugate Preparation
For storage purposes, purified antibodies were dialyzed into 0.1 M carbonate–bicarbonate buffer (cat. #C3041, Sigma-Aldrich, St. Louis, MO). For ELISA applications, a portion of these antibodies was conjugated with (+)-biotin N-hydroxysuccinimide ester (cat. #35013-72-0, Sigma-Aldrich, St. Louis, MO). All antibody preparations were adjusted to a concentration of 2 mg/mL. To achieve a biotin-to-IgG molar ratio of approximately 5:1, 300 µL of 1 mg/mL biotin ester (dissolved in DMSO) was added to each 10 mL of purified antibody solution. The conjugation was carried out according to the manufacturer’s instructions. Following biotinylation, the conjugated antibodies were dialyzed into 20 mM phosphate-buffered saline (PBS), pH 7.0.
2.8. ELISA Procedure
High-binding 96-well plates (Corning, NY, USA, cat. #9018) were coated with 90 µL of type-specific polyclonal rabbit IgG (concentration in range of 3–5 mg/mL, diluted 1:500 in 0.05 M carbonate–bicarbonate buffer, cat. #C3041, Sigma-Aldrich, St. Louis, MO) and incubated overnight at 4 °C. The following day, wells were blocked with 200 µL of blocking buffer (20 mM PBS containing 1% FBS, Gibco, cat. #10091148) for 1 h at 37 °C to prevent nonspecific binding. After each step, plates were washed twice with washing buffer (20 mM PBS with 0.05% Tween-80, cat. #P1379, Sigma-Aldrich, St. Louis, MO).
Next, 90 µL of D- or H-antigen diluted in dilution buffer (20 mM PBS, 0.05% Tween-80, and 1% FBS) was added to each well in a three-step dilution series. Wells containing dilution buffer alone served as background controls. Plates were incubated for 2 h at 37 °C.
Following this, 90 µL of biotin-conjugated polyclonal rabbit IgG (concentration in range of 1.2–1.9 mg/mL diluted 1:200 in dilution buffer) was added and incubated for 1 h at 37 °C. Then, 90 µL of streptavidin–peroxidase conjugate (cat. #S5512, Sigma-Aldrich, St. Louis, MO; diluted 1:10,000 in dilution buffer) was added to each well and incubated for 30 min at 37 °C.
For color development, 90 µL of TMB substrate (cat. #T8665, Sigma-Aldrich, St. Louis, MO) was added to each well and incubated at room temperature in the dark for 5–15 min. The reaction was stopped by adding 45 µL of stop solution (2 M H2SO4, Honeywell, Perth, Australia, cat. #00646), and absorbance was measured at 450 nm using a Multiskan FC Microplate Spectrophotometer (Thermo Fisher Scientific, USA).
2.9. Poliovirus RNA Extraction
Poliovirus RNA was extracted from PV samples using QIAamp Viral RNA Mini Kit (cat #52904, Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. The concentration of extracted viral RNA samples was determined by using a spectrophotometer at 260 nm (iMark microplate absorbance reader, BioRad, Singapore).
2.10. Numerical and Statistical Analysis of Data
All calculations were performed by using standard formulas in Microsoft Excel (linear regression, standard deviation, etc.). To demonstrate antibody specifity for the D- and H- antigens, graphs were plotted showing the mean absorbance at 450 nm (minus background) versus reverse dilution of antigens. All data are presented as averages with standard deviation. Multiple comparisons in two-way ANOVA were used to compare the differences between viruses in each specific dilution in ELISA with the scientific software GraphPad Prism version 8.2.1.
2.11. Biosafety and Biosecurity Measures
FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis), Moscow, Russia, is an OPV producer and scientific research institute. It is fully accredited by the national authorities for work with BSL 1–3 agents. Additionally, it has a Certificate of participation in poliovirus containment (RUS-CP-20191202-007) issued by GCC.
4. Discussion
The efficacy of poliovirus vaccines critically depends on the precise quantification of the immunogenic D-antigen, which is responsible for eliciting neutralizing antibodies. Accurate measurement of the D-antigen is essential for vaccine evaluation, particularly due to its potential conversion into the non-protective H-antigen.
For vaccine quality control, traditionally D-antigen ELISA is used. It is worth mentioning that polyclonal antibody-based ELISA, such as the one developed in our study, and monoclonal antibody-based ELISA (widespread D-antigen detection method) represent two distinct technical paradigms for antigen detection. Each approach carries advantages and limitations that must be carefully considered in the context of vaccine quality control.
Polyclonal antibodies constitute a heterogeneous mixture of immunoglobulin molecules that recognize multiple epitopes across the target antigen, while monoclonal antibodies represent a homogeneous population of immunoglobulins that bind to a single, specific epitope. This broad recognition profile of polyclonal antibodies confers a significant advantage in detecting potentially diverse antigenic configurations that may arise from slight variations in vaccine preparation or viral strain differences. Diversity in polyclonal antibodies makes the detection system more robust against minor structural variations in the D-antigen, which is particularly valuable when working with Sabin strains that may exhibit subtle structural differences compared to wild-type poliovirus strains. In contrast, monoclonal antibody-based ELISA offer specificity for a single epitope, providing precision in targeting a defined antigenic determinant. This high specificity can be advantageous when targeting a functionally critical epitope.
Our polyclonal antibody-based ELISA offers substantial cost advantages in initial development. In contrast, the initial development phase for monoclonal antibodies usually takes several months and requires significant personnel resources for hybridoma generation, screening, and cloning. However, once developed, monoclonal antibodies can be produced indefinitely from stable cell lines.
One of the most significant challenges associated with polyclonal antibody-based ELISA is the inherent batch-to-batch variability. Each animal immunization event produces a unique antibody repertoire, even when using identical antigen preparations and immunization protocols. This variability requires strict quality control measures and batch validation protocols to minimize this variability.
Each approach offers distinct advantages that must be weighed against specific application requirements, resource constraints, and performance priorities. Our polyclonal antibody-based ELISA demonstrates particular strengths in detection robustness and cost-effectiveness for initial implementation, making it especially suitable for quality control applications where broad antigen recognition and rapid method development are prioritized.
Despite the fact that the primary method for detecting and quantifying D-antigen in poliovirus vaccine production is the ELISA alternative methods are being developed. For example, surface plasmon resonance (SPR) technology measures binding interactions in real time without the need to label the interacting molecules [
28].
Among available methods, ELISA remains the gold standard for D-antigen quantification owing to its high sensitivity and specificity, ensuring the reliability of vaccine potency assessment.
In this study, we developed and characterized a panel of ELISA systems for the detection and differentiation of poliovirus D- and H-antigens derived from Sabin strains. Our approach was based on the purification of structurally distinct viral particles from Vero cell-derived preparations using cesium chloride gradient ultracentrifugation. The D-antigen fractions corresponded to mature, genome-containing virions, while the H-antigen fractions contained immature, genome-free empty capsids, as confirmed by electron microscopy, SDS-PAGE profiling of capsid proteins, and RNA quantification.
The ELISA systems based on polyclonal antibodies raised against D- or H-antigens of poliovirus Sabin strains exhibited high serotype and antigen-form specificity. Each D-antigen-specific ELISA (types I-D, II-D, III-D) demonstrated significantly stronger reactivity with its homologous antigen compared to heterologous serotypes, indicating minimal cross-reactivity.
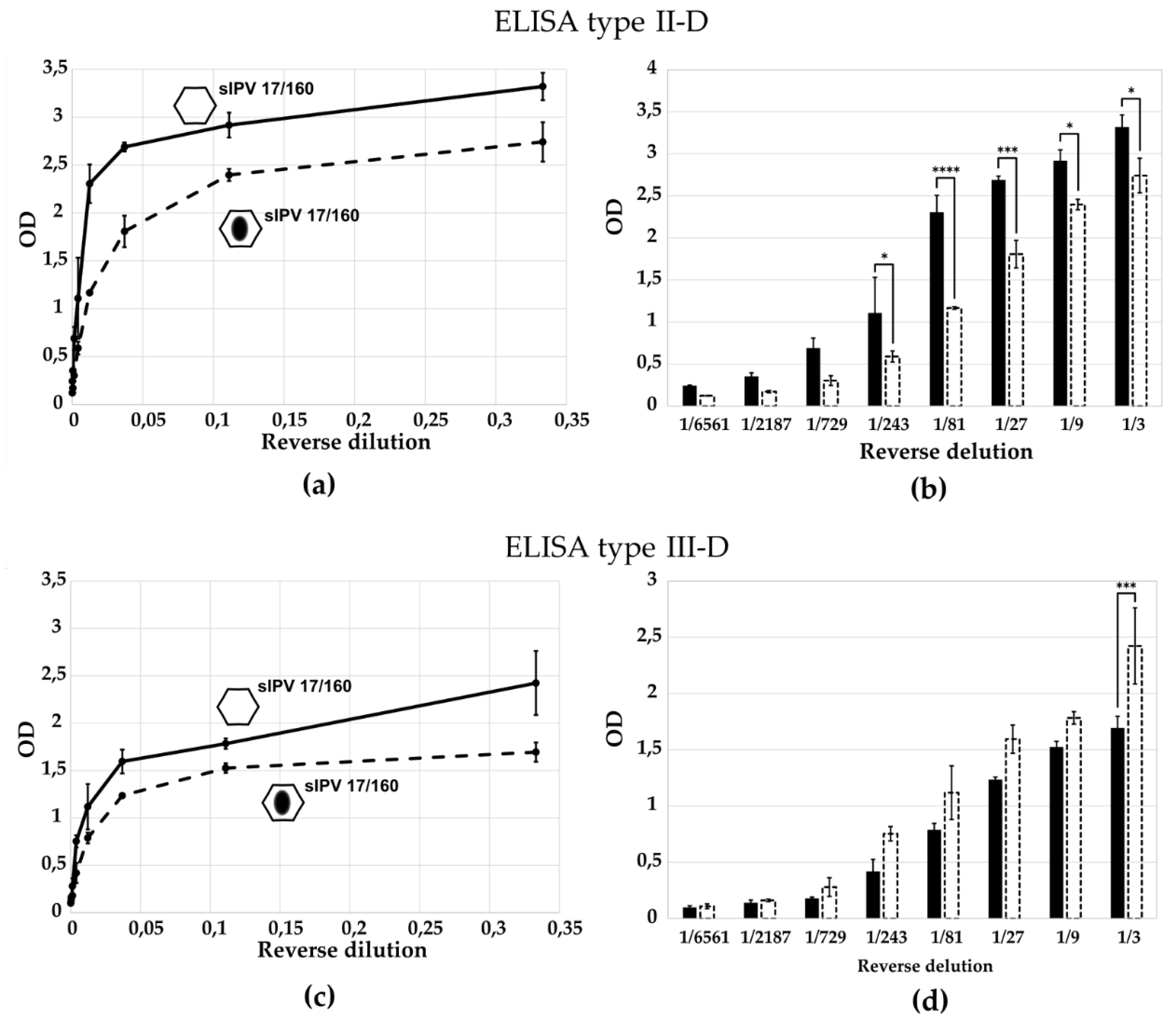
ELISA targeting D-antigens clearly discriminated between native and H-antigen (heat-treated:
Supplemental Figure S3 and
Figure 7) or between native and H-antigen (empty capsid:
Figure 5) preparations, confirming their ability to selectively detect immuno-genic virions.
ELISA test systems constructed with antibodies against H-antigen showed preferential binding to heat-treated (
Supplemental Figure S3 and
Figure 8) or empty capsid-rich (
Figure 6) preparations, while displaying minimal cross-reactivity with D-antigen-containing virions.
It was shown the specificity of the ELISA tests for sIPV 17/160 (from Sabin strains) in form of D-antigen and H-antigen (by the heat treatment). This specificity emphasizes the critical role of D-antigens in eliciting an effective immune reaction, particularly in the context of vaccine development. Furthermore, the heat treatment not only affects the antigenic profile but also raises questions regarding the stability and efficacy of the vaccine. These findings underscore the importance of optimizing vaccine preparation processes to ensure that the immunogenic properties are preserved. It is worth mentioning that for ELISA type III-D test the difference in the optical density value between D- and H-form sIPV 17/160 is statistically significant only for the first virus dilution (
Figure 7c,d). Probably the reason is the sufficient stability of Sabin type 3 in sIPV 17/160 and heat treatment at 56 °C for 1 h is not enough for the formation of H-antigens. Therefore, we did not observe a significant difference in this case, likely reflecting the relative thermal stability of Sabin type 3 particles in sIPV 17/160.
Also it was shown greater specificity of the developed ELISA tests in recognizing the standard sIPV 17/160 than in recognizing IPV 12/104. The observed differences in assay highlight the need for tailored potency testing approaches, as traditional assays developed for wild-type IPV may not adequately reflect the potency of sIPV vaccines.
ELISA tests for D-antigen reliably measure the antigen content in samples of trivalent sIPV. The system is satisfactory and can be further used to control vaccine production. However, to accurately quantify D-antigen in the presence of H-antigen (and vice versa) further experiments are needed. Completion of the validation of the obtained ELISA systems for D- and H-antigens is our goal for further research.
Overall, our results demonstrate that the developed ELISA are capable of accurately detecting and distinguishing D- and H-antigen forms of poliovirus across all three Sabin serotypes. They provide a valuable tool for assessing antigen quality and content in Sabin-based IPV formulations and support the need for homologous antigen–antibody systems when evaluating vaccine potency. The ability to selectively detect H-antigens also enables monitoring of non-immunogenic components that may affect vaccine efficacy or stability, thereby contributing to improved quality control and standardization in sIPV production.