Immune Persistence Following a Single Dose of Varicella Vaccine: 5-Year and 8-Year Follow-Up of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Vaccines
2.4. Immunogenicity Assessment
2.5. Sample Size Determination and Statistical Analysis
3. Results
3.1. Study Participants
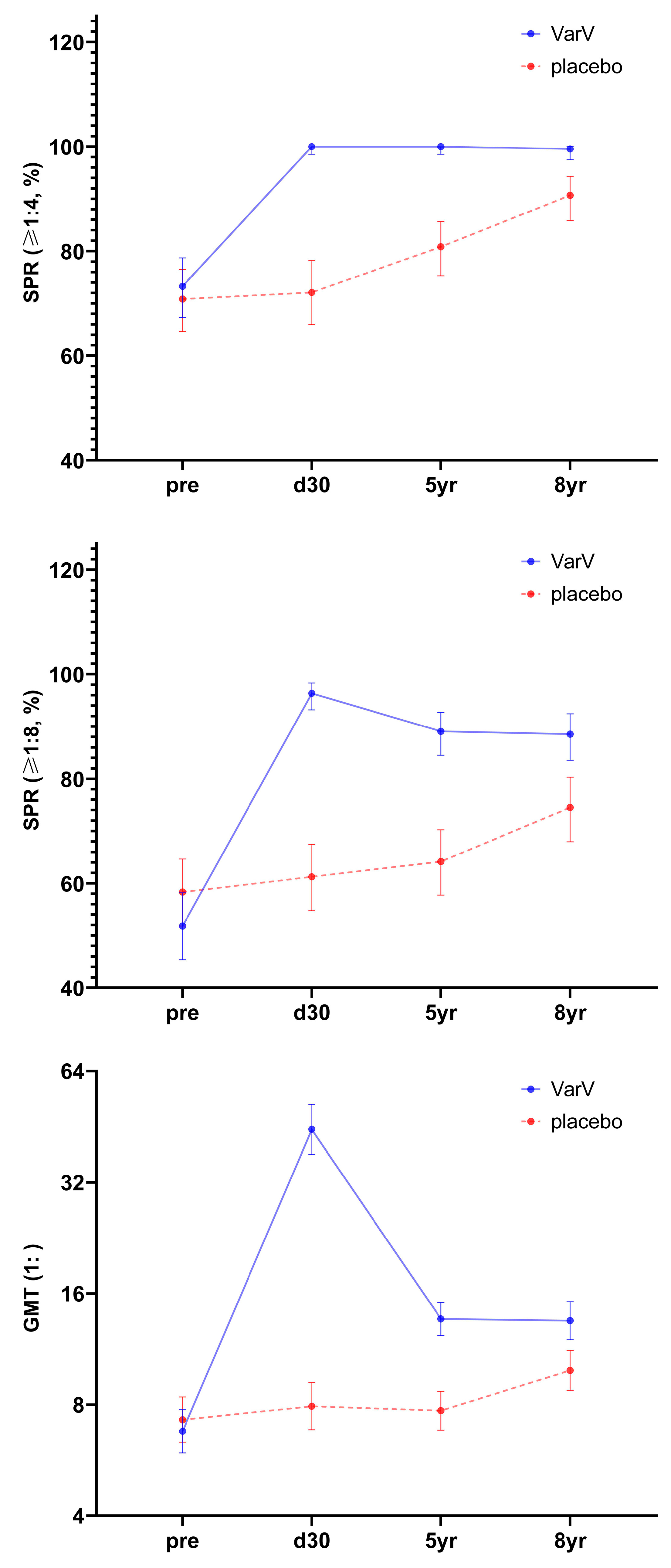
3.2. Immunogenicity and Immune Persistence in Overall Population
3.2.1. 5-Year Immune Persistence Analysis
3.2.2. 8-Year Immune Persistence Analysis
3.2.3. Full Series Immune Persistence Analysis
3.3. Immunogenicity and Immune Persistence in Different Age Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef]
- Fiordelisi, D.; Poliseno, M.; De Gennaro, N.; Milano, E.; Santoro, C.R.; Segala, F.V.; Franco, C.F.; Cesari, G.M.; Frallonardo, L.; Guido, G.; et al. Varicella-Zoster Virus Reactivation and Increased Vascular Risk in People Living with HIV: Data from a Retrospective Cohort Study. Viruses 2023, 15, 2217. [Google Scholar] [CrossRef]
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- Helmuth, I.G.; Poulsen, A.; Suppli, C.H.; Mølbak, K. Varicella in Europe—A review of the epidemiology and experience with vaccination. Vaccine 2015, 33, 2406–2413. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; AbuKhamsin, A.; Memish, Z.A. Epidemiology and impact of varicella vaccination: A longitudinal study 1994–2011. Travel Med. Infect. Dis. 2013, 11, 310–314. [Google Scholar] [CrossRef]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014--Recommendations. Vaccine 2016, 34, 198–199. [Google Scholar]
- Takahashi, M.; Otsuka, T.; Okuno, Y.; Asano, Y.; Yazaki, T.; Isomura, S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974, 304, 1288–1290. [Google Scholar] [CrossRef]
- Ozaki, T.; Asano, Y. Development of varicella vaccine in Japan and future prospects. Vaccine 2016, 34, 3427–3433. [Google Scholar] [CrossRef]
- Shu, M.; Zhang, D.; Ma, R.; Yang, T.; Pan, X. Long-term vaccine efficacy of a 2-dose varicella vaccine in China from 2011 to 2021: A retrospective observational study. Front. Public Heal. 2022, 10, 1039537. [Google Scholar] [CrossRef]
- Zhang, Z.; Suo, L.; Pan, J.; Zhao, D.; Lu, L. Two-dose varicella vaccine effectiveness in China: A meta-analysis and evidence quality assessment. BMC Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Luan, G.; Yao, H.; Yin, D.; Liu, J. Trends and Age-Period-Cohort Effect on Incidence of Varicella Under Age 35 — China, 2005–2021. China CDC Wkly. 2024, 6, 390–395. [Google Scholar] [CrossRef]
- Ruprecht, A.; Marin, M.; Strain, A.K.; Harry, K.; Kenyon, C. Notes from the Field: Expanded Laboratory Testing for Varicella - Minnesota, 2016-2023. MMWR Morb Mortal Wkly Rep. 2024, 73, 245–246. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, K.; Zhang, A.; Feng, Z.; Seward, J.F.; Bialek, S.R.; Wang, C. Single-dose varicella vaccine effectiveness in school settings in China. Vaccine 2013, 31, 3834–3838. [Google Scholar] [CrossRef]
- Xiu, S.; Xu, Z.; Wang, X.; Zhang, L.; Wang, Q.; Yang, M.; Shen, Y. Varicella vaccine effectiveness evaluation in Wuxi, China: A retrospective cohort study. Epidemiology Infect. 2024, 152, e105. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, S.; Zhang, M.; Zhong, G.; Peng, S.; Quan, J.; Lin, H.; Hu, X.; Zhu, K.; Huang, X.; et al. The epidemiology of varicella and effectiveness of varicella vaccine in Ganyu, China: A long-term community surveillance study. BMC Public Heal. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Fu, C.; Wang, M.; Liang, J.; Xu, J.; Wang, C.; Bialek, S. The Effectiveness of Varicella Vaccine in China. Pediatr. Infect. Dis. J. 2010, 29, 690–693. [Google Scholar] [CrossRef]
- Yang, L.; Yang, C.; Duan, Q. Epidemic characteristics analysis of breakthrough cases of varicella in Shapingba, Chongqing, 2019-2023. Mod. Prev. Med. 2024, 51, 3677–3681. (In Chinese) [Google Scholar]
- Li, R.; Hu, Y.; Wan, K.; Gu, Q.; Wu, Z.; Wu, X.; Shen, Z. Epidemiological analysis of breakthrough cases in varicella outbreak during 2007 ⁃ 2023 in Fengxian District of Shanghai. Chin. J. Biol. 2025, 38, 685–690. (In Chinese) [Google Scholar]
- Chen, Z.; Yao, K.; Ji, Y.; Zhang, Z. The epidemiological characteristics of chickenpox and the protective effect of vaccines among people under 25 years old in Nantong Economic Development Zone from 2018 to 2023. Jiangsu J. Health Care 2025, 27, 1–3. (In Chinese) [Google Scholar]
- Lu, X.; Jiang, X.; Guo, L.; Zhong, M. Epidemiological characteristics of varicella and vaccine immunization efficacy analysis in Pinghu city from 2010 to 2020. Mod. Pract. Med. 2024, 36, 45–48. (In Chinese) [Google Scholar]
- Liang, J.; Zhu, Q.; Hu, P.; Liu, M. A cross-sectional study on the immune persistence of 99 children vaccinated with live attenuated varicella vaccine. Mod. Prev. Med. 2017, 44, 642–645. (In Chinese) [Google Scholar]
- Huang, L.; Jin, S.; Geng, Z.; Mo, Z.; Tao, H. A study on the immune persistence of live attenuated varicella vaccine 11 years after the initial immunization. Chin. J. Immunol. 2020, 36, 2558–2560. (In Chinese) [Google Scholar]
- Hao, B.; Chen, Z.; Zeng, G.; Huang, L.; Luan, C.; Xie, Z.; Chen, J.; Bao, M.; Tian, X.; Xu, B.; et al. Efficacy, safety and immunogenicity of live attenuated varicella vaccine in healthy children in China: Double-blind, randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect 2019, 25, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Zhang, J.; Wang, C. Survey of antibody level against varicella-zoster virus in healthy population in Licheng district, Jinan. Dis. Surveill. 2016, 31, 586–590. (In Chinese) [Google Scholar]
- Meng, P.; Huang, J.; Cai, L.; Yang, X. Investigation on varicella zoster virus antibody level in healthy population in Wuhan. J. Pub. Health Prev. Med. 2022, 33, 60–62. (In Chinese) [Google Scholar]
- Marin, M.; Marti, M.; Kambhampati, A.; Jeram, S.M.; Seward, J.F. Global Varicella Vaccine Effectiveness: A Meta-analysis. Pediatrics 2016, 137, e20153741. [Google Scholar] [CrossRef]
- Choi, J.K.; Park, S.H.; Park, S.; Choi, S.M.; Kim, S.H.; Lee, D.G.; Yoo, J.H.; Choi, J.H.; Kang, J.H. Trends in varicella and herpes zoster epidemiology before and after the implementation of universal one-dose varicella vaccination over one decade in South Korea, 2003-2015. Hum. Vaccin. Immunother. 2019, 15, 2554–2560. [Google Scholar] [CrossRef]
- Su, J.; Huang, Z.; Chen, H.; Huang, Q.; Tang, Y.; Xia, Y.; Liu, W.; Jian, Z.; Huang, Y.; Feng, S.; et al. Observation on the immunogenicity and safety of booster immunization with Domestic freeze-dried varicella attenuated live vaccine. Chin. J. Vaccines Immun. 2016, 22, 366–370. (In Chinese) [Google Scholar]
- Liu, Y.; Gao, S.; Zhao, J.; Wang, P.; Xu, J.; Li, L.; Xu, X.; Li, Z.; Ha, X.; Li, K. Observation on the immune persistence of freeze-dried live attenuated varicella vaccine. Chin. J. Biol. 2006, 19, 3. (In Chinese) [Google Scholar]
- Ma, M.; Liu, W.; Yang, J.; Lin, X.; Fu, D.; He, L. A cross-sectional study on antibody levels of children vaccinated with varicella in a certain district of Shenzhen City in 2013. Pract. Prev. Med. 2015, 22, 3. (In Chinese) [Google Scholar]
- Asano, Y.; Nagai, T.; Miyata, T.; Yazaki, T.; Ito, S.; Yamanishi, K.; Takahashi, M. Long-Term Protective Immunity of Recipients of the OKA Strain of Live Varicella Vaccine. Pediatrics 1985, 75, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Vessey, S.; Chan, C.Y.; Kuter, B.J.; Kaplan, K.M.; Waters, M.; Kutzler, D.P.; Carfagno, P.A.; Sadoff, J.C.; Heyse, J.F.; Matthews, H.; et al. Childhood vaccination against varicella: Persistence of antibody, duration of protection, and vaccine efficacy. J. Pediatr. 2001, 139, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kuter, B.; Matthews, H.; Shinefield, H.; Black, S.; Dennehy, P.; Watson, B.; Reisinger, K.; Kim, L.L.; Lupinacci, L.; Hartzel, J.; et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr. Infect. Dis. J. 2004, 23, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Luo, F.; Li, S.; Chen, T.; Li, K. Investigation of varicella outbreak in a kindergarten with high varicella vaccine coverage. Mod. Prev. Med. 2010, 37, 3. (In Chinese) [Google Scholar]
- Zhang, L.; Wang, Y.; Liu, Y.; Sun, X.; Tang, F.; Zhou, M. Study on epidemic factors of varicella outbreak under high varicella vaccine coverage. Chin. J. Prev. Med. 2019, 23, 417–420. (In Chinese) [Google Scholar]

| Analysis Dataset | Variable | VarV Group (N = 247) | Placebo Group (N = 240) | Total (N = 487) | p-Value |
|---|---|---|---|---|---|
| IPS | N | 247 | 240 | 487 | |
| Age of vaccination (years, mean ± SD) | 6.5 ± 2.8 | 6.2 ± 2.8 | 6.3 ± 2.8 | 0.2898 | |
| Gender (male, %) | 51.4% | 48.8% | 50.1% | 0.5562 | |
| IPS2 | N | 218 | 204 | 422 | |
| Age of vaccination (years, mean ± SD) | 6.3 ± 2.7 | 6.0 ± 2.8 | 6.1 ± 2.7 | 0.2185 | |
| Gender (male, %) | 51.8% | 49.5% | 50.7% | 0.6331 |
| Time Point | Variable | 1–4 Years Old | 5–8 Years Old | 9–12 Years Old | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VarV Group | Placebo Group | p-Value | VarV Group | Placebo Group | p-Value | VarV Group | Placebo Group | p-Value | ||
| Before vaccination | N | 51 | 63 | 141 | 126 | 55 | 51 | |||
| Seropositive (≧1:4) rate % (95%CI) | 39.22 (25.84, 53.89) | 47.62 (34.88, 60.59) | 0.3686 | 79.43 (71.82, 85.77) | 74.60 (66.08, 81.93) | 0.3481 | 89.09 (77.75, 95.89) | 90.20 (78.59, 96.74) | 0.8521 | |
| Seropositive (≧1:8) rate % (95%CI) | 29.41 (17.49, 43.83) | 38.10 (26.15, 51.20) | 0.3312 | 53.90 (45.31, 62.32) | 57.94 (48.82, 66.67) | 0.5074 | 67.27 (53.29, 79.32) | 84.31 (71.41, 92.98) | 0.0416 | |
| GMT (1:) (95%CI) | 3.44 (2.79, 4.25) | 4.23 (3.36, 5.31) | 0.1998 | 7.50 (6.25, 9.01) | 7.21 (5.98, 8.68) | 0.7586 | 9.79 (7.43, 12.90) | 14.75 (10.78, 20.18) | 0.0508 | |
| 30 days after vaccination | Seropositive (≧1:4) rate % (95%CI) | 100.00 (93.02, 100.00) | 52.38 (39.41, 65.12) | <0.0001 | 100.00 (97.42, 100.00) | 74.60 (66.08, 81.93) | <0.0001 | 100.00 (93.51, 100.00) | 90.20 (78.59, 96.74) | 0.0548 |
| Seropositive (≧1:8) rate % (95%CI) | 96.08 (86.54, 99.52) | 42.86 (30.46, 55.95) | <0.0001 | 95.74 (90.97, 98.42) | 61.11 (52.02, 69.66) | <0.0001 | 98.18 (90.28, 99.95) | 84.31 (71.41, 92.98) | 0.0271 | |
| GMT (1:) (95%CI) | 23.09 (18.83, 28.32) | 4.82 (3.77, 6.18) | <0.0001 | 48.36 (38.94, 60.07) | 7.66 (6.34, 9.25) | <0.0001 | 66.47 (47.26, 93.48) | 16.00 (11.41, 22.43) | <0.0001 | |
| 5 years after vaccination | Seropositive (≧1:4) rate % (95%CI) | 100.00 (93.02, 100.00) | 77.78% (65.54, 87.28) | 0.0003 | 100.00 (97.42, 100.00) | 82.54 (74.77, 88.72) | <0.0001 | 100.00 (93.51, 100.00) | 80.39 (66.88, 90.18) | 0.0004 |
| Seropositive (≧1:8) rate % (95%CI) | 88.24 (76.13, 95.56) | 60.32% (47.20, 72.43) | 0.0009 | 87.94 (81.40, 92.82) | 65.08 (56.08, 73.35) | <0.0001 | 92.73 (82.41, 97.98) | 66.67 (52.08, 79.24) | 0.0008 | |
| GMT (1:) (95%CI) | 10.79 (9.01, 12.91) | 6.93 (5.55, 8.66) | 0.0025 | 13.60 (11.81, 15.68) | 7.61 (6.47, 8.95) | <0.0001 | 17.26 (13.71, 21.72) | 9.04 (6.71, 12.19) | 0.0008 | |
| 8 years after vaccination | N | 47 | 60 | 128 | 106 | 43 | 38 | |||
| Seropositive (≧1:4) rate % (95%CI) | 100 (92.45, 100.00) | 86.67 (75.41, 94.06) | 0.0088 | 99.22 (95.72, 99.98) | 92.45 (85.67, 96.69) | 0.0123 | 100 (91.78, 100.00) | 92.11 (78.62, 98.34) | 0.0989 | |
| Seropositive (≧1:8) rate % (95%CI) | 87.23 (74.26, 95.17) | 63.33 (49.90, 75.41) | 0.0052 | 86.72 (79.59, 92.07) | 76.42 (67.18, 84.12) | 0.0409 | 95.35 (84.19, 99.43) | 86.84 (71.91, 95.59) | 0.2440 | |
| GMT (1:) (95%CI) | 11.74 (8.84, 15.59) | 8.48 (6.60, 10.88) | 0.0866 | 13.17 (11.29, 15.35) | 9.80 (8.30, 11.56) | 0.0103 | 17.07 (13.29, 21.91) | 13.09 (9.80, 17.49) | 0.1628 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lei, X.; Huang, L.; Ma, Y.; Yuan, H.; Zhao, D.; Meng, F. Immune Persistence Following a Single Dose of Varicella Vaccine: 5-Year and 8-Year Follow-Up of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial. Vaccines 2025, 13, 1024. https://doi.org/10.3390/vaccines13101024
Wang Y, Lei X, Huang L, Ma Y, Yuan H, Zhao D, Meng F. Immune Persistence Following a Single Dose of Varicella Vaccine: 5-Year and 8-Year Follow-Up of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial. Vaccines. 2025; 13(10):1024. https://doi.org/10.3390/vaccines13101024
Chicago/Turabian StyleWang, Yanxia, Xiangling Lei, Lili Huang, Yuehong Ma, Hongxue Yuan, Dongyang Zhao, and Fanhong Meng. 2025. "Immune Persistence Following a Single Dose of Varicella Vaccine: 5-Year and 8-Year Follow-Up of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial" Vaccines 13, no. 10: 1024. https://doi.org/10.3390/vaccines13101024
APA StyleWang, Y., Lei, X., Huang, L., Ma, Y., Yuan, H., Zhao, D., & Meng, F. (2025). Immune Persistence Following a Single Dose of Varicella Vaccine: 5-Year and 8-Year Follow-Up of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial. Vaccines, 13(10), 1024. https://doi.org/10.3390/vaccines13101024




