A CpG 1018S/QS-21-Adjuvanted HBsAg Therapeutic Vaccine as a Novel Strategy Against HBV
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
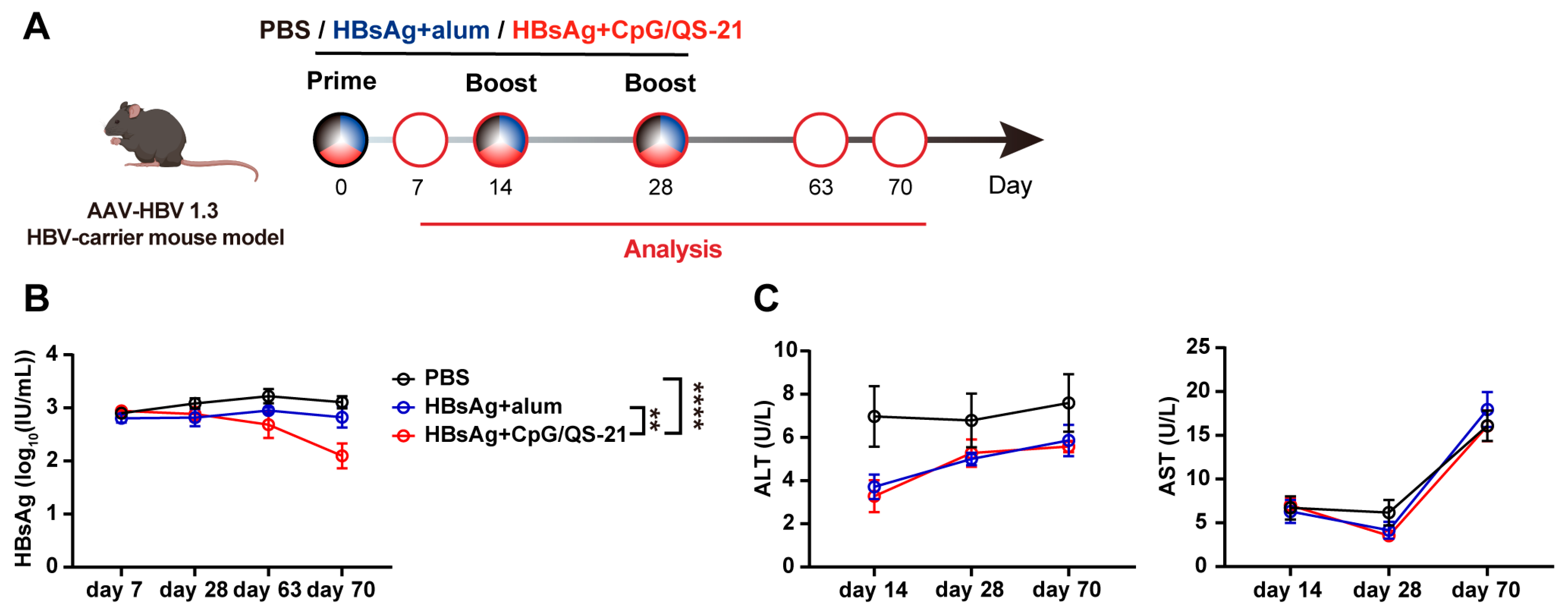
2.2. HBV-Carrier Mouse Model
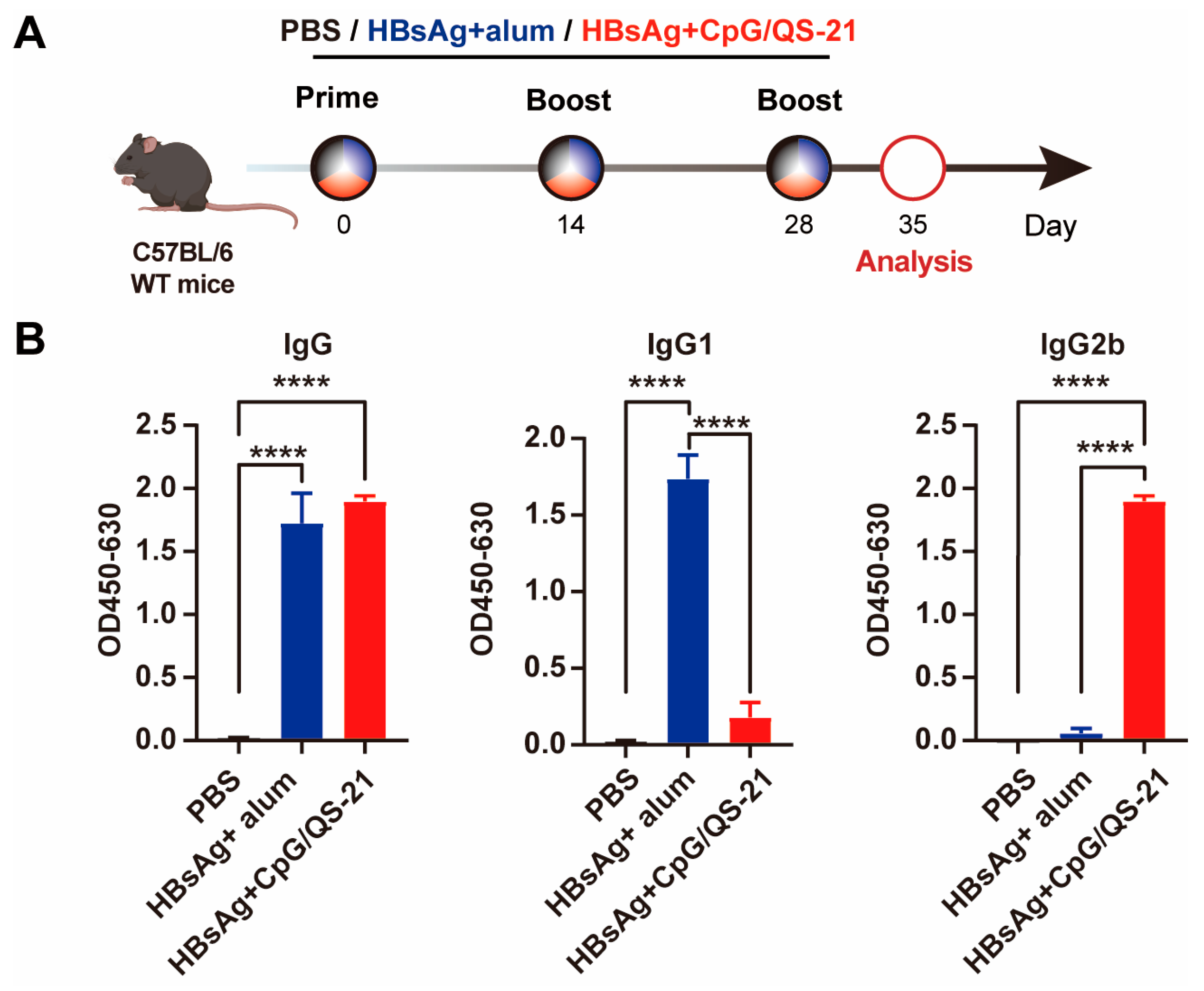
2.3. Vaccination
2.4. Serum HBsAg and Anti-HBs IgG Subclass (IgG1/IgG2b) and ALT/AST Detection
2.5. Mononuclear Cell (MNC) Isolation
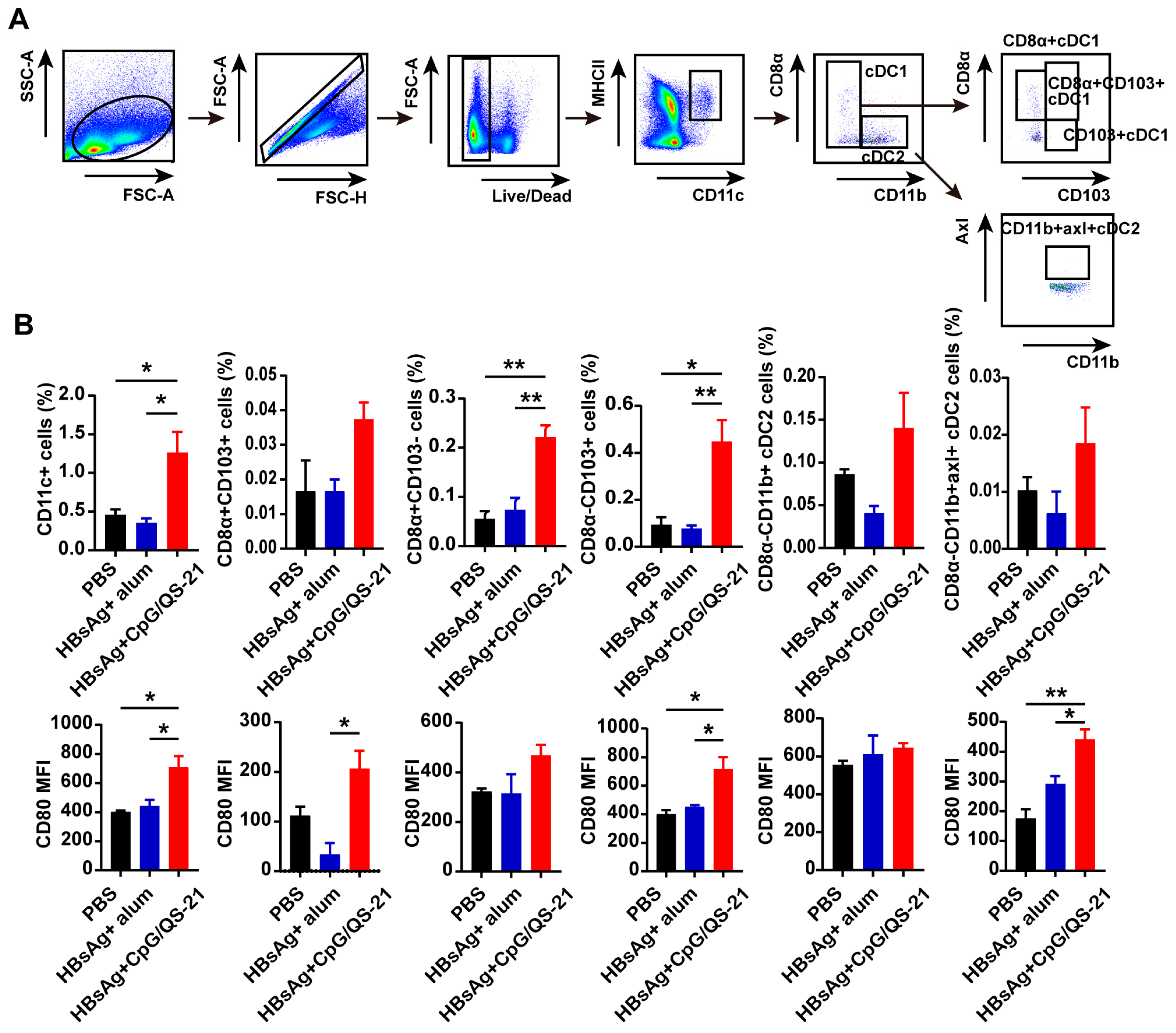
2.6. Flow Cytometry
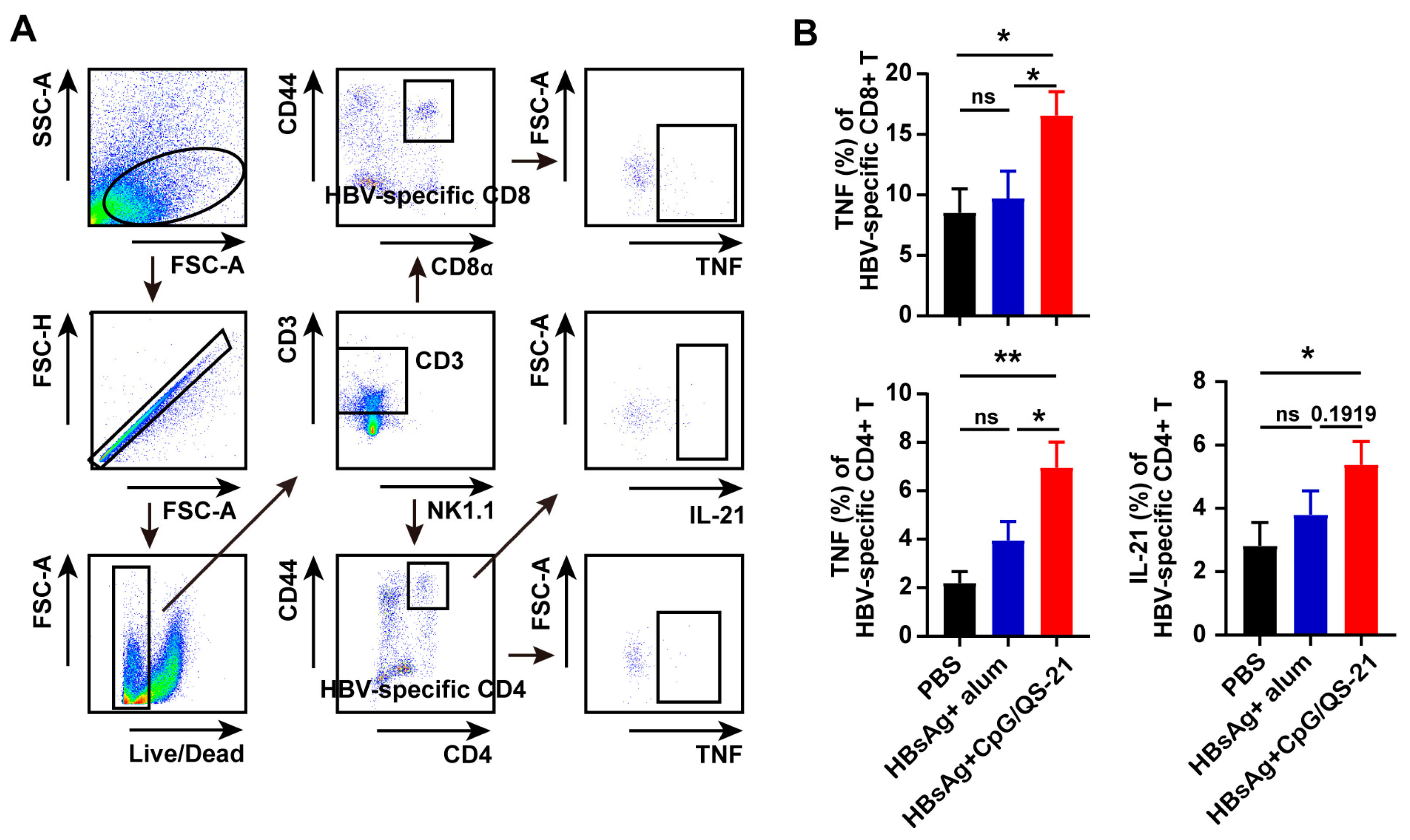
2.7. Intracellular Cytokine Analysis
2.8. Statistical Analysis
3. Results
3.1. CpG 1018S/QS-21-Adjuvanted HBsAg Elicits Robust Th1-Skewed Antibody Responses
3.2. CpG 1018S/QS-21-Adjuvanted HBsAg Reduces Circulating HBsAg in HBV-Carrier Mice
3.3. CpG 1018S/QS-21-Adjuvanted HBsAg Promotes Dendritic Cell Expansion and Activation
3.4. CpG 1018S/QS-21-Adjuvanted HBsAg Alleviates T Cell Exhaustion and Enhances HBV-Specific T Cell Effector Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef]
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2022, 22, 19–32. [Google Scholar] [CrossRef]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.; Lau, D.T.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18035. [Google Scholar] [CrossRef]
- Tu, T.; Budzinska, M.A.; Shackel, N.A.; Urban, S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses 2017, 9, 75. [Google Scholar] [CrossRef]
- Shih, C.; Yang, C.C.; Choijilsuren, G.; Chang, C.H.; Liou, A.T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387. [Google Scholar] [CrossRef]
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef]
- Lang-Meli, J.; Neumann-Haefelin, C.; Thimme, R. Immunotherapy and therapeutic vaccines for chronic HBV infection. Curr. Opin. Virol. 2021, 51, 149–157. [Google Scholar] [CrossRef]
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; Ma, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial. Hum. Vaccin. Immunother. 2020, 16, 388–399. [Google Scholar] [CrossRef]
- Khanam, A.; Chua, J.V.; Kottilil, S. Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression. Int. J. Mol. Sci. 2021, 22, 5497. [Google Scholar] [CrossRef]
- Peeridogaheh, H.; Meshkat, Z.; Habibzadeh, S.; Arzanlou, M.; Shahi, J.M.; Rostami, S.; Gerayli, S.; Teimourpour, R. Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Res. 2018, 245, 29–43. [Google Scholar] [CrossRef]
- Li, T.Y.; Yang, Y.; Zhou, G.; Tu, Z.K. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J. Gastroenterol. 2019, 25, 3527–3537. [Google Scholar] [CrossRef]
- Kitagawa, S.; Matsuda, T.; Washizaki, A.; Murakami, H.; Yamamoto, T.; Yoshioka, Y. Elucidation of the role of nucleolin as a cell surface receptor for nucleic acid-based adjuvants. NPJ Vaccines 2022, 7, 115. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garcon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert. Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine 2019, 60, 152905. [Google Scholar] [CrossRef]
- Lee, G.H.; Lim, S.G. CpG-Adjuvanted Hepatitis B Vaccine (HEPLISAV-B(R)) Update. Expert. Rev. Vaccines 2021, 20, 487–495. [Google Scholar] [CrossRef]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef]
- Luan, N.; Cao, H.; Wang, Y.; Lin, K.; Liu, C. Ionizable Lipid Nanoparticles Enhanced the Synergistic Adjuvant Effect of CpG ODNs and QS21 in a Varicella Zoster Virus Glycoprotein E Subunit Vaccine. Pharmaceutics 2022, 14, 973. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, X.; Cheng, J.; Li, Y.; Luan, N.; Hu, J.; Liang, B.; Zhang, H.; Gao, D.; Lei, Z.; et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines 2025, 13, 497. [Google Scholar] [CrossRef]
- Zhao, H.; Han, Q.; Yang, A.; Wang, Y.; Wang, G.; Lin, A.; Wang, X.; Yin, C.; Zhang, J. CpG-C ODN M362 as an immunoadjuvant for HBV therapeutic vaccine reverses the systemic tolerance against HBV. Int. J. Biol. Sci. 2022, 18, 154–165. [Google Scholar] [CrossRef]
- Lv, S.; Wang, J.; Dou, S.; Yang, X.; Ni, X.; Sun, R.; Tian, Z.; Wei, H. Nanoparticles encapsulating hepatitis B virus cytosine-phosphate-guanosine induce therapeutic immunity against HBV infection. Hepatology 2014, 59, 385–394. [Google Scholar] [CrossRef]
- Aillot, L.; Bonnin, M.; Ait-Goughoulte, M.; Bendriss-Vermare, N.; Maadadi, S.; Dimier, L.; Subic, M.; Scholtes, C.; Najera, I.; Zoulim, F.; et al. Interaction between Toll-Like Receptor 9-CpG Oligodeoxynucleotides and Hepatitis B Virus Virions Leads to Entry Inhibition in Hepatocytes and Reduction of Alpha Interferon Production by Plasmacytoid Dendritic Cells. Antimicrob. Agents Chemother. 2018, 62, e01741-17. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Han, Q.-J.; Wang, G.; Lin, A.; Xu, D.-Q.; Wang, Y.-Q.; Zhao, L.-H.; Tian, Z.-G.; Zhang, J. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8(+) effector memory T cells. Gut 2019, 68, 2032–2043. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Hu, Y.; Xu, D.; Yin, C.; Han, Q.; Zhang, J. Chitosan Nanovaccines as Efficient Carrier Adjuvant System for IL-12 with Enhanced Protection Against HBV. Int. J. Nanomed. 2021, 16, 4913–4928. [Google Scholar] [CrossRef]
- Ye, B.; Liu, X.; Li, X.; Kong, H.; Tian, L.; Chen, Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015, 6, e1694. [Google Scholar] [CrossRef]
- Zheng, J.R.; Wang, Z.L.; Feng, B. Hepatitis B functional cure and immune response. Front. Immunol. 2022, 13, 1075916. [Google Scholar] [CrossRef]
- Li, H.; Xiao, S.; Huo, C.; Yang, S.; Wang, J.; Lan, X.; Li, M.; Shi, L.; Zhuo, L.; Zhang, J.; et al. Targeting Ferroptosis Restores the Antiviral Activity of CD8(+) T Cells During Chronic Hepatitis B Virus Infection. Cell Mol. Gastroenterol. Hepatol. 2025, 19, 101612. [Google Scholar] [CrossRef]
- Weiner, G.J. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J. Leukoc. Biol. 2000, 68, 455–463. [Google Scholar] [CrossRef]
- Hanagata, N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017, 12, 515–531. [Google Scholar] [CrossRef]
- Cancel, J.C.; Crozat, K.; Dalod, M.; Mattiuz, R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019, 177, 556–571.e16. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef]
- Yang, L.; Anderson, D.E.; Kuchroo, J.; Hafler, D.A. Lack of TIM-3 immunoregulation in multiple sclerosis. J. Immunol. 2008, 180, 4409–4414. [Google Scholar] [CrossRef]
- Gefen, T.; Castro, I.; Muharemagic, D.; Puplampu-Dove, Y.; Patel, S.; Gilboa, E. A TIM-3 Oligonucleotide Aptamer Enhances T Cell Functions and Potentiates Tumor Immunity in Mice. Mol. Ther. 2017, 25, 2280–2288. [Google Scholar] [CrossRef]
- Kosinska, A.D.; Zhang, E.; Johrden, L.; Liu, J.; Seiz, P.L.; Zhang, X.; Ma, Z.; Kemper, T.; Fiedler, M.; Glebe, D.; et al. Combination of DNA prime--adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013, 9, e1003391. [Google Scholar] [CrossRef]
- Lok, A.S.; Pan, C.Q.; Han, S.-H.B.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef]
- Boni, C.; Janssen, H.L.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Yoshida, E.M.; Trinh, H.; Rodell, T.C.; Laccabue, D.; et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients with Chronic Hepatitis. Gastroenterology 2019, 157, 227–241.e7. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Ha, S.-J.; Mueller, S.N.; Wherry, E.J.; Barber, D.L.; Aubert, R.D.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 2008, 205, 543–555. [Google Scholar] [CrossRef]
- Fochesato, M.; Dendouga, N.; Boxus, M. Comparative preclinical evaluation of AS01 versus other Adjuvant Systems in a candidate herpes zoster glycoprotein E subunit vaccine. Hum. Vaccin. Immunother. 2016, 12, 2092–2095. [Google Scholar] [CrossRef]
- Campbell, J.D. Development of the CpG Adjuvant 1018: A Case Study. Methods Mol. Biol. 2017, 1494, 15–27. [Google Scholar]
- Champion, C.R. Heplisav-B: A Hepatitis B Vaccine with a Novel Adjuvant. Ann. Pharmacother. 2021, 55, 783–791. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wu, J.; Meng, X.; Weng, H.; Li, Q.; Li, L.; Ma, Z.; Bi, S.; Han, Q.; Zhao, H.; et al. A CpG 1018S/QS-21-Adjuvanted HBsAg Therapeutic Vaccine as a Novel Strategy Against HBV. Vaccines 2025, 13, 1014. https://doi.org/10.3390/vaccines13101014
Wang Z, Wu J, Meng X, Weng H, Li Q, Li L, Ma Z, Bi S, Han Q, Zhao H, et al. A CpG 1018S/QS-21-Adjuvanted HBsAg Therapeutic Vaccine as a Novel Strategy Against HBV. Vaccines. 2025; 13(10):1014. https://doi.org/10.3390/vaccines13101014
Chicago/Turabian StyleWang, Zixuan, Jing Wu, Xiaohan Meng, He Weng, Qiang Li, Lin Li, Zhenhao Ma, Sirong Bi, Qiuju Han, Huajun Zhao, and et al. 2025. "A CpG 1018S/QS-21-Adjuvanted HBsAg Therapeutic Vaccine as a Novel Strategy Against HBV" Vaccines 13, no. 10: 1014. https://doi.org/10.3390/vaccines13101014
APA StyleWang, Z., Wu, J., Meng, X., Weng, H., Li, Q., Li, L., Ma, Z., Bi, S., Han, Q., Zhao, H., Liu, C., & Meng, D. (2025). A CpG 1018S/QS-21-Adjuvanted HBsAg Therapeutic Vaccine as a Novel Strategy Against HBV. Vaccines, 13(10), 1014. https://doi.org/10.3390/vaccines13101014







