Age-Specific Differences in the Dynamics of Neutralizing Antibody to Emerging SARS-CoV-2 Variants Following Breakthrough Infections: A Longitudinal Cohort Study
Abstract
1. Introduction
2. Materials and Methods
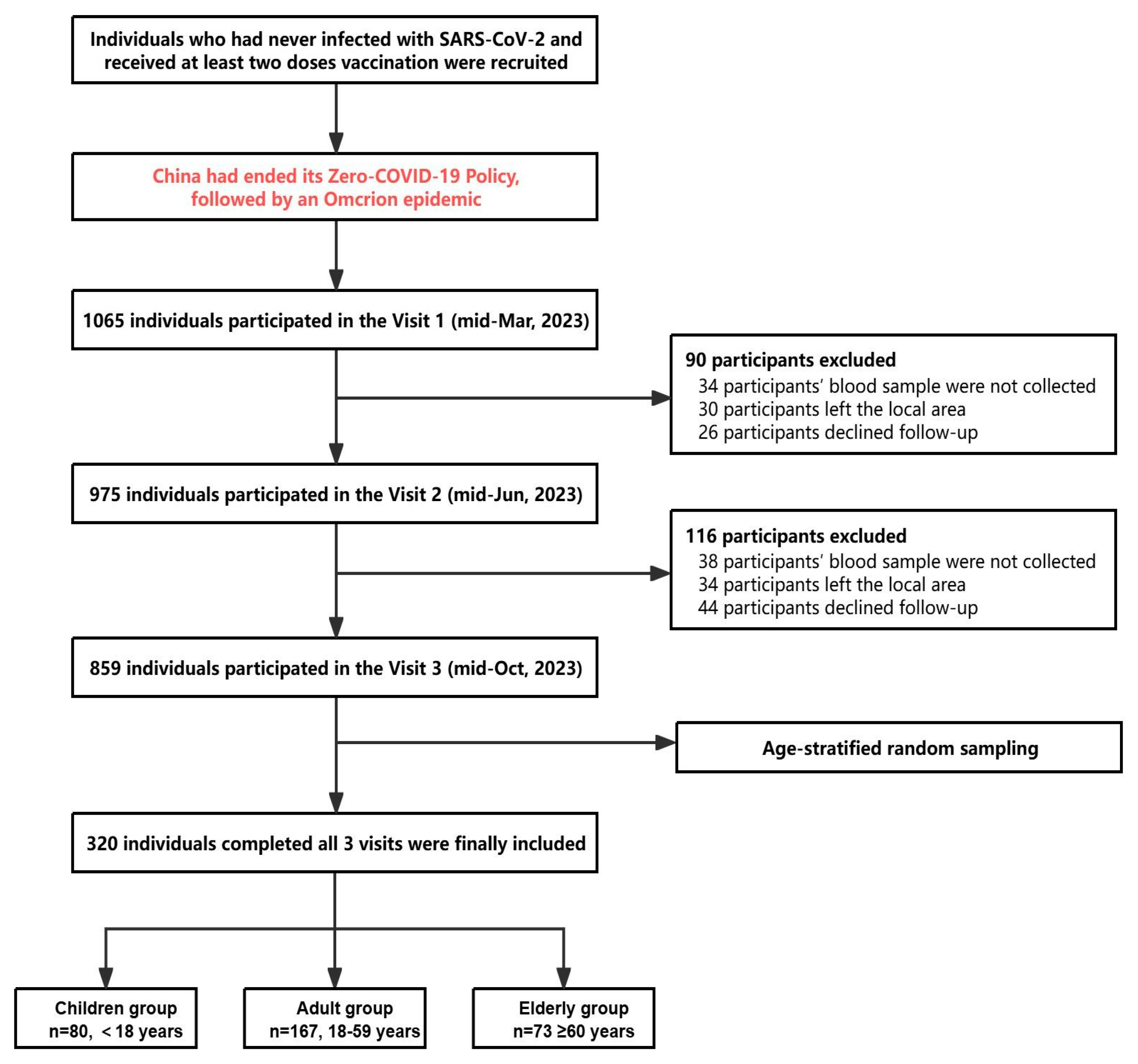
2.1. Participants and Design
2.2. Preparation of Pseudotyped Viruses
2.3. Serum NAb Detection Based on Pseudotyped Virus Neutralization Assay
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Longitudinal Assessment of Antibody Levels Against SARS-CoV-2 Variants Across Age Groups
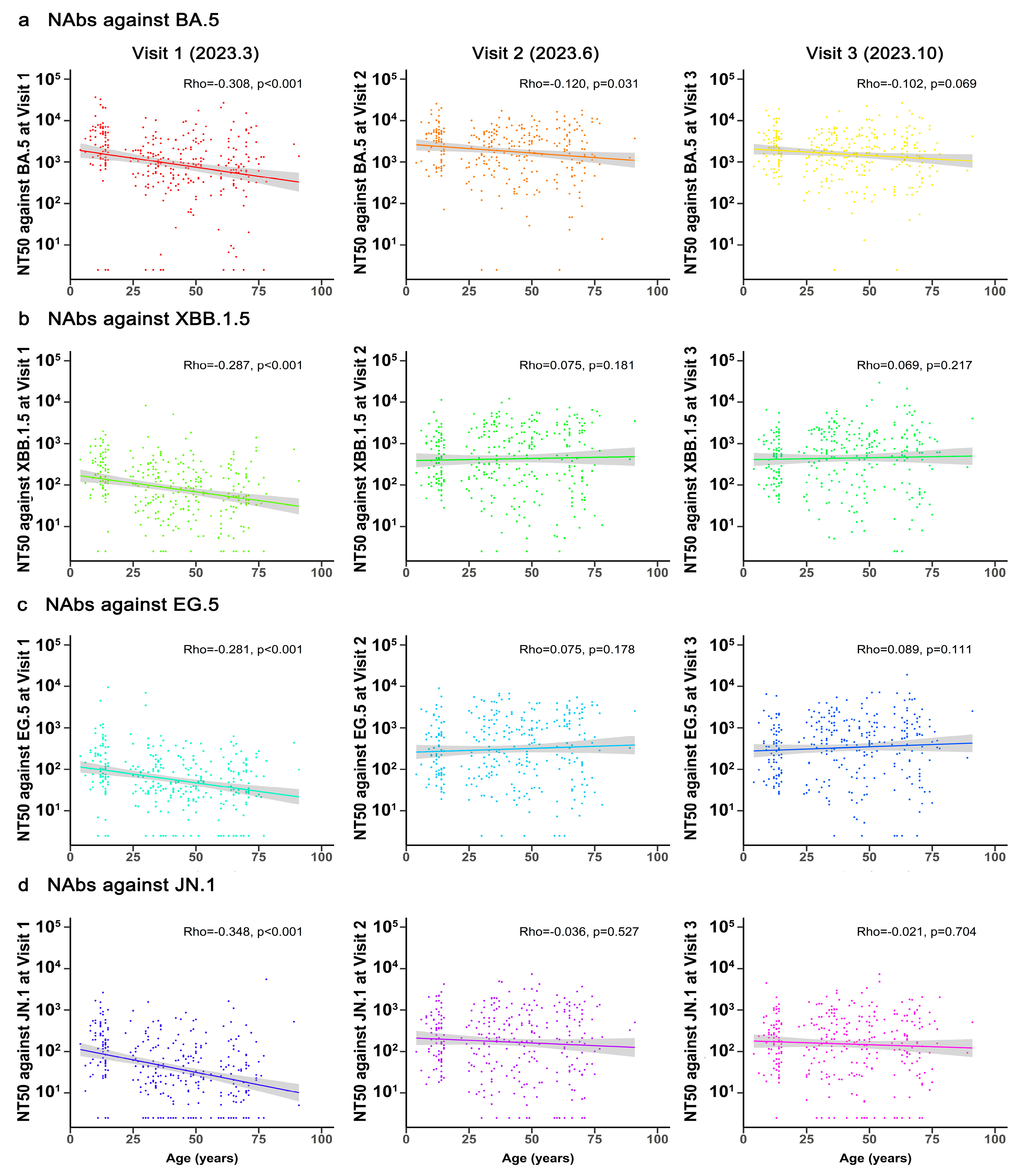
3.3. Correlation Between Age and NAbs Against Different SARS-CoV-2 Variants
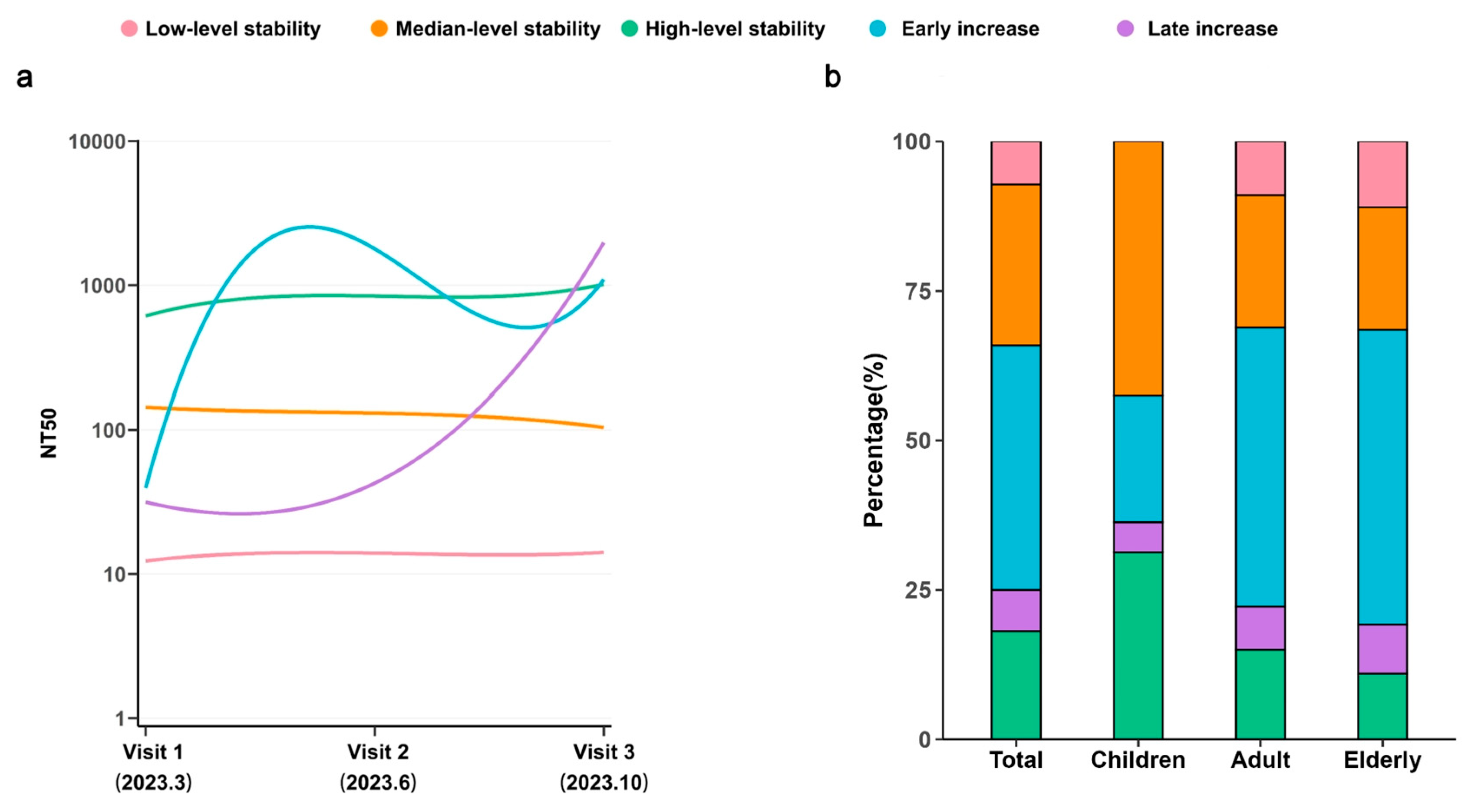
3.4. Longitudinal Dynamics of NAbs Across Age Groups
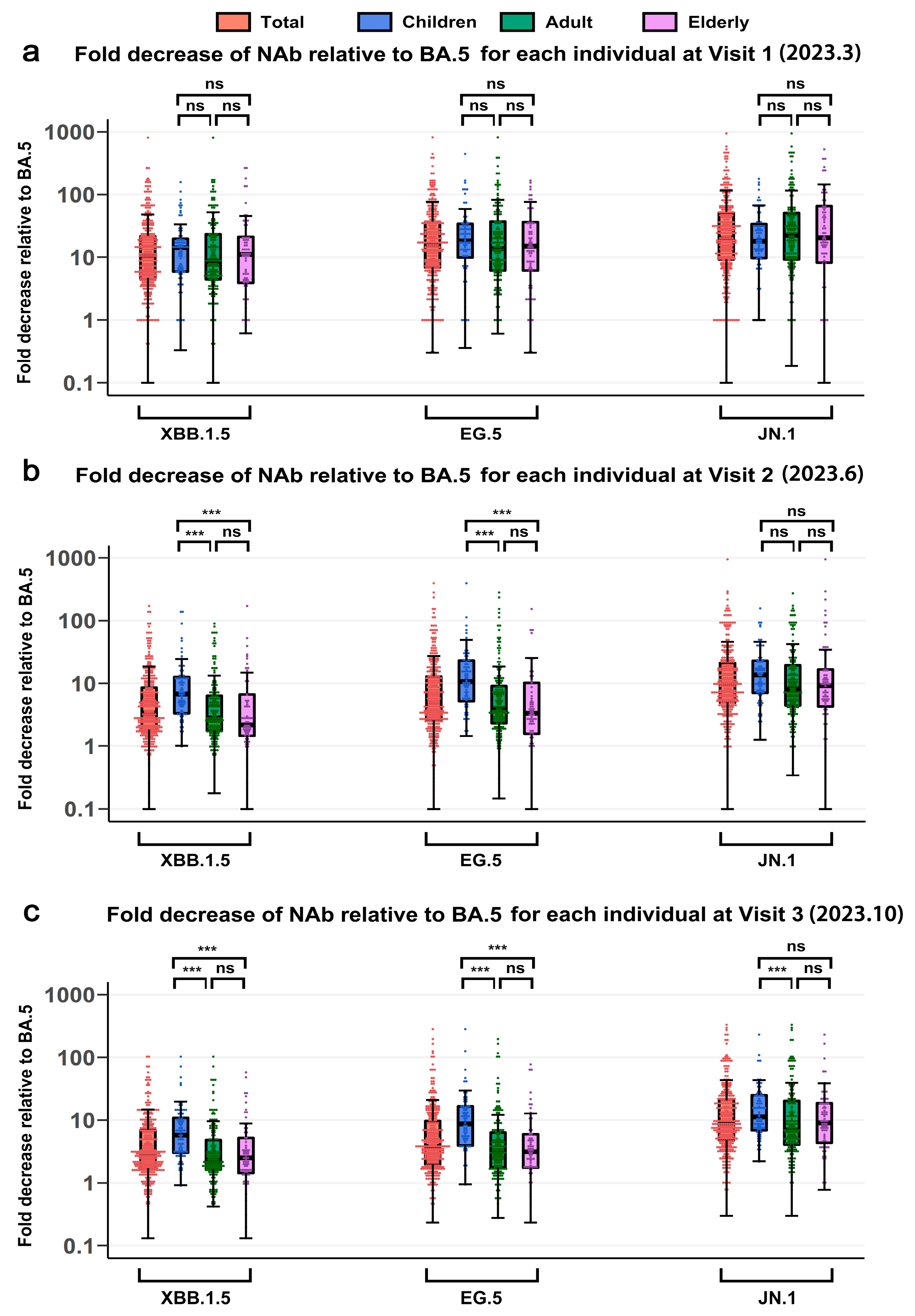
3.5. Cross-Neutralizing Activity Against SARS-CoV-2 Variants Across Age Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 19 September 2024).
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Zarandi, P.K.; Ghiasi, M.; Kooshki, H.; Mohammadi, M.; Amani, J.; Rezaei, N. Immunosenescence and inflamm-ageing in COVID-19. Ageing Res. Rev. 2023, 84, 101818. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef]
- Bellusci, L.; Grubbs, G.; Sait, S.; Yonker, L.M.; Randolph, A.G.; Novak, T.; Kobayashi, T.; Khurana, S. Neutralization of SARS-CoV-2 Omicron BQ.1, BQ.1.1 and XBB.1 variants following SARS-CoV-2 infection or vaccination in children. Nat. Commun. 2023, 14, 7952. [Google Scholar] [CrossRef]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Harahsheh, A.S.; Handoko, R.; Raghuveer, G.; Portman, M.A.; Khoury, M.; Newburger, J.W.; Lee, S.; Jain, S.S.; Khare, M.; et al. SARS-CoV-2 Variants and Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2023, 388, 1624–1626. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, Y.; Xiao, M.; Chen, Q.; Pinpin, L.; Wang, X.; Wang, J.; Shao, Z.; Qiu, Y.; Lu, Y.; et al. Health Consequences Among COVID-19 Convalescent Patients 30 Months Post-Infection in China. Zoonoses 2024, 4, 999. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Cao, C.; Ji, T.; Zheng, T.; Dai, Y.; Liu, M.; Jiang, J.; Sun, D.; Bai, Z.; Lu, X.; et al. Longitudinal analysis of memory Tfh cells and antibody response following CoronaVac vaccination. JCI Insight 2023, 8, e168437. [Google Scholar] [CrossRef]
- Shapiro, J.R.; Sitaras, I.; Park, H.S.; Aytenfisu, T.Y.; Caputo, C.; Li, M.; Lee, J.; Johnston, T.S.; Li, H.; Wouters, C.; et al. Association of Frailty, Age, and Biological Sex With Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine-Induced Immunity in Older Adults. Clin. Infect. Dis. 2022, 75, S61–S71. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Q.; Zhang, H.; Yuan, G.; Wang, X.; Sheng, K.; Li, C.; Cai, J.; Sun, Y.; Zhao, J.; et al. Neutralization against Omicron subvariants after BA.5/BF.7 breakthrough infection weakened as virus evolution and aging despite repeated prototype-based vaccination(1). Emerg. Microbes Infect. 2023, 12, 2249121. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, F.; Chitwood, M.H.; Cohen, T.; Pitzer, V.E.; Russi, M.; Swartwood, N.A.; Salomon, J.A.; Menzies, N.A. Changes in Population Immunity Against Infection and Severe Disease From Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variants in the United States Between December 2021 and November 2022. Clin. Infect. Dis. 2023, 77, 355–361. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Haiwei, Z.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef]
- Jacobsen, E.-M.; Fabricius, D.; Class, M.; Topfstedt, F.; Lorenzetti, R.; Janowska, I.; Schmidt, F.; Staniek, J.; Zernickel, M.; Stamminger, T.; et al. High antibody levels and reduced cellular response in children up to one year after SARS-CoV-2 infection. Nat. Commun. 2022, 13, 7315. [Google Scholar] [CrossRef]
- Xiao, C.; Ren, Z.; Zhang, B.; Mao, L.; Zhu, G.; Gao, L.; Su, J.; Ye, J.; Long, Z.; Zhu, Y.; et al. Insufficient epitope-specific T cell clones are responsible for impaired cellular immunity to inactivated SARS-CoV-2 vaccine in older adults. Nat. Aging 2023, 3, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Toh, Z.Q.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults With Mild COVID-19. JAMA Netw. Open 2022, 5, e221313. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef]
- Vanetti, C.; Lampasona, V.; Stracuzzi, M.; Fenizia, C.; Biasin, M.; Saulle, I.; Limanaqi, F.; Abdelsalam, A.; Loretelli, C.; Paradiso, L.; et al. The Immunological Profile of SARS-CoV-2 Infection in Children Is Linked to Clinical Severity and Age. Int. J. Mol. Sci. 2023, 24, 6779. [Google Scholar] [CrossRef]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hong, W.; Lei, H.; He, C.; Lei, W.; Zhou, Y.; Zhao, T.; Alu, A.; Ma, X.; Li, J.; et al. Low levels of neutralizing antibodies against XBB Omicron subvariants after BA.5 infection. Signal Transduct. Target. Ther. 2023, 8, 252. [Google Scholar] [CrossRef]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Lau, E.H.Y.; Wong, C.K.H.; Leung, G.M.; Wu, J.T. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after adjustment of the zero-COVID policy in November-December 2022. Nat. Med. 2023, 29, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, S.; Lu, F. Impact of National Omicron Outbreak at the end of 2022 on the future outlook of COVID-19 in China. Emerg. Microbes Infect. 2023, 12, 2191738. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ma, J.; Wang, H.; Lan, Y.; Tang, X. Epidemiological features of SARS-CoV-2 Omicron infection under new control strategy: A cross-sectional study of the outbreak since December 2022 in Sichuan, China. BMC Public Health 2023, 23, 2463. [Google Scholar] [CrossRef]
- Pérez-Alós, L.; Hansen, C.B.; Almagro Armenteros, J.J.; Madsen, J.R.; Heftdal, L.D.; Hasselbalch, R.B.; Pries-Heje, M.M.; Bayarri-Olmos, R.; Jarlhelt, I.; Hamm, S.R.; et al. Previous immunity shapes immune responses to SARS-CoV-2 booster vaccination and Omicron breakthrough infection risk. Nat. Commun. 2023, 14, 5624. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wu, P.; Lu, W.; Liu, K.; Ma, K.; Huang, L.; Cai, J.; Zhang, H.; Qin, Y.; Sun, H.; et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS Pathog. 2020, 16, e1008520. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 2023, 614, 521–529. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Pang, D.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: A national cohort study. Lancet Infect. Dis. 2023, 23, 799–805. [Google Scholar] [CrossRef]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Long, Q.; Wu, Y.; Hu, X.; He, Y.; Jiang, M.; Li, J.; Zhao, L.; Yang, S.; et al. Comprehensive Humoral and Cellular Immune Responses to SARS-CoV-2 Variants in Diverse Chinese Population. Research 2022, 2022, 9873831. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhuang, X.; Zhang, S.; Chen, Z.; Zou, Y.; Sheng, J.; Li, T.; Tai, W.; Yu, J.; et al. Inactivated vaccine-elicited potent antibodies can broadly neutralize SARS-CoV-2 circulating variants. Nat. Commun. 2023, 14, 2179. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Chen, C.; Yiu, K.; Chan, T.O.; Lai, K.C.; Ling, K.C.; Sun, Y.; Hui, D.S.; Cheng, S.M.S.; Peiris, M. A Randomized Clinical Trial Using CoronaVac or BNT162b2 Vaccine as a Third Dose in Adults Vaccinated with Two Doses of CoronaVac. Am. J. Respir. Crit. Care Med. 2022, 205, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. Jama 2022, 327, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; He, L.; Bao, Y.; Chen, Y.; Lu, G.; Zhang, Y.; Xu, Y.; Su, B.; Xu, J.; Wang, Y.; et al. Repeated vaccination of inactivated SARS-CoV-2 vaccine dampens neutralizing antibodies against Omicron variants in breakthrough infection. Cell Res. 2023, 33, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Wu, S.; Wang, Y.; Wang, B.; Zhang, Z.; Zhang, J.; Song, X.; Hou, L.; Chen, W. Monovalent XBB.1.5 booster vaccination induces a broad spectrum of SARS-CoV-2 neutralizing antibodies. Emerg. Microbes Infect. 2024, 13, 2286260. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kang, X.; Zhao, X.; Zhu, S.; Feng, S.; Du, Y.; Wang, Z.; Zhao, Y.; Song, X.; Li, X.; et al. Age-Specific Differences in the Dynamics of Neutralizing Antibody to Emerging SARS-CoV-2 Variants Following Breakthrough Infections: A Longitudinal Cohort Study. Vaccines 2025, 13, 1013. https://doi.org/10.3390/vaccines13101013
Zhang Z, Kang X, Zhao X, Zhu S, Feng S, Du Y, Wang Z, Zhao Y, Song X, Li X, et al. Age-Specific Differences in the Dynamics of Neutralizing Antibody to Emerging SARS-CoV-2 Variants Following Breakthrough Infections: A Longitudinal Cohort Study. Vaccines. 2025; 13(10):1013. https://doi.org/10.3390/vaccines13101013
Chicago/Turabian StyleZhang, Zhihao, Xiaoyu Kang, Xin Zhao, Sijia Zhu, Shuo Feng, Yin Du, Zhen Wang, Yingying Zhao, Xuemei Song, Xinlian Li, and et al. 2025. "Age-Specific Differences in the Dynamics of Neutralizing Antibody to Emerging SARS-CoV-2 Variants Following Breakthrough Infections: A Longitudinal Cohort Study" Vaccines 13, no. 10: 1013. https://doi.org/10.3390/vaccines13101013
APA StyleZhang, Z., Kang, X., Zhao, X., Zhu, S., Feng, S., Du, Y., Wang, Z., Zhao, Y., Song, X., Li, X., Cai, H., Liu, M., Long, P., Yuan, Y., Cheng, S., Wang, C., Yang, G., Wei, S., Wu, T., ... Wang, H. (2025). Age-Specific Differences in the Dynamics of Neutralizing Antibody to Emerging SARS-CoV-2 Variants Following Breakthrough Infections: A Longitudinal Cohort Study. Vaccines, 13(10), 1013. https://doi.org/10.3390/vaccines13101013





