Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Laboratory Animals
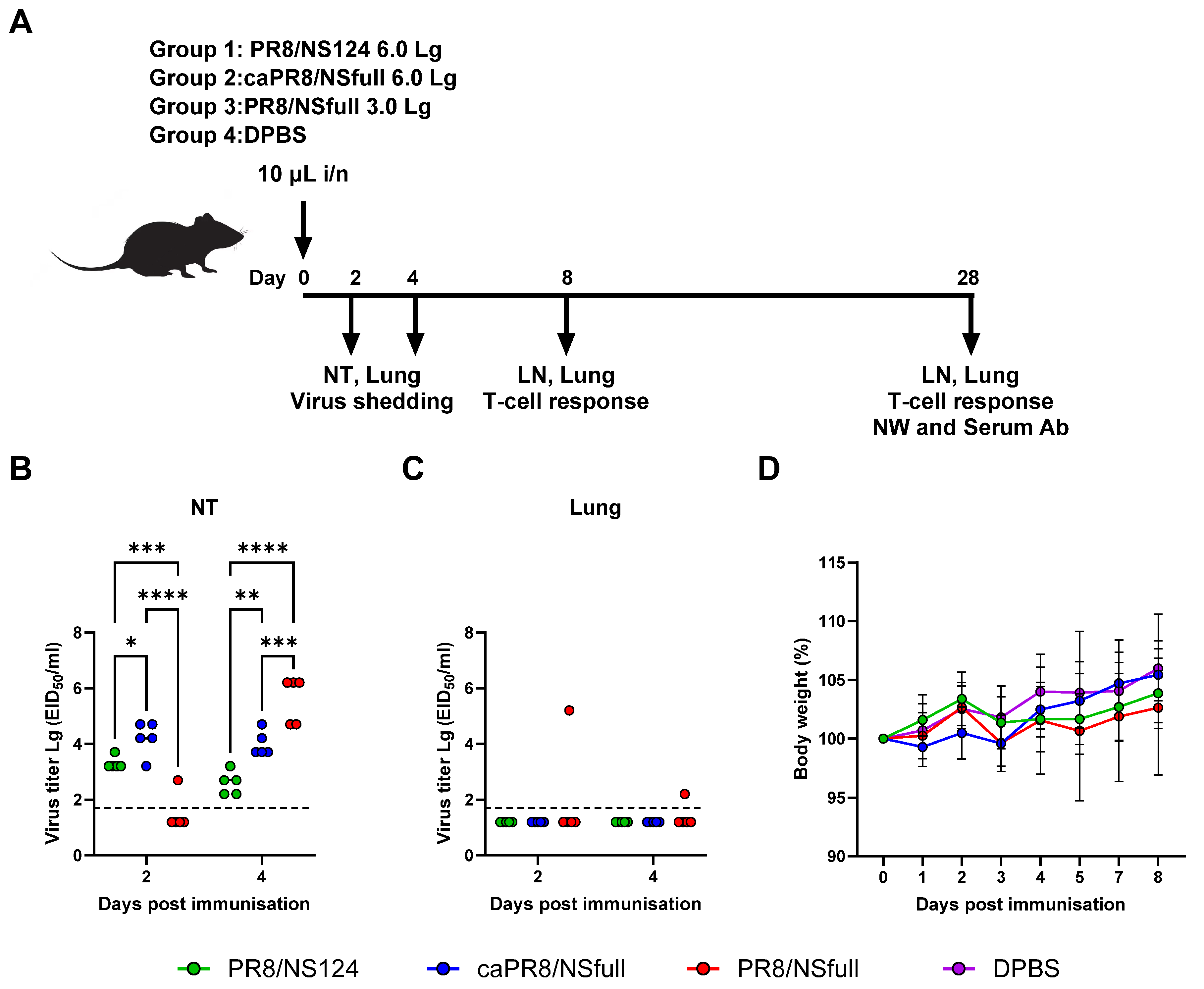
2.3. Immunisation and Challenge Infection
2.4. Viral Infectious Activity Analysis
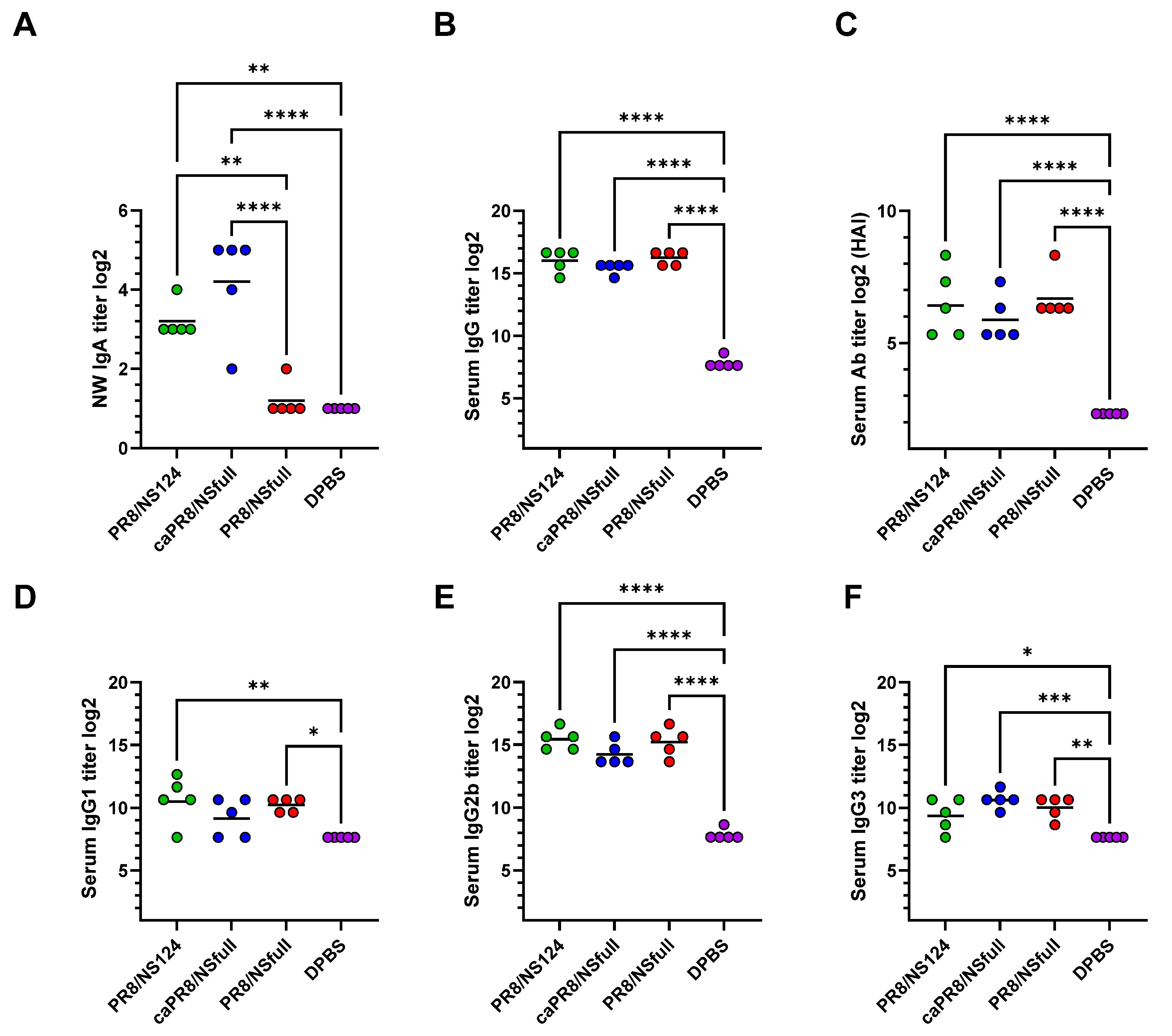
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Hemagglutination Inhibition Assay (HAI)
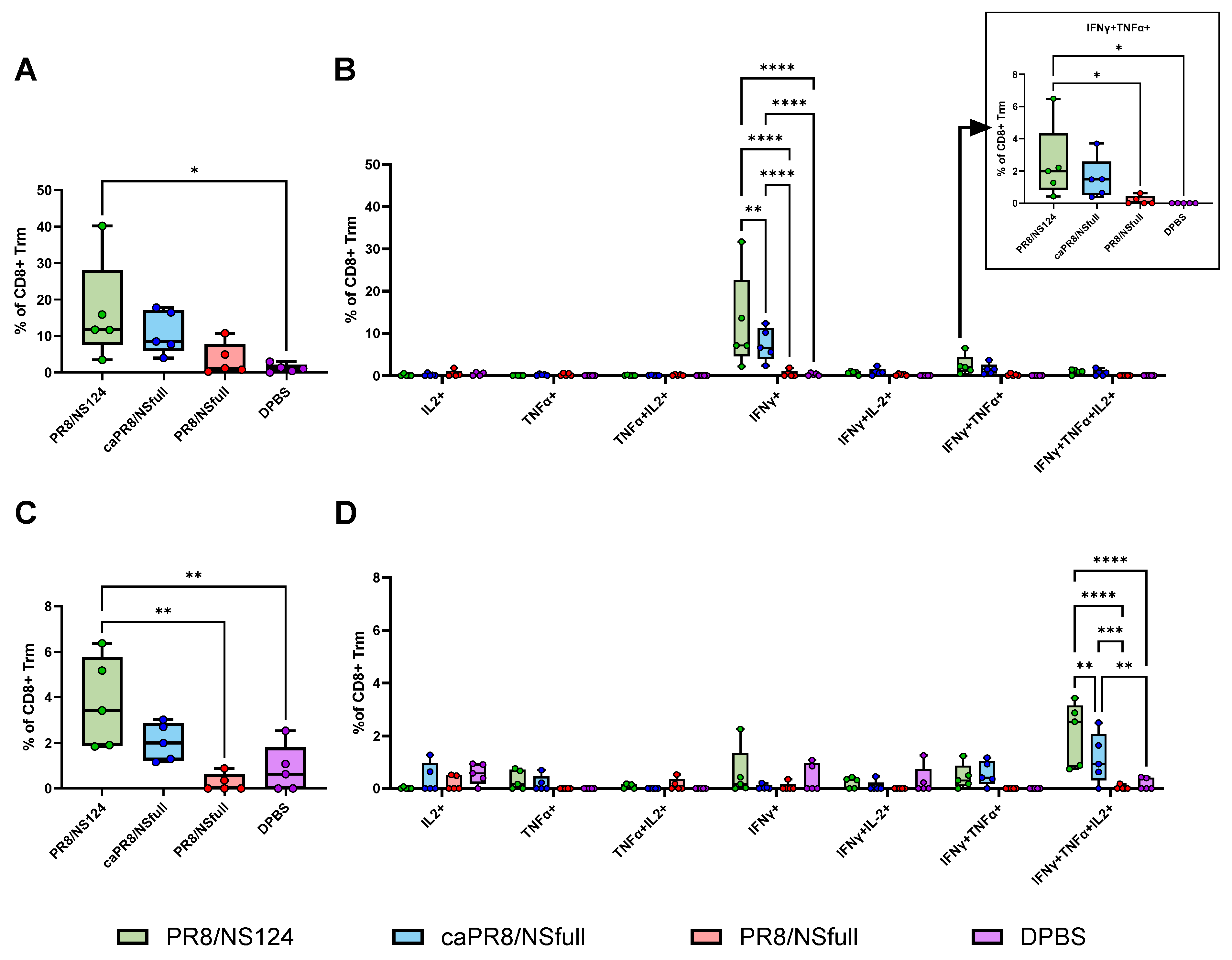
2.7. Lymphocyte Isolation and Stimulation
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Safety and Reproductive Activity of PR8/NSfull, caPR8/NSfull, and PR8/NS124 Viruses Following Intranasal Immunisation
3.2. Local and Systemic Humoral Responses Following Intranasal Immunization
3.3. Tissue-Resident T-Cell Response to Intranasal Immunisation
3.4. Tfh Response Activation and iBALT Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Influenza (Seasonal). Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Influenza-(Seasonal) (accessed on 3 October 2023).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Palese, P. Is a Universal Influenza Virus Vaccine Possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Weir, J.P.; Engelhardt, O.; Katz, J.M.; Cox, R.J. Meeting Report and Review: Immunological Assays and Correlates of Protection for next-Generation Influenza Vaccines. Influenza Other Respir. Viruses 2020, 14, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and Pandemic Influenza: 100 Years of Progress, Still Much to Learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune Responses after Live Attenuated Influenza Vaccination. Hum. Vaccin. Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef]
- Grebe, K.M.; Yewdell, J.W.; Bennink, J.R. Heterosubtypic Immunity to Influenza A Virus: Where Do We Stand? Microbes Infect. 2008, 10, 1024–1029. [Google Scholar] [CrossRef]
- Hurwitz, E.S.; Haber, M.; Chang, A.; Shope, T.; Teo, S.; Ginsberg, M.; Waecker, N.; Cox, N.J. Effectiveness of Influenza Vaccination of Day Care Children in Reducing Influenza-Related Morbidity among Household Contacts. JAMA 2000, 284, 1677–1682. [Google Scholar] [CrossRef]
- Rudenko, L.; Isakova-Sivak, I. Pandemic Preparedness with Live Attenuated Influenza Vaccines Based on A/Leningrad/134/17/57 Master Donor Virus. Expert Rev. Vaccines 2015, 14, 395–412. [Google Scholar] [CrossRef]
- Thwaites, R.S.; Uruchurtu, A.S.S.; Negri, V.A.; Cole, M.E.; Singh, N.; Poshai, N.; Jackson, D.; Hoschler, K.; Baker, T.; Scott, I.C.; et al. Early Mucosal Events Promote Distinct Mucosal and Systemic Antibody Responses to Live Attenuated Influenza Vaccine. Nat. Commun. 2023, 14, 8053. [Google Scholar] [CrossRef]
- Ramirez, S.I.; Faraji, F.; Hills, L.B.; Lopez, P.G.; Goodwin, B.; Stacey, H.D.; Sutton, H.J.; Hastie, K.M.; Saphire, E.O.; Kim, H.J.; et al. Immunological Memory Diversity in the Human Upper Airway. Nature 2024, 632, 630–636. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The Relative Efficacy of Trivalent Live Attenuated and Inactivated Influenza Vaccines in Children and Adults. Influenza Other Respi. Viruses 2011, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Albrecht, R.A.; García-Sastre, A. Innate Immune Evasion Strategies of Influenza Viruses. Future Microbiol. 2010, 5, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, K.; Shurygina, A.-P.; Sergeeva, M.; Stukova, M.; Egorov, A. Intranasal Immunization with the Influenza A Virus Encoding Truncated NS1 Protein Protects Mice from Heterologous Challenge by Restraining the Inflammatory Response in the Lungs. Microorganisms 2021, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Ferko, B.; Stasakova, J.; Romanova, J.; Kittel, C.; Sereinig, S.; Katinger, H.; Egorov, A. Immunogenicity and Protection Efficacy of Replication-Deficient Influenza A Viruses with Altered NS1 Genes. J. Virol. 2004, 78, 13037–13045. [Google Scholar] [CrossRef] [PubMed]
- Romanova, J.; Krenn, B.M.; Wolschek, M.; Ferko, B.; Romanovskaja-Romanko, E.; Morokutti, A.; Shurygina, A.-P.; Nakowitsch, S.; Ruthsatz, T.; Kiefmann, B.; et al. Preclinical Evaluation of a Replication-Deficient Intranasal ΔNS1 H5N1 Influenza Vaccine. PLoS ONE 2009, 4, e5984. [Google Scholar] [CrossRef]
- Vasilyev, K.; Yukhneva, M.; Shurygina, A.-P.; Stukova, M.; Egorov, A. Enhancement of the Immunogenicity of Influenza A Virus by the Inhibition of Immunosuppressive Function of NS1 Protein. Microbiol. Indep. Res. J. 2018, 5, 48–58. [Google Scholar] [CrossRef]
- Prokopenko, P.; Matyushenko, V.; Rak, A.; Stepanova, E.; Chistyakova, A.; Goshina, A.; Kudryavtsev, I.; Rudenko, L.; Isakova-Sivak, I. Truncation of NS1 Protein Enhances T Cell-Mediated Cross-Protection of a Live Attenuated Influenza Vaccine Virus Expressing Wild-Type Nucleoprotein. Vaccines 2023, 11, 501. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, M.; Lau, S.-Y.; Chen, P.; Mok, B.W.-Y.; Liu, S.; Liu, H.; Huang, X.; Cremin, C.J.; Song, W.; et al. Generation of DelNS1 Influenza Viruses: A Strategy for Optimizing Live Attenuated Influenza Vaccines. mBio 2019, 10, e02180-19. [Google Scholar] [CrossRef]
- AYu, E.; Medvedeva, T.E.; Polezhaev, F.I.; Alexandrova, G.I.; YuZ, G. Peculiarities of Obtaining and Characterization of a Cold-Adapted A/PR/8/34 Influenza Virus Variant. Acta Virol. 1984, 28, 19–25. [Google Scholar]
- Ferko, B.; Stasakova, J.; Sereinig, S.; Romanova, J.; Katinger, D.; Niebler, B.; Katinger, H.; Egorov, A. Hyperattenuated Recombinant Influenza A Virus Nonstructural-Protein-Encoding Vectors Induce Human Immunodeficiency Virus Type 1 Nef-Specific Systemic and Mucosal Immune Responses in Mice. J. Virol. 2001, 75, 8899–8908. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method Of Estimating Fifty Percent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Shurygina, A.-P.; Zabolotnykh, N.; Vinogradova, T.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sarsenbayeva, G.; Sansyzbay, A.; Vasilyev, K.; Buzitskaya, J.; et al. Preclinical Evaluation of TB/FLU-04L-An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis. Int. J. Mol. Sci. 2023, 24, 7439. [Google Scholar] [CrossRef] [PubMed]
- Pizzolla, A.; Nguyen, T.H.O.; Smith, J.M.; Brooks, A.G.; Kedzierska, K.; Heath, W.R.; Reading, P.C.; Wakim, L.M. Resident Memory CD8+ T Cells in the Upper Respiratory Tract Prevent Pulmonary Influenza Virus Infection. Sci. Immunol. 2017, 2, eaam6970. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yeung, K.K.; Watts, T.H. Tissue-Resident Memory T Cells in Protective Immunity to Influenza Virus. Curr. Opin. Virol. 2024, 65, 101397. [Google Scholar] [CrossRef]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. 2017, 24, e00414-16. [Google Scholar] [CrossRef]
- Stukova, M.A.; Sereinig, S.; Zabolotnyh, N.V.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.I.; Katinger, H.; Kiselev, O.I.; Egorov, A. Vaccine Potential of Influenza Vectors Expressing Mycobacterium Tuberculosis ESAT-6 Protein. Tuberculosis 2006, 86, 236–246. [Google Scholar] [CrossRef]
- Sergeeva, M.; Romanovskaya-Romanko, E.; Zabolotnyh, N.; Pulkina, A.; Vasilyev, K.; Shurigina, A.P.; Buzitskaya, J.; Zabrodskaya, Y.; Fadeev, A.; Vasin, A.; et al. Mucosal Influenza Vector Vaccine Carrying TB10.4 and HspX Antigens Provides Protection against Mycobacterium Tuberculosis in Mice and Guinea Pigs. Vaccines 2021, 9, 394. [Google Scholar] [CrossRef]
- Pulkina, A.; Vasilyev, K.; Muzhikyan, A.; Sergeeva, M.; Romanovskaya-Romanko, E.; Shurygina, A.-P.; Shuklina, M.; Vasin, A.; Stukova, M.; Egorov, A. IgGκ Signal Peptide Enhances the Efficacy of an Influenza Vector Vaccine against Respiratory Syncytial Virus Infection in Mice. Int. J. Mol. Sci. 2023, 24, 11445. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and Immunogenicity of a Live-Attenuated Influenza Virus Vector-Based Intranasal SARS-CoV-2 Vaccine in Adults: Randomised, Double-Blind, Placebo-Controlled, Phase 1 and 2 Trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and Efficacy of the Intranasal Spray SARS-CoV-2 Vaccine DNS1-RBD: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.; Brandt, S.; Sereinig, S.; Romanova, J.; Ferko, B.; Katinger, D.; Grassauer, A.; Alexandrova, G.; Katinger, H.; Muster, T. Transfectant Influenza A Viruses with Long Deletions in the NS1 Protein Grow Efficiently in Vero Cells. J. Virol. 1998, 72, 6437–6441. [Google Scholar] [CrossRef]
- García-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A Virus Lacking the NS1 Gene Replicates in Interferon-Deficient Systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wacheck, V.; Egorov, A.; Groiss, F.; Pfeiffer, A.; Fuereder, T.; Hoeflmayer, D.; Kundi, M.; Popow-Kraupp, T.; Redlberger-Fritz, M.; Mueller, C.A.; et al. A Novel Type of Influenza Vaccine: Safety and Immunogenicity of Replication-Deficient Influenza Virus Created by Deletion of the Interferon Antagonist NS1. J. Infect. Dis. 2010, 201, 354–362. [Google Scholar] [CrossRef]
- Stasakova, J.; Ferko, B.; Kittel, C.; Sereinig, S.; Romanova, J.; Katinger, H.; Egorov, A. Influenza A Mutant Viruses with Altered NS1 Protein Function Provoke Caspase-1 Activation in Primary Human Macrophages, Resulting in Fast Apoptosis and Release of High Levels of Interleukins 1β and 18. J. Gen. Virol. 2005, 86, 185–195. [Google Scholar] [CrossRef]
- Kandasamy, M.; Suryawanshi, A.; Tundup, S.; Perez, J.T.; Schmolke, M.; Manicassamy, S.; Manicassamy, B. RIG-I Signaling Is Critical for Efficient Polyfunctional T Cell Responses during Influenza Virus Infection. PLoS Pathog. 2016, 12, e1005754. [Google Scholar] [CrossRef]
- Trieu, M.-C.; Zhou, F.; Lartey, S.L.; Sridhar, S.; Mjaaland, S.; Cox, R.J. Augmented CD4+ T-Cell and Humoral Responses after Repeated Annual Influenza Vaccination with the Same Vaccine Component A/H1N1pdm09 over 5 Years. Npj Vaccines 2018, 3, 37. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Ueno, H. Circulating T Follicular Helper Subsets in Human Blood. Methods Mol. Biol. 2022, 2380, 29–39. [Google Scholar] [CrossRef]
- Heit, A.; Schmitz, F.; Gerdts, S.; Flach, B.; Moore, M.S.; Perkins, J.A.; Robins, H.S.; Aderem, A.; Spearman, P.; Tomaras, G.D.; et al. Vaccination Establishes Clonal Relatives of Germinal Center T Cells in the Blood of Humans. J. Exp. Med. 2017, 214, 2139–2152. [Google Scholar] [CrossRef]
- He, J.; Tsai, L.M.; Leong, Y.A.; Hu, X.; Ma, C.S.; Chevalier, N.; Sun, X.; Vandenberg, K.; Rockman, S.; Ding, Y.; et al. Circulating Precursor CCR7(Lo)PD-1(Hi) CXCR5+ CD4+ T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity 2013, 39, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Swarnalekha, N.; Schreiner, D.; Litzler, L.C.; Iftikhar, S.; Kirchmeier, D.; Künzli, M.; Son, Y.M.; Sun, J.; Moreira, E.A.; King, C.G. T Resident Helper Cells Promote Humoral Responses in the Lung. Sci. Immunol. 2021, 6, eabb6808. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Hutloff, A. Analysis of T-Cells in Inflamed Nonlymphoid Tissues. Methods Mol. Biol. 2021, 2285, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-X.; Esterbauer, R.; Vanderven, H.A.; Juno, J.A.; Kent, S.J.; Wheatley, A.K. Inducible Bronchus-Associated Lymphoid Tissues (IBALT) Serve as Sites of B Cell Selection and Maturation Following Influenza Infection in Mice. Front. Immunol. 2019, 10, 611. [Google Scholar] [CrossRef]
- Adachi, Y.; Onodera, T.; Yamada, Y.; Daio, R.; Tsuiji, M.; Inoue, T.; Kobayashi, K.; Kurosaki, T.; Ato, M.; Takahashi, Y. Distinct Germinal Center Selection at Local Sites Shapes Memory B Cell Response to Viral Escape. J. Exp. Med. 2015, 212, 1709–1723. [Google Scholar] [CrossRef]
- Bentebibel, S.-E.; Lopez, S.; Obermoser, G.; Schmitt, N.; Mueller, C.; Harrod, C.; Flano, E.; Mejias, A.; Albrecht, R.A.; Blankenship, D.; et al. Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination. Sci. Transl. Med. 2013, 5, 176ra32. [Google Scholar] [CrossRef]
- Sircy, L.M.; Ramstead, A.G.; Gibbs, L.C.; Joshi, H.; Baessler, A.; Mena, I.; García-Sastre, A.; Emerson, L.L.; Fairfax, K.C.; Williams, M.A.; et al. Generation of Antigen-Specific Memory CD4 T Cells by Heterologous Immunization Enhances the Magnitude of the Germinal Center Response upon Influenza Infection. PLoS Pathog. 2024, 20, e1011639. [Google Scholar] [CrossRef]
- Baiyegunhi, O.; Ndlovu, B.; Ogunshola, F.; Ismail, N.; Walker, B.D.; Ndung’u, T.; Ndhlovu, Z.M. Frequencies of Circulating Th1-Biased T Follicular Helper Cells in Acute HIV-1 Infection Correlate with the Development of HIV-Specific Antibody Responses and Lower Set Point Viral Load. J. Virol. 2018, 92, e00659-18. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Kurata, I.; Matsumoto, I.; Sumida, T. T Follicular Helper Cell Subsets: A Potential Key Player in Autoimmunity. Immunol. Med. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.-L.; Yu, D.; Liu, Z. Roles of Follicular Helper and Regulatory T Cells in Allergic Diseases and Allergen Immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef]
- Gao, X.; Luo, K.; Wang, D.; Wei, Y.; Yao, Y.; Deng, J.; Yang, Y.; Zeng, Q.; Dong, X.; Xiong, L.; et al. T Follicular Helper 17 (Tfh17) Cells Are Superior for Immunological Memory Maintenance. eLife 2023, 12, e82217. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurygina, A.-P.; Shuklina, M.; Ozhereleva, O.; Romanovskaya-Romanko, E.; Kovaleva, S.; Egorov, A.; Lioznov, D.; Stukova, M. Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice. Vaccines 2025, 13, 58. https://doi.org/10.3390/vaccines13010058
Shurygina A-P, Shuklina M, Ozhereleva O, Romanovskaya-Romanko E, Kovaleva S, Egorov A, Lioznov D, Stukova M. Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice. Vaccines. 2025; 13(1):58. https://doi.org/10.3390/vaccines13010058
Chicago/Turabian StyleShurygina, Anna-Polina, Marina Shuklina, Olga Ozhereleva, Ekaterina Romanovskaya-Romanko, Sofia Kovaleva, Andrej Egorov, Dmitry Lioznov, and Marina Stukova. 2025. "Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice" Vaccines 13, no. 1: 58. https://doi.org/10.3390/vaccines13010058
APA StyleShurygina, A.-P., Shuklina, M., Ozhereleva, O., Romanovskaya-Romanko, E., Kovaleva, S., Egorov, A., Lioznov, D., & Stukova, M. (2025). Truncated NS1 Influenza A Virus Induces a Robust Antigen-Specific Tissue-Resident T-Cell Response and Promotes Inducible Bronchus-Associated Lymphoid Tissue Formation in Mice. Vaccines, 13(1), 58. https://doi.org/10.3390/vaccines13010058






