Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent
Abstract
1. Introduction
2. Materials and Methods
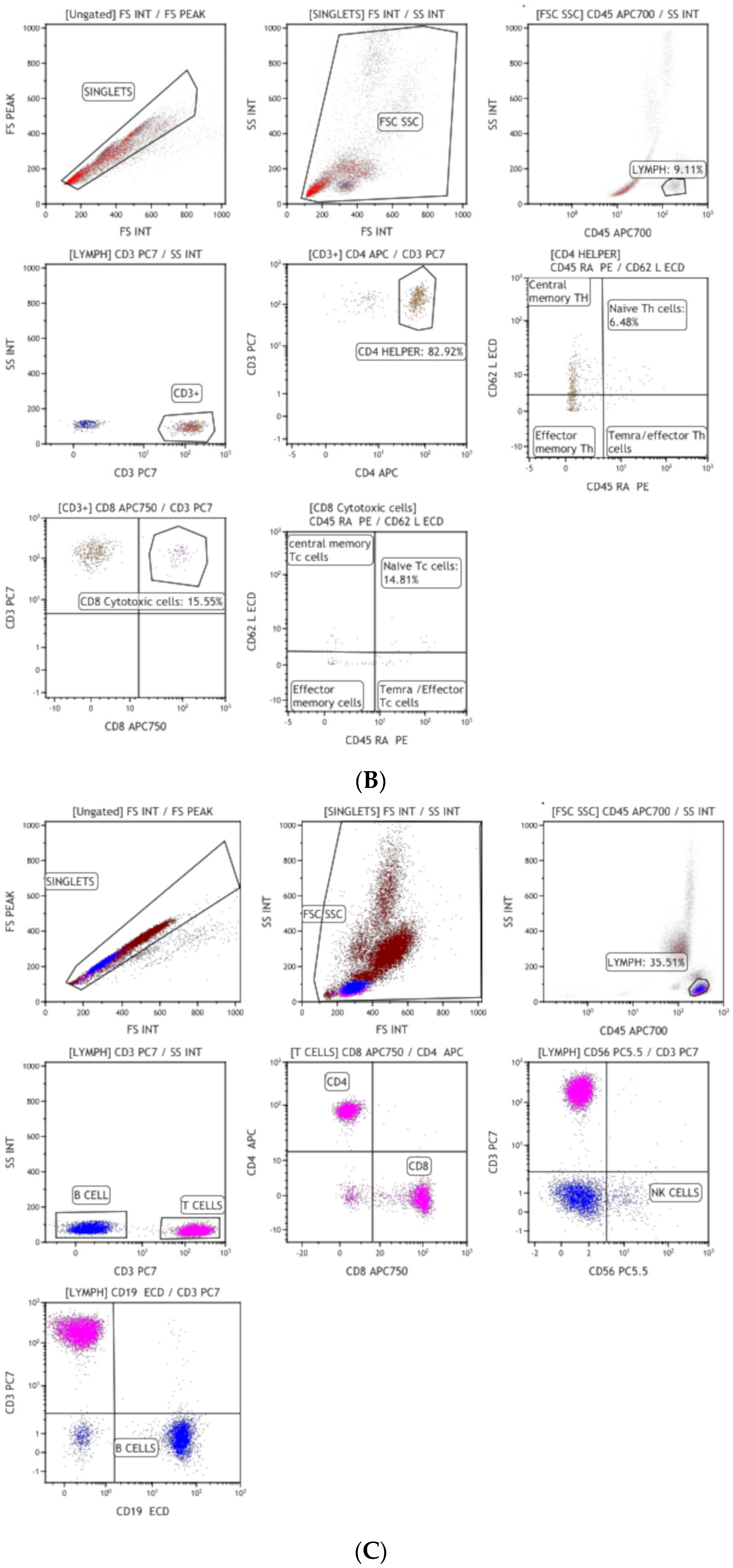
2.1. Immunophenotyping
2.2. Stool Sample Collection, Processing, and Enterovirus Isolation
2.3. RT-PCR and Sanger Sequencing
2.4. Comparative Analysis of Nucleotide and Amino Acid Sequences
2.5. NGS
2.6. Poliovirus Neutralizing Antibody Titer
2.7. Cytokine Assay
3. Results
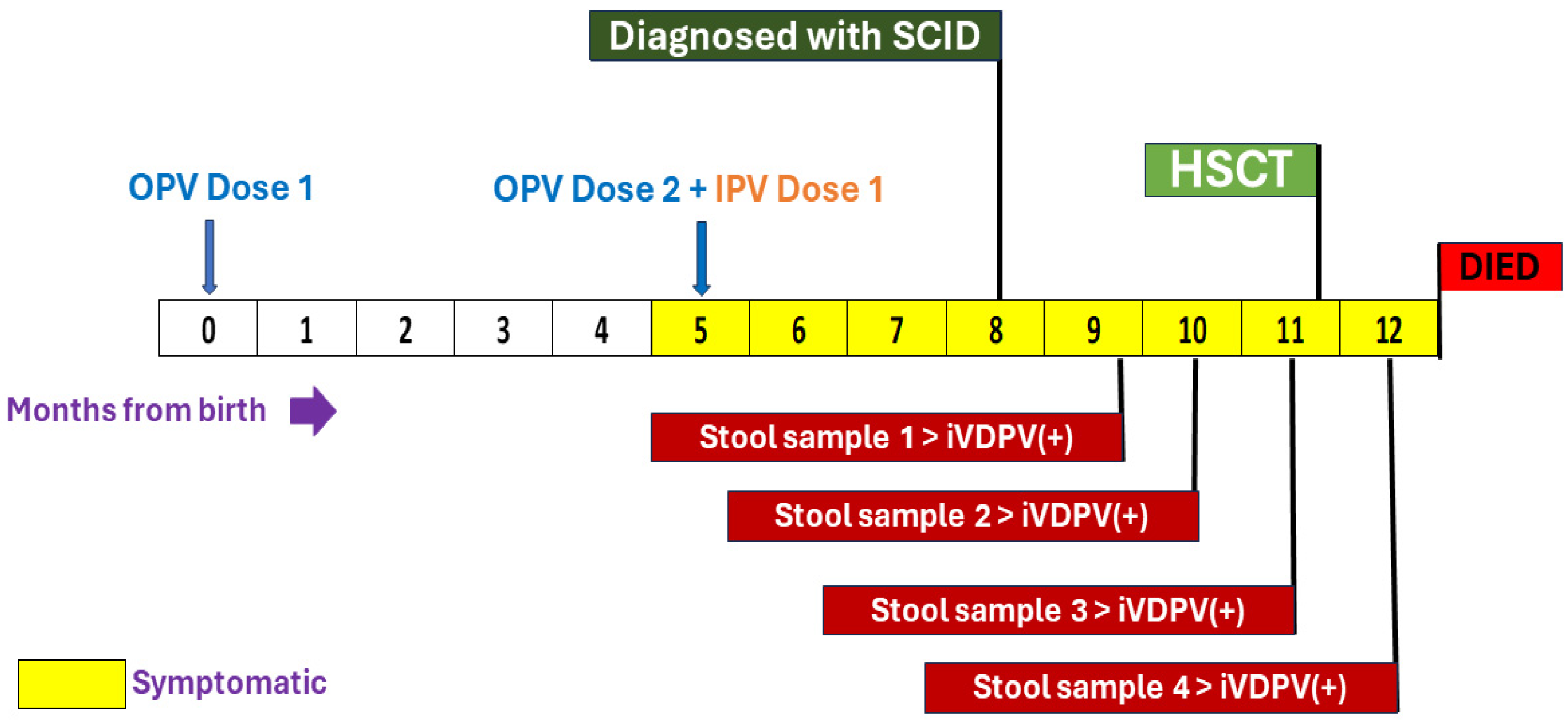
3.1. Clinical Suspicion and Diagnosis of Inborn Errors of Immunity (IEI)
3.2. Stool Sample Analysis for Poliovirus Infection
3.3. Action Taken by the Surveillance Program and Details of the Epidemiological Investigation
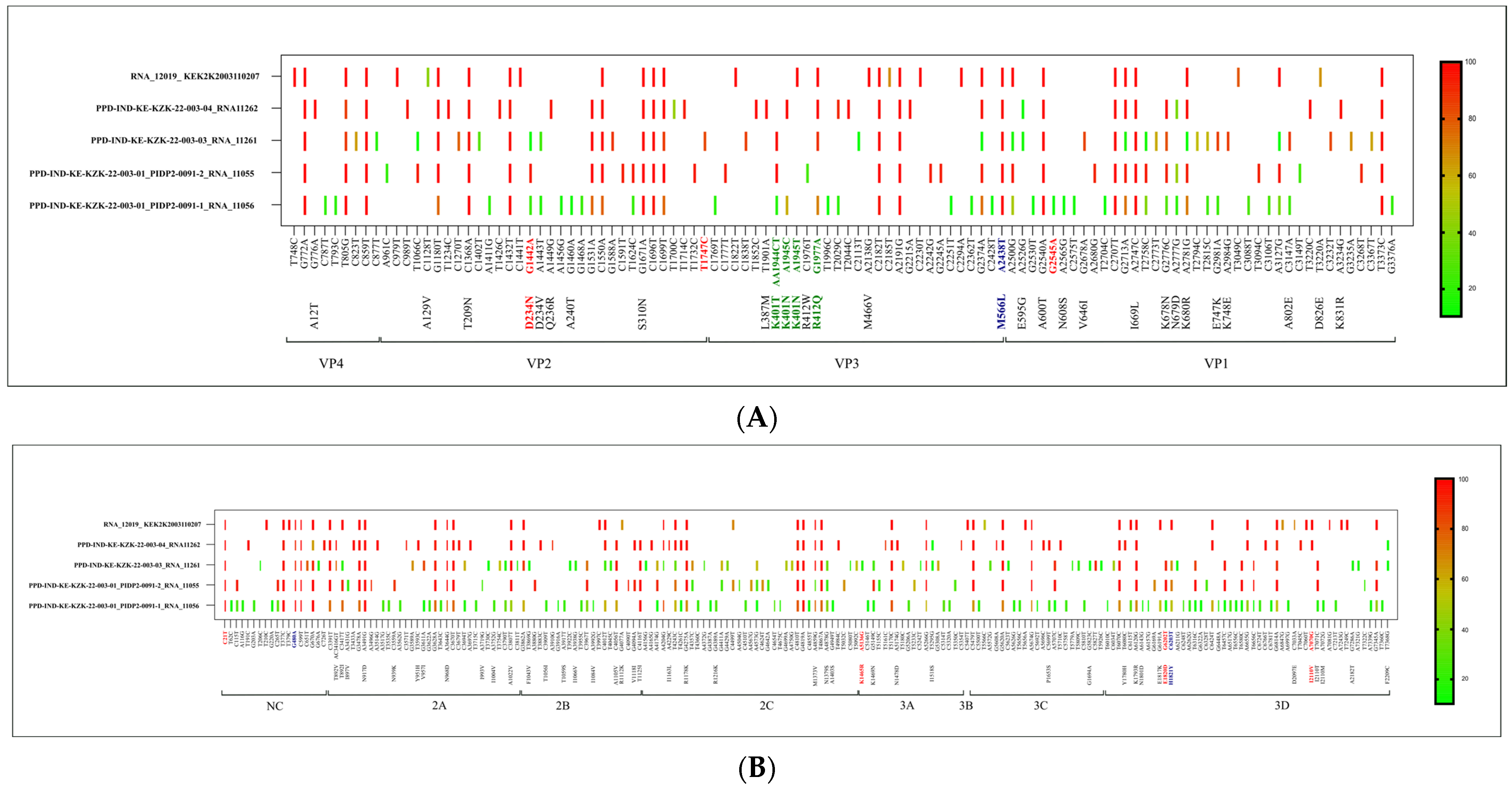
3.4. Molecular Characterization of iVDPV by Whole Genome Sequencing
3.5. Anti-Polio Antibody and Cytokine Production
3.6. Hematopoietic Stem Cell Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.E.; Greene, S.A.; Burns, C.C.; Tallis, G.; Wassilak, S.G.F.; Bolu, O. Progress Toward Poliomyelitis Eradication—Worldwide, January 2021–March 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Zomahoun, D.J.; Burman, A.L.; Snider, C.J.; Chauvin, C.; Gardner, T.; Lickness, J.S.; Ahmed, J.A.; Diop, O.; Gerber, S.; Anand, A. Impact of COVID-19 pandemic on global poliovirus surveillance. Morb. Mortal. Wkly. Rep. 2021, 69, 1648–1652. [Google Scholar] [CrossRef]
- Bigouette, J.P.; Henderson, E.; Traoré, M.A.; Wassilak, S.G.F.; Jorba, J.; Mahoney, F.; Bolu, O.; Diop, O.M.; Burns, C.C. Update on vaccine-derived poliovirus outbreaks—Worldwide, January 2021–December 2022. Morb. Mortal. Wkly. Rep. 2023, 72, 366–371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Poliomyelitis (Polio). Available online: https://www.who.int/health-topics/poliomyelitis#tab=tab_1 (accessed on 1 December 2023).
- Initiative GPE. Guidelines for Implementing Poliovirus Surveillance among Patients with Primary Immunodeficiency Disorders (PIDs); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Aluri, J.; Desai, M.; Gupta, M.; Dalvi, A.; Terance, A.; Rosenzweig, S.D.; Stoddard, J.L.; Niemela, J.E.; Tamankar, V.; Mhatre, S.; et al. Clinical, immunological, and molecular findings in 57 patients with severe combined immunodeficiency (SCID) from India. Front. Immunol. 2019, 10, 23. [Google Scholar] [CrossRef]
- Galal, N.M.; Meshaal, S.; ElHawary, R.; Nasr, E.; Bassiouni, L.; Ashghar, H.; Farag, N.H.; Mach, O.; Burns, C.; Iber, J.; et al. Poliovirus excretion following vaccination with live poliovirus vaccine in patients with primary immunodeficiency disorders: Clinicians’ perspectives in the endgame plan for polio eradication. BMC Res. Notes 2018, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Liu, Y.; Xu, J.-W.; Wang, Q.; Yin, Z.-D.; Wen, N.; Yang, H.; Rodewald, L.E.; Zhang, Z.-Y. Detection of a highly divergent type 3 vaccine-derived poliovirus in a child with a severe primary immunodeficiency disorder—Chongqing, China, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1148–1150. [Google Scholar] [CrossRef]
- Macklin, G.; Liao, Y.; Takane, M.; Dooling, K.; Gilmour, S.; Mach, O.; Kew, O.M.; Sutter, R.W.; iVDPV Working Group. Prolonged excretion of poliovirus among individuals with primary immunodeficiency disorder: An analysis of the World Health Organization registry. Front. Immunol. 2017, 8, 1103. [Google Scholar] [CrossRef]
- World Health Organization; Global Polio Eradication Initiative. Polio Eradication Strategy 2022–2026 Delivering on a Promise; Global Strategy; WHO Publications: Geneva, Switzerland, 2021; ISBN 9789240031937. [Google Scholar]
- Mohanty, M.C.; Desai, M.; Mohammad, A.; Aggarwal, A.; Govindaraj, G.; Bhattad, S.; Lashkari, H.P.; Rajasekhar, L.; Verma, H.; Kumar, A.; et al. Assessment of Enterovirus Excretion and Identification of VDPVs in Patients with Primary Immunodeficiency in India: Outcome of ICMR–WHO Collaborative Study Phase-I. Vaccines 2023, 11, 1211. [Google Scholar] [CrossRef]
- Mohanty, M.C.; Madkaikar, M.R.; Desai, M.; Aluri, J.; Varose, S.Y.; Taur, P.; Sharma, D.K.; Nalavade, U.P.; Rane, S.V.; Gupta, M.; et al. Natural Clearance of Prolonged VDPV Infection in a Child with Primary Immunodeficiency Disorder. Front. Immunol. 2019, 10, 1567. [Google Scholar] [CrossRef]
- WHO. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/bitstream/handle/10665/68762/WHO_IVB_04.10.pdf;jsessionid=993D46DCF3B5F9174C6E759967C83384?sequence=1 (accessed on 1 December 2023).
- Mohanty, M.C.; Mohammad, A.; Verma, H.; Kumar, A.; Madkaikar, M.R.; Desai, M.; Varose, S.Y.; Sawant, U.; Yadav, R.M.; Taur, P.; et al. Poliovirus surveillance in patients with primary immunodeficiencies, India. Bull. World Health Organ. 2023, 101, 346–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kilpatrick, D.R.; Iber, J.C.; Chen, Q.; Ching, K.; Yang, S.-J.; De, L.; Mandelbaum, M.D.; Emery, B.; Campagnoli, R.; Burns, C.C.; et al. Poliovirus serotype-specific VP1 sequencing primers. Virol. Methods 2011, 174, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Colbere-Garapin, F.; Macadam, A.; Taffs, L.F.; Marsden, S.; Minor, P.; Horaud, F. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J. Virol. 1990, 64, 4922–4929. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D.; Ferguson, M.; Evans, D.M.A.; Almond, J.W.; Icenogle, J.P. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J. Gen. Virol. 1986, 67 Pt 7, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, G.M.; Vellarikkal, S.K.; Jayarajan, R.; Ravi, R.; Verma, A.; Chakkiyar, K.; Jayakrishnan, M.P.; Arakkal, R.; Raj, R.; Sivasubbu, S.; et al. Case Report: Whole exome sequencing identifies variation c.2308G>A p.E770K in RAG1 associated with B- T- NK+ severe combined immunodeficiency. F1000Research 2016, 5, 2532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macklin, G.; Diop, O.M.; Humayun, A.; Shahmahmoodi, S.; El-Sayed, Z.A.; Triki, H.; Rey, G.; Avagyan, T.; Grabovac, V.; Jorba, J.; et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses—Worldwide, July 2018-December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 913–917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Govindaraj, G.M.; Krishnakumar, P.; Scaria, V.; Athulya, E.; Ajithkumar, V.T.; Dongre, A.R. Building on an Ad Hoc COVID-19 Response to Enhance Community-based Care for Vulnerable Children in Kerala, India. NEJM Catal. Innov. Care Deliv. 2020. [Google Scholar]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine Derived Polioviruses and the endgame statergy for global polio eradication. Annu. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; De Coster, I.; Bandyopadhyay, A.S.; Revets, H.; Withanage, K.; De Smedt, P.; Suykens, L.; Oberste, M.S.; Weldon, W.C.; Costa-Clemens, S.A.; et al. The safety and immunogenicity of 2 novellive attenuated monovalent oral poliovaccines in healthy adults: A double-blind single center Phase 1 study. Lancet 2019, 394, 148–158. [Google Scholar] [CrossRef]
- Muslin, C.; Mac Kain, A.; Bessaud, M.; Blondel, B.; Delpeyroux, F. Recombination in Enteroviruses, a Multi-Step Modular Evolutionary Process. Viruses 2019, 11, 859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexander, J.P.; Ehresmann, K.; Seward, J.; Wax, G.; Harriman, K.; Fuller, S.; Cebelinski, E.A.; Chen, Q.; Jorba, J.; Kew, O.M.; et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J. Infect. Dis. 2009, 199, 391–397. [Google Scholar] [CrossRef]
- Avellón, A.; Cabrerizo, M.; de Miguel, T.; Pérez-Breña, P.; Tenorio, A.; Pérez, J.L.; de Aragón, M.V.M.; Trallero, G. Paralysis case and contact spread of recombinant vaccine-derived poliovirus, Spain. Emerg. Infect. Dis. 2008, 14, 1807–1809. [Google Scholar] [CrossRef]
- Copelyn, J.; Hincks, J.R.; Wilmshurst, J.M.; Petersen, W.; Howard, W.; Jallow, S.; Moonsamy, S.; Seakamela, L.; Suchard, M.; Collett, M.S.; et al. Clearance of immunodeficiency-associated vaccine-derived poliovirus infection with pocapavir. Pediatr. Infect. Dis. J. 2020, 39, 435–437. [Google Scholar] [CrossRef]
- Collett, M.S.; Hincks, J.R.; Benschop, K.; Duizer, E.; van der Avoort, H.; Rhoden, E.; Liu, H.; Oberste, M.S.; McKinlay, M.A.; Hartford, M. Antiviral Activity of Pocapavir in a Randomized, Blinded, Placebo-Controlled Human Oral Poliovirus Vaccine Challenge Model. J. Infect. Dis. 2017, 215, 335–343. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Irannejad, M.; Abolhassani, H.; Shahmahmoodi, S.; Hamidieh, A.A.; Soleyman-Jahi, S.; Yazdani, R.; Azizi, G.; Aghamohammadi, A. Clearing vaccine-derived poliovirus infection following hematopoietic stem cell transplantation: A case report and review of literature. J. Clin. Immunol. 2018, 38, 610–616. [Google Scholar] [CrossRef]
- Singanayagam, A.; Klapsa, D.; Burton-Fanning, S.; Hand, J.; Wilton, T.; Stephens, L.; Mate, R.; Shillitoe, B.; Celma, C.; Slatter, M.; et al. Asymptomatic immunodeficiency-associated vaccine-derived poliovirus infections in two UK children. Nat. Commun. 2023, 14, 3413. [Google Scholar] [CrossRef]


| Characteristics | VDPV Patient |
|---|---|
| Age at hospitalization, months | 7 months |
| Sex | Male |
| Immunodeficiency type | Severe combined immunodeficiency (SCID) |
| Diagnosis of immunodeficiency (months) | 7 months |
| OPV * doses | 2 |
| IPV * dose (age at vaccination in months) | 1 |
| Period of poliovirus excretion after 1st detection, months | 3 months |
| Estimated total time of virus excretion at the time of detection, months | 3 months |
| Estimated total time of poliovirus excretion, months | 3 months |
| Maximum nucleotide differences | 16 |
| Neutralizing antibody tires § | |
| Against poliovirus type 1 | 3.5 |
| Against poliovirus type 3 | 2.83 |
| Samples | ITD Results | Sequencing Results | Nucleotide Changes |
|---|---|---|---|
| Sample 1 | P1 Discordant | iVDPV1 (16 nucleotide) | A21G, T45C, G51T, G61A, C96T, C228T, G234A, A268C, T279C, G297C, A298G, A302G, C489T, A648G, C665T, T894C |
| Sample 2 | P1SL | iVDPV1 (15 nucleotide) | A21G, G61A, A201G, C228T, G234A, A268C, T279C, G297C, A298G, A302G, C405T, T615C, A648G, C789T, T894C |
| Sample 3 | P1SL | iVDPV1 (15 nucleotide) | A21G, G61A, A133G, A201G, C228T, G234A, A268C, T279C, G297C, A298G, A302G, T615C, A648G, C789T, T894C |
| Sample 4 | P1SL | iVDPV1 (11 nucleotide) | G61A, C228T, G234A, A268C, G297C, A298G, A302G, C489T, T741C, C753G, T894C |
| Contact 1 | P1SL | iVDPV1 (11 nucleotide) | A21G, G61A, C228T, G234A, A268C, G297C, A298G, A302G, T570C, A648G, T894C |
| Samples | Sample 1 | Sample 2 | Sample 3 | Sample 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide | Amino Acid | Previously Reported Neurovirulent Mutations | Poliovirus Strain Sabin-1 to Mahoney Strain | Nucleotide | Amino Acid | Previously Reported Neurovirulent Mutations | Poliovirus Strain Sabin-1 to Mahoney Strain | Nucleotide | Amino Acid | Previously Reported Neurovirulent Mutations | Poliovirus Strain Sabin-1 to Mahoney Strain | Nucleotide | Amino Acid | Previously Reported Neurovirulent Mutations | Polio-virus Strain Sabin-1 to Mahoney Strain | |
| NC | 11 | 0 | G480A | C21T | 7 | 0 | G480A | C21T | 8 | 0 | G480A | C21T | 7 | 0 | G480A | C21T |
| VP4 | 5 | 0 | 3 | 0 | 5 | 0 | 4 | 1 | ||||||||
| VP2 | 15 | 5 | G1442A (AA: D234N) | 14 | 3 | G1442A (AA: D234N) | 15 | 4 | G1442A (AA: D234N), T1747C | 14 | 3 | |||||
| VP3 | 13 | 4 | A2438T (AA:M566L) | 9 | 3 | A2438T (AA:M566L) | 8 | 3 | A2438T (AA:M566L) | 11 | 4 | A2438T (AA:M566L) | ||||
| VP1 | 22 | 8 | G2545A | 15 | 5 | 21 | 9 | 11 | 7 | |||||||
| 2A | 18 | 6 | 12 | 7 | 16 | 6 | 14 | 5 | ||||||||
| 2B | 10 | 5 | 6 | 3 | 9 | 4 | 7 | 3 | ||||||||
| 2C | 19 | 5 | 17 | 3 | 19 | 3 | 11 | 3 | ||||||||
| 3A | 7 | 1 | 6 | 1 | 8 | 3 | A5136G (AA: K1465R) | 6 | 2 | |||||||
| 3C | 11 | 1 | 4 | 0 | 11 | 1 | 7 | 1 | ||||||||
| 3D | 31 | 7 | C6203T (AA:H1821Y) | G6202T (AA: E1820D) | 14 | 4 | C6203T (AA:H1821Y) | G6202T (AA: E1820D) | 16 | 6 | C6203T (AA:H1821Y) | G6202T (AA: E1820D) | 10 | 4 | A7070G (AA: I2110V) | |
| 162 | 42 | 107 | 29 | 136 | 39 | 102 | 33 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, M.C.; Govindaraj, G.; Ahmad, M.; Varose, S.Y.; Tatkare, M.; Shete, A.; Yadav, S.; Joshi, Y.; Yadav, P.; Sharma, D.; et al. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent. Vaccines 2024, 12, 759. https://doi.org/10.3390/vaccines12070759
Mohanty MC, Govindaraj G, Ahmad M, Varose SY, Tatkare M, Shete A, Yadav S, Joshi Y, Yadav P, Sharma D, et al. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent. Vaccines. 2024; 12(7):759. https://doi.org/10.3390/vaccines12070759
Chicago/Turabian StyleMohanty, Madhu Chhanda, Geeta Govindaraj, Mohammad Ahmad, Swapnil Y. Varose, Manogat Tatkare, Anita Shete, Savita Yadav, Yash Joshi, Pragya Yadav, Deepa Sharma, and et al. 2024. "Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent" Vaccines 12, no. 7: 759. https://doi.org/10.3390/vaccines12070759
APA StyleMohanty, M. C., Govindaraj, G., Ahmad, M., Varose, S. Y., Tatkare, M., Shete, A., Yadav, S., Joshi, Y., Yadav, P., Sharma, D., Kumar, A., Verma, H., Patil, A. P., Edavazhipurath, A., Dhanasooraj, D., Othayoth Kandy, S., Puthenpurayil, J. M., Chakyar, K., Melarcode Ramanan, K., & Madkaikar, M. (2024). Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent. Vaccines, 12(7), 759. https://doi.org/10.3390/vaccines12070759








