Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccination Campaigns
2.2. Immunity Estimation
2.3. Virus Surveillance
2.4. Estimating Seeding Date
2.5. Estimating Time to Detection
2.6. Estimating Number of Emergences Expected
3. Results
3.1. Description of Post-Switch OPV2 Use
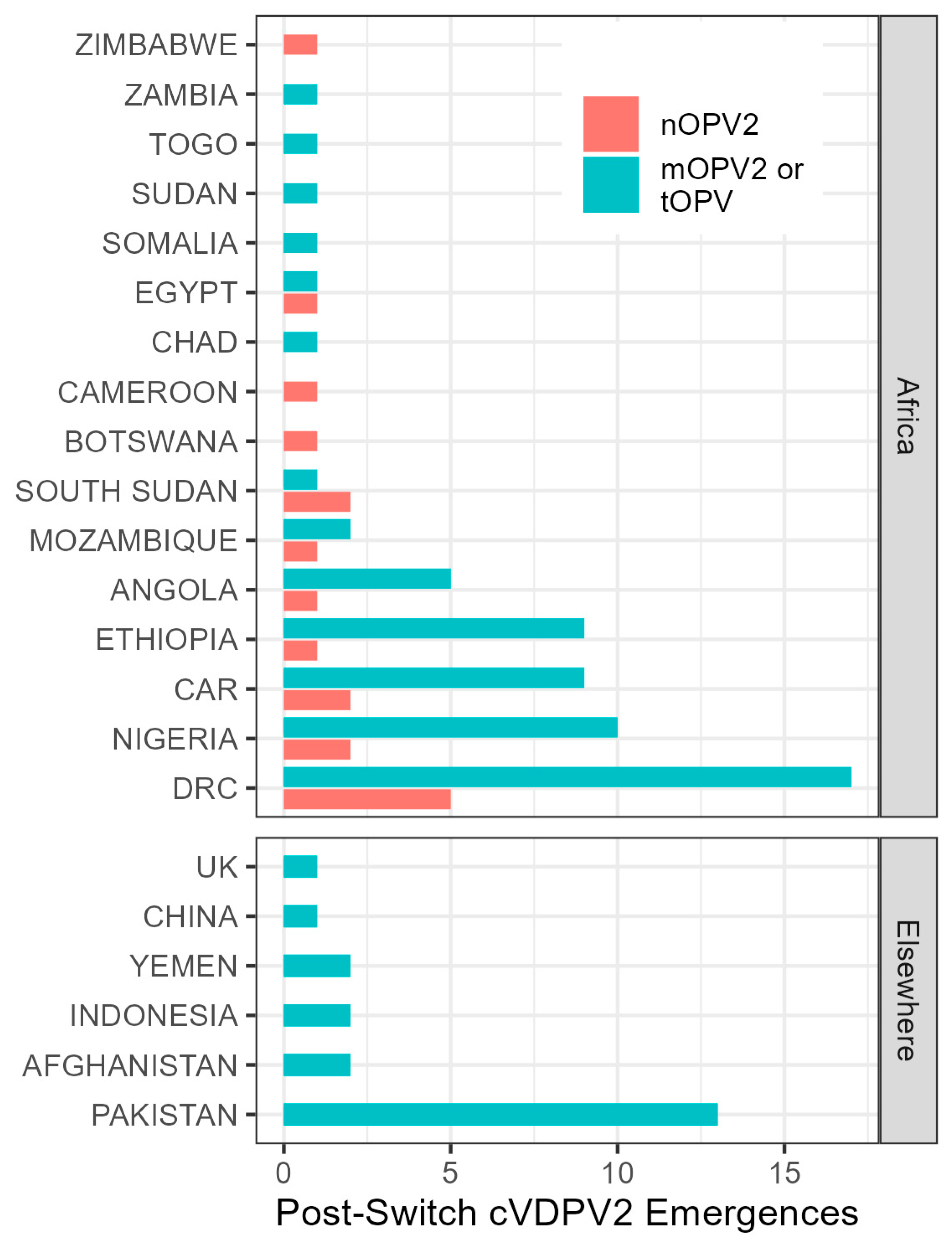
3.2. Observation of Post-Switch cVDPV2 Emergences
3.3. Estimating Emergence Expectation for nOPV2
3.4. Crude Global Analysis
3.5. Adjusted Africa Analysis
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A




References
- Sabin, A.B. Oral Poliovirus Vaccine: History of Its Development and Prospects for Eradication of Poliomyelitis. JAMA 1965, 194, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Geiger, K. Progress Toward Poliomyelitis Eradication—Worldwide, January 2022–December 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-Derived Polioviruses. J. Infect. Dis. 2014, 210 (Suppl. S1), S283–S293. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C. Eradicating Polio with a Vaccine We Must Stop Using. Lancet Infect. Dis. 2018, 18, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Namageyo-Funa, A. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2023–June 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 909–916. [Google Scholar] [CrossRef]
- Garon, J.; Seib, K.; Orenstein, W.A.; Ramirez Gonzalez, A.; Chang Blanc, D.; Zaffran, M.; Patel, M. Polio Endgame: The Global Switch from tOPV to bOPV. Expert Rev. Vaccines 2016, 15, 693–708. [Google Scholar] [CrossRef]
- Gray, E.J.; Cooper, L.V.; Bandyopadhyay, A.S.; Blake, I.M.; Grassly, N.C. The Origins and Risk Factors for Serotype-2 Vaccine-Derived Poliovirus Emergences in Africa During 2016–2019. J. Infect. Dis. 2023, 228, 80–88. [Google Scholar] [CrossRef]
- Yeh, M.T.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.S.; Van Damme, P.; De Coster, I.; et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 2020, 27, 736–751.e8. [Google Scholar] [CrossRef]
- Macadam, A.J.; Ferguson, G.; Stone, D.M.; Meredith, J.; Knowlson, S.; Auda, G.; Almond, J.W.; Minor, P.D. Rational Design of Genetically Stable, Live-Attenuated Poliovirus Vaccines of All Three Serotypes: Relevance to Poliomyelitis Eradication. J. Virol. 2006, 80, 8653–8663. [Google Scholar] [CrossRef]
- Famulare, M.; Chang, S.; Iber, J.; Zhao, K.; Adeniji, J.A.; Bukbuk, D.; Baba, M.; Behrend, M.; Burns, C.C.; Oberste, M.S. Sabin Vaccine Reversion in the Field: A Comprehensive Analysis of Sabin-Like Poliovirus Isolates in Nigeria. J. Virol. 2015, 90, 317–331. [Google Scholar] [CrossRef]
- Wahid, R.; Mercer, L.; Gast, C.; De Leon, T.; Sáez-Llorens, X.; Fix, A.; Macadam, A.; Stephens, L.; Chumakov, K.; Smits, S.L.; et al. Evaluating Stability of Attenuated Sabin and Two Novel Type 2 Oral Poliovirus Vaccines in Children. npj Vaccines 2022, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Ochoge, M.; Futa, A.C.; Umesi, A.; Affleck, L.; Kotei, L.; Daffeh, B.; Saidy-Jah, E.; Njie, A.; Oyadiran, O.; Edem, B.; et al. Safety of the Novel Oral Poliovirus Vaccine Type 2 (nOPV2) in Infants and Young Children Aged 1 to <5 Years and Lot-to-Lot Consistency of the Immune Response to nOPV2 in Infants in The Gambia: A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet 2024, 403, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Statement of the Thirty-Ninth Meeting of the Polio IHR Emergency Committee. Available online: https://www.who.int/news/item/13-08-2024-statement-of-the-thirty-ninth-meeting-of-the-polio-ihr-emergency-committee (accessed on 12 November 2024).
- World Health Organization. First Ever Vaccine Listed Under WHO Emergency Use. Available online: https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use (accessed on 5 November 2024).
- Macklin, G.R.; Peak, C.; Eisenhawer, M.; Kurji, F.; Mach, O.; Konz, J.; Gast, C.; Bachtiar, N.S.; Bandyopadhyay, A.S.; Zipursky, S. Enabling Accelerated Vaccine Roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine Type 2 (nOPV2) Experience. Vaccine 2023, 41 (Suppl. S1), A122–A127. [Google Scholar] [CrossRef] [PubMed]
- Martin, J. Genetic Characterization of Novel Oral Polio Vaccine Type 2 Viruses During Initial Use Phase Under Emergency Use Listing—Worldwide, March–October 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 786–790. [Google Scholar] [CrossRef]
- GACVS Sub-Committee on nOPV2. Summary Note for Record Meeting of the GACVS (Global Advisory Committee on Vaccine Safety) Sub-Committee on Novel Oral Polio Vaccine Type 2 (nOPV2) Safety—July 18, 2024. Available online: https://polioeradication.org/wp-content/uploads/2024/09/GACVS-nOPV2-sub-committee-meeting-20240718.pdf (accessed on 21 November 2024).
- World Health Organization. Polio Information System (POLIS). Available online: https://extranet.who.int/polis (accessed on 5 November 2024).
- Voorman, A.; O’Reilly, K.; Lyons, H.; Goel, A.K.; Touray, K.; Okiror, S. Real-Time Prediction Model of cVDPV2 Outbreaks to Aid Outbreak Response Vaccination Strategies. Vaccine 2023, 41, A105–A112. [Google Scholar] [CrossRef]
- World Health Organization. Global Polio Surveillance Action Plan 2022–2024. 2022. Available online: https://polioeradication.org/wp-content/uploads/2022/05/GPSAP-2022-2024-EN.pdf (accessed on 5 November 2024).
- Joffret, M.-L.; Doté, J.W.; Gumede, N.; Vignuzzi, M.; Bessaud, M.; Gouandjika-Vasilache, I. Vaccine-Derived Polioviruses, Central African Republic, 2019. Emerg. Infect. Dis. 2021, 27, 620–623. [Google Scholar] [CrossRef]
- Davlantes, E. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use—Six African Countries, 2021–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1041–1042. [Google Scholar] [CrossRef]
- McCarthy, K.A.; Chabot-Couture, G.; Famulare, M.; Lyons, H.M.; Mercer, L.D. The Risk of Type 2 Oral Polio Vaccine Use in Post-Cessation Outbreak Response. BMC Med. 2017, 15, 175. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Wassilak, S.G.; Pallansch, M.A.; Burns, C.C.; Wiesen, E.; Durry, E.; Badizadegan, K.; Thompson, K.M. Outbreak Response Strategies with Type 2-Containing Oral Poliovirus Vaccines. Vaccine 2022, 41 (Suppl. S1), A142. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, September 2023: Conclusions and Recommendations. Wkly. Epidemiol. Rec. 2023, 47, 599–620. [Google Scholar]
- Razafindratsimandresy, R.; Joffret, M.-L.; Andriamandimby, S.F.; Andriamamonjy, S.; Rabemanantsoa, S.; Richard, V.; Delpeyroux, F.; Heraud, J.-M.; Bessaud, M. Enterovirus Detection in Different Regions of Madagascar Reveals a Higher Abundance of Enteroviruses of Species C in Areas Where Several Outbreaks of Vaccine-Derived Polioviruses Occurred. BMC Infect. Dis. 2022, 22, 821. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N. Surveillance To Track Progress Toward Polio Eradication—Worldwide, 2022–2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Lyons, H.; Shuaib, F.; Adamu, U.S.; Korir, C.; Erbeto, T.; Bandyopadhyay, A.S.; Okiror, S. Impact of Supplementary Immunization Activities Using Novel Oral Polio Vaccine Type 2 during a Large Outbreak of Circulating Vaccine-Derived Poliovirus in Nigeria. J. Infect. Dis. 2024, 229, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bashorun, A.O.; Kotei, L.; Jawla, O.; Jallow, A.F.; Saidy, A.J.; Kinteh, M.-A.; Kujabi, A.; Jobarteh, T.; Kanu, F.J.; Donkor, S.A.; et al. Tolerability, Safety, and Immunogenicity of the Novel Oral Polio Vaccine Type 2 in Children Aged 6 Weeks to 59 Months in an Outbreak Response Campaign in The Gambia: An Observational Cohort Study. Lancet Infect. Dis. 2024, 24, 417–426. [Google Scholar] [CrossRef]
- Mirzoev, A.; Macklin, G.R.; Zhang, Y.; Mainou, B.A.; Sadykova, U.; Olsavszky, V.S.; Huseynov, S.; Ruziev, M.; Saidzoda, F.; Bobokhonova, M.; et al. Assessment of Serological Responses Following Vaccination Campaigns with Type 2 Novel Oral Polio Vaccine: A Population-Based Study in Tajikistan in 2021. Lancet Glob. Health 2022, 10, e1807–e1814. [Google Scholar] [CrossRef]
- Nathanson, N.; Kew, O.M. From Emergence to Eradication: The Epidemiology of Poliomyelitis Deconstructed. Am. J. Epidemiol. 2010, 172, 1213–1229. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Meeting of the WHO Global Advisory Committee on Vaccine Safety and the WHO Advisory Committee on Safety of Medicinal, 13–15 November 2023. Wkly. Epidemiol. Rec. 2024, 99, 95–104. [Google Scholar]
- Donato, C.M.; Ch’ng, L.S.; Boniface, K.F.; Crawford, N.W.; Buttery, J.P.; Lyon, M.; Bishop, R.F.; Kirkwood, C.D. Identification of Strains of RotaTeq Rotavirus Vaccine in Infants With Gastroenteritis Following Routine Vaccination. J. Infect. Dis. 2012, 206, 377–383. [Google Scholar] [CrossRef]
- Chang, S.Y.; Bisht, A.; Faysman, K.; Schiller, G.J.; Uslan, D.Z.; Multani, A. Vaccine-Associated Measles in a Hematopoietic Cell Transplant Recipient: Case Report and Comprehensive Review of the Literature. Open Forum Infect. Dis. 2021, 8, ofab326. [Google Scholar] [CrossRef]
- Greenwood, K.P.; Hafiz, R.; Ware, R.S.; Lambert, S.B. A Systematic Review of Human-to-Human Transmission of Measles Vaccine Virus. Vaccine 2016, 34, 2531–2536. [Google Scholar] [CrossRef]
- Gordillo-Marañón, M.; Candore, G.; Hedenmalm, K.; Browne, K.; Flynn, R.; Piccolo, L.; Santoro, A.; Zaccaria, C.; Kurz, X. Lessons Learned on Observed-to-Expected Analysis Using Spontaneous Reports During Mass Vaccination. Drug Saf. 2024, 47, 607. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, P.C.; Hu, M.; Wong, H.-L.; Shoaibi, A.; Zhou, C.K.; Lo, A.-C.; Amend, K.; Beachler, D.C.; McMahill-Walraven, C.N.; Smith, E.R.; et al. Near Real-Time Surveillance of Safety Outcomes in US COVID-19 Vaccine Recipients Aged 12 to 64 Years. Vaccine 2022, 40, 6481. [Google Scholar] [CrossRef] [PubMed]



| Country i | Vaccine Doses (Million) ii | cVDPV2 Emergences iii | |||
|---|---|---|---|---|---|
| mOPV2 or tOPV | nOPV2 | Derived from mOPV2 or tOPV | Derived from nOPV2 | ||
| Africa | 483 | 1170 | 59 | 18 | |
| Nigeria | 163 | 657 | 10 | 2 | |
| DRC | 53 | 89 | 17 | 5 | |
| Elsewhere | 196 | 20 | 21 | 0 | |
| Total | 679 | 1190 | 80 | 18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peak, C.M.; Lyons, H.; Voorman, A.; Gray, E.J.; Cooper, L.V.; Blake, I.M.; Hawes, K.M.; Bandyopadhyay, A.S. Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine. Vaccines 2024, 12, 1308. https://doi.org/10.3390/vaccines12121308
Peak CM, Lyons H, Voorman A, Gray EJ, Cooper LV, Blake IM, Hawes KM, Bandyopadhyay AS. Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine. Vaccines. 2024; 12(12):1308. https://doi.org/10.3390/vaccines12121308
Chicago/Turabian StylePeak, Corey M., Hil Lyons, Arend Voorman, Elizabeth J. Gray, Laura V. Cooper, Isobel M. Blake, Kaija M. Hawes, and Ananda S. Bandyopadhyay. 2024. "Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine" Vaccines 12, no. 12: 1308. https://doi.org/10.3390/vaccines12121308
APA StylePeak, C. M., Lyons, H., Voorman, A., Gray, E. J., Cooper, L. V., Blake, I. M., Hawes, K. M., & Bandyopadhyay, A. S. (2024). Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine. Vaccines, 12(12), 1308. https://doi.org/10.3390/vaccines12121308