SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma
Abstract
1. Introduction
2. Materials and Methods
3. Results
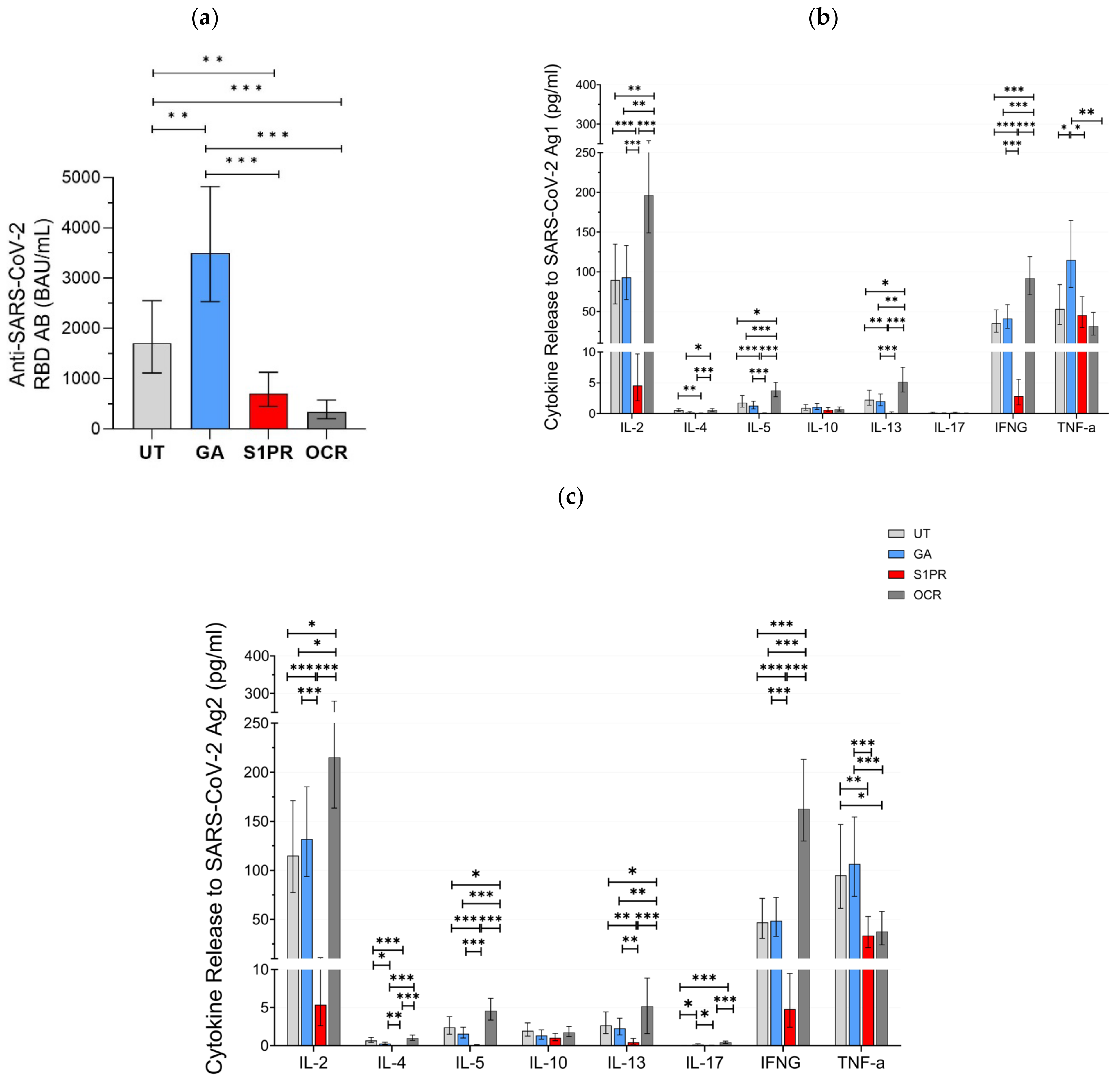
3.1. SARS-CoV-2-Specific Immune Responses in Fully Vaccinated Patients
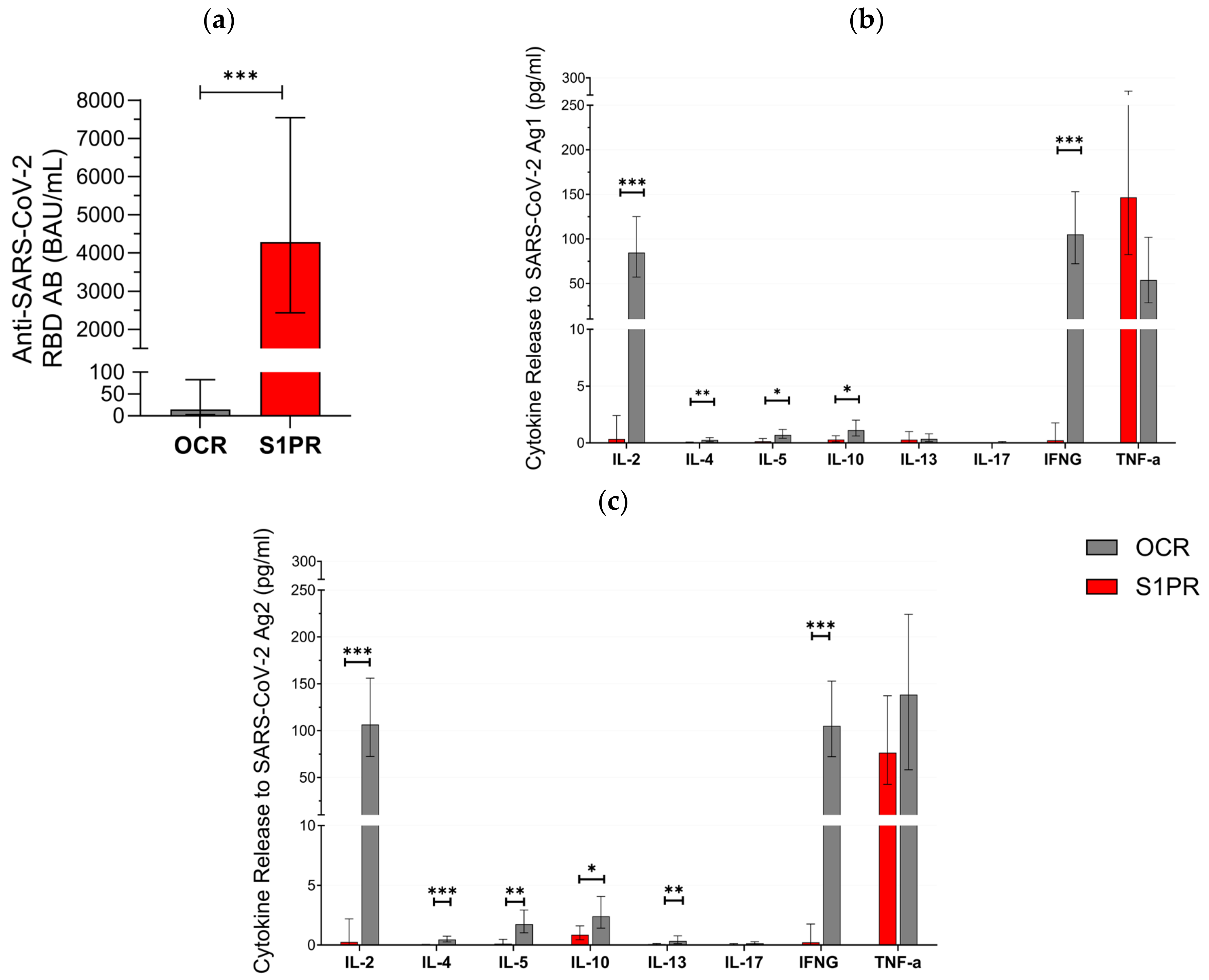
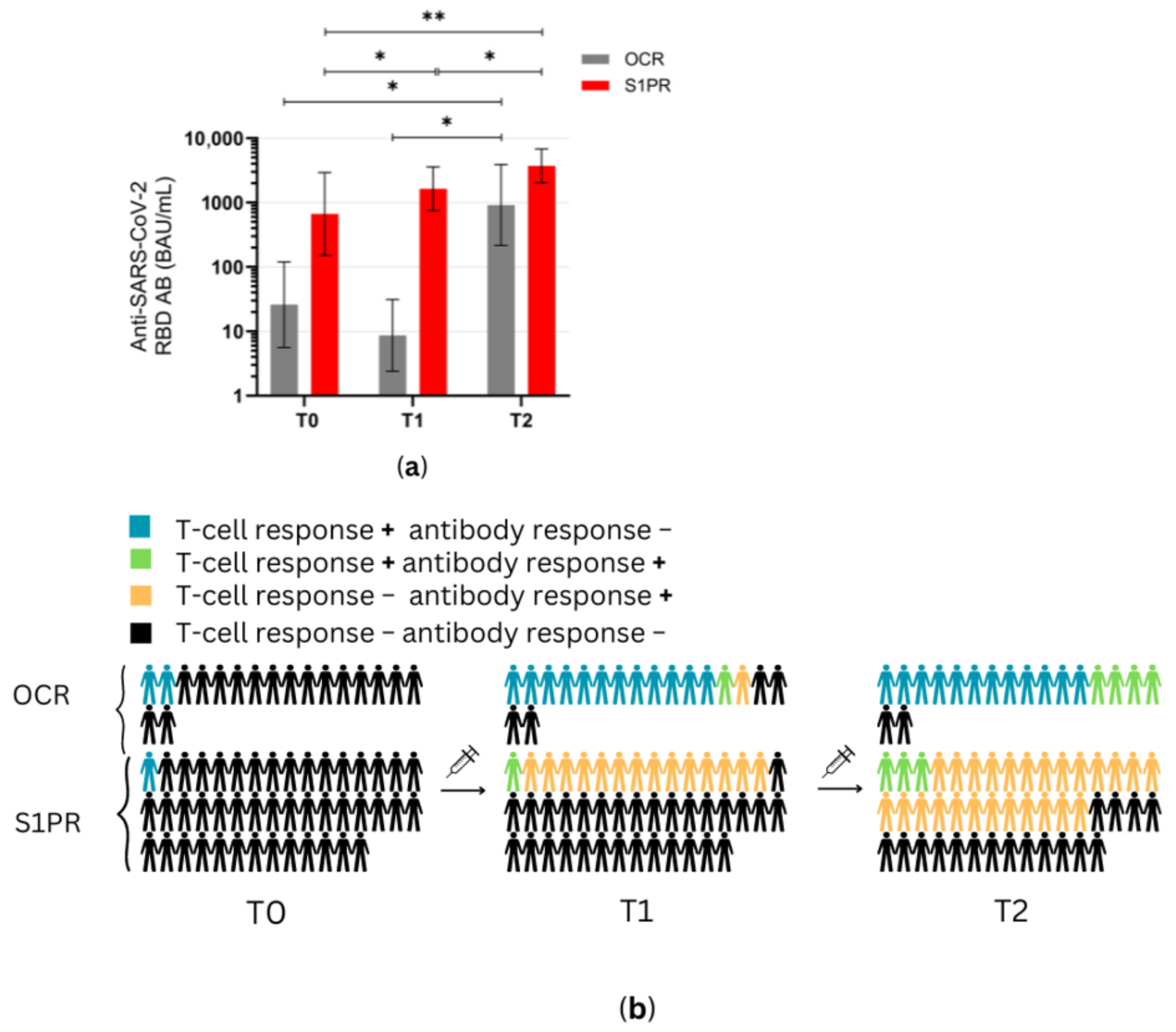
3.2. SARS-CoV-2-Specific Immune Responses after Booster Vaccination
3.3. The Protein-Based Vaccine Cohort: Population and Patient Characteristics
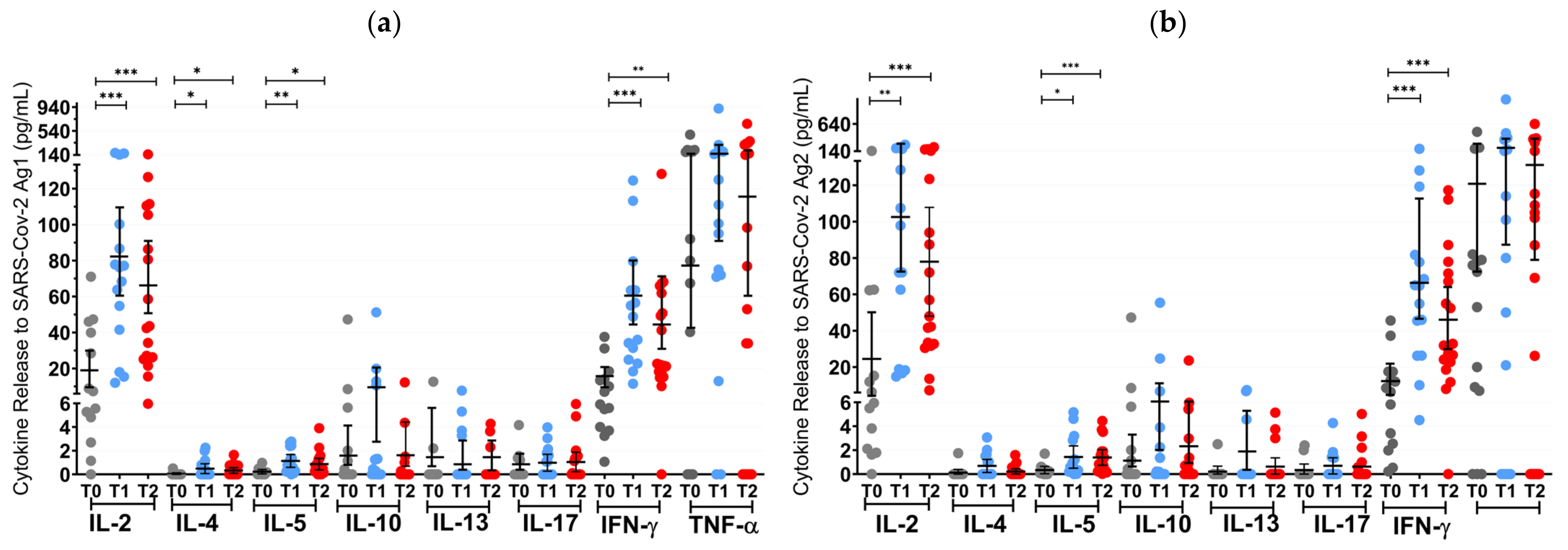
3.4. SARS-CoV-2-Specific Immune Responses after Protein-Based Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassmann, H.; Mahad, D.H.; Trapp, B.D. Progressive Multiple Sclerosis 1 Pathological Mechanisms in Progressive Multiple Sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Holstiege, J.; Akmatov, M.K.; Klimke, K.; Dammertz, L.; Kohring, C.; Marx, C.; Frahm, N.; Peters, M.; Ellenberger, D.; Zettl, U.K.; et al. Trends in Administrative Prevalence of Multiple Sclerosis and Utilization Patterns of Disease Modifying Drugs in Germany. Mult. Scler. Relat. Disord. 2022, 59, 103534. [Google Scholar] [CrossRef]
- Flachenecker, P.; Kobelt, G.; Berg, J.; Capsa, D.; Gannedahl, M. New Insights into the Burden and Costs of Multiple Sclerosis in Europe: Results for Germany. Mult. Scler. J. 2017, 23 (Suppl. S2), 78–90. [Google Scholar] [CrossRef]
- Woopen, C.; Schleußner, K.; Akgün, K.; Ziemssen, T. Approach to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis. Front. Immunol. 2021, 12, 701752. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Atta, Y.; Dillenseger, A.; Proschmann, U.; Schleußner, K.; Woopen, C.; Ziemssen, T.; Akgün, K. Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort. Vaccines 2024, 12, 265. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 10 May 2024).
- Golshani, M.; Hrdý, J. Multiple Sclerosis Patients and Disease Modifying Therapies: Impact on Immune Responses against COVID-19 and SARS-CoV-2 Vaccination. Vaccines 2022, 10, 279. [Google Scholar] [CrossRef]
- Gombolay, G.Y.; Dutt, M.; Tyor, W. Immune Responses to SARS-CoV-2 Vaccination in Multiple Sclerosis: A Systematic Review/Meta-Analysis. Ann. Clin. Transl. Neurol. 2022, 9, 1321–1331. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Braun, J.; Fauchere, F.; Vanshylla, K.; Loyal, L.; Henze, L.; Kruse, B.; Dingeldey, M.; Jürchott, K.; Mangold, M.; et al. SARS-CoV-2 MRNA Vaccinations Fail to Elicit Humoral and Cellular Immune Responses in Patients with Multiple Sclerosis Receiving Fingolimod. J. Neurol. Neurosurg. Psychiatry 2022, 93, 960–971. [Google Scholar] [CrossRef]
- Baker, D.; Forte, E.; Pryce, G.; Kang, A.S.; James, L.K.; Giovannoni, G.; Schmierer, K. The Impact of Sphinogosine-1-Phosphate Receptor Modulators on COVID-19 and SARS-CoV-2 Vaccination. Mult. Scler. Relat. Disord. 2022, 69, 104425. [Google Scholar] [CrossRef]
- Alfonso-Dunn, R.; Lin, J.; Kirschner, V.; Lei, J.; Feuer, G.; Malin, M.; Liu, J.; Roche, M.; Sadiq, S.A. Strong T-Cell Activation in Response to COVID-19 Vaccination in Multiple Sclerosis Patients Receiving B-Cell Depleting Therapies. Front. Immunol. 2022, 13, 926318. [Google Scholar] [CrossRef]
- Woopen, C.; Dunsche, M.; Haase, R.; Raposo, C.; Pedotti, R.; Akgün, K.; Ziemssen, T. Timing of SARS-CoV-2 Vaccination Matters in People with Multiple Sclerosis on Pulsed Anti-CD20 Treatment. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200031. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 MRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Van Kempen, Z.L.E.; Hogenboom, L.; Toorop, A.A.; Steenhuis, M.; Stalman, E.W.; Kummer, L.Y.L.; van Dam, K.P.J.; Bloem, K.; ten Brinke, A.; van Ham, S.M.; et al. Ocrelizumab Concentration Is a Good Predictor of SARS-CoV-2 Vaccination Response in Patients with Multiple Sclerosis. Ann. Neurol. 2023, 93, 103–108. [Google Scholar] [CrossRef]
- Nytrova, P.; Stastna, D.; Tesar, A.; Menkyova, I.; Posova, H.; Koprivova, H.; Mikulova, V.; Hrdy, J.; Smela, G.; Horakova, D.; et al. Immunity Following SARS-CoV-2 Vaccination in Autoimmune Neurological Disorders Treated with Rituximab or Ocrelizumab. Front. Immunol. 2023, 14, 1149629. [Google Scholar] [CrossRef]
- Pompsch, M.; Fisenkci, N.; Horn, P.A.; Kraemer, M.; Lindemann, M. Evidence of Extensive Cellular Immune Response after SARS-CoV-2 Vaccination in Ocrelizumab-Treated Patients with Multiple Sclerosis. Neurol. Res. Pract. 2021, 3, 60. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Receiving Disease Modifying Therapies: Immunological Patterns and Clinical Implications. Front. Immunol. 2022, 12, 796482. [Google Scholar] [CrossRef]
- Lv, D.; Liu, Y.; Guo, F.; Wu, A.; Mo, Y.; Wang, S.; Chu, J. Combining Interferon-γ Release Assays with Lymphocyte Enumeration for Diagnosis of Mycobacterium tuberculosis Infection. J. Int. Med. Res. 2020, 48, 030006052092566. [Google Scholar] [CrossRef]
- Hussain, S.; Afzal, N.; Javaid, K.; Ullah, M.I.; Ahmad, T.; Zaman, S.U. Level of Interferon Gamma in the Blood of Tuberculosis Patients. Iran. J. Immunol. 2010, 7, 240–246. [Google Scholar]
- Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Kanjevac, T.; Arsenijevic, D.; Radosavljevic, G.; Milovanovic, M.; Arsenijevic, N. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Front. Immunol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Faissner, S.; Heitmann, N.; Plaza-Sirvent, C.; Trendelenburg, P.; Ceylan, U.; Motte, J.; Bessen, C.; Urlaub, D.; Watzl, C.; Overheu, O.; et al. Immune Response in Ofatumumab Treated Multiple Sclerosis Patients after SARS-CoV-2 Vaccination. Front. Immunol. 2022, 13, 980526. [Google Scholar] [CrossRef]
- Safont, G.; Villar-Hernández, R.; Smalchuk, D.; Stojanovic, Z.; Marín, A.; Lacoma, A.; Pérez-Cano, C.; López-Martínez, A.; Molina-Moya, B.; Solis, A.J.; et al. Measurement of IFN-γ and IL-2 for the Assessment of the Cellular Immunity against SARS-CoV-2. Sci. Rep. 2024, 14, 1137. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Millington, K.A.; Innes, J.A.; Hackforth, S.; Hinks, T.S.C.; Deeks, J.J.; Dosanjh, D.P.S.; Guyot-Revol, V.; Gunatheesan, R.; Klenerman, P.; Lalvani, A. Dynamic Relationship between IFN-γ and IL-2 Profile of Mycobacterium tuberculosis-Specific T Cells and Antigen Load. J. Immunol. 2007, 178, 5217–5226. [Google Scholar] [CrossRef]
- Fong, C.C.; Spencer, J.; Howlett-Prieto, Q.; Feng, X.; Reder, A.T. Adaptive and Innate Immune Responses in Multiple Sclerosis with Anti-CD20 Therapy: Gene Expression and Protein Profiles. Front. Neurol. 2023, 14, 1158487. [Google Scholar] [CrossRef]
- Killestein, J.; van Kempen, Z.L.E. Vaccination Responses in B-cell-depleted Multiple Sclerosis Patients: The Role of Drug Pharmacokinetics. Eur. J. Neurol. 2022, 29, 3137–3138. [Google Scholar] [CrossRef]
- Kornek, B.; Leutmezer, F.; Rommer, P.S.; Koblischke, M.; Schneider, L.; Haslacher, H.; Thalhammer, R.; Zimprich, F.; Zulehner, G.; Bsteh, G.; et al. B Cell Depletion and SARS-CoV-2 Vaccine Responses in Neuroimmunologic Patients. Ann. Neurol. 2022, 91, 342–352. [Google Scholar] [CrossRef]
- Meltendorf, S.; Vogel, K.; Thurm, C.; Prätsch, F.; Reinhold, A.; Färber, J.; Heuft, H.; Kaasch, A.J.; Hachenberg, T.; Weinzierl, S.; et al. IL-13 Determines Specific IgE Responses and SARS-CoV-2 Immunity after Mild COVID-19 and Novel MRNA Vaccination. Eur. J. Immunol. 2022, 52, 1972–1979. [Google Scholar] [CrossRef]
- Kratzer, B.; Schlax, L.C.; Gattinger, P.; Waidhofer-Söllner, P.; Trapin, D.; Tauber, P.A.; Sehgal, A.N.A.; Körmöczi, U.; Rottal, A.; Feichter, M.; et al. Combined Assessment of S- and N-Specific IL-2 and IL-13 Secretion and CD69 Neo-Expression for Discrimination of Post–Infection and Post-Vaccination Cellular SARS-CoV-2-Specific Immune Response. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 3408–3425. [Google Scholar] [CrossRef]
- Mueller-Enz, M.; Woopen, C.; Katoul Al Rahbani, G.; Haase, R.; Dunsche, M.; Ziemssen, T.; Akgün, K. NVX-CoV2373-Induced T- and B-Cellular Immunity in Immunosuppressed People with Multiple Sclerosis That Failed to Respond to MRNA and Viral Vector SARS-CoV-2 Vaccines. Front. Immunol. 2023, 14, 1081933. [Google Scholar] [CrossRef]
- Bellamkonda, N.; Lambe, U.P.; Sawant, S.; Nandi, S.S.; Chakraborty, C.; Shukla, D. Immune Response to SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1464. [Google Scholar] [CrossRef]
- Matrix-MTM Adjuvant Technology. Novavax. Available online: https://www.novavax.de/en/science-technology/matrix-m (accessed on 1 June 2024).
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of Ocrelizumab on Vaccine Responses in Patients with Multiple Sclerosis. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef]
- Mehling, M.; Hilbert, P.; Fritz, S.; Durovic, B.; Eichin, D.; Gasser, O.; Kuhle, J.; Klimkait, T.; Lindberg, R.L.P.; Kappos, L.; et al. Antigen-Specific Adaptive Immune Responses in Fingolimod-Treated Multiple Sclerosis Patients. Ann. Neurol. 2011, 69, 408–413. [Google Scholar] [CrossRef]
- Metze, C.; Winkelmann, A.; Loebermann, M.; Hecker, M.; Schweiger, B.; Reisinger, E.C.; Zettl, U.K. Immunogenicity and Predictors of Response to a Single Dose Trivalent Seasonal Influenza Vaccine in Multiple Sclerosis Patients Receiving Disease-Modifying Therapies. CNS Neurosci. Ther. 2019, 25, 245–254. [Google Scholar] [CrossRef]
- Zhou, F.; Hansen, L.; Pedersen, G.; Grødeland, G.; Cox, R. Matrix M Adjuvanted H5N1 Vaccine Elicits Broadly Neutralizing Antibodies and Neuraminidase Inhibiting Antibodies in Humans That Correlate with In Vivo Protection. Front. Immunol. 2021, 12, 747774. [Google Scholar] [CrossRef]
- Bengtsson, K.L.; Song, H.; Stertman, L.; Liu, Y.; Flyer, D.C.; Massare, M.J.; Xu, R.-H.; Zhou, B.; Lu, H.; Kwilas, S.A.; et al. Matrix-M Adjuvant Enhances Antibody, Cellular and Protective Immune Responses of a Zaire Ebola/Makona Virus Glycoprotein (GP) Nanoparticle Vaccine in Mice. Vaccine 2016, 34, 1927–1935. [Google Scholar] [CrossRef]

| Characteristic | Fully Vaccinated Cohort (n = 126) | Booster Cohort (n = 28) |
|---|---|---|
| Sex, n (%) | ||
| Female | 93 (73.8) | 18 (64.3) |
| Age, years | ||
| Mean ± sd | 48.37 ± 12.32 | 50.68 ± 9.9 |
| Median | 48 | 49 |
| Range | 21-77 | 32-68 |
| MS subtype, n (%) | ||
| CIS | 2 (1.6) | 0 (0.0) |
| RRMS | 97 (75.8) | 17 (60.7) |
| PPMS | 13 (10.2) | 3 (10.7) |
| SPMS | 16 (12.5) | 8 (28.6) |
| DMT modality | ||
| UT | 28 (22.2) | - |
| GA | 27 (21.1) | - |
| OCR | 39 (30.5) | 16 (57.1) |
| S1PR | 32 (25.4) | 12 (42.9) |
| Treatment duration, days: mean ± SD | ||
| GA | ||
| OCR | 3440 (2510) | |
| Time since last infusion | 912 (616) | 1295 (762) |
| S1PR | 157 (124) | 133 (130) |
| 1440 (1321) | 1180 (1080) | |
| Time vaccination–sampling | ||
| days, mean ± sd | 63.75 ± 39.1 | 74.96 ± 44.1 |
| days, range | 6-203 | 12-147 |
| Previous COVID-19 Infections, n (%) | ||
| Yes | 10 (7.9) | 8 (28.6) |
| No | 116 (92.1) | 20 (71.4) |
| Vaccines, n (%) | 2x mRNA, 114 (90.4) | 3x mRNA, 22 (78.6) |
| 2x VVV, 6 (4.8) | 2 mRNA + 1 VVV, 5 (17.9) | |
| 1x mRNA + 1 VVV, 6 (4.8) | 1x mRNA + 2 VVV, 1 (3.6) |
| Characteristic | Protein-Based Vaccine Cohort (n = 63) |
|---|---|
| Sex, n (%) | |
| Female | 33 (52.38) |
| Age, years | |
| Mean ± sd | 49.74 ± 11.22 |
| Median | 51 |
| Range | 24–72 |
| MS subtype, n (%) | |
| RRMS | 53 (84.1) |
| PPMS | 2 (3.2) |
| SPMS | 8 (12.7) |
| DMT modality | |
| OCR | 18 (28.57) |
| S1PR | 45 (71.43) |
| Treatment duration, days: mean ± SD | |
| OCR | 901 (462) |
| Time since last infusion | 96.61 (69.29) |
| S1PR | 2201 (1096) |
| Previous vaccines, n (%) | |
| 2x mRNA/VVV | 2 (3.2) |
| 3x mRNA/VVV | 59 (93.6) |
| COVID-19 + 2x mRNA/VVV | 2 (3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Rahbani, G.K.; Woopen, C.; Dunsche, M.; Proschmann, U.; Ziemssen, T.; Akgün, K. SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma. Vaccines 2024, 12, 684. https://doi.org/10.3390/vaccines12060684
Al Rahbani GK, Woopen C, Dunsche M, Proschmann U, Ziemssen T, Akgün K. SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma. Vaccines. 2024; 12(6):684. https://doi.org/10.3390/vaccines12060684
Chicago/Turabian StyleAl Rahbani, Georges Katoul, Christina Woopen, Marie Dunsche, Undine Proschmann, Tjalf Ziemssen, and Katja Akgün. 2024. "SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma" Vaccines 12, no. 6: 684. https://doi.org/10.3390/vaccines12060684
APA StyleAl Rahbani, G. K., Woopen, C., Dunsche, M., Proschmann, U., Ziemssen, T., & Akgün, K. (2024). SARS-CoV-2-Specific Immune Cytokine Profiles to mRNA, Viral Vector and Protein-Based Vaccines in Patients with Multiple Sclerosis: Beyond Interferon Gamma. Vaccines, 12(6), 684. https://doi.org/10.3390/vaccines12060684






