Eosinophils Play a Surprising Leading Role in Recurrent Urticaria in Horses
Abstract
1. Introduction
2. Materials & Methods
2.1. Horses
2.2. Exploratory Urticaria Activity Score (E-UAS)
2.3. Blood Collection and Differential Blood Analysis
2.4. Skin Punch Biopsies
2.5. eIL-5-CuMV-TT Vaccine Production and Vaccination of Horses
2.6. Statistics
3. Results
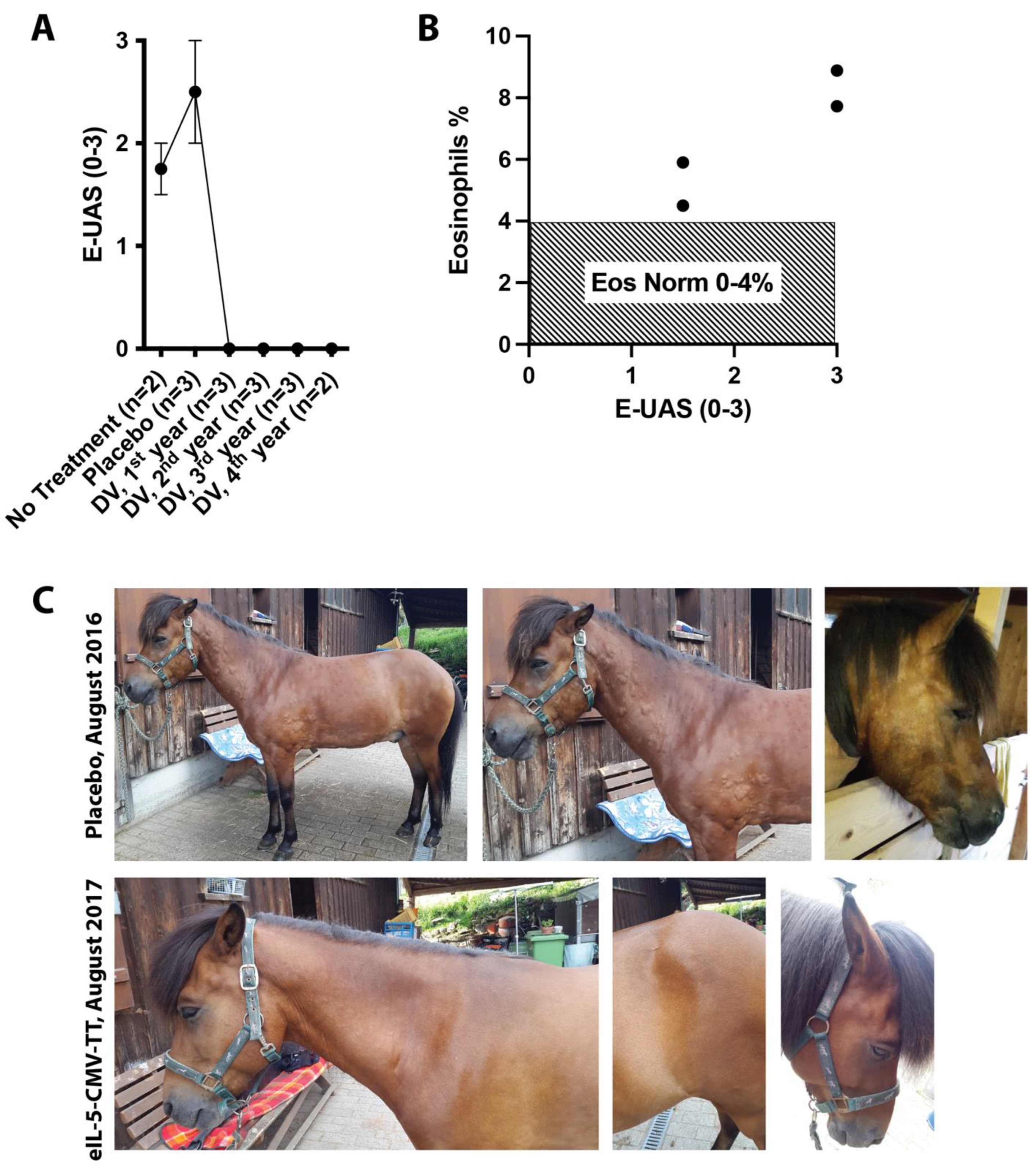
3.1. Clinical Effect on Seasonal Urticaria in Interleukin (IL)-5 Vaccinated IBH Horses
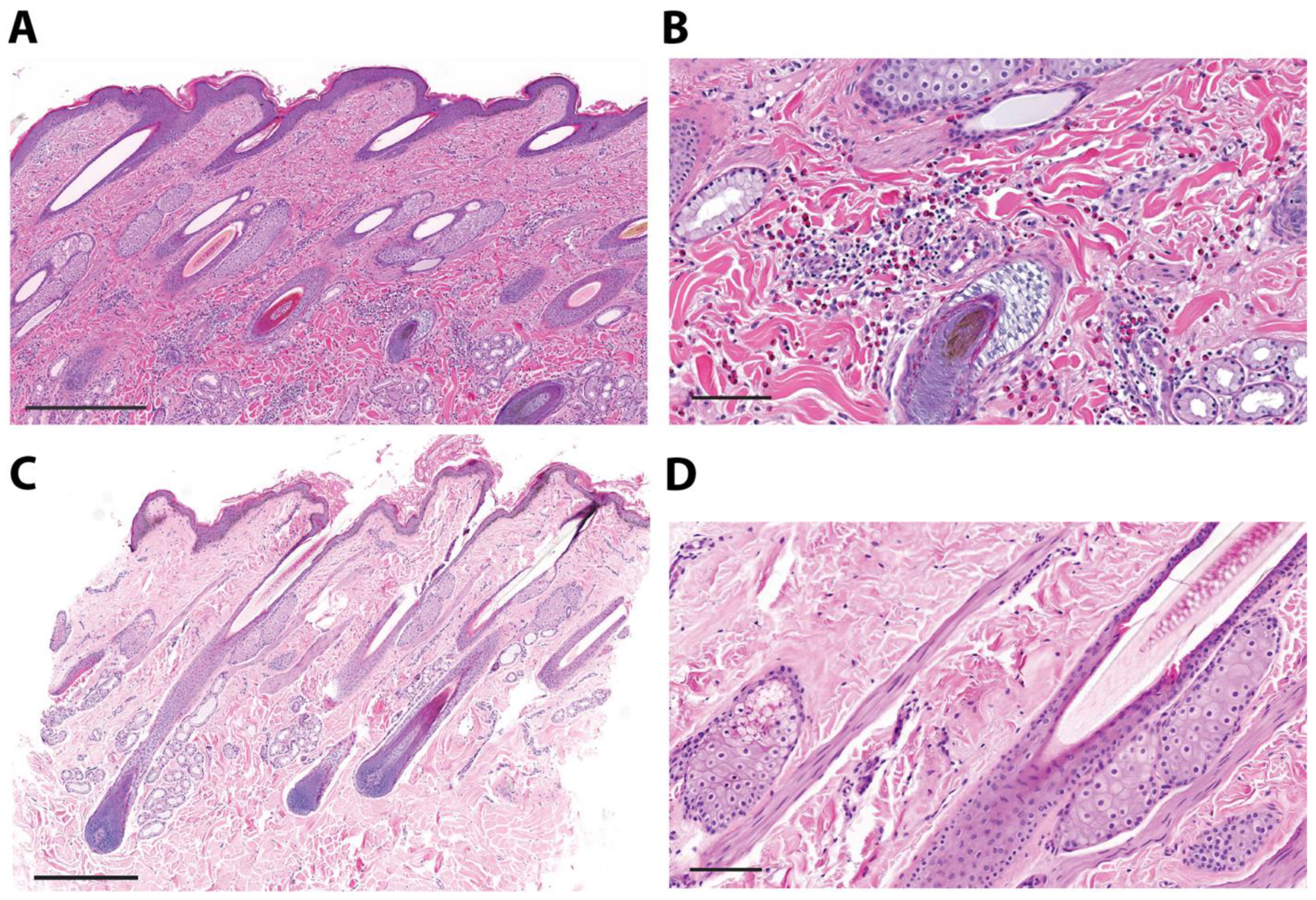
3.2. Eosinophilic Gene Expression and Eosinophil Infiltration into Urticaria-Affected Skin
3.3. Two Case Reports of IL-5 Vaccination in Non-IBH Horses with Non-Seasonal Recurrent Urticaria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, A.A.; Rosychuk, R.A. Equine Dermatology. Vet. Clin. North Am. Equine Pract. 2013, 29, xi–xii. [Google Scholar] [CrossRef] [PubMed]
- Akucewich, L.; Kunkle, G. Urticaria. Compendium: Equine Edition 2007, 100–111. Available online: https://www.vetfolio.com/learn/article/urticaria (accessed on 14 May 2024).
- Rüfenacht, S.; Marti, E.; von Tscharner, C.; Doherr, M.G.; Forster, U.; Welle, M.; Roosje, P.J. Immunoglobulin E-bearing cells and mast cells in skin biopsies of horses with urticaria. Vet. Dermatol. 2005, 16, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller, M.W. Skin immune system and allergic skin diseases. In Equine Dermatology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 436–467. [Google Scholar] [CrossRef]
- Cunningham, F.M.; Dunkel, B. Equine recurrent airway obstruction and insect bite hypersensitivity: Understanding the diseases and uncovering possible new therapeutic approaches. Vet. J. 2008, 177, 334–344. [Google Scholar] [CrossRef]
- Fettelschoss-Gabriel, A.; Fettelschoss, V.; Thoms, F.; Giese, C.; Daniel, M.; Olomski, F.; Kamarachev, J.; Birkmann, K.; Bühler, M.; Kummer, M.; et al. Treating insect-bite hypersensitivity in horses with active vaccination against IL-5. J. Allergy Clin. Immunol. 2018, 142, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Fettelschoss-Gabriel, A.; Fettelschoss, V.; Olomski, F.; Birkmann, K.; Thoms, F.; Bühler, M.; Kummer, M.; Zeltins, A.; Kündig, T.M.; Bachmann, M.F. Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy 2018, 74, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, S.; Fettelschoss, V.; Olomski, F.; Talker, S.C.; Mirkovitch, J.; Rhiner, T.; Birkmann, K.; Thoms, F.; Wagner, B.; Bachmann, M.F.; et al. Safety Profile of a Virus-Like Particle-Based Vaccine Targeting Self-Protein Interleukin-5 in Horses. Vaccines 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Aberer, W.; Asero, R.; Bindslev-Jensen, C.; Brzoza, Z.; Canonica, G.W.; Church, M.K.; Ensina, L.F.; Giménez-Arnau, A.; Godse, K.; et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy 2014, 69, 868–887. [Google Scholar] [CrossRef] [PubMed]
- Olomski, F.; Fettelschoss, V.; Jonsdottir, S.; Birkmann, K.; Thoms, F.; Marti, E.; Bachmann, M.F.; Kundig, T.M.; Fettelschoss-Gabriel, A. Interleukin 31 in insect bite hypersensitivity-Alleviating clinical symptoms by active vaccination against itch. Allergy 2020, 75, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Jebbawi, F.; Chemintzer, A.; Pantelyushin, S.; Lam, J.; Rhiner, T.; Keller, G.; Waldern, N.; Canonica, F.; Fettelschoss-Gabriel, A. Cytokines and chemokines in insect bite hypersensitivity (submitted).
- Kehrli, D.; Jandova, V.; Fey, K.; Jahn, P.; Gerber, V. Multiple hypersensitivities including recurrent airway obstruction, insect bite hypersensitivity, and urticaria in 2 warmblood horse populations. J. Vet. Intern. Med. 2015, 29, 320–326. [Google Scholar] [CrossRef]
- Mueller, S.; Aigner, T.; Neureiter, D.; Stolte, M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: A retrospective and comparative study on pathological biopsy. J. Clin. Pathol. 2006, 59, 1175–1180. [Google Scholar] [CrossRef]
- Fettrelet, T.; Gigon, L.; Karaulov, A.; Yousefi, S.; Simon, H.-U. The Enigma of Eosinophil Degranulation. Int. J. Mol. Sci. 2021, 22, 7091. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kephart, G.M.; Talley, N.J.; Wagner, J.M.; Sarr, M.G.; Bonno, M.; McGovern, T.W.; Gleich, G.J. Eosinophil infiltration and degranulation in normal human tissue. Anat. Rec. 1998, 252, 418–425. [Google Scholar] [CrossRef]
- Kocatürk, E.; Maurer, M.; Metz, M.; Grattan, C. Looking forward to new targeted treatments for chronic spontaneous urticaria. Clin. Transl. Allergy 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Latiff, A.H.A.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Staubach, P.; Kurzen, H.; Strömer, K.; Ostendorf, R.; Maurer, M. Chronic spontaneous urticaria—A management pathway for patients with chronic spontaneous urticaria. JDDG: J. der Dtsch. Dermatol. Ges. 2015, 13, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Terhorst, D.; Metz, M.; Altrichter, S.; Zuberbier, T.; Maurer, M.; Bergmann, K.C. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. JDDG: J. der Dtsch. Dermatol. Ges. 2018, 16, 477–478. [Google Scholar] [CrossRef]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef]
- Altrichter, S.; Giménez-Arnau, A.M.; A Bernstein, J.; Metz, M.; Bahadori, L.; Bergquist, M.; Brooks, L.; Ho, C.N.; Jain, P.; Lukka, P.B.; et al. Benralizumab does not elicit therapeutic effect in patients with chronic spontaneous urticaria: Results from the phase 2b multinational, randomised, double-blind, placebo-controlled ARROYO trial. Br. J. Dermatol. 2024. [Google Scholar] [CrossRef]
- Khoury, P.; Makiya, M.A.; Rahim, R.; Bowman, A.; Espinoza, D.; Schiffenbauer, A.; Koch, M.; Anderson, C.; Constantine, G.; Maric, I.; et al. Mepolizumab incompletely suppresses clinical flares in a pilot study of episodic angioedema with eosinophilia. J. Allergy Clin. Immunol. 2024, 153, 821–830.e6. [Google Scholar] [CrossRef]
- Rhiner, T.; Fettelschoss, V.; Schoster, A.; Birkmann, K.; Fettelschoss-Gabriel, A. Targeting eosinophils by active vaccination against interleukin-5 reduces basophil counts in horses with insect bite hypersensitivity in the 2nd year of vaccination. Vet. J. 2022, 288, 105896. [Google Scholar] [CrossRef]
- Ochensberger, B.; Tassera, L.; Bifrare, D.; Rihs, S.; Dahinden, C.A. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur. J. Immunol. 1999, 29, 11–22. [Google Scholar] [CrossRef]
- Yoshimura-Uchiyama, C.; Yamaguchi, M.; Nagase, H.; Fujisawa, T.; Ra, C.; Matsushima, K.; Iwata, T.; Igarashi, T.; Yamamoto, K.; Hirai, K. Comparative effects of basophil-directed growth factors. Biochem. Biophys. Res. Commun. 2003, 302, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K.; Weston, C.; Rana, B.M.; Brightling, C.E.; Cousins, D.J. Human group 2 innate lymphoid cells do not express the IL-5 receptor. J. Allergy Clin. Immunol. 2017, 140, 1430–1433.e4. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti–IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar] [CrossRef] [PubMed]
- Guillet, C.; Steinmann, S.; Lang, C.; Maul, J.-T.; Schmid-Grendelmeier, P. Eosinophil-mast cell interaction: Mepolizumab leads to a reduction of clinical symptoms and serum tryptase in a patient with eosinophilic asthma and idiopathic mast cell activation. J. Allergy Clin. Immunol. Pract. 2020, 9, 1393–1395.e1. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Pavord, I.D. Living without eosinophils: Evidence from mouse and man. Eur. Respir. J. 2023, 61, 2201217. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Klion, A.D. Biologic therapies targeting eosinophils: Current status and future prospects. J. Allergy Clin. Immunol. Pract. 2015, 3, 167–174. [Google Scholar] [CrossRef]
- Loeffler, A.; Herrick, D.; Allen, S.; Littlewood, J.D. Long-term management of horses with atopic dermatitis in southeastern England: A retrospective questionnaire study of owners’ perceptions. Vet. Dermatol. 2018, 29, 526-e176. [Google Scholar] [CrossRef]
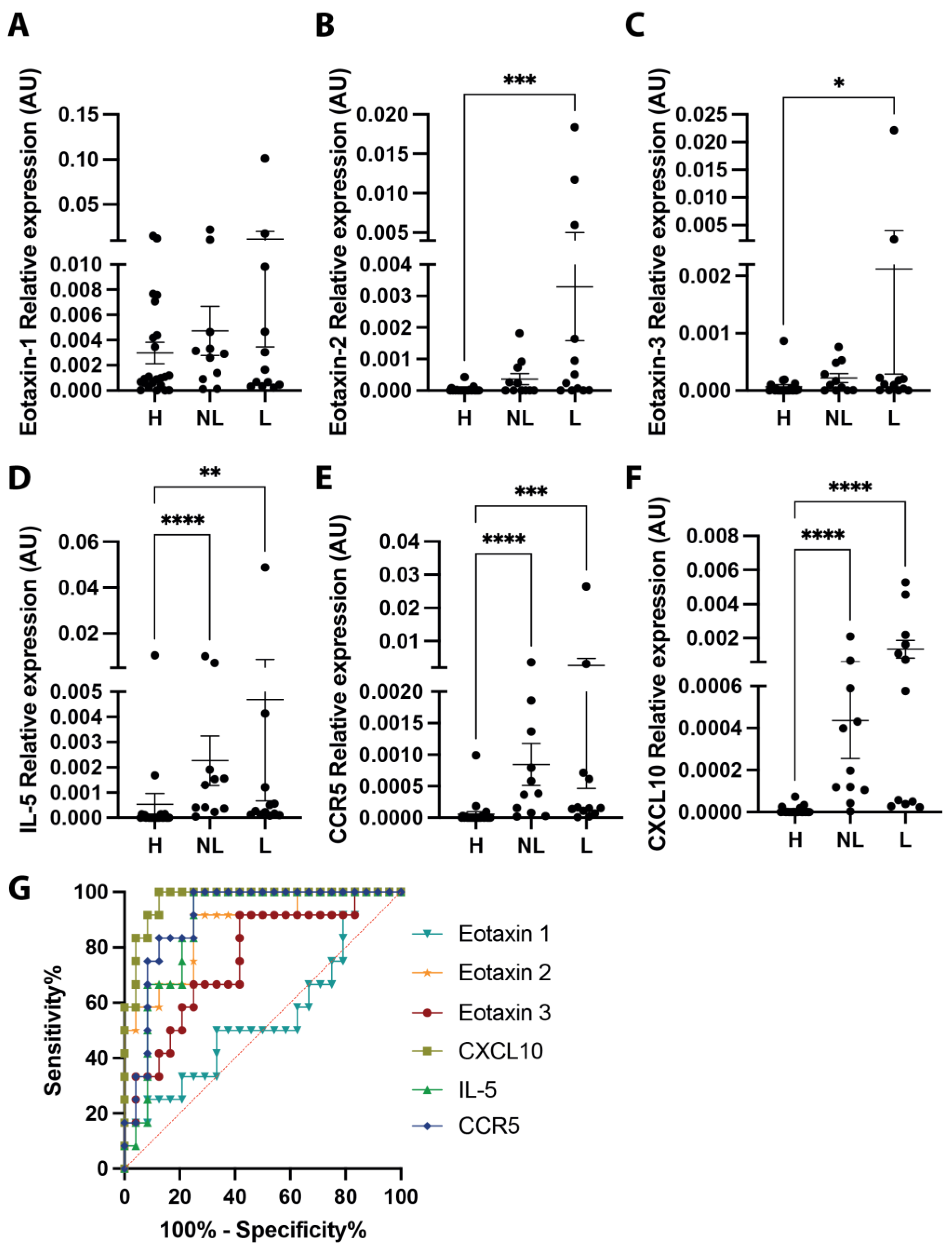

| Gene/AUC | Area | Standard Error | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Eotaxin 1 | 0.5486 | 0.1069 | 0.3391–0.7581 | 0.6385 |
| Eotaxin 2 | 0.8715 | 0.06312 | 0.7478–0.9952 | 0.0003 |
| Eotaxin 3 | 0.7569 | 0.08557 | 0.5892–0.9247 | 0.013 |
| CXCL10 | 0.9722 | 0.02278 | 0.9276–1 | <0.0001 |
| IL-5 | 0.8785 | 0.05782 | 0.7652–0.9918 | 0.0003 |
| CCR5 | 0.9063 | 0.04992 | 0.8084–1 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birkmann, K.; Jebbawi, F.; Waldern, N.; Hug, S.; Inversini, V.; Keller, G.; Holm, A.; Grest, P.; Canonica, F.; Schmid-Grendelmeier, P.; et al. Eosinophils Play a Surprising Leading Role in Recurrent Urticaria in Horses. Vaccines 2024, 12, 562. https://doi.org/10.3390/vaccines12060562
Birkmann K, Jebbawi F, Waldern N, Hug S, Inversini V, Keller G, Holm A, Grest P, Canonica F, Schmid-Grendelmeier P, et al. Eosinophils Play a Surprising Leading Role in Recurrent Urticaria in Horses. Vaccines. 2024; 12(6):562. https://doi.org/10.3390/vaccines12060562
Chicago/Turabian StyleBirkmann, Katharina, Fadi Jebbawi, Nina Waldern, Sophie Hug, Victoria Inversini, Giulia Keller, Anja Holm, Paula Grest, Fabia Canonica, Peter Schmid-Grendelmeier, and et al. 2024. "Eosinophils Play a Surprising Leading Role in Recurrent Urticaria in Horses" Vaccines 12, no. 6: 562. https://doi.org/10.3390/vaccines12060562
APA StyleBirkmann, K., Jebbawi, F., Waldern, N., Hug, S., Inversini, V., Keller, G., Holm, A., Grest, P., Canonica, F., Schmid-Grendelmeier, P., & Fettelschoss-Gabriel, A. (2024). Eosinophils Play a Surprising Leading Role in Recurrent Urticaria in Horses. Vaccines, 12(6), 562. https://doi.org/10.3390/vaccines12060562






