Live Attenuated Vaccines against Tuberculosis: Targeting the Disruption of Genes Encoding the Secretory Proteins of Mycobacteria
Abstract
1. Introduction
2. Secretory Systems of Mycobacteria

3. Mycobacterial Vaccines Deficient in Secreted Protein(s)
3.1. Ag85 Complex
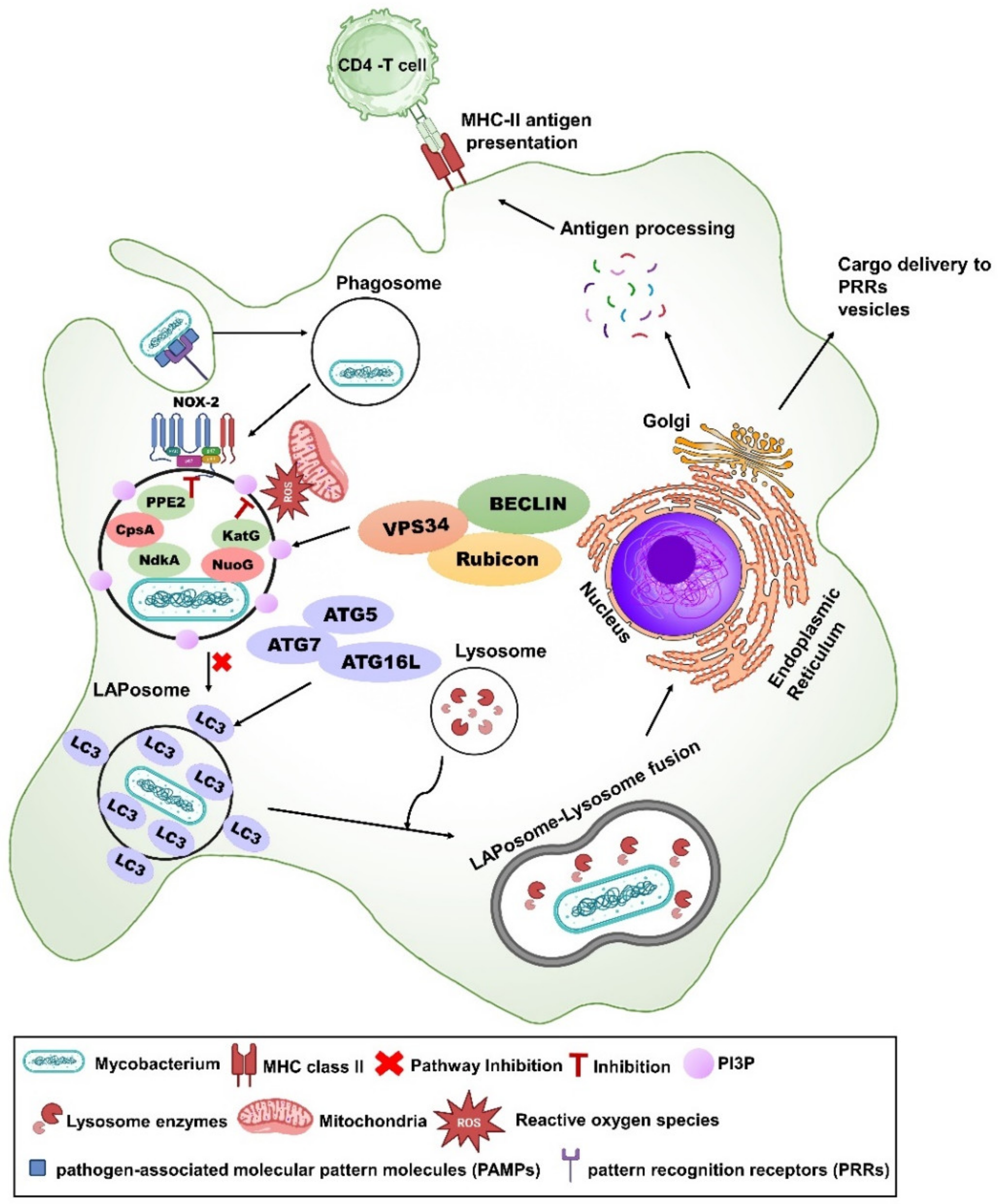
3.2. LpqH
3.3. LprG
3.4. BfrB
3.5. CpsA
3.6. BioA
3.7. Gln Proteins
3.8. SapM
3.9. Ptp
3.10. Zmp1
3.11. Eis
3.12. Esx5
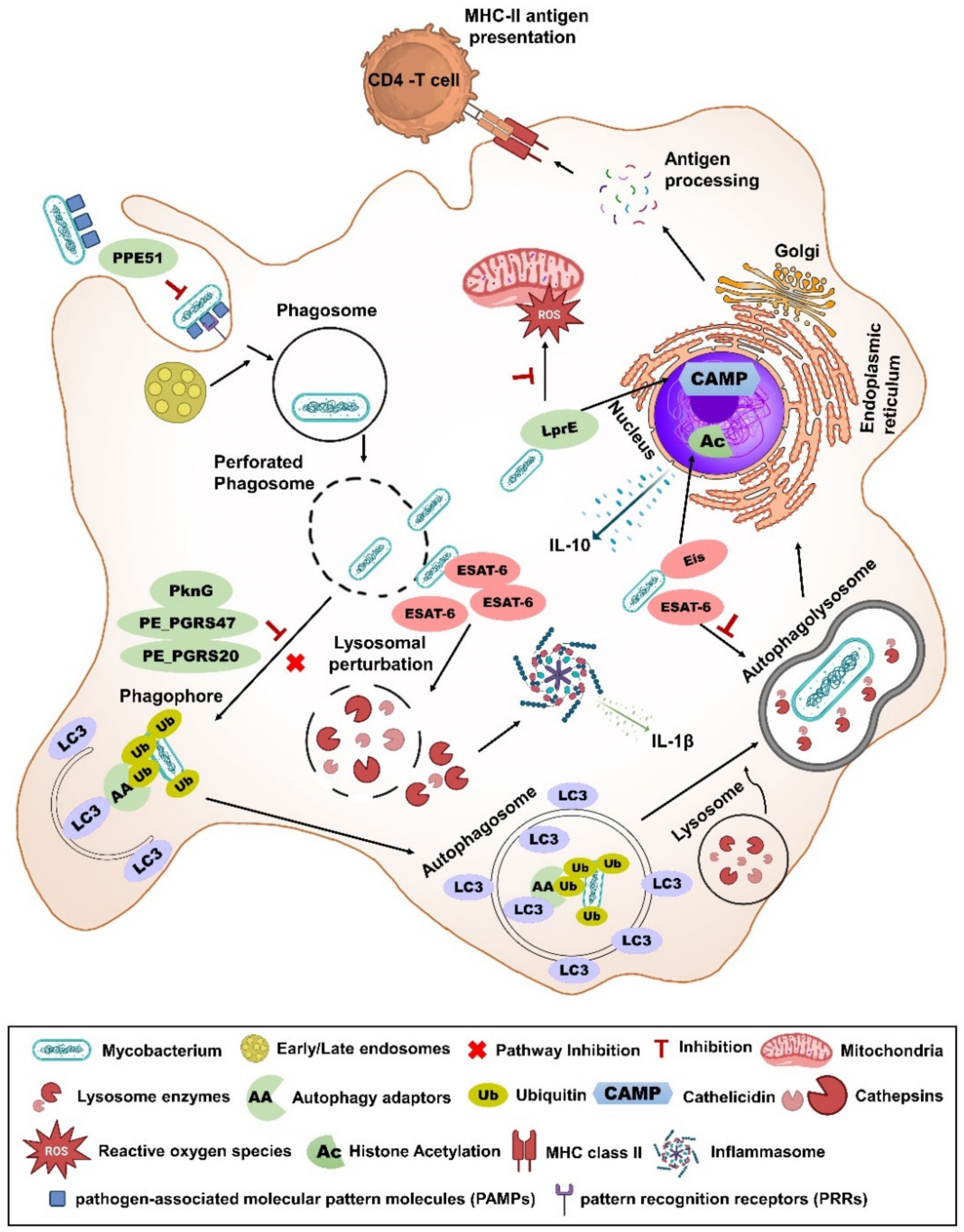
3.13. UreC
3.14. NuoG
| Vaccine Name | Vaccine Components |
Secreted Protein(s) Absent | Immunization Route/Dose | Challenge Mtb Strain | Challenge Route/Dose | Animal Model (Strain) | Efficacy in Relation to BCG | log10 CFU/LUNGS Reduction Than BCG | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ΔfbpA | H37Rv strain with single gene (fbpA) knockout. | FbpA or Ag85A | SC/105 CFU/mouse | Erdman | Aerosol/2.5 log10 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~1.5 | [115] |
| ΔglnA1 | H37Rv strain with single gene (glnA1) knockout. | Glutamine synthetase A1 | SC/106 CFU/mouse | Erdman | Aerosol/200 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [148] |
| ΔglnA1EA2 | H37Rv strain with 3 genes (glnA1, glnE, and glnA2) knockout. | Glutamine synthetase A, E and A2 | SC/106 CFU/mouse | Erdman | Aerosol/200 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [148] |
| Δ19 | H37Rv strain with single gene (lpqH) knockout. | Lipoprotein LpqH | SC/106 CFU/mouse | H37Rv | Aerosol/100 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [130] |
| Δmms | H37Rv strain with 3 genes (ptpA, ptpB, and sapM) knockout. | Phosphatases PtpA, PtpB, and SapM | ID/5 × 105 CFU/guinea pig | H37Rv | Aerosol/10–30 CFU per guinea pig | Guinea pigs | Better than BCG | 0.83–4 weeks post-challenge; 1.41–12 weeks post-challenge | [154] |
| ΔbfrB | H37Rv strain with single gene (bfrB) knockout | Bacterio-ferritin B | SC/106 CFU/mouse | H37Rv | Aerosol/100 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [140] |
| ΔbioA | H37Rv strain with single gene (bioA) knockout | BioA or 7,8-diaminopelargonic acid synthase | ID/106 CFU/guinea pig (single or double dose with 6 week interval) | Erdman | Aerosol/50 CFU per guinea pig | Guinea pigs | Equal to BCG | - | [145] |
| Δmmsb | H37Rv strain with 4 genes (ptpA, ptpB, sapM, and bioA) knockout. | Phosphatases PtpA, PtpB, SapM and BioA | ID/5 × 105 CFU/guinea pig | H37Rv | Aerosol/10–30 CFU per guinea pig | Guinea pigs | Less than BCG | - | [155] |
| mc26206ΔcpsA | H37Rv strain with 3 genes (leuD, panCD, and cpsA) knockout. | CpsA | SC/106 CFU/mouse | H37Rv | Aerosol/400 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [142] |
| ΔlprG | H37Rv strain with two genes (lprG and Rv1410c) knockout. | Lipoprotein LprG | SC/~106 CFU/mouse | H37Rv and Erdman | Aerosol/75 CFU per mouse or Aerosol/1 Median Infectious Dose (1MID50). | Mouse (C57BL/6, BALB/c and C3HeB/FeJ) | Equal or better than BCG | 0.67–0.9 (in C3HeB/ FeJ mice); | [134,135] |
| SO2 | MT103 strain with single gene (phoP) knockout. | All secreted proteins that are affected by PhoP | SC/107 CFU/mouse | H37Rv | IV/2.5 × 105 CFU per mouse | Mouse (BALB/c) | Equal to BCG | - | [180,181] |
| SC/5 × 104 CFU/ guinea pig | H37Rv | Aerosol/10–50 CFU or 500 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Better than BCG in high-dose challenge | >1 | ||||
| ID/5 × 105 CFU/macaques | Erdman | IT/1000 CFU per macaques | Rhesus macaques (Macaca mulatta) | Better than BCG | 0.77 | ||||
| Δppe25-pe19 | H37Rv strain with 5 genes (ppe25, pe18, ppe26, ppe27, and pe19) knock out. | PPE25, PE18, PPE26, PPE27 and PE19 | SC/106 CFU/mouse | H37Rv | Aerosol/100 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~0.5 | [169] |
| ΔsecA2 | mc23112 strain with single gene (secA2) knock out. | SC/106 CFU/mouse | Beijing/W (HN878) or Erdman | Aerosol/50–100 CFU per mouse | Mouse (C57BL/6) | Better than BCG | 0.72 | [182] | |
| ID/103 CFU/guinea pig | H37Rv | Aerosol/10–30 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Better than BCG in lymph node but not in lungs | - | ||||
| ΔsecA2ΔlysA | mc23112 strain with double gene (secA2 and lysA) knockout. | Proteins secreted by SecA2 secretion system | SC/106 CFU/mouse | Erdman | Aerosol/50–100 CFU per mouse | Mouse (C57BL/6) | Better than BCG | 0.66 | [183] |
| MTBVAC | MT103 strain with double gene (phoP and fadD26) knockout. | All secreted proteins affected by PhoP | SC/5 × 105 CFU/mouse | H37Rv | IN/100 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~0.5 | [184,185] |
| SC/5 × 103 − 5 × 105 − CFU/guinea pig | H37Rv | Aerosol/10–50 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Equal to BCG | - | ||||
| ID/8.2 × 105 CFU/macaques | Erdman | Aerosol/14–30 CFU per macaques | Rhesus macaques (Macaca mulatta) | Better than BCG but not in CFU | - | ||||
| MTBVAC erp- | MT103 strain with triple gene (phoP, fadD26, and erp) knock out. | All secreted proteins which are affected by PhoP and Erp | ID/105 CFU/mouse | H37Rv | IT/103 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [186] |
| Δesx-5 | H37Rv strain with 17 genes (eccB5, eccc5, cyp143, Rv1786, ppe25, pe18, ppe26, ppe27, pe19, esxM, esxN, ncRv11793, Rv1794, eccD5, mycP5, eccE5, and eccA5) knock out. | ECCB5, ECCC5, CYP143, RV1786, PPE25, PE18, PPE26, PPE27, PE19, ESXM, ESXN, NCRV11793, RV1794, ECCD5, MYCP5, ECCE5, and ECCA5 | IM/106 CFU/mouse (2 dose with 6 week interval) | HN878, and H37Rv | Aerosol/40–100 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [170] |
| IM/104 CFU/guinea pig | Beijing 212 | Aerosol/10–20 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Equal to BCG | - | ||||
| Δesx-3 | H37Rv strain with 11 genes (eccA3, eccB3, eccC3, pe5, ppe4, esxG, esxH, espG3, eccD3, mycP3, and eccE3) knock out | ECCA3, ECCB3, ECCC3, PE5, PPE4, ESXG, ESXH, ESPG3, ECCD3, MYCP3, and ECCE3 | IM /104 CFU/guinea pig | Beijing 212 | Aerosol/10–20 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Not mentioned | - | [170] |
3.15. SecA2
3.16. PhoP
3.17. Mpt
3.18. Erp
3.19. BCG_1419c
| Vaccine Name | Vaccine Components |
Secreted Protein(s) Absent |
Immunization Route/Dose | Challenge Mtb Strain | Challenge Route/Dose | Animal Model (Strain) |
Efficacy in Relation to BCG |
log10 CFU/LUNGS Reduction Than BCG | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| VPM1002 (ΔureC::hly) | BCG Pasteur strain with single gene (ureC) knockout, which expresses listeriolysin (hly). | Urease C | IV/106 CFU/mouse | H37Rv or Beijing/W | Aerosol/30 or 200 CFU per mouse | Mouse (BALB/c) | Better than BCG | ~0.5–2 | [177] |
| sapM::T | BCG 1721 strain with single gene (sapM) knockout. | SapM phosphatase | SC/105 CFU/mouse | H37Rv | IV/5 × 104 CFU per mouse (or) IT/ 2 × 105 CFU per mouse | Mouse (BALB/c) | Better than BCG | ~0.5 (Luminescence) | [156] |
| Δzmp1 | BCG Pasteur or Denmark strain with single gene (zmp1) knockout. | Zmp1 or Zinc containing metalloprotease 1 | SC/5 × 104 CFU/ guinea pig | H37Rv | Aerosol/10–50 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Better than BCG | ~0.91 | [164] |
| BCG:Δ85B | BCG Pasteur strain with single gene (fbpB) knockout. | FbpB/Ag85B | SC/5 × 105 CFU/mouse | H37Rv | Aerosol/100 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [118] |
| ΔnuoG | BCG Pasteur strain with single gene (nuoG) knockout. | NuoG type-I NADH dehydrogenase subunit G | SC/106 CFU/mouse | H37Rv | Aerosol/100–200 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~0.5 | [101] |
| ΔureC::hly ΔnuoG | BCG Pasteur strain with double gene (ureC, nuoG) knockout, which expresses listeriolysin. | UreC and NuoG | SC/106 CFU/mouse | H37Rv or Beijing/W | Aerosol/100–200 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~0.8–2 | [101] |
| ΔBCG TK (triple knock-out) | BCG Danish strain with five gene (esxS, mpt70, mpt83, espC and espA) knockout | EsxS, Mpt70, Mpt83, EspC, and EspA | SC/5 × 104 CFU/guinea pig | M. bovis AF2122/97 | Aerosol/10–20 CFU per guinea pig | Guinea pigs (Dunkin Hartley) | Equal to BCG | - | [201] |
| ΔBCG2432c | BCG China strain with eis gene (BCG2432c) knockout | EIS or Enhanced Intracellular Survival protein | SC/106 CFU/mouse | H37Rv | IN/100 CFU per mouse | Mouse (C57BL/6) | Better than BCG | ~1–2 | [168] |
| ΔBCG3174 | BCG China strain with nuoG gene knockout (BCG3174) | NuoG | SC/106 CFU/mouse | H37Rv | Intranasal/100 CFU per mouse | Mouse (C57BL/6) | Equal to BCG | - | [168] |
| ΔBCG 1419c | BCG Pasteur strain with single gene (c-di-GMP phosphodiesterase) knockout. | All secreted proteins affected by (c-di-GMP phosphodiesterase). | SC/8 × 103 or 2.5 × 102 or 5 × 104 or 106 or ~105 or 107 CFU/mouse | H37Rv or M2 or HN878 | IT/2.5 × 105 or 103 or ~170 CFU per mouse or Aerosol/100–200 CFU per mouse. | Mouse (BALB/c, B6D2F1, C57BL/6, I/StSnEgYCit) | Equal to BCG or Better than BCG in chronic infection model | ~0.8 (chronic infection model) | [207,208,209,210,211,212,213] |
| ID/103 CFU per guinea pig | H37Rv | Aerosol/10–20 CFU per guinea pig | Guinea pigs | Equal to BCG | - |
4. Status of Mycobacterial Vaccines Deficient in Secreted Protein(s)
5. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brennan, P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- Ahmed, A.; Rakshit, S.; Adiga, V.; Dias, M.; Dwarkanath, P.; D’Souza, G.; Vyakarnam, A. A century of BCG: Impact on tuberculosis control and beyond. Immunol. Rev. 2021, 301, 98–121. [Google Scholar] [CrossRef]
- Andersen, P.; Doherty, T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005, 3, 656–662. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Vaccination Against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Front. Immunol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Fine, P.E. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346, 1339–1345. [Google Scholar] [CrossRef]
- Prentice, S.; Dockrell, H.M. Antituberculosis BCG vaccination: More reasons for varying innate and adaptive immune responses. J. Clin. Investig. 2020, 130, 5121–5123. [Google Scholar] [CrossRef]
- Behr, M.A. Comparative genomics of BCG vaccines. Tuberculosis 2001, 81, 165–168. [Google Scholar] [CrossRef]
- Rook, G.A.; Dheda, K.; Zumla, A. Do successful tuberculosis vaccines need to be immunoregulatory rather than merely Th1-boosting? Vaccine 2005, 23, 2115–2120. [Google Scholar] [CrossRef][Green Version]
- Elias, D.; Akuffo, H.; Pawlowski, A.; Haile, M.; Schon, T.; Britton, S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 2005, 23, 1326–1334. [Google Scholar] [CrossRef]
- Elias, D.; Britton, S.; Aseffa, A.; Engers, H.; Akuffo, H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine 2008, 26, 3897–3902. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- Zhuang, L.; Ye, Z.; Li, L.; Yang, L.; Gong, W. Next-Generation TB Vaccines: Progress, Challenges, and Prospects. Vaccines 2023, 11, 1304. [Google Scholar] [CrossRef]
- Sable, S.B.; Posey, J.E.; Scriba, T.J. Tuberculosis Vaccine Development: Progress in Clinical Evaluation. Clin. Microbiol. Rev. 2019, 33, 10–1128. [Google Scholar] [CrossRef]
- Andersen, P.; Scriba, T.J. Moving tuberculosis vaccines from theory to practice. Nat. Rev. Immunol. 2019, 19, 550–562. [Google Scholar] [CrossRef]
- Tang, J.; Yam, W.C.; Chen, Z. Mycobacterium tuberculosis infection and vaccine development. Tuberculosis 2016, 98, 30–41. [Google Scholar] [CrossRef]
- Ng, T.W.; Saavedra-Avila, N.A.; Kennedy, S.C.; Carreno, L.J.; Porcelli, S.A. Current efforts and future prospects in the development of live mycobacteria as vaccines. Expert Rev. Vaccines 2015, 14, 1493–1507. [Google Scholar] [CrossRef]
- Schrager, L.K.; Vekemens, J.; Drager, N.; Lewinsohn, D.M.; Olesen, O.F. The status of tuberculosis vaccine development. Lancet Infect. Dis. 2020, 20, e28–e37. [Google Scholar] [CrossRef]
- Larsen, S.E.; Erasmus, J.H.; Reese, V.A.; Pecor, T.; Archer, J.; Kandahar, A.; Hsu, F.C.; Nicholes, K.; Reed, S.G.; Baldwin, S.L.; et al. An RNA-Based Vaccine Platform for Use against Mycobacterium tuberculosis. Vaccines 2023, 11, 130. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Yaghoubi, A.; Sadat Seddighinia, F.; Rezaee, S.A.R. A century of attempts to develop an effective tuberculosis vaccine: Why they failed? Int. Immunopharmacol. 2022, 109, 108791. [Google Scholar] [CrossRef]
- Scriba, T.J.; Kaufmann, S.H.; Henri Lambert, P.; Sanicas, M.; Martin, C.; Neyrolles, O. Vaccination Against Tuberculosis with Whole-Cell Mycobacterial Vaccines. J. Infect. Dis. 2016, 214, 659–664. [Google Scholar] [CrossRef]
- Seder, R.A.; Hill, A.V. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef]
- Porcelli, S.; Morita, C.T.; Brenner, M.B. CD1b restricts the response of human CD4-8-T lymphocytes to a microbial antigen. Nature 1992, 360, 593–597. [Google Scholar] [CrossRef]
- Van Rhijn, I.; Moody, D.B. CD1 and mycobacterial lipids activate human T cells. Immunol. Rev. 2015, 264, 138–153. [Google Scholar] [CrossRef]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334.e325. [Google Scholar] [CrossRef]
- Mourits, V.P.; Koeken, V.; de Bree, L.C.J.; Moorlag, S.; Chu, W.C.; Xu, X.; Dijkstra, H.; Lemmers, H.; Joosten, L.A.B.; Wang, Y.; et al. BCG-Induced Trained Immunity in Healthy Individuals: The Effect of Plasma Muramyl Dipeptide Concentrations. J. Immunol. Res. 2020, 2020, 5812743. [Google Scholar] [CrossRef]
- Schoenen, H.; Huber, A.; Sonda, N.; Zimmermann, S.; Jantsch, J.; Lepenies, B.; Bronte, V.; Lang, R. Differential control of Mincle-dependent cord factor recognition and macrophage responses by the transcription factors C/EBPbeta and HIF1alpha. J. Immunol. 2014, 193, 3664–3675. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhu, L.L.; Chang, Q.; Jiang, C.; You, Y.; Luo, T.; Jia, X.M.; Lin, X. C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-kappaB activation. J. Biol. Chem. 2014, 289, 30052–30062. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Matsumoto, M.; Takeuchi, O.; Akira, S.; Azuma, I.; Hayashi, A.; Toyoshima, K.; Seya, T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: Involvement of toll-like receptors. Infect. Immun. 2000, 68, 6883–6890. [Google Scholar] [CrossRef]
- Blanc, L.; Gilleron, M.; Prandi, J.; Song, O.R.; Jang, M.S.; Gicquel, B.; Drocourt, D.; Neyrolles, O.; Brodin, P.; Tiraby, G.; et al. Mycobacterium tuberculosis inhibits human innate immune responses via the production of TLR2 antagonist glycolipids. Proc. Natl. Acad. Sci. USA 2017, 114, 11205–11210. [Google Scholar] [CrossRef]
- Tran, V.; Ahn, S.K.; Ng, M.; Li, M.; Liu, J. Loss of Lipid Virulence Factors Reduces the Efficacy of the BCG Vaccine. Sci. Rep. 2016, 6, 29076. [Google Scholar] [CrossRef]
- Walker, K.B.; Brennan, M.J.; Ho, M.M.; Eskola, J.; Thiry, G.; Sadoff, J.; Dobbelaer, R.; Grode, L.; Liu, M.A.; Fruth, U.; et al. The second Geneva Consensus: Recommendations for novel live TB vaccines. Vaccine 2010, 28, 2259–2270. [Google Scholar] [CrossRef]
- Vergne, I.; Chua, J.; Lee, H.H.; Lucas, M.; Belisle, J.; Deretic, V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2005, 102, 4033–4038. [Google Scholar] [CrossRef]
- Master, S.S.; Rampini, S.K.; Davis, A.S.; Keller, C.; Ehlers, S.; Springer, B.; Timmins, G.S.; Sander, P.; Deretic, V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 2008, 3, 224–232. [Google Scholar] [CrossRef]
- Velmurugan, K.; Chen, B.; Miller, J.L.; Azogue, S.; Gurses, S.; Hsu, T.; Glickman, M.; Jacobs, W.R., Jr.; Porcelli, S.A.; Briken, V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007, 3, e110. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Majlessi, L.; Prados-Rosales, R.; Casadevall, A.; Brosch, R. Release of mycobacterial antigens. Immunol. Rev. 2015, 264, 25–45. [Google Scholar] [CrossRef]
- Sambandamurthy, V.K.; Jacobs, W.R., Jr. Live attenuated mutants of Mycobacterium tuberculosis as candidate vaccines against tuberculosis. Microbes Infect. 2005, 7, 955–961. [Google Scholar] [CrossRef]
- Watt, J.; Liu, J. Preclinical Progress of Subunit and Live Attenuated Mycobacterium tuberculosis Vaccines: A Review following the First in Human Efficacy Trial. Pharmaceutics 2020, 12, 848. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4, 213–239. [Google Scholar] [CrossRef]
- Tsirigotaki, A.; De Geyter, J.; Sostaric, N.; Economou, A.; Karamanou, S. Protein export through the bacterial Sec pathway. Nat. Rev. Microbiol. 2017, 15, 21–36. [Google Scholar] [CrossRef]
- Berks, B.C. The twin-arginine protein translocation pathway. Annu. Rev. Biochem. 2015, 84, 843–864. [Google Scholar] [CrossRef]
- Converse, S.E.; Cox, J.S. A protein secretion pathway critical for Mycobacterium tuberculosis virulence is conserved and functional in Mycobacterium smegmatis. J. Bacteriol. 2005, 187, 1238–1245. [Google Scholar] [CrossRef]
- Bitter, W.; Houben, E.N.; Luirink, J.; Appelmelk, B.J. Type VII secretion in mycobacteria: Classification in line with cell envelope structure. Trends Microbiol. 2009, 17, 337–338. [Google Scholar] [CrossRef]
- Augenstreich, J.; Briken, V. Host Cell Targets of Released Lipid and Secreted Protein Effectors of Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2020, 10, 595029. [Google Scholar] [CrossRef]
- Ates, L.S.; Houben, E.N.G.; Bitter, W. Type VII Secretion: A Highly Versatile Secretion System. Microbiol. Spectr. 2016, 4, 357–384. [Google Scholar] [CrossRef]
- Tran, H.R.; Grebenc, D.W.; Klein, T.A.; Whitney, J.C. Bacterial type VII secretion: An important player in host-microbe and microbe-microbe interactions. Mol. Microbiol. 2021, 115, 478–489. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Gey van Pittius, N.C.; Champion, P.A.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.; Appelmelk, B.J.; Bitter, W. Type VII secretion—Mycobacteria show the way. Nat. Rev. Microbiol. 2007, 5, 883–891. [Google Scholar] [CrossRef]
- Daleke, M.H.; Ummels, R.; Bawono, P.; Heringa, J.; Vandenbroucke-Grauls, C.M.; Luirink, J.; Bitter, W. General secretion signal for the mycobacterial type VII secretion pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 11342–11347. [Google Scholar] [CrossRef]
- Dumas, E.; Christina Boritsch, E.; Vandenbogaert, M.; Rodriguez de la Vega, R.C.; Thiberge, J.M.; Caro, V.; Gaillard, J.L.; Heym, B.; Girard-Misguich, F.; Brosch, R.; et al. Mycobacterial Pan-Genome Analysis Suggests Important Role of Plasmids in the Radiation of Type VII Secretion Systems. Genome Biol. Evol. 2016, 8, 387–402. [Google Scholar] [CrossRef]
- Newton-Foot, M.; Warren, R.M.; Sampson, S.L.; van Helden, P.D.; Gey van Pittius, N.C. The plasmid-mediated evolution of the mycobacterial ESX (Type VII) secretion systems. BMC Evol. Biol. 2016, 16, 62. [Google Scholar] [CrossRef]
- Brodin, P.; Majlessi, L.; Marsollier, L.; de Jonge, M.I.; Bottai, D.; Demangel, C.; Hinds, J.; Neyrolles, O.; Butcher, P.D.; Leclerc, C.; et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 2006, 74, 88–98. [Google Scholar] [CrossRef]
- Pym, A.S.; Brodin, P.; Brosch, R.; Huerre, M.; Cole, S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002, 46, 709–717. [Google Scholar] [CrossRef]
- Lewis, K.N.; Liao, R.; Guinn, K.M.; Hickey, M.J.; Smith, S.; Behr, M.A.; Sherman, D.R. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 2003, 187, 117–123. [Google Scholar] [CrossRef]
- Hsu, T.; Hingley-Wilson, S.M.; Chen, B.; Chen, M.; Dai, A.Z.; Morin, P.M.; Marks, C.B.; Padiyar, J.; Goulding, C.; Gingery, M.; et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 12420–12425. [Google Scholar] [CrossRef]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Roy, S.; Ghatak, D.; Das, P.; BoseDasgupta, S. ESX secretion system: The gatekeepers of mycobacterial survivability and pathogenesis. Eur. J. Microbiol. Immunol. 2020, 10, 202–209. [Google Scholar] [CrossRef]
- Portal-Celhay, C.; Tufariello, J.M.; Srivastava, S.; Zahra, A.; Klevorn, T.; Grace, P.S.; Mehra, A.; Park, H.S.; Ernst, J.D.; Jacobs, W.R., Jr.; et al. Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4(+) T-cell activation. Nat. Microbiol. 2016, 2, 16232. [Google Scholar] [CrossRef]
- Tufariello, J.M.; Chapman, J.R.; Kerantzas, C.A.; Wong, K.W.; Vilcheze, C.; Jones, C.M.; Cole, L.E.; Tinaztepe, E.; Thompson, V.; Fenyo, D.; et al. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc. Natl. Acad. Sci. USA 2016, 113, E348–E357. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Bestebroer, J.; Savage, N.D.; de Punder, K.; van Zon, M.; Wilson, L.; Korbee, C.J.; van der Sar, A.M.; Ottenhoff, T.H.; van der Wel, N.N.; et al. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J. Immunol. 2011, 187, 4744–4753. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Savage, N.D.; van Zon, M.; Wilson, L.; Vandenbroucke-Grauls, C.M.; van der Wel, N.N.; Ottenhoff, T.H.; Bitter, W. The ESX-5 secretion system of Mycobacterium marinum modulates the macrophage response. J. Immunol. 2008, 181, 7166–7175. [Google Scholar] [CrossRef]
- Shah, S.; Briken, V. Modular Organization of the ESX-5 Secretion System in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2016, 6, 49. [Google Scholar] [CrossRef]
- Gray, T.A.; Clark, R.R.; Boucher, N.; Lapierre, P.; Smith, C.; Derbyshire, K.M. Intercellular communication and conjugation are mediated by ESX secretion systems in mycobacteria. Science 2016, 354, 347–350. [Google Scholar] [CrossRef]
- Dong, D.; Wang, D.; Li, M.; Wang, H.; Yu, J.; Wang, C.; Liu, J.; Gao, Q. PPE38 modulates the innate immune response and is required for Mycobacterium marinum virulence. Infect. Immun. 2012, 80, 43–54. [Google Scholar] [CrossRef]
- Beresford, N.; Patel, S.; Armstrong, J.; Szoor, B.; Fordham-Skelton, A.P.; Tabernero, L. MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem. J. 2007, 406, 13–18. [Google Scholar] [CrossRef]
- Wong, D.; Bach, H.; Sun, J.; Hmama, Z.; Av-Gay, Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. USA 2011, 108, 19371–19376. [Google Scholar] [CrossRef]
- Mittal, E.; Kumar, S.; Rahman, A.; Krishnasastry, M.V. Modulation of phagolysosome maturation by bacterial tlyA gene product. J. Biosci. 2014, 39, 821–834. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Lau, A.; Liao, T.Y.; Bucci, C.; Hmama, Z. Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS ONE 2010, 5, e8769. [Google Scholar] [CrossRef]
- Pradhan, G.; Shrivastva, R.; Mukhopadhyay, S. Mycobacterial PknG Targets the Rab7l1 Signaling Pathway to Inhibit Phagosome-Lysosome Fusion. J. Immunol. 2018, 201, 1421–1433. [Google Scholar] [CrossRef]
- Clemens, D.L.; Lee, B.Y.; Horwitz, M.A. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 1995, 177, 5644–5652. [Google Scholar] [CrossRef]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef]
- Kim, K.H.; An, D.R.; Song, J.; Yoon, J.Y.; Kim, H.S.; Yoon, H.J.; Im, H.N.; Kim, J.; Kim, D.J.; Lee, S.J.; et al. Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. Proc. Natl. Acad. Sci. USA 2012, 109, 7729–7734. [Google Scholar] [CrossRef]
- Miller, J.L.; Velmurugan, K.; Cowan, M.J.; Briken, V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010, 6, e1000864. [Google Scholar] [CrossRef]
- Sun, J.; Singh, V.; Lau, A.; Stokes, R.W.; Obregon-Henao, A.; Orme, I.M.; Wong, D.; Av-Gay, Y.; Hmama, Z. Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity. PLoS Pathog. 2013, 9, e1003499. [Google Scholar] [CrossRef]
- Piddington, D.L.; Fang, F.C.; Laessig, T.; Cooper, A.M.; Orme, I.M.; Buchmeier, N.A. Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 2001, 69, 4980–4987. [Google Scholar] [CrossRef]
- Fan, L.; Wu, X.; Jin, C.; Li, F.; Xiong, S.; Dong, Y. MptpB Promotes Mycobacteria Survival by Inhibiting the Expression of Inflammatory Mediators and Cell Apoptosis in Macrophages. Front. Cell. Infect. Microbiol. 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.H.; Srivastava, S.; Kotturu, S.K.; Ghosh, S.; Mukhopadhyay, S. The PPE2 protein of Mycobacterium tuberculosis translocates to host nucleus and inhibits nitric oxide production. Sci. Rep. 2017, 7, 39706. [Google Scholar] [CrossRef] [PubMed]
- Thi, E.P.; Hong, C.J.; Sanghera, G.; Reiner, N.E. Identification of the Mycobacterium tuberculosis protein PE-PGRS62 as a novel effector that functions to block phagosome maturation and inhibit iNOS expression. Cell. Microbiol. 2013, 15, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, S.; van Wyk, N.; Warren, R.M.; Sampson, S.L. Phylogeny to function: PE/PPE protein evolution and impact on Mycobacterium tuberculosis pathogenicity. Mol. Microbiol. 2015, 96, 901–916. [Google Scholar] [CrossRef]
- Yaseen, I.; Kaur, P.; Nandicoori, V.K.; Khosla, S. Mycobacteria modulate host epigenetic machinery by Rv1988 methylation of a non-tail arginine of histone H3. Nat. Commun. 2015, 6, 8922. [Google Scholar] [CrossRef]
- Sharma, G.; Upadhyay, S.; Srilalitha, M.; Nandicoori, V.K.; Khosla, S. The interaction of mycobacterial protein Rv2966c with host chromatin is mediated through non-CpG methylation and histone H3/H4 binding. Nucleic Acids Res. 2015, 43, 3922–3937. [Google Scholar] [CrossRef]
- Duan, L.; Yi, M.; Chen, J.; Li, S.; Chen, W. Mycobacterium tuberculosis EIS gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3. Biochem. Biophys. Res. Commun. 2016, 473, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Jose, L.; Ramachandran, R.; Bhagavat, R.; Gomez, R.L.; Chandran, A.; Raghunandanan, S.; Omkumar, R.V.; Chandra, N.; Mundayoor, S.; Kumar, R.A. Hypothetical protein Rv3423.1 of Mycobacterium tuberculosis is a histone acetyltransferase. FEBS J. 2016, 283, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.X.; Ge, P.P.; Li, J.; Wang, Q.; Gao, G.F.; Qiu, X.B.; Liu, C.H. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat. Immunol. 2015, 16, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Jeon, B.Y.; Lee, H.M.; Jin, H.S.; Yuk, J.M.; Song, C.H.; Lee, S.H.; Lee, Z.W.; Cho, S.N.; Kim, J.M.; et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010, 6, e1001230. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.K.; Basu, S.; Basu, K.K.; Banerjee, A.; Pathak, S.; Bhattacharyya, A.; Kaisho, T.; Kundu, M.; Basu, J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 2007, 8, 610–618. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Li, J.; Yang, H.; Tang, T.; Liang, H.; Zuo, M.; Wang, J.; Liu, H.; Liu, F.; et al. Host-mediated ubiquitination of a mycobacterial protein suppresses immunity. Nature 2020, 577, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Liu, Y.; Zhang, H.N.; Jiang, H.W.; Cheng, L.; Guo, S.J.; Deng, J.Y.; Bi, L.J.; Zhang, X.E.; Gao, H.F.; et al. Global Profiling of PknG Interactions Using a Human Proteome Microarray Reveals Novel Connections with CypA. Proteomics 2018, 18, e1800265. [Google Scholar] [CrossRef]
- Vemula, M.H.; Medisetti, R.; Ganji, R.; Jakkala, K.; Sankati, S.; Chatti, K.; Banerjee, S. Mycobacterium tuberculosis Zinc Metalloprotease-1 Assists Mycobacterial Dissemination in Zebrafish. Front. Microbiol. 2016, 7, 1347. [Google Scholar] [CrossRef]
- Sun, J.; Siroy, A.; Lokareddy, R.K.; Speer, A.; Doornbos, K.S.; Cingolani, G.; Niederweis, M. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat. Struct. Mol. Biol. 2015, 22, 672–678. [Google Scholar] [CrossRef]
- Tundup, S.; Mohareer, K.; Hasnain, S.E. Mycobacterium tuberculosis PE25/PPE41 protein complex induces necrosis in macrophages: Role in virulence and disease reactivation? FEBS Open Bio. 2014, 4, 822–828. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, Y.; Lei, Z.; Lu, Z.; Tan, S.; Ge, P.; Chai, Q.; Zhao, M.; Zhang, X.; Li, B.; et al. A mycobacterial effector promotes ferroptosis-dependent pathogenicity and dissemination. Nat. Commun. 2023, 14, 1430. [Google Scholar] [CrossRef]
- Wang, J.; Teng, J.L.; Zhao, D.; Ge, P.; Li, B.; Woo, P.C.; Liu, C.H. The ubiquitin ligase TRIM27 functions as a host restriction factor antagonized by Mycobacterium tuberculosis PtpA during mycobacterial infection. Sci. Rep. 2016, 6, 34827. [Google Scholar] [CrossRef]
- Danelishvili, L.; Yamazaki, Y.; Selker, J.; Bermudez, L.E. Secreted Mycobacterium tuberculosis Rv3654c and Rv3655c proteins participate in the suppression of macrophage apoptosis. PLoS ONE 2010, 5, e10474. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Q.; Dong, Y.; Yue, Y.; Xiong, S. Rv3033, as an Emerging Anti-apoptosis Factor, Facilitates Mycobacteria Survival via Inhibiting Macrophage Intrinsic Apoptosis. Front. Immunol. 2018, 9, 2136. [Google Scholar] [CrossRef]
- Chai, Q.; Yu, S.; Zhong, Y.; Lu, Z.; Qiu, C.; Yu, Y.; Zhang, X.; Zhang, Y.; Lei, Z.; Qiang, L.; et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 2022, 378, eabq0132. [Google Scholar] [CrossRef]
- Rastogi, S.; Ellinwood, S.; Augenstreich, J.; Mayer-Barber, K.D.; Briken, V. Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLoS Pathog. 2021, 17, e1009712. [Google Scholar] [CrossRef]
- Danelishvili, L.; Everman, J.L.; McNamara, M.J.; Bermudez, L.E. Inhibition of the Plasma-Membrane-Associated Serine Protease Cathepsin G by Mycobacterium tuberculosis Rv3364c Suppresses Caspase-1 and Pyroptosis in Macrophages. Front. Microbiol. 2011, 2, 281. [Google Scholar] [CrossRef]
- Hu, D.; Wu, J.; Wang, W.; Mu, M.; Zhao, R.; Xu, X.; Chen, Z.; Xiao, J.; Hu, F.; Yang, Y.; et al. Autophagy regulation revealed by SapM-induced block of autophagosome-lysosome fusion via binding RAB7. Biochem. Biophys. Res. Commun. 2015, 461, 401–407. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Nieuwenhuizen, N.; Vogelzang, A.; Liu, H.; Kaiser, P.; Schuerer, S.; Lazar, D.; Wagner, I.; Mollenkopf, H.J.; Kaufmann, S.H. Deletion of nuoG from the Vaccine Candidate Mycobacterium bovis BCG DeltaureC::hly Improves Protection against Tuberculosis. mBio 2016, 7. [Google Scholar] [CrossRef]
- Strong, E.J.; Ng, T.W.; Porcelli, S.A.; Lee, S. Mycobacterium tuberculosis PE_PGRS20 and PE_PGRS47 Proteins Inhibit Autophagy by Interaction with Rab1A. mSphere 2021, 6, e0054921. [Google Scholar] [CrossRef]
- Ge, P.; Lei, Z.; Yu, Y.; Lu, Z.; Qiang, L.; Chai, Q.; Zhang, Y.; Zhao, D.; Li, B.; Pang, Y.; et al. M. tuberculosis PknG manipulates host autophagy flux to promote pathogen intracellular survival. Autophagy 2022, 18, 576–594. [Google Scholar] [CrossRef]
- Padhi, A.; Pattnaik, K.; Biswas, M.; Jagadeb, M.; Behera, A.; Sonawane, A. Mycobacterium tuberculosis LprE Suppresses TLR2-Dependent Cathelicidin and Autophagy Expression to Enhance Bacterial Survival in Macrophages. J. Immunol. 2019, 203, 2665–2678. [Google Scholar] [CrossRef]
- Strong, E.J.; Wang, J.; Ng, T.W.; Porcelli, S.A.; Lee, S. Mycobacterium tuberculosis PPE51 Inhibits Autophagy by Suppressing Toll-Like Receptor 2-Dependent Signaling. mBio 2022, 13, e0297421. [Google Scholar] [CrossRef]
- Horwitz, M.A.; Harth, G.; Dillon, B.J.; Maslesa-Galic, S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 2000, 97, 13853–13858. [Google Scholar] [CrossRef]
- Jagannath, C.; Lindsey, D.R.; Dhandayuthapani, S.; Xu, Y.; Hunter, R.L., Jr.; Eissa, N.T. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat. Med. 2009, 15, 267–276. [Google Scholar] [CrossRef]
- Khan, A.; Bakhru, P.; Saikolappan, S.; Das, K.; Soudani, E.; Singh, C.R.; Estrella, J.L.; Zhang, D.; Pasare, C.; Ma, Y.; et al. An autophagy-inducing and TLR-2 activating BCG vaccine induces a robust protection against tuberculosis in mice. NPJ Vaccines 2019, 4, 34. [Google Scholar] [CrossRef]
- Singh, A.K.; Srikrishna, G.; Bivalacqua, T.J.; Bishai, W.R. Recombinant BCGs for tuberculosis and bladder cancer. Vaccine 2021, 39, 7321–7331. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E.; Kaufmann, S.H.E. Next-Generation Vaccines Based on Bacille Calmette-Guerin. Front. Immunol. 2018, 9, 121. [Google Scholar] [CrossRef]
- Wiker, H.G.; Harboe, M. The antigen 85 complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef]
- Karbalaei Zadeh Babaki, M.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef]
- Belisle, J.T.; Vissa, V.D.; Sievert, T.; Takayama, K.; Brennan, P.J.; Besra, G.S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 1997, 276, 1420–1422. [Google Scholar] [CrossRef]
- Armitige, L.Y.; Jagannath, C.; Wanger, A.R.; Norris, S.J. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: Effect on growth in culture and in macrophages. Infect. Immun. 2000, 68, 767–778. [Google Scholar] [CrossRef]
- Copenhaver, R.H.; Sepulveda, E.; Armitige, L.Y.; Actor, J.K.; Wanger, A.; Norris, S.J.; Hunter, R.L.; Jagannath, C. A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential. Infect. Immun. 2004, 72, 7084–7095. [Google Scholar] [CrossRef]
- Roche, C.M.; Smith, A.; Lindsey, D.R.; Meher, A.; Schluns, K.; Arora, A.; Armitige, L.Y.; Jagannath, C. The DeltafbpA attenuated candidate vaccine from Mycobacterium tuberculosis, H37Rv primes for a stronger T-bet dependent Th1 immunity in mice. Tuberculosis 2011, 91 (Suppl. S1), S96–S104. [Google Scholar] [CrossRef]
- Jain, R.; Dey, B.; Dhar, N.; Rao, V.; Singh, R.; Gupta, U.D.; Katoch, V.M.; Ramanathan, V.D.; Tyagi, A.K. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS ONE 2008, 3, e3869. [Google Scholar] [CrossRef]
- Prendergast, K.A.; Counoupas, C.; Leotta, L.; Eto, C.; Bitter, W.; Winter, N.; Triccas, J.A. The Ag85B protein of the BCG vaccine facilitates macrophage uptake but is dispensable for protection against aerosol Mycobacterium tuberculosis infection. Vaccine 2016, 34, 2608–2615. [Google Scholar] [CrossRef]
- Copin, R.; Coscolla, M.; Efstathiadis, E.; Gagneux, S.; Ernst, J.D. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG). Vaccine 2014, 32, 5998–6004. [Google Scholar] [CrossRef]
- Armitige, L.Y. Role of FbpA and FbpB in the Pathogenesis and Mycolyltransferase Activity of Mycobacterium Tuberculosis. Ph.D. Thesis, University of Texas Health Sciences Center Houston, Houston, TX, USA, 2002. [Google Scholar]
- Patin, E.C.; Geffken, A.C.; Willcocks, S.; Leschczyk, C.; Haas, A.; Nimmerjahn, F.; Lang, R.; Ward, T.H.; Schaible, U.E. Trehalose dimycolate interferes with FcgammaR-mediated phagosome maturation through Mincle, SHP-1 and FcgammaRIIB signalling. PLoS ONE 2017, 12, e0174973. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, V.; Saikolappan, S.; Das, K.; Veerapandian, R.; Granica, O.; Khan, A.; Dhandayuthapani, S.; Jagannath, C. The ΔfbpAΔsapM candidate vaccine derived from Mycobacterium tuberculosis H37Rv is markedly immunogenic in macrophages and induces robust immunity to tuberculosis in mice. Front. Immunol. 2023. submitted. [Google Scholar]
- Solans, L.; Gonzalo-Asensio, J.; Sala, C.; Benjak, A.; Uplekar, S.; Rougemont, J.; Guilhot, C.; Malaga, W.; Martin, C.; Cole, S.T. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1004183. [Google Scholar] [CrossRef]
- Romao, S.; Munz, C. LC3-associated phagocytosis. Autophagy 2014, 10, 526–528. [Google Scholar] [CrossRef]
- Koster, S.; Upadhyay, S.; Chandra, P.; Papavinasasundaram, K.; Yang, G.; Hassan, A.; Grigsby, S.J.; Mittal, E.; Park, H.S.; Jones, V.; et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. USA 2017, 114, E8711–E8720. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Brightbill, H.D.; Libraty, D.H.; Krutzik, S.R.; Yang, R.B.; Belisle, J.T.; Bleharski, J.R.; Maitland, M.; Norgard, M.V.; Plevy, S.E.; Smale, S.T.; et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999, 285, 732–736. [Google Scholar] [CrossRef]
- Ciaramella, A.; Cavone, A.; Santucci, M.B.; Garg, S.K.; Sanarico, N.; Bocchino, M.; Galati, D.; Martino, A.; Auricchio, G.; D’Orazio, M.; et al. Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J. Infect. Dis. 2004, 190, 1167–1176. [Google Scholar] [CrossRef]
- Stewart, G.R.; Wilkinson, K.A.; Newton, S.M.; Sullivan, S.M.; Neyrolles, O.; Wain, J.R.; Patel, J.; Pool, K.L.; Young, D.B.; Wilkinson, R.J. Effect of deletion or overexpression of the 19-kilodalton lipoprotein Rv3763 on the innate response to Mycobacterium tuberculosis. Infect. Immun. 2005, 73, 6831–6837. [Google Scholar] [CrossRef]
- Henao-Tamayo, M.; Junqueira-Kipnis, A.P.; Ordway, D.; Gonzales-Juarrero, M.; Stewart, G.R.; Young, D.B.; Wilkinson, R.J.; Basaraba, R.J.; Orme, I.M. A mutant of Mycobacterium tuberculosis lacking the 19-kDa lipoprotein Rv3763 is highly attenuated in vivo but retains potent vaccinogenic properties. Vaccine 2007, 25, 7153–7159. [Google Scholar] [CrossRef][Green Version]
- Gehring, A.J.; Dobos, K.M.; Belisle, J.T.; Harding, C.V.; Boom, W.H. Mycobacterium tuberculosis LprG (Rv1411c): A novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 2004, 173, 2660–2668. [Google Scholar] [CrossRef]
- Drage, M.G.; Tsai, H.C.; Pecora, N.D.; Cheng, T.Y.; Arida, A.R.; Shukla, S.; Rojas, R.E.; Seshadri, C.; Moody, D.B.; Boom, W.H.; et al. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat. Struct. Mol. Biol. 2010, 17, 1088–1095. [Google Scholar] [CrossRef]
- Martinot, A.J.; Farrow, M.; Bai, L.; Layre, E.; Cheng, T.Y.; Tsai, J.H.; Iqbal, J.; Annand, J.W.; Sullivan, Z.A.; Hussain, M.M.; et al. Mycobacterial Metabolic Syndrome: LprG and Rv1410 Regulate Triacylglyceride Levels, Growth Rate and Virulence in Mycobacterium tuberculosis. PLoS Pathog. 2016, 12, e1005351. [Google Scholar] [CrossRef]
- Martinot, A.J.; Blass, E.; Yu, J.; Aid, M.; Mahrokhian, S.H.; Cohen, S.B.; Plumlee, C.R.; Larocca, R.A.; Siddiqi, N.; Wakabayashi, S.; et al. Protective efficacy of an attenuated Mtb DeltaLprG vaccine in mice. PLoS Pathog. 2020, 16, e1009096. [Google Scholar] [CrossRef]
- Vidal, S.J.; Sellers, D.; Yu, J.; Wakabayashi, S.; Sixsmith, J.; Aid, M.; Barrett, J.; Stevens, S.F.; Liu, X.; Li, W.; et al. Attenuated Mycobacterium tuberculosis vaccine protection in a low-dose murine challenge model. iScience 2023, 26, 106963. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Sonnweber, T.; Weiss, G. The struggle for iron—A metal at the host-pathogen interface. Cell. Microbiol. 2010, 12, 1691–1702. [Google Scholar] [CrossRef]
- Malen, H.; Berven, F.S.; Fladmark, K.E.; Wiker, H.G. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics 2007, 7, 1702–1718. [Google Scholar] [CrossRef]
- de Souza, G.A.; Leversen, N.A.; Malen, H.; Wiker, H.G. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. J. Proteomics 2011, 75, 502–510. [Google Scholar] [CrossRef]
- Pandey, R.; Rodriguez, G.M. A ferritin mutant of Mycobacterium tuberculosis is highly susceptible to killing by antibiotics and is unable to establish a chronic infection in mice. Infect. Immun. 2012, 80, 3650–3659. [Google Scholar] [CrossRef]
- Subbian, S.; Pandey, R.; Soteropoulos, P.; Rodriguez, G.M. Vaccination with an Attenuated Ferritin Mutant Protects Mice against Virulent Mycobacterium tuberculosis. J. Immunol. Res. 2015, 2015, 385402. [Google Scholar] [CrossRef]
- Stefanovic, C.; Hager, F.F.; Schaffer, C. LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly. Int. J. Mol. Sci. 2021, 22, 908. [Google Scholar] [CrossRef]
- Koster, S.; Klevorn, T.; Papavinasasundaram, K.; Sassetti, C.M.; Portal-Celhay, C.; Philips, J.A. Consequence of enhanced LC3-trafficking for a live, attenuated M. tuberculosis vaccine. Vaccine 2018, 36, 939–944. [Google Scholar] [CrossRef]
- Dey, S.; Lane, J.M.; Lee, R.E.; Rubin, E.J.; Sacchettini, J.C. Structural characterization of the Mycobacterium tuberculosis biotin biosynthesis enzymes 7,8-diaminopelargonic acid synthase and dethiobiotin synthetase. Biochemistry 2010, 49, 6746–6760. [Google Scholar] [CrossRef]
- Woong Park, S.; Klotzsche, M.; Wilson, D.J.; Boshoff, H.I.; Eoh, H.; Manjunatha, U.; Blumenthal, A.; Rhee, K.; Barry, C.E., 3rd; Aldrich, C.C.; et al. Evaluating the sensitivity of Mycobacterium tuberculosis to biotin deprivation using regulated gene expression. PLoS Pathog. 2011, 7, e1002264. [Google Scholar] [CrossRef]
- Kar, R.; Nangpal, P.; Mathur, S.; Singh, S.; Tyagi, A.K. bioA mutant of Mycobacterium tuberculosis shows severe growth defect and imparts protection against tuberculosis in guinea pigs. PLoS ONE 2017, 12, e0179513. [Google Scholar] [CrossRef]
- Harth, G.; Clemens, D.L.; Horwitz, M.A. Glutamine synthetase of Mycobacterium tuberculosis: Extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 1994, 91, 9342–9346. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, B.Y.; Bardarov, S.; Chen, M.; Morris, S.L.; Jacobs, W.R., Jr. Protection elicited by two glutamine auxotrophs of Mycobacterium tuberculosis and in vivo growth phenotypes of the four unique glutamine synthetase mutants in a murine model. Infect. Immun. 2006, 74, 6491–6495. [Google Scholar] [CrossRef]
- Schirmer, S.; Rauh, L.; Alebouyeh, S.; Delgado-Velandia, M.; Salgueiro, V.C.; Lerma, L.; Serrano-Mestre, J.L.; Azkargorta, M.; Elortza, F.; Lavin, J.L.; et al. Immunogenicity of Mycobacterial Extracellular Vesicles Isolated from Host-Related Conditions Informs About Tuberculosis Disease Status. Front. Microbiol. 2022, 13, 907296. [Google Scholar] [CrossRef]
- Saleh, M.T.; Belisle, J.T. Secretion of an acid phosphatase (SapM) by Mycobacterium tuberculosis that is similar to eukaryotic acid phosphatases. J. Bacteriol. 2000, 182, 6850–6853. [Google Scholar] [CrossRef]
- Pal, R.; Bisht, M.K.; Mukhopadhyay, S. Secretory proteins of Mycobacterium tuberculosis and their roles in modulation of host immune responses: Focus on therapeutic targets. FEBS J. 2022, 289, 4146–4171. [Google Scholar] [CrossRef]
- Saikolappan, S.; Estrella, J.; Sasindran, S.J.; Khan, A.; Armitige, L.Y.; Jagannath, C.; Dhandayuthapani, S. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS ONE 2012, 7, e36198. [Google Scholar] [CrossRef]
- Puri, R.V.; Reddy, P.V.; Tyagi, A.K. Secreted acid phosphatase (SapM) of Mycobacterium tuberculosis is indispensable for arresting phagosomal maturation and growth of the pathogen in guinea pig tissues. PLoS ONE 2013, 8, e70514. [Google Scholar] [CrossRef]
- Chauhan, P.; Reddy, P.V.; Singh, R.; Jaisinghani, N.; Gandotra, S.; Tyagi, A.K. Secretory phosphatases deficient mutant of Mycobacterium tuberculosis imparts protection at the primary site of infection in guinea pigs. PLoS ONE 2013, 8, e77930. [Google Scholar] [CrossRef]
- Bahal, R.K.; Mathur, S.; Chauhan, P.; Tyagi, A.K. An attenuated quadruple gene mutant of Mycobacterium tuberculosis imparts protection against tuberculosis in guinea pigs. Biol. Open 2018, 7, bio029546. [Google Scholar] [CrossRef]
- Festjens, N.; Bogaert, P.; Batni, A.; Houthuys, E.; Plets, E.; Vanderschaeghe, D.; Laukens, B.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; et al. Disruption of the SapM locus in Mycobacterium bovis BCG improves its protective efficacy as a vaccine against M. tuberculosis. EMBO Mol. Med. 2011, 3, 222–234. [Google Scholar] [CrossRef]
- Festjens, N.; Vandewalle, K.; Houthuys, E.; Plets, E.; Vanderschaeghe, D.; Borgers, K.; Van Hecke, A.; Tiels, P.; Callewaert, N. SapM mutation to improve the BCG vaccine: Genomic, transcriptomic and preclinical safety characterization. Vaccine 2019, 37, 3539–3551. [Google Scholar] [CrossRef]
- Wang, J.; Ge, P.; Qiang, L.; Tian, F.; Zhao, D.; Chai, Q.; Zhu, M.; Zhou, R.; Meng, G.; Iwakura, Y.; et al. The mycobacterial phosphatase PtpA regulates the expression of host genes and promotes cell proliferation. Nat. Commun. 2017, 8, 244. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell. Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Bach, H.; Papavinasasundaram, K.G.; Wong, D.; Hmama, Z.; Av-Gay, Y. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 2008, 3, 316–322. [Google Scholar] [CrossRef]
- Singh, R.; Rao, V.; Shakila, H.; Gupta, R.; Khera, A.; Dhar, N.; Singh, A.; Koul, A.; Singh, Y.; Naseema, M.; et al. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol. Microbiol. 2003, 50, 751–762. [Google Scholar] [CrossRef]
- Zhou, B.; He, Y.; Zhang, X.; Xu, J.; Luo, Y.; Wang, Y.; Franzblau, S.G.; Yang, Z.; Chan, R.J.; Liu, Y.; et al. Targeting mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc. Natl. Acad. Sci. USA 2010, 107, 4573–4578. [Google Scholar] [CrossRef]
- Johansen, P.; Fettelschoss, A.; Amstutz, B.; Selchow, P.; Waeckerle-Men, Y.; Keller, P.; Deretic, V.; Held, L.; Kundig, T.M.; Bottger, E.C.; et al. Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin. Vaccine Immunol. 2011, 18, 907–913. [Google Scholar] [CrossRef]
- Sander, P.; Clark, S.; Petrera, A.; Vilaplana, C.; Meuli, M.; Selchow, P.; Zelmer, A.; Mohanan, D.; Andreu, N.; Rayner, E.; et al. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine 2015, 33, 1353–1359. [Google Scholar] [CrossRef]
- Dahl, J.L.; Wei, J.; Moulder, J.W.; Laal, S.; Friedman, R.L. Subcellular localization of the Iitracellular survival-enhancing Eis protein of Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 4295–4302. [Google Scholar] [CrossRef][Green Version]
- Wei, J.; Dahl, J.L.; Moulder, J.W.; Roberts, E.A.; O’Gaora, P.; Young, D.B.; Friedman, R.L. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J. Bacteriol. 2000, 182, 377–384. [Google Scholar] [CrossRef]
- Samuel, L.P.; Song, C.H.; Wei, J.; Roberts, E.A.; Dahl, J.L.; Barry, C.E.; Jo, E.K.; Friedman, R.L. Expression, production and release of the Eis protein by Mycobacterium tuberculosis during infection of macrophages and its effect on cytokine secretion. Microbiology 2007, 153, 529–540. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, M.; Zhang, Y.; Peng, H.; Lei, Q.; Yuan, X.; Liu, S.; Xiong, Y.; Lin, X.; Jo-Lewis, B.N.; et al. Deletion of BCG_2432c from the Bacillus Calmette-Guerin vaccine enhances autophagy-mediated immunity against tuberculosis. Allergy 2022, 77, 619–632. [Google Scholar] [CrossRef]
- Sayes, F.; Sun, L.; Di Luca, M.; Simeone, R.; Degaiffier, N.; Fiette, L.; Esin, S.; Brosch, R.; Bottai, D.; Leclerc, C.; et al. Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion: Encoded PE-PPE proteins predicts vaccine potential. Cell Host Microbe 2012, 11, 352–363. [Google Scholar] [CrossRef]
- Tiwari, S.; Dutt, T.S.; Chen, B.; Chen, M.; Kim, J.; Dai, A.Z.; Lukose, R.; Shanley, C.; Fox, A.; Karger, B.R.; et al. BCG-Prime and boost with Esx-5 secretion system deletion mutant leads to better protection against clinical strains of Mycobacterium tuberculosis. Vaccine 2020, 38, 7156–7165. [Google Scholar] [CrossRef]
- Saini, N.K.; Baena, A.; Ng, T.W.; Venkataswamy, M.M.; Kennedy, S.C.; Kunnath-Velayudhan, S.; Carreno, L.J.; Xu, J.; Chan, J.; Larsen, M.H.; et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 2016, 1, 16133. [Google Scholar] [CrossRef]
- Amaral, E.P.; Riteau, N.; Moayeri, M.; Maier, N.; Mayer-Barber, K.D.; Pereira, R.M.; Lage, S.L.; Kubler, A.; Bishai, W.R.; D’Imperio-Lima, M.R.; et al. Lysosomal Cathepsin Release Is Required for NLRP3-Inflammasome Activation by Mycobacterium tuberculosis in Infected Macrophages. Front. Immunol. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Romagnoli, A.; Etna, M.P.; Giacomini, E.; Pardini, M.; Remoli, M.E.; Corazzari, M.; Falasca, L.; Goletti, D.; Gafa, V.; Simeone, R.; et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 2012, 8, 1357–1370. [Google Scholar] [CrossRef]
- Schaible, U.E.; Sturgill-Koszycki, S.; Schlesinger, P.H.; Russell, D.G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 1998, 160, 1290–1296. [Google Scholar] [CrossRef]
- Honer zu Bentrup, K.; Russell, D.G. Mycobacterial persistence: Adaptation to a changing environment. Trends Microbiol. 2001, 9, 597–605. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jethwaney, D.; Schilling, B.; Clemens, D.L.; Gibson, B.W.; Horwitz, M.A. The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome. Mol. Cell. Proteomics 2010, 9, 32–53. [Google Scholar] [CrossRef]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Nasser Eddine, A.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef]
- Desel, C.; Dorhoi, A.; Bandermann, S.; Grode, L.; Eisele, B.; Kaufmann, S.H. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 2011, 204, 1573–1584. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Cotton, M.F.; Eisele, B.; Gengenbacher, M.; Grode, L.; Hesseling, A.C.; Walzl, G. The BCG replacement vaccine VPM1002: From drawing board to clinical trial. Expert Rev. Vaccines 2014, 13, 619–630. [Google Scholar] [CrossRef]
- Martin, C.; Williams, A.; Hernandez-Pando, R.; Cardona, P.J.; Gormley, E.; Bordat, Y.; Soto, C.Y.; Clark, S.O.; Hatch, G.J.; Aguilar, D.; et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine 2006, 24, 3408–3419. [Google Scholar] [CrossRef]
- Verreck, F.A.; Vervenne, R.A.; Kondova, I.; van Kralingen, K.W.; Remarque, E.J.; Braskamp, G.; van der Werff, N.M.; Kersbergen, A.; Ottenhoff, T.H.; Heidt, P.J.; et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS ONE 2009, 4, e5264. [Google Scholar] [CrossRef]
- Hinchey, J.; Lee, S.; Jeon, B.Y.; Basaraba, R.J.; Venkataswamy, M.M.; Chen, B.; Chan, J.; Braunstein, M.; Orme, I.M.; Derrick, S.C.; et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 2007, 117, 2279–2288. [Google Scholar] [CrossRef]
- Hinchey, J.; Jeon, B.Y.; Alley, H.; Chen, B.; Goldberg, M.; Derrick, S.; Morris, S.; Jacobs, W.R., Jr.; Porcelli, S.A.; Lee, S. Lysine auxotrophy combined with deletion of the SecA2 gene results in a safe and highly immunogenic candidate live attenuated vaccine for tuberculosis. PLoS ONE 2011, 6, e15857. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.; Gullick, J.; Clark, S.; Gleeson, F.; McIntyre, A.; Arlehamn, C.L.; Sette, A.; et al. MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies. NPJ Vaccines 2021, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Arbues, A.; Aguilo, J.I.; Gonzalo-Asensio, J.; Marinova, D.; Uranga, S.; Puentes, E.; Fernandez, C.; Parra, A.; Cardona, P.J.; Vilaplana, C.; et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 2013, 31, 4867–4873. [Google Scholar] [CrossRef]
- Solans, L.; Uranga, S.; Aguilo, N.; Arnal, C.; Gomez, A.B.; Monzon, M.; Badiola, J.J.; Gicquel, B.; Martin, C. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine 2014, 32, 5192–5197. [Google Scholar] [CrossRef]
- Braunstein, M.; Espinosa, B.J.; Chan, J.; Belisle, J.T.; Jacobs, W.R., Jr. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 453–464. [Google Scholar] [CrossRef]
- Kurtz, S.; McKinnon, K.P.; Runge, M.S.; Ting, J.P.; Braunstein, M. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect. Immun. 2006, 74, 6855–6864. [Google Scholar] [CrossRef]
- Perez, E.; Samper, S.; Bordas, Y.; Guilhot, C.; Gicquel, B.; Martin, C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 2001, 41, 179–187. [Google Scholar] [CrossRef]
- Gonzalo-Asensio, J.; Mostowy, S.; Harders-Westerveen, J.; Huygen, K.; Hernandez-Pando, R.; Thole, J.; Behr, M.; Gicquel, B.; Martin, C. PhoP: A missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS ONE 2008, 3, e3496. [Google Scholar] [CrossRef]
- Lee, J.S.; Krause, R.; Schreiber, J.; Mollenkopf, H.J.; Kowall, J.; Stein, R.; Jeon, B.Y.; Kwak, J.Y.; Song, M.K.; Patron, J.P.; et al. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe 2008, 3, 97–103. [Google Scholar] [CrossRef]
- Frigui, W.; Bottai, D.; Majlessi, L.; Monot, M.; Josselin, E.; Brodin, P.; Garnier, T.; Gicquel, B.; Martin, C.; Leclerc, C.; et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008, 4, e33. [Google Scholar] [CrossRef]
- Anil Kumar, V.; Goyal, R.; Bansal, R.; Singh, N.; Sevalkar, R.R.; Kumar, A.; Sarkar, D. EspR-dependent ESAT-6 Protein Secretion of Mycobacterium tuberculosis Requires the Presence of Virulence Regulator PhoP. J. Biol. Chem. 2016, 291, 19018–19030. [Google Scholar] [CrossRef]
- Broset, E.; Martin, C.; Gonzalo-Asensio, J. Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: Implications for virulence regulation and application to vaccine development. mBio 2015, 6, e01289-15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Asensio, J.; Malaga, W.; Pawlik, A.; Astarie-Dequeker, C.; Passemar, C.; Moreau, F.; Laval, F.; Daffe, M.; Martin, C.; Brosch, R.; et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl. Acad. Sci. USA 2014, 111, 11491–11496. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, N.; Gonzalo-Asensio, J.; Alvarez-Arguedas, S.; Marinova, D.; Gomez, A.B.; Uranga, S.; Spallek, R.; Singh, M.; Audran, R.; Spertini, F.; et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat. Commun. 2017, 8, 16085. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.T.; Fruth, U.; Brennan, M.J.; Dobbelaer, R.; Hubrechts, P.; Ho, M.M.; Mayner, R.E.; Thole, J.; Walker, K.B.; Liu, M.; et al. New live mycobacterial vaccines: The Geneva consensus on essential steps towards clinical development. Vaccine 2005, 23, 3753–3761. [Google Scholar] [CrossRef]
- Clark, S.; Lanni, F.; Marinova, D.; Rayner, E.; Martin, C.; Williams, A. Revaccination of Guinea Pigs With the Live Attenuated Mycobacterium tuberculosis Vaccine MTBVAC Improves BCG’s Protection Against Tuberculosis. J. Infect. Dis. 2017, 216, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef]
- Clemmensen, H.S.; Dube, J.Y.; McIntosh, F.; Rosenkrands, I.; Jungersen, G.; Aagaard, C.; Andersen, P.; Behr, M.A.; Mortensen, R. In vivo antigen expression regulates CD4 T cell differentiation and vaccine efficacy against Mycobacterium tuberculosis infection. bioRxiv 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Chandran, A.; Williams, K.; Mendum, T.; Stewart, G.; Clark, S.; Zadi, S.; Lanni, F.; McLeod, N.; Williams, A.; Villarreal-Ramos, B.; et al. Development of a diagnostic compatible BCG vaccine against Bovine tuberculosis. Sci. Rep. 2019, 9, 17791. [Google Scholar] [CrossRef]
- Bigi, F.; Alito, A.; Fisanotti, J.C.; Romano, M.I.; Cataldi, A. Characterization of a novel Mycobacterium bovis secreted antigen containing PGLTS repeats. Infect. Immun. 1995, 63, 2581–2586. [Google Scholar] [CrossRef]
- Berthet, F.X.; Lagranderie, M.; Gounon, P.; Laurent-Winter, C.; Ensergueix, D.; Chavarot, P.; Thouron, F.; Maranghi, E.; Pelicic, V.; Portnoï, D.; et al. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science 1998, 282, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.L.; Klein, K.; Kim, R.; Beery, D.; Ramakrishnan, L. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect. Immun. 2006, 74, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Flores-Valdez, M.A.; Aceves-Sanchez Mde, J.; Pedroza-Roldan, C.; Vega-Dominguez, P.J.; Prado-Montes de Oca, E.; Bravo-Madrigal, J.; Laval, F.; Daffe, M.; Koestler, B.; Waters, C.M. The Cyclic Di-GMP Phosphodiesterase Gene Rv1357c/BCG1419c Affects BCG Pellicle Production and In Vivo Maintenance. IUBMB Life 2015, 67, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Fernandez, J.B.; Ferreira-Souza, G.H.M.; Rodriguez-Campos, J.; Aceves-Sanchez, M.J.; Bravo-Madrigal, J.; Vallejo-Cardona, A.A.; Flores-Valdez, M.A. Proteomic characterization of a second-generation version of the BCGDeltaBCG1419c vaccine candidate by means of electrospray-ionization quadrupole time-of-flight mass spectrometry. Pathog. Dis. 2021, 79, ftaa070. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Sanchez, M.J.; Flores-Valdez, M.A.; Pedroza-Roldan, C.; Creissen, E.; Izzo, L.; Silva-Angulo, F.; Dawson, C.; Izzo, A.; Bielefeldt-Ohmann, H.; Segura-Cerda, C.A.; et al. Vaccination with BCGDeltaBCG1419c protects against pulmonary and extrapulmonary TB and is safer than BCG. Sci. Rep. 2021, 11, 12417. [Google Scholar] [CrossRef]
- Korotetskaya, M.; Baikuzina, P.; Segura-Cerda, C.A.; Aceves-Sánchez, M.J.; Apt, A.; Flores-Valdez, M.A. BCG and BCGΔBCG1419c transiently protect hypersusceptible I/St mice and induce different influx of macrophages and neutrophils during pulmonary tuberculosis. J. Med. Microbiol. 2022, 71, 001485. [Google Scholar] [CrossRef]
- Pedroza-Roldán, C.; Guapillo, C.; Barrios-Payán, J.; Mata-Espinosa, D.; Aceves-Sánchez Mde, J.; Marquina-Castillo, B.; Hernández-Pando, R.; Flores-Valdez, M.A. The BCGΔBCG1419c strain, which produces more pellicle in vitro, improves control of chronic tuberculosis in vivo. Vaccine 2016, 34, 4763–4770. [Google Scholar] [CrossRef] [PubMed]
- Flores-Valdez, M.A.; Pedroza-Roldán, C.; Aceves-Sánchez, M.J.; Peterson, E.J.R.; Baliga, N.S.; Hernández-Pando, R.; Troudt, J.; Creissen, E.; Izzo, L.; Bielefeldt-Ohmann, H.; et al. The BCGΔBCG1419c Vaccine Candidate Reduces Lung Pathology, IL-6, TNF-α, and IL-10 During Chronic TB Infection. Front. Microbiol. 2018, 9, 1281. [Google Scholar] [CrossRef]
- Kwon, K.W.; Aceves-Sánchez, M.J.; Segura-Cerda, C.A.; Choi, E.; Bielefeldt-Ohmann, H.; Shin, S.J.; Flores-Valdez, M.A. BCGΔBCG1419c increased memory CD8(+) T cell-associated immunogenicity and mitigated pulmonary inflammation compared with BCG in a model of chronic tuberculosis. Sci. Rep. 2022, 12, 15824. [Google Scholar] [CrossRef]
- Aceves-Sanchez, M.J.; Barrios-Payan, J.A.; Segura-Cerda, C.A.; Flores-Valdez, M.A.; Mata-Espinosa, D.; Pedroza-Roldan, C.; Yadav, R.; Saini, D.K.; de la Cruz, M.A.; Ares, M.A.; et al. BCG∆BCG1419c and BCG differ in induction of autophagy, c-di-GMP content, proteome, and progression of lung pathology in Mycobacterium tuberculosis HN878-infected male BALB/c mice. Vaccine 2023, 41, 3824–3835. [Google Scholar] [CrossRef]
- Segura-Cerda, C.A.; Marquina-Castillo, B.; Lozano-Ordaz, V.; Mata-Espinosa, D.; Barrios-Payán, J.A.; López-Torres, M.O.; Aceves-Sánchez, M.J.; Bielefeldt-Ohmann, H.; Hernández-Pando, R.; Flores-Valdez, M.A. BCG and BCGΔBCG1419c protect type 2 diabetic mice against tuberculosis via different participation of T and B lymphocytes, dendritic cells and pro-inflammatory cytokines. NPJ Vaccines 2020, 5, 21. [Google Scholar] [CrossRef]
- Zulauf, K.E.; Sullivan, J.T.; Braunstein, M. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog. 2018, 14, e1007011. [Google Scholar] [CrossRef] [PubMed]
- Katti, M.K.; Dai, G.; Armitige, L.Y.; Rivera Marrero, C.; Daniel, S.; Singh, C.R.; Lindsey, D.R.; Dhandayuthapani, S.; Hunter, R.L.; Jagannath, C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008, 10, 1286–1303. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019, 348, 69–121. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef]
- Kuan, R.; Muskat, K.; Peters, B.; Lindestam Arlehamn, C.S. Is mapping the BCG vaccine-induced immune responses the key to improving the efficacy against tuberculosis? J. Intern. Med. 2020, 288, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Brazier, B.; McShane, H. Towards new TB vaccines. Semin. Immunopathol. 2020, 42, 315–331. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Lewinsohn, D.M.; Scriba, T.J. Polyfunctional CD4(+) T Cells As Targets for Tuberculosis Vaccination. Front. Immunol. 2017, 8, 1262. [Google Scholar] [CrossRef]
- Satti, I.; McShane, H. Current approaches toward identifying a correlate of immune protection from tuberculosis. Expert Rev. Vaccines 2019, 18, 43–59. [Google Scholar] [CrossRef]
- Andersen, P.; Woodworth, J.S. Tuberculosis vaccines--rethinking the current paradigm. Trends Immunol. 2014, 35, 387–395. [Google Scholar] [CrossRef]
- Andersen, P.; Kaufmann, S.H. Novel vaccination strategies against tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 4, a018523. [Google Scholar] [CrossRef]
- Lin, Y.; Slight, S.R.; Khader, S.A. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 2010, 32, 79–90. [Google Scholar] [CrossRef]
- Khader, S.A.; Divangahi, M.; Hanekom, W.; Hill, P.C.; Maeurer, M.; Makar, K.W.; Mayer-Barber, K.D.; Mhlanga, M.M.; Nemes, E.; Schlesinger, L.S.; et al. Targeting innate immunity for tuberculosis vaccination. J. Clin. Investig. 2019, 129, 3482–3491. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Bettencourt, P.J.G. The 100(th) anniversary of bacille Calmette-Guérin (BCG) and the latest vaccines against COVID-19. Int. J. Tuberc. Lung Dis. 2021, 25, 611–613. [Google Scholar] [CrossRef]
- Buffen, K.; Oosting, M.; Quintin, J.; Ng, A.; Kleinnijenhuis, J.; Kumar, V.; van de Vosse, E.; Wijmenga, C.; van Crevel, R.; Oosterwijk, E.; et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014, 10, e1004485. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, W. Acetylation in the regulation of autophagy. Autophagy 2023, 19, 379–387. [Google Scholar] [CrossRef]
- DiNardo, A.R.; Rajapakshe, K.; Nishiguchi, T.; Grimm, S.L.; Mtetwa, G.; Dlamini, Q.; Kahari, J.; Mahapatra, S.; Kay, A.; Maphalala, G.; et al. DNA hypermethylation during tuberculosis dampens host immune responsiveness. J. Clin. Investig. 2020, 130, 3113–3123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veerapandian, R.; Gadad, S.S.; Jagannath, C.; Dhandayuthapani, S. Live Attenuated Vaccines against Tuberculosis: Targeting the Disruption of Genes Encoding the Secretory Proteins of Mycobacteria. Vaccines 2024, 12, 530. https://doi.org/10.3390/vaccines12050530
Veerapandian R, Gadad SS, Jagannath C, Dhandayuthapani S. Live Attenuated Vaccines against Tuberculosis: Targeting the Disruption of Genes Encoding the Secretory Proteins of Mycobacteria. Vaccines. 2024; 12(5):530. https://doi.org/10.3390/vaccines12050530
Chicago/Turabian StyleVeerapandian, Raja, Shrikanth S. Gadad, Chinnaswamy Jagannath, and Subramanian Dhandayuthapani. 2024. "Live Attenuated Vaccines against Tuberculosis: Targeting the Disruption of Genes Encoding the Secretory Proteins of Mycobacteria" Vaccines 12, no. 5: 530. https://doi.org/10.3390/vaccines12050530
APA StyleVeerapandian, R., Gadad, S. S., Jagannath, C., & Dhandayuthapani, S. (2024). Live Attenuated Vaccines against Tuberculosis: Targeting the Disruption of Genes Encoding the Secretory Proteins of Mycobacteria. Vaccines, 12(5), 530. https://doi.org/10.3390/vaccines12050530









