New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review
Abstract
1. Introduction
2. Methods
3. Results
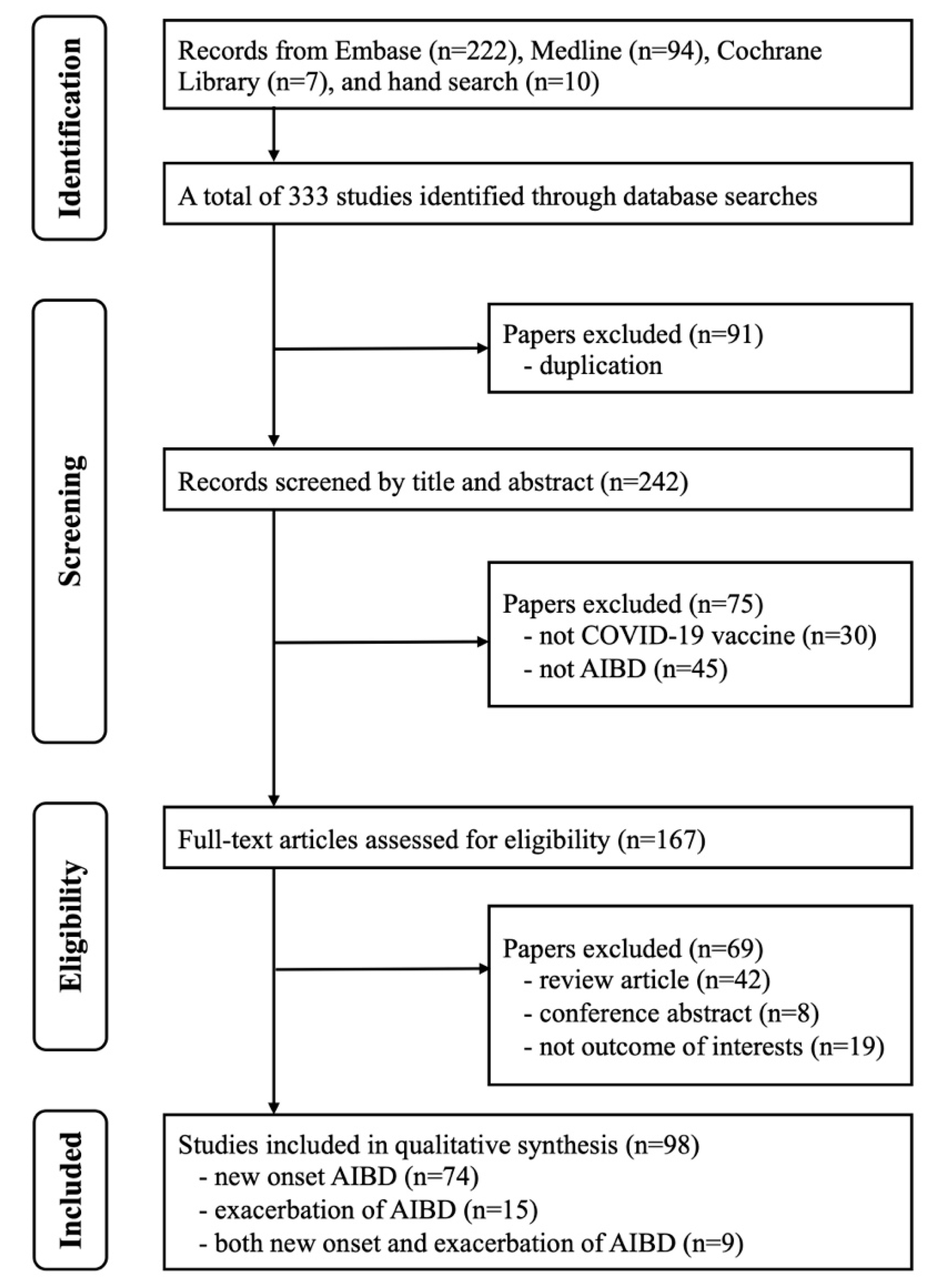
3.1. Literature Search
3.2. Patient Characteristics
3.3. Vaccine Type, Vaccine Dose, and Time to AIBD Onset Following Vaccination
3.4. Other Potential Non-Vaccine Triggers
3.5. The Assessment of Naranjo Scores for New-Onset AIBD or AIBD Flares
3.6. Treatment and Outcomes for New-Onset AIBD or AIBD Flares
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Chi, C.C. Aiming at a bright future. Dermatol. Sin. 2022, 40, 1–2. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 April 2023).
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef]
- Agharbi, F.Z.; Eljazouly, M.; Basri, G.; Faik, M.; Benkirane, A.; Albouzidi, A.; Chiheb, S. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann. Dermatol. Venereol. 2022, 149, 56–57. [Google Scholar] [CrossRef]
- Akoglu, G. Pemphigus vulgaris after SARS-CoV-2 vaccination: A case with new-onset and two cases with severe aggravation. Dermatol. Ther. 2022, 35, e15396. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Freeman, E.E.; Sun, Q.; McMahon, D.E.; Singh, R.; Fathy, R.; Tyagi, A.; Blumenthal, K.; Hruza, G.J.; French, L.E.; Fox, L.P. Skin reactions to COVID-19 vaccines: An American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J. Am. Acad. Dermatol. 2022, 86, e165–e167. [Google Scholar] [CrossRef]
- Hung, W.-K.; Chi, C.-C.; Wang, S.-H. AZ arm: Delayed cutaneous reaction to ChAdOx1 nCoV-19 (AZD1222) vaccine. Dermatol. Sin. 2022, 40, 52–53. [Google Scholar] [CrossRef]
- Lin, P.T.; Chi, C.C. Erythrodermic psoriasis following ChAdOx1 nCOV-19 vaccination: A case report. Dermatol. Sin. 2022, 40, 62–63. [Google Scholar] [CrossRef]
- Grieco, T.; Maddalena, P.; Sernicola, A.; Muharremi, R.; Basili, S.; Alvaro, D.; Cangemi, R.; Rossi, A.; Pellacani, G. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol. Ther. 2021, 34, e15153. [Google Scholar] [CrossRef]
- Hsieh, T.S.; Chen, J.S.; Tsai, T.F. Dyshidrotic bullous pemphigoid developing after Moderna mRNA-1273 vaccination. Dermatol. Sin. 2023, 41, 52–53. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Tang, H.; Fan, M.; Wang, W.; Ding, Y.; Shen, S.; Zhou, W.; Zhang, Y.; Wang, Z. Bullous Pemphigoid After Vaccination With the Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine: Two Cases in China. Wound. Manag. Prev. 2022, 68, 22–25. [Google Scholar] [CrossRef]
- Afacan, E.; Edek, Y.C.; Ilter, N.; Gulekon, A. Can COVID-19 vaccines cause or exacerbate bullous pemphigoid? A report of seven cases from one center. Int. J. Dermatol. 2022, 61, 626–627. [Google Scholar] [CrossRef]
- Hung, W.K.; Chi, C.C. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e407–e409. [Google Scholar] [CrossRef]
- Witte, M.; Zillikens, D.; Schmidt, E. Diagnosis of Autoimmune Blistering Diseases. Front. Med. 2018, 5, 296. [Google Scholar] [CrossRef]
- Holtsche, M.M.; Boch, K.; Schmidt, E. Autoimmune bullous dermatoses. J. Dtsch. Dermatol. Ges. 2023, 21, 405–412. [Google Scholar] [CrossRef]
- Damiani, G.; Pacifico, A.; Pelloni, F.; Iorizzo, M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: Is the second dose therefore contraindicated? J. Eur. Acad. Dermatol. Venereol. 2021, 35, e645–e647. [Google Scholar] [CrossRef]
- Coto-Segura, P.; Fernandez-Prada, M.; Mir-Bonafe, M.; Garcia-Garcia, B.; Gonzalez-Iglesias, I.; Alonso-Penanes, P.; Gonzalez-Guerrero, M.; Gutierrez-Palacios, A.; Miranda-Martinez, E.; Martinon-Torres, F. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: Report of four cases and review of the literature. Clin. Exp. Dermatol. 2022, 47, 141–143. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kuo, L.T.; Shao, S.H.; Chi, C.C. Ten essential steps for performing a systematic review: A quick tutorial. Dermatol. Sin. 2022, 40, 204–206. [Google Scholar] [CrossRef]
- Shao, S.C.; Kuo, L.T.; Huang, Y.T.; Lai, P.C.; Chi, C.C. Using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to rate the certainty of evidence of study outcomes from systematic reviews: A quick tutorial. Dermatol. Sin. 2023, 41, 3–7. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- National Institutes of Health. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 16 January 2023).
- Khalid, M.; Lipka, O.; Becker, C. Moderna COVID-19 vaccine induced skin rash. Vis. J. Emerg. Med. 2021, 25, 101108. [Google Scholar] [CrossRef]
- Nakamura, K.; Kosano, M.; Sakai, Y.; Saito, N.; Takazawa, Y.; Omodaka, T.; Kiniwa, Y.; Okuyama, R. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J. Dermatol. 2021, 48, e606–e607. [Google Scholar] [CrossRef]
- Perez-Lopez, I.; Moyano-Bueno, D.; Ruiz-Villaverde, R. Bullous pemphigoid and COVID-19 vaccine. Med. Clin. 2021, 157, e333–e334. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Damsky, W.; Fathy, R.; McMahon, D.E.; Turner, N.; Valentin, M.N.; Rallis, T.; Aivaz, O.; Fox, L.P.; Freeman, E.E. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J. Allergy Clin. Immunol. 2021, 148, 750–751. [Google Scholar] [CrossRef]
- Alshammari, F.; Abuzied, Y.; Korairi, A.; Alajlan, M.; Alzomia, M.; AlSheef, M. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) COVID-19 vaccine: A case report. Ann. Med. Surg. 2022, 75, 103420. [Google Scholar] [CrossRef]
- Avallone, G.; Cavallo, F.; Astrua, C.; Caldarola, G.; Conforti, C.; De Simone, C.; di Meo, N.; di Stefani, A.; Genovese, G.; Maronese, C.A.; et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: A real-life multicentre experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e876–e879. [Google Scholar] [CrossRef]
- Bailly-Caille, B.; Jouen, F.; Dompmartin, A.; Morice, C. A case report of anti-P200 pemphigoid following COVID-19 vaccination. JAAD Case Rep. 2022, 23, 83–86. [Google Scholar] [CrossRef]
- Bardazzi, F.; Carpanese, M.A.; Abbenante, D.; Filippi, F.; Sacchelli, L.; Loi, C. New-onset bullous pemphigoid and flare of pre-existing bullous pemphigoid after the third dose of the COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15555. [Google Scholar] [CrossRef]
- Birabaharan, M.; Kaelber, D.C.; Orme, C.M.; Paravar, T.; Karris, M.Y. Evaluating risk of bullous pemphigoid after mRNA COVID-19 vaccination. Br. J. Dermatol. 2022, 187, 271–273. [Google Scholar] [CrossRef]
- Bostan, E.; Yel, B.; Akdogan, N.; Gokoz, O. New-onset bullous pemphigoid after inactivated COVID-19 vaccine: Synergistic effect of the COVID-19 vaccine and vildagliptin. Dermatol. Ther. 2022, 35, e15241. [Google Scholar] [CrossRef]
- Daines, B.; Madigan, L.M.; Vitale, P.A.; Khalighi, M.; Innes, M. A new eruption of bullous pemphigoid following mRNA COVID-19 vaccination. Dermatol. Online J. 2022, 28, 11. [Google Scholar] [CrossRef]
- Darrigade, A.S.; Oules, B.; Sohier, P.; Jullie, M.L.; Moguelet, P.; Barbaud, A.; Soria, A.; Vignier, N.; Lebrun-Vignes, B.; Sanchez-Pena, P.; et al. Sweet-like syndrome and multiple COVID arm syndrome following COVID-19 vaccines: ‘specific’ patterns in a series of 192 patients. Br. J. Dermatol. 2022, 187, 615–617. [Google Scholar] [CrossRef]
- Dell’Antonia, M.; Anedda, S.; Usai, F.; Atzori, L.; Ferreli, C. Bullous pemphigoid triggered by COVID-19 vaccine: Rapid resolution with corticosteroid therapy. Dermatol. Ther. 2022, 35, e15208. [Google Scholar] [CrossRef]
- Desai, A.D.; Shah, R.; Haroon, A.; Wassef, C. Bullous Pemphigoid Following the Moderna mRNA-1273 Vaccine. Cureus 2022, 14, e24126. [Google Scholar] [CrossRef]
- Fu, P.A.; Chen, C.W.; Hsu, Y.T.; Wei, K.C.; Lin, P.C.; Chen, T.Y. A case of acquired hemophilia A and bullous pemphigoid following SARS-CoV-2 mRNA vaccination. J. Formos. Med. Assoc. 2022, 121, 1872–1876. [Google Scholar] [CrossRef]
- Gambichler, T.; Hamdani, N.; Budde, H.; Sieme, M.; Skrygan, M.; Scholl, L.; Dickel, H.; Behle, B.; Ganjuur, N.; Scheel, C.; et al. Bullous pemphigoid after SARS-CoV-2 vaccination: Spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br. J. Dermatol. 2022, 186, 728–731. [Google Scholar] [CrossRef]
- Hali, F., Sr.; Araqi, L., Jr.; Marnissi, F.; Meftah, A.; Chiheb, S. Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Series of Five Cases. Cureus 2022, 14, e23127. [Google Scholar] [CrossRef]
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2022, 49, 34–41. [Google Scholar] [CrossRef]
- Maronese, C.A.; Caproni, M.; Moltrasio, C.; Genovese, G.; Vezzoli, P.; Sena, P.; Previtali, G.; Cozzani, E.; Gasparini, G.; Parodi, A.; et al. Bullous Pemphigoid Associated With COVID-19 Vaccines: An Italian Multicentre Study. Front. Med. 2022, 9, 841506. [Google Scholar] [CrossRef]
- Maronese, C.A.; Di Zenzo, G.; Genovese, G.; Barei, F.; Monestier, A.; Pira, A.; Moltrasio, C.; Marzano, A.V. Reply to “New-onset bullous pemphigoid after inactivated COVID-19 vaccine: Synergistic effect of the COVID-19 vaccine and vildagliptin”. Dermatol. Ther. 2022, 35, e15496. [Google Scholar] [CrossRef]
- Nakahara, Y.; Yamane, M.; Sunada, M.; Aoyama, Y. SARS-CoV-2 vaccine-triggered conversion from systemic lupus erythematosus (SLE) to bullous SLE and dipeptidyl peptidase 4 inhibitors-associated bullous pemphigoid. J. Dermatol. 2022, 50, 162–165. [Google Scholar] [CrossRef]
- Nida, S.S.; Tobon, G.J.; Wilson, M.; Chauhan, K. A patient develops bullous rash after receiving the second dose of COVID-19 vaccine. Cureus 2022, 14, e29786. [Google Scholar] [CrossRef]
- Pauluzzi, M.; Stinco, G.; Errichetti, E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: A report and brief literature review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e257–e259. [Google Scholar] [CrossRef]
- Russo, R.; Gasparini, G.; Cozzani, E.; D’Agostino, F.; Parodi, A. Absolving COVID-19 Vaccination of Autoimmune Bullous Disease Onset. Front. Immunol. 2022, 13, 834316. [Google Scholar] [CrossRef]
- Savoldy, M.A.; Tadicherla, T.; Moureiden, Z.; Ayoubi, N.; Baldwin, B.T. The Successful Treatment of COVID-19-Induced Bullous Pemphigoid With Dupilumab. Cureus 2022, 14, e30541. [Google Scholar] [CrossRef]
- Schmidt, V.; Blum, R.; Mohrenschlager, M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e88–e90. [Google Scholar] [CrossRef]
- Shakoei, S.; Kalantari, Y.; Nasimi, M.; Tootoonchi, N.; Ansari, M.S.; Razavi, Z.; Etesami, I. Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases. Dermatol. Ther. 2022, 35, e15651. [Google Scholar] [CrossRef]
- Shanshal, M. Dyshidrosiform Bullous Pemphigoid Triggered by COVID-19 Vaccination. Cureus 2022, 14, e26383. [Google Scholar] [CrossRef]
- Wan, V.; Chen, D.; Shiau, C.J.; Jung, G.W. Association between COVID-19 vaccination and bullous pemphigoid—A case series and literature review. SAGE Open Med. Case Rep. 2022, 10, 2050313X221131868. [Google Scholar] [CrossRef]
- Young, J.; Mercieca, L.; Ceci, M.; Pisani, D.; Betts, A.; Boffa, M.J. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e13–e16. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, X.; Guo, S.; He, H.; Cui, H. Bullous pemphigoid after inactivated COVID-19 vaccination: Case report. Dermatol. Ther. 2022, 35, e15595. [Google Scholar] [CrossRef]
- Baffa, M.E.; Maglie, R.; Montefusco, F.; Pipito, C.; Senatore, S.; Antiga, E. Severe bullous pemphigoid following COVID-19 vaccination resistant to rituximab and successfully treated with dupilumab. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e135–e137. [Google Scholar] [CrossRef]
- Cowan, T.L.; Huang, C.; Murrell, D.F. Autoimmune blistering skin diseases triggered by COVID-19 vaccinations: An Australian case series. Front. Med. 2023, 9, 1117176. [Google Scholar] [CrossRef]
- Dawoud, N.M.; Aslam, H.; Alshehri, M.A.; Dawoud, M.M. COVID-19 Vaccine-Triggered Bullous Pemphigoid: Two New Cases from Saudi Arabia. Indian J. Dermatol. 2023, 68, 590. [Google Scholar] [PubMed]
- Mulianto, N.; Hashfi, A.F. Bullous pemphigoid associated with COVID-19 vaccine in child: A case report. J. Pak. Assoc. Dermatol. 2023, 33, 730–735. [Google Scholar]
- Sun, L.; Brazão, C.; Mancha, D.; Soares-de-Almeida, L.; Filipe, P. Reply to: ‘Severe bullous pemphigoid following COVID-19 vaccination resistant to rituximab and successfully treated with dupilumab’ by Baffa et al. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e578–e580. [Google Scholar] [CrossRef] [PubMed]
- Oguz Topal, I.; Tokmak, A.; Kurmuş, G.I.; Kalkan, G.; Demirseren, D.D.; Tosun, M.; Emre, S.; Özkök Akbulut, T.; Kaya Özden, H.; Koska, M.; et al. Skin manifestations following anti-COVID-19 vaccination: A multicentricstudy from Turkey. J. Cosmet. Dermatol. 2023, 22, 354–363. [Google Scholar] [CrossRef]
- Üstün, P.; Satılmış, A.; Kılıç, İ.İ.; Adışen, E. COVID-19 Vaccine Induced Bullous Pemphigoid: Case Report and Review of the Literature. J. Turk. Acad. Dermatol. 2023, 17, 27–30. [Google Scholar] [CrossRef]
- Diab, R.; Rakhshan, A.; Salarinejad, S.; Pourani, M.R.; Ansar, P.; Abdollahimajd, F. Clinicopathological characteristics of cutaneous complications following COVID-19 vaccination: A case series. J. Cosmet. Dermatol. 2024, 23, 725–730. [Google Scholar] [CrossRef]
- Yamamoto, S.; Koga, H.; Tsutsumi, M.; Ishii, N.; Nakama, T. Bullous pemphigoid associated with prodromal-phase by repeated COVID-19 vaccinations. J. Dermatol. 2024, 51, e6–e7. [Google Scholar] [CrossRef]
- Mustin, D.E.; Huffaker, T.B.; Feldman, R.J. New-Onset Pemphigoid Gestationis Following COVID-19 Vaccination. Cutis 2023, 111, E2–E4. [Google Scholar] [CrossRef]
- Rungraungrayabkul, D.; Rattanasiriphan, N.; Juengsomjit, R. Mucous Membrane Pemphigoid Following the Administration of COVID-19 Vaccine. Head Neck Pathol. 2023, 17, 587–588. [Google Scholar] [CrossRef]
- Calabria, E.; Antonelli, A.; Lavecchia, A.; Giudice, A. Oral mucous membrane pemphigoid after SARS-CoV-2 vaccination. Oral Diseases 2024, 30, 782–783. [Google Scholar] [CrossRef]
- Hali, F.; Kerouach, A.; Alatawna, H.; Chiheb, S.; Lakhdar, H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2022, 47, 611–613. [Google Scholar] [CrossRef]
- Han, J.; Russo, G.; Stratman, S.; Psomadakis, C.E.; Rigo, R.; Owji, S.; Luu, Y.; Mubasher, A.; Gonzalez, B.R.; Ungar, J.; et al. Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine. JAAD Case Rep. 2022, 24, 101–104. [Google Scholar] [CrossRef]
- Nahm, W.J.; Juarez, M.; Wu, J.; Kim, R.H. Eosinophil-rich linear IgA bullous dermatosis induced by mRNA COVID-19 booster vaccine. J. Cutan. Pathol. 2023, 50, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Mansour, Y.; Didona, D.; Dilling, A.; Ghoreschi, K.; Meier, K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e649–e651. [Google Scholar] [CrossRef] [PubMed]
- Agharbi, F.Z.; Basri, G.; Chiheb, S. Pemphigus vulgaris following second dose of mRNA-(Pfizer-BioNTech) COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15769. [Google Scholar] [CrossRef] [PubMed]
- Aryanian, Z.; Balighi, K.; Azizpour, A.; Kamyab Hesari, K.; Hatami, P. Coexistence of Pemphigus Vulgaris and Lichen Planus following COVID-19 Vaccination. Case Rep. Dermatol. Med. 2022, 2022, 2324212. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Canfora, F.; Mascolo, M.; Varricchio, S.; Mignogna, M.D.; Adamo, D. Autoimmune mucocutaneous blistering diseases after SARS-CoV-2 vaccination: A Case report of Pemphigus Vulgaris and a literature review. Pathol. Res. Pract. 2022, 232, 153834. [Google Scholar] [CrossRef]
- Corra, A.; Barei, F.; Genovese, G.; Zussino, M.; Spigariolo, C.B.; Mariotti, E.B.; Quintarelli, L.; Verdelli, A.; Caproni, M.; Marzano, A.V. Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019. J. Dermatol. 2022, 50, 229–233. [Google Scholar] [CrossRef]
- Das, P.; Arora, S.; Singh, G.K.; Bellad, P.; Rahman, R.; Bahuguna, A.; Sapra, D.; Shrivastav, R.; Gupta, A. A study of COVID-19 vaccine (Covishield) induced dermatological adverse effects from India. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e402–e404. [Google Scholar] [CrossRef] [PubMed]
- Hatami, P.; Balighi, K.; Nicknam Asl, H.; Aryanian, Z. COVID vaccination in patients under treatment with rituximab: A presentation of two cases from Iran and a review of the current knowledge with a specific focus on pemphigus. Dermatol. Ther. 2022, 35, e15216. [Google Scholar] [CrossRef]
- Knechtl, G.V.; Seyed Jafari, S.M.; Berger, T.; Rammlmair, A.; Feldmeyer, L.; Borradori, L. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e251–e253. [Google Scholar] [CrossRef]
- Koutlas, I.G.; Camara, R.; Argyris, P.P.; Davis, M.D.P.; Miller, D.D. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-CoV-2 vaccine. Oral Dis. 2022, 28 (Suppl. S2), 2612–2613. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, Y.; Yoshizaki, A.; Yamada, T.; Akiyama, Y.; Toyama, S.; Sato, S. Pemphigus vulgaris with advanced hypopharyngeal and gastric cancer following SARS-CoV-2 vaccination. J Dermatol 2022, 50, e74–e75. [Google Scholar] [CrossRef]
- Saffarian, Z.; Samii, R.; Ghanadan, A.; Vahidnezhad, H. De novo severe pemphigus vulgaris following SARS-CoV-2 vaccination with BBIBP-CorV. Dermatol. Ther. 2022, 35, e15448. [Google Scholar] [CrossRef]
- Singh, A.; Bharadwaj, S.J.; Chirayath, A.G.; Ganguly, S. Development of severe pemphigus vulgaris following ChAdOx1 nCoV-19 vaccination and review of literature. J. Cosmet. Dermatol. 2022, 21, 2311–2314. [Google Scholar] [CrossRef]
- Thongprasom, K.; Pengpis, N.; Phattarataratip, E.; Samaranayake, L. Oral pemphigus after COVID-19 vaccination. Oral. Dis. 2022, 28 (Suppl. S2), 2597–2598. [Google Scholar] [CrossRef]
- Hui, H.Z.; Wang, Y.J.; Cheng, J.R.; Mao, H.; Guo, H.X.; Diao, Q.C.; Shi, B.J. Rituximab for COVID-19 Vaccine-Associated Pemphigus Vulgaris. Am. J. Ther. 2023, 30, E544–E546. [Google Scholar] [CrossRef]
- Khalayli, N.; Omar, A.; Kudsi, M. Pemphigus vulgaris after the second dose of COVID-19 vaccination: A case report. J. Med. Case Rep. 2023, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Alami, S.; Benzekri, L.; Senouci, K.; Meziane, M. Pemphigus foliaceus triggered after inactivated SARS-CoV-2 vaccine: Coincidence or causal link? Dermatol. Ther. 2022, 35, e15775. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Young, P.A.; So, J.Y.; Pol-Rodriguez, M.; Rieger, K.E.; Lewis, M.A.; Winge, M.C.G.; Bae, G.H. New-onset pemphigus vegetans and pemphigus foliaceus after SARS-CoV-2 vaccination: A report of 2 cases. JAAD Case Rep. 2022, 27, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Lua, A.C.Y.; Ong, F.L.L.; Choo, K.J.L.; Yeo, Y.W.; Oh, C.C. An unusual presentation of pemphigus foliaceus following COVID-19 vaccination. Australas. J. Dermatol. 2022, 63, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Pourani, M.; Bidari-Zerehpoosh, F.; Ayatollahi, A.; Robati, R.M. New onset of pemphigus foliaceus following BBIBP COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15816. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Nogueira, M.; Figueiras, O.; Coelho, A.; Cunha Velho, G.; Raposo, I. Pemphigus foliaceous after mRNA COVID-19 vaccine. Eur. J. Dermatol. 2022, 32, 428–429. [Google Scholar] [CrossRef]
- Rouatbi, J.; Aounallah, A.; Lahouel, M.; Sriha, B.; Belajouza, C.; Denguezli, M. Two cases with new onset of pemphigus foliaceus after SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15827. [Google Scholar] [CrossRef]
- Yildirici, S.; Yayli, S.; Demirkesen, C.; Vural, S. New onset of pemphigus foliaceus following BNT162b2 vaccine. Dermatol. Ther. 2022, 35, e15381. [Google Scholar] [CrossRef]
- Almasi-Nasrabadi, M.; Ayyalaraju, R.S.; Sharma, A.; Elsheikh, S.; Ayob, S. New onset pemphigus foliaceus following AstraZeneca COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.N.; Nguyen, T.T.P.; Vu, T.T.P.; Nguyen, H.T. Pemphigus Foliaceus after COVID-19 Vaccination: A Report of Two Cases. Case Rep. Dermatol. Med. 2023, 2023, 1218388. [Google Scholar] [CrossRef] [PubMed]
- Weschawalit, S.; Pongcharoen, P.; Suthiwartnarueput, W.; Srivilaithon, W.; Daorattanachai, K.; Jongrak, P.; Chakkavittumrong, P. Cutaneous Adverse Events After COVID-19 Vaccination. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, F.; Lamberti, A.; Cota, C.; Rubegni, P.; Cinotti, E. Reply to ‘development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani F et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e976–e978. [Google Scholar] [CrossRef]
- Lansang, R.P.; Amdemichael, E.; Sajic, D. IgA pemphigus following COVID-19 vaccination: A case report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231181022. [Google Scholar] [CrossRef]
- Kianfar, N.; Dasdar, S.; Salehi Farid, A.; Balighi, K.; Mahmoudi, H.; Daneshpazhooh, M. Exacerbation of Autoimmune Bullous Diseases After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: Is There Any Association? Front. Med. 2022, 9, 957169. [Google Scholar] [CrossRef]
- Happaerts, M.; Vanassche, T. Acquired hemophilia following COVID-19 vaccination: Case report and review of literature. Res. Pract. Thromb. Haemost. 2022, 6, e12785. [Google Scholar] [CrossRef]
- Juay, L.; Chandran, N.S. Three cases of vesiculobullous non-IgE-mediated cutaneous reactions to tozinameran (Pfizer-BioNTech COVID-19 vaccine). J. Eur. Acad. Dermatol. Venereol. 2021, 35, e855–e857. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Ruggiero, A.; Battista, T.; Fabbrocini, G.; Megna, M. Bullous pemphigoid and COVID-19 vaccination: Management and treatment reply to ‘Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: A report and brief literature review’ by Pauluzzi et al. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e35–e36. [Google Scholar] [CrossRef]
- Massip, E.; Marcant, P.; Font, G.; Faiz, S.; Duvert-Lehembre, S.; Alcaraz, I.; Vermersch-Langlin, A.; Veron, M.; Macaire, C.; Faure, K.; et al. Cutaneous manifestations following COVID-19 vaccination: A multicentric descriptive cohort. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e253–e255. [Google Scholar] [CrossRef]
- Rasner, C.J.; Schultz, B.; Bohjanen, K.; Pearson, D.R. Autoimmune bullous disorder flares following severe acute respiratory syndrome coronavirus 2 vaccination: A case series. J. Med. Case Rep. 2023, 17, 408. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, S.; Matsuzaki, Y.; Yao, S.; Sagara, C.; Akasaka, E.; Koga, H.; Ishii, N.; Hashimoto, T.; Sawamura, D. Case report: A case of epidermolysis bullosa acquisita with IgG and IgM anti-basement membrane zone antibodies relapsed after COVID-19 mRNA vaccination. Front. Med. 2023, 10, 1093827. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Giordano, S.; Astrua, C.; Merli, M.; Senetta, R.; Conforti, C.; Ribero, S.; Marzano, A.V.; Quaglino, P. Reply to ‘The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: Is the second dose therefore contraindicated?’ by Damiani G et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e433–e435. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Fabbrocini, G.; Nappa, P.; Megna, M. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e750–e751. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.K.; Darji, K.; Chaudhry, S.B. Severe flare of pemphigus vulgaris after first dose of COVID-19 vaccine. JAAD Case Rep. 2022, 22, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Saleh, N.A. Pemphigus vulgaris relapse during the coronavirus disease pandemic. Dermatol. Ther. 2022, 35, e15354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Ma, S.H.; Wang, L.H.; Chang, Y.T.; Wu, C.Y. Pemphigus aggravation following Pfizer-BioNTech vaccination: A case report and review of literature. Int. J. Rheum. Dis. 2023, 26, 1187–1190. [Google Scholar] [CrossRef]
- Ligrone, L.; Lembo, S.; Cillo, F.; Spennato, S.; Fabbrocini, G.; Raimondo, A. A severe relapse of pemphigus vulgaris after SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1369–e1371. [Google Scholar] [CrossRef]
- Al Salmi, A.; Al Khamisani, M.; Al Shibli, A.; Al Maqbali, S. Adverse cutaneous reactions reported post COVID-19 vaccination in Al Buraimi governorate, Sultanate of Oman. Dermatol. Ther. 2022, 35, e15820. [Google Scholar] [CrossRef]
- Ozgen, Z.; Aksoy, H.; Akin Cakici, O.; Koku Aksu, A.E.; Erdem, O.; Kara Polat, A.; Gurel, M.S. COVID-19 severity and SARS-CoV-2 vaccine safety in pemphigus patients. Dermatol. Ther. 2022, 35, e15417. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Strong, R.; Yale, M.; Dunn, P.; Woodley, D.T. Safety of the COVID-19 vaccine booster in patients with immunobullous diseases: A cross-sectional study of the International Pemphigus and Pemphigoid Foundation. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Wu, P.C.; Liu, C.W.; Huang, Y.C. Association between bullous pemphigoid and psychiatric disorders: A systematic review and meta-analysis. J. Dtsch. Dermatol. Ges. 2022, 20, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, K.; Chi, C.C.; Vincent, A.; Groves, R.W.; Venning, V.; Wojnarowska, F. The association of bullous pemphigoid with cerebrovascular disease and dementia: A case-control study. Arch. Dermatol. 2010, 146, 1251–1254. [Google Scholar] [CrossRef]
- Liu, S.D.; Chen, W.T.; Chi, C.C. Association between medication use and bullous pemphigoid: A systematic review and meta-analysis. JAMA Dermatol. 2020, 156, 891–900. [Google Scholar] [CrossRef]
- Murayama, H.; Sakuma, M.; Takahashi, Y.; Morimoto, T. Improving the assessment of adverse drug reactions using the Naranjo Algorithm in daily practice: The Japan Adverse Drug Events Study. Pharmacol. Res. Perspect. 2018, 6, e00373. [Google Scholar] [CrossRef]
- Ujiie, I.; Ujiie, H.; Iwata, H.; Shimizu, H. Clinical and immunological features of pemphigus relapse. Br. J. Dermatol. 2019, 180, 1498–1505. [Google Scholar] [CrossRef]
- Vadala, M.; Poddighe, D.; Laurino, C.; Palmieri, B. Vaccination and autoimmune diseases: Is prevention of adverse health effects on the horizon? EPMA J. 2017, 8, 295–311. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Woodley, D.T. Association between vaccination and autoimmune bullous diseases: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Aashish; Rai, A.; Khatri, G.; Priya; Hasan, M.M. Bullous pemphigoid following COVID-19 vaccine: An autoimmune disorder. Ann. Med. Surg. 2022, 80, 104266. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Gasparini, G.; Russo, R.; Parodi, A. May bullous pemphigoid be worsened by COVID-19 vaccine? Front. Med. 2022, 9, 931872. [Google Scholar] [CrossRef]
- Hertl, M.; Eming, R.; Veldman, C. T cell control in autoimmune bullous skin disorders. J. Clin. Investig. 2006, 116, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sernicola, A.; Mazzetto, R.; Tartaglia, J.; Ciolfi, C.; Miceli, P.; Alaibac, M. Role of Human Leukocyte Antigen Class II in Antibody-Mediated Skin Disorders. Medicina 2023, 59, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Bednarek, M.; Tukaj, S. Case Report: Circulating Anti-SARS-CoV-2 Antibodies Do Not Cross-React With Pemphigus or Pemphigoid Autoantigens. Front. Med. 2021, 8, 807711. [Google Scholar] [CrossRef]
- Kasperkiewicz, M. Association between COVID-19 vaccination and autoimmune bullous diseases: A random coincidence or rare event. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e665–e666. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Woodley, D.T. Association between vaccination and immunobullous disorders: A brief, updated systematic review with focus on COVID-19. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e498–e500. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Fania, L.; Sinagra, J.L.M.; Salemme, A.; Di Zenzo, G. Bullous pemphigoid: Trigger and predisposing factors. Biomolecules 2020, 10, 1432. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chi, C.C. Levels of evidence and study designs: A brief introduction to dermato-epidemiologic research methodology. Dermatol. Sin. 2023, 41, 199–205. [Google Scholar] [CrossRef]
| Author, Year | Country | Age, Sex | Blister Sites | Vaccine (Dose) | Onset | Other Triggers | Pathology | DIF/IIF | ELISA | Treatment | Outcome (Time) | Further Vaccine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP | ||||||||||||
| Khalid 2021 [25] | US | 62 M | 1st: trunk 2nd: trunk, limbs, genitalia | MOD (both) | 1st: 14 d 2nd: 4 d | No new/change in meds, allergic hx | eos | NR | NR | NR | NR | Flare after both doses |
| Nakamura 2021 [26] | Japan | 83 F | Trunk and limbs | BNT (2nd) | 3 d | No DPP4i use | SubE, eos | DIF: IgG (linear) IIF: NR | BP180+ | SC, IVIG | Improved (NR) | NR |
| Pérez-López 2021 [27] | Spain | 78 F | Face, trunk, and limbs | BNT (both) | 1st: 3 d 2nd: NR | NR | NR | NR | NR | TC, SC | Improved (NR) | Flare after both doses |
| Tomayko 2021 [28] | US | 97 F | NR | BNT (2nd) | 2 d | NR | SubE, eos | DIF: C3/IgG/IgA (linear) IIF: NR | BP180+/230+ | TC, DOX, NAM | Improved (2 w) | NR |
| US | 75 M | NR | BNT (2nd) | 10 d | NR | SubE, eos | DIF: C3 (linear) IIF: NR | BP180+ | TC, SC, DOX, NAM | Improved (3 w) | NR | |
| US | 64 M | NR | BNT (2nd) | 14 d | NR | SubE, eos | DIF: C3 (linear) IIF: NR | BP180+/230+ | TC | Improved (4 w) | NR | |
| US | 82 M | NR | BNT (2nd) | 1 d | NR | SubE, eos | DIF: C3/IgG/IgA (linear) IIF: NR | BP180−/230− | TC | Resolved (2 w) | NR | |
| US | 95 F | NR | BNT (1st) | 5 d | NR | SubE, eos | DIF: C3/IgG/IgA (linear) IIF: NR | BP180−/230− | TC, DOX, NAM | Resolved (8 w) | No flare | |
| US | 87 M | NR | MOD (2nd) | 21 d | Alzheimer’s disease | SubE, eos | DIF: C3 (linear) IIF: NR | BP180+/230+ | SC, DOX, NAM | Ongoing (105 d) | NR | |
| US | 42 F | NR | MOD (2nd) | 3 d | NR | SubE, eos | DIF: C3/IgG/IgM (granular) IIF: NR | BP180+/230+ | TC, SC | Ongoing (23 d) | NR | |
| US | 85 M | NR | BNT (1st) | 5 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | SC | Ongoing (59 d) | Not received | |
| US | 83 F | NR | MOD (1st) | 8 d | Major depression | SubE, eos | DIF: Negative IIF: Negative | BP180−/230− | TC, SC | Ongoing (2 m) | Not received | |
| US | 66 F | NR | BNT (both) | 1st: 7 d 2nd: NR | NR | SubE, eos | DIF: Negative IIF: Negative | BP180−/230− | TC, SC | Resolved (3 w) | Flare after both doses | |
| US | 70 F | NR | MOD (1st) | 9 d | NR | SubE, eos | DIF: Negative IIF: NR | NR | SC | Resolved (15 d) | No flare | |
| US | 83 F | NR | BNT (2nd) | 7 d | Dementia | SubE, eos | NR | NR | TC, SC, DOX, NAM | Ongoing (6 w) | NR | |
| Afacan 2022 [14] | Turkey | 88 F | NR | SINV (2nd) | 30 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC, MTX | COVID-19 infection while tx | NR |
| Turkey | 82 F | NR | BNT (3rd) | 14 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC, Dapsone | Improved (NR) | NR | |
| Turkey | 65 M | NR | BNT (3rd) | 14 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | TC, DOX | Improved (NR) | NR | |
| Turkey | 82 F | NR | SINV (2nd) | 14 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC | Improved (NR) | NR | |
| Agharbi 2022 (1) [5] | Morocco | 77 M | Scalp, trunk, and limbs | AZ (1st) | 1 d | No past hx | SubE | DIF: IgG (linear) IIF: IgG (linear) | NR | TC, DOX | Improved (NR) | Not received |
| Alshammari 2022 [29] | Saudi Arabia | 78 M | Limbs | BNT (2nd) | 1 d | NR | Eos | DIF: C3/IgG/IgM (linear) IIF: NR | NR | TC, SC | Died (2 m) | NR |
| Avallone 2022 [30] | Italy | 72 M | Trunk, lower limbs | MOD (3rd) | 20 d | No predisposing factor | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | NR | NR | NR |
| Bailly-Caille 2022 [31] | France | 74 M | Limbs | MOD (both) | 1st: 10 d 2nd: 2 d | No new meds | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180−/230−/COL7−/P200+ | TC, Colchicine | Resolved (6 m) | NR |
| Bardazzi 2022 [32] | Italy | 76 F | Back, right leg | BNT (3rd) | 12 d | NR | NR | NR | BP180+/230+ | TC, SC | Resolved (1 m) | NR |
| Italy | 79 F | Trunk | BNT (3rd) | 9 d | NR | NR | NR | BP180+/230+ | TC, SC, NAM | Resolved (1 m) | NR | |
| Birabaharan 2022 [33] | US | 57 pts | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Bostan 2022 [34] | Turkey | 67 M | Generalized | Inactivated (1st) | 35 d | Under vildagliptin, no past skin hx | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | Stop vildagliptin, SC, OMA | Ongoing (8 m) | Flare after both doses |
| Coto-Segura 2022 [19] | Spain | 86 M | Trunk and limbs | BNT (2nd) | 17 d | NR | SubE, intraE, eos | DIF: Negative IIF: NR | NR | TC, SC | Resolved (NR) | NR |
| Spain | 85 M | Trunk and limbs | BNT (2nd) | 8 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC | Resolved (NR) | NR | |
| Spain | 84 M | Trunk and limbs | BNT (2nd) | 7 d | NR | SubC, eos | DIF: C3/IgG/IgM (linear) IIF: NR | NR | TC, SC | Resolved (NR) | NR | |
| Daines 2022 [35] | US | 70s M | Trunk, limbs, palms | BNT (2nd) | 1 d | No new meds, DPP4i use | SubE, eos | DIF: C3/IgG (linear) IIF: positive | BP180+/230− | TC, SC, CYSP, MTX | Improved (5 m) | NR |
| Darrigade 2022 [36] | France | 4 pts | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Dell’Antonia 2022 [37] | Italy | 83 M | 1st: legs 2nd: trunk and limbs | BNT (both) | 1st: 7 d 2nd: 3 d | No new meds or family hx, DPP4i use | SubE, eos, lym | DIF: C3 (linear) IIF: NR | NR | TC, SC | Resolved (3 w) | Flare after both doses |
| Desai 2022 [38] | US | 73 F | 1st: NR 2nd: face, trunk, limbs | MOD (both) | 1st: 1 d 2nd: 1 d | No allergic hx, recent illness, or family hx, no new meds | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | SC, MMF | Improved (7 d) | Flare after both doses |
| Fu 2022 [39] | Taiwan | 77 M | Trunk and hands | MOD (2nd) | 21 d | NR | SubE, neu | DIF: C3/IgG (linear) IIF: negative | NR | SC, CTX | Improved (5 w) | NR |
| Gambichler 2022 [40] | Germany | 80 M | 1st: lower legs 2nd: trunk | BNT (both) | 1st: 14 d 2nd: NR | No new meds | SubE | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230+ | SC | NR | Flare after both doses |
| Germany | 89 M | Entire integument | BNT (1st) | 2 d | No new meds | SubE | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230+ | SC | NR | NR | |
| Guo 2022 [13] | China | 67 F | Generalized | SINV (1st) | 7 d | No new meds, no family hx | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | BP180+ | TC, SC | Improved (2 w) | Flare after both doses |
| China | 66 F | Generalized | SINV (1st) | 10 d | No past hx, no new meds | SubE, eos, neu | DIF: C3 (linear) IIF: IgG (linear) | BP180+ | TC, SC | Improved (2 w) | NR | |
| Hali (1) 2022 [41] | Morocco | 51 M | Trunk, lower limbs, oral mucosa | AZ (2nd) | 7 d | No past hx, no new meds | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | BP180+ | SC | Resolved (4 w) | NR |
| Morocco | 54 F | Trunk, limbs, oral mucosa | AZ (1st) | 3 d | No past hx, no new meds | SubE, eos | DIF: C3/IgG (linear) IIF: C3/IgG (linear) | NR | TC | Improved (NR) | Not received | |
| Morocco | 68 M | 1st: vaccination site 2nd: trunk, limbs, oral, genital mucosa | AZ (both) | 1st: 14 d 2nd: 7 d | No new meds, no family hx | SubE, eos | DIF: C3 (linear) IIF: NR | NR | SC | Improved (1 m) | NR | |
| Hung 2022 [15] | Taiwan | 39 M | Trunk, hands, and feet | MOD (1st) | 1 m | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | NR | SC, DOX | Resolved (NR) | NR |
| Larson 2021 [42] | US | 76 M | Legs | BNT (both) | 1st: 21 d 2nd: NR | No new meds, DPP4i use | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | NR | TC, SC, DOX, NAM | Improved (NR) | Flare after both doses |
| US | 84 M | Trunk and limbs | MOD (2nd) | 14 d | No new/change in meds, DPP4i use | IntraE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC | Improved (NR) | NR | |
| McMahon 2022 [4] | US | 12 pts | Trunk, limbs, oral/genital mucosa | MOD (n = 4) BNT (n = 8) | NR | NR | SubE, eos | DIF: C3/IgG (linear) (n = 5); DIF: IgG (linear) (n = 1) IIF: NR | BP180+ (n = 1) | NR | NR | NR |
| Maronese 2022 (1) [43] | Italy | 84 F | NR | BNT (1st) | 25 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230− | TC, SC, DOX | Resolved (3 m) | NR |
| Italy | 83 M | NR | BNT (1st) | 32 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230+ | TC, SC, DOX | Resolved (3 m) | NR | |
| Italy | 56 F | NR | MOD (1st) | 7 d | NR | SubE, eos | DIF: negative IIF: IgG (linear) | BP180+/230+ | TC, DOX | Resolved (3 m) | NR | |
| Italy | 79 M | NR | BNT (1st) | 4 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230− | TC, DOX | Resolved (3 m) | NR | |
| Italy | 86 M | NR | BNT (1st) | 37 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230− | TC | Resolved (3 m) | NR | |
| Italy | 91 M | NR | BNT (1st) | 28 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180−/230− | TC, SC | Resolved (3 m) | NR | |
| Italy | 86 M | NR | BNT (1st) | 36 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC, DOX | Resolved (3 m) | NR | |
| Italy | 84 F | NR | MOD (1st) | 7 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/230− | TC, SC, DOX | Resolved (3 m) | NR | |
| Italy | 84 M | NR | BNT (1st) | 23 d | NR | SubE, eos | DIF: C3 (linear) IIF: NR | BP180−/230− | SC | Resolved (3 m) | NR | |
| Italy | 82 F | NR | BNT (1st) | 34 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | BP180−/230− | SC | Improved (3 m) | NR | |
| Italy | 76 M | NR | BNT (1st) | 34 d | NR | SubE, eos | DIF: C3 (linear) IIF: NR | BP180−/230− | SC | NR | NR | |
| Italy | 78 M | NR | BNT (1st) | 4 d | NR | SubE, eos | DIF: NR IIF: IgG (linear) | BP180+/230+ | TC | Resolved (3 m) | NR | |
| Italy | 90 F | NR | BNT (1st) | 28 d | NR | SubE, eos | DIF: IgG (linear) IIF: IgG (linear) | BP180+/230− | TC, SC | Improved (3 m) | NR | |
| Italy | 90 M | NR | BNT (1st) | 64 d | NR | SubE, eos | DIF: C3 (linear) IIF: negative | BP180−/230− | SC | Resolved (3 m) | NR | |
| Italy | 72 M | NR | BNT (1st) | 16 d | NR | SubE, eos | DIF: C3 (linear) IIF: negative | BP180+/230− | TC, SC, MTX | Improved (3 m) | NR | |
| Italy | 80 M | NR | BNT (1st) | 6 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | NR | TC, SC | Improved (3 m) | NR | |
| Italy | 77 F | NR | AZ (1st) | 3 d | NR | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | BP180+/230+ | MTX | Resolved (3 m) | NR | |
| Italy | 60 F | NR | BNT (1st) | 75 d | NR | SubE, eos | DIF: C3 (granular) IIF: IgG (linear) | BP180+/230+ | SC | Resolved (3 m) | NR | |
| Italy | 70 F | NR | BNT (1st) | 27 d | NR | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | BP180−/230− | SC | Improved (3 m) | NR | |
| Italy | 72 F | NR | AZ (1st) | 7 d | NR | SubE, eos | NR | NR | SC, Dapsone | Improved (3 m) | NR | |
| Italy | 85 M | NR | BNT (1st) | 27 d | NR | SubE, eos | NR | NR | SC | Ongoing (3 m) | NR | |
| Maronese 2022 (2) [44] | Italy | 85 M | NR | BNT (2nd) | 28 d | DPP4i use | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | BP180+/230+ | Stop DPP4i, TC, DOX | Improved (1 m) | NR |
| Italy | 84 F | NR | BNT (1st) | 28 d | DPP4i use for years | NR | NR | BP180−/230− | Stop DPP4i, TC, SC, DOX | Improved (1 m) | NR | |
| Italy | 86 M | NR | BNT (2nd) | 14 d | DPP4i use for years | NR | NR | BP180+/230− | Stop DPP4i, TC, SC, DOX | Improved (1 m) | NR | |
| Nakahara 2022 [45] | Japan | 71 M | Neck and arms | BNT (2nd) | 40 d | DPP4i use for years | SubE, lym | DIF: IgG (linear) IIF: IgG (linear) | BP180+/COL7− | Stop DPP4i, TC, SC, HCQ | Resolved (4 w) | NR |
| Nida 2022 [46] | US | 70 M | Trunk and hands | BNT (2nd) | 2 d | New meds of pimavanserin for PD | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC | Improved (NR) | NR |
| Pauluzzi 2022 [47] | Italy | 46 M | Trunk and upper limbs | BNT (1st) | 15 d | No past hx, no new meds | SubE, eos | DIF: C3 (linear) IIF: NR | BP180+ | SC, AZA | Improved (7 w) | Not received |
| Russo 2022 [48] | Italy | 75 M | Cutaneous | BNT (1st) | 2 d | DPP4i use | NR | NR | NR | Stop DPP4i, TC | Improved (NR) | NR |
| Savoldy 2022 [49] | US | 78 M | 1st: back 2nd: trunk, limbs | NR (both) | 1st: 7 d 2nd: NR | No new meds, but polypharmacy | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC, DOX, Dupi | Improved (3 m) | Flare after both doses |
| Schmidt 2022 [50] | Switzerland | 84 F | Both: trunk and limbs | MOD (both) | 1st: days 2nd: NR | No new meds, but polypharmacy | SubE, eos | NR | BP180+/230+ | NR | NR | Flare after both doses |
| Shakoei 2022 [51] | Iran | 85 F | Trunk and limbs | SINP (1st) | 20 d | No allergic, past hx, no new meds | NR | NR | NR | TC, DOX | Improved (NR) | NR |
| Iran | 91 M | Mucocutaneous | SINP (1st) | 19 d | No allergic, past hx, no new meds | NR | NR | NR | TC, RIX | Improved (NR) | NR | |
| Shanshal 2022 [52] | The UK | 90 F | Both: trunk, limbs | BNT (both) | 1st: 7 d 2nd: NR | No past skin hx, no new meds | SubE, eos | DIF: C3 (linear) IIF: IgG (linear) | NR | 1st: TC 2nd: SC | Ongoing (2 m) | Flare after both doses |
| Wan 2022 [53] | Canada | 50 F | 3rd: face, neck, trunk, limbs, oral and genital mucosa | BNT (2nd) MOD (3rd) | 2nd: 14 d 3rd: 1 d | No new meds | SubE, eos, lym | DIF: C3/IgG (linear) IIF: NR | NR | SC, MTX | Improved (16 w) | NR |
| Canada | 82 M | Limbs | BNT (both) | 1st: 10 d 2nd: 3 d | No new meds | SubE, eos, neu, lym | DIF: C3/IgG (linear) IIF: NR | NR | TC | Resolved (2 w) | Flare after both doses, no flare after the 3rd dose of MOD | |
| Young 2022 [54] | Malta | 68 M | Trunk and oral mucosa | BNT (both) | 1st: 3 d 2nd: NR | No past hx | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | SC, TC | Resolved (3 m) | Flare after both doses |
| Zhang 2022 [55] | China | 23 M | Generalized | SINP (3rd) | 1 d | NR | SubE, eos | DIF: C3/IgG (linear) IIF: positive | BP180+/230+ | SC | Improved (7 d) | NR |
| China | 81 M | Limbs and oral mucosa | SINP (3rd) | 15 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | BP180+ | SC, IVIG | Improved (NR) | NR | |
| Baffa 2023 [56] | Italy | 91 F | Trunk, limbs, and oral mucosa | BNT (2nd) | 10 d | No new meds | SubE, eos | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+ | TC, SC, AZA, RIX, Dupi | Resolved (3 m) | NR |
| Cowan 2023 [57] | Australia | 82 M | NR | AZ (2nd) | 31 d | NR | NR | NR | NR | NR | NR | NR |
| Australia | 62 M | NR | BNT (3rd) | 123 d | NR | NR | NR | NR | NR | NR | NR | |
| Australia | 71 M | NR | AZ (2nd) | 26 d | NR | NR | NR | NR | NR | NR | NR | |
| Australia | 60 F | NR | AZ (2nd) | 5 d | NR | NR | NR | NR | NR | NR | NR | |
| Dawoud 2023 [58] | Saudi Arabia | 86 M | Generalized | AZ (1st) | 1 m | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | BP180+/230+ | TC, DOX, SC | Improved (7 w) | NR |
| Saudi Arabia | 76 M | Hands and feet | BNT (1st) | 2 wk | NR | SubE, eos | DIF: C3/IgG (linear) IIF: NR | BP180+/230+ | TC, DOX, SC | Improved (7 w) | NR | |
| Hsieh 2023 [12] | Taiwan | 94 F | Feet, palms, thigh | MOD (1st) | 18 d | No new meds | Lym, eos | DIF: C3 (linear) IIF: negative | BP180+ | TC, SC, KMnO4 | Improved (NR) | NR |
| Mulianto 2023 [59] | Indonesia | 11 M | Generalized | SINV (NR) | 4 d | No allergic history or family hx | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | SC, ERY | Improved (2 m) | NR |
| Sun 2023 [60] | Portugal | 79 F | Trunk, limbs, mucosa | BNT (2nd) | 3 d | No past skin hx, no new meds | SubE, eos, neu | DIF: C3/IgG (linear) IIF: NR | BP180+ | TC, SC, IVIG, DOX, MMF | Improved (2 w) | NR |
| Topal 2023 [61] | Turkey | 6 pts (>50 y, 4 F, 2 M) | NR | BNT (2nd) (n = 1) SINV (1st) (n = 2) SINV (2nd) (n = 3) | NR | NR | NR | NR | NR | NR | NR | NR |
| Üstün 2023 [62] | Turkey | 41 F | Trunk, limbs | BNT (1st) | 2 wk | No hx of infection or drug use | SubE, eos | DIF: C3/IgG (linear) IIF: NR | NR | TC, SC | Resolved (3.5 m) | NR |
| Diab 2024 [63] | Iran | 70 F | NR | SINP (1st) | 20 d | NR | NR | NR | NR | SC | Improved (60 d) | NR |
| Iran | 77 F | NR | SINP (2nd) | 30 d | NR | NR | NR | NR | SC, RIX | Improved (45 d) | NR | |
| Yamamoto 2024 [64] | Japan | 72 M | Thigh | BNT (3rd) | 1 d | NR | SubE, eos | DIF: C3/IgG IIF: NR | BP180+ | SC | Improved (NR) | NR |
| PGes | ||||||||||||
| Mustin 2023 [65] | Georgia | 36 F | Trunk and limbs | BNT (2nd) | 10 d | Pregnancy, no past skin hx | SpD | DIF: C3/IgG (linear) IIF: IgG (linear) | BP180+/BP230− | TC, SC, IVIG | Resolved (7 m) | NR |
| MMP | ||||||||||||
| Darrigade 2022 [36] | France | 1 pt | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Rungraungrayabkul 2023 [66] | Thailand | 74 F | Oral mucosa | BNT (1st) | 3 wk | No past medical hx, no meds | SubE | DIF: C3/IgG (linear) IIF: NR | NR | TC, DOX | Improved (2 w) | Not received |
| Calabria 2024 [67] | Italy | 72 F | Oral mucosa | BNT (3rd) | 9 d | Breast cancer treated with aromatase inhibitor, osteoporosis treated with denosumab | SubE | DIF: IgA/IgG (linear), C3 (granular) IIF: NR | BP180+/BP230− | TC, SC | Resolved (6 w) | NR |
| LABD | ||||||||||||
| Coto-Segura 2022 [19] | Spain | 71 M | Thighs | BNT (2nd) | 3 d | No concomitant meds | SubE, eos | DIF: IgA (linear) | NR | TC | Resolved (NR) | NR |
| Hali (2) 2022 [68] | Morocco | 61 M | Trunk, lower limbs, and oral and genital mucosa | AZ (2nd) | 3 d | No infection, no new meds | SubE, eos, lym | DIF: IgA (linear) IIF: IgA (linear) | Dsg1−/3−/BP180− | SC | Improved (NR) | NR |
| Han 2022 [69] | US | 86 F | Neck, trunk, and limbs | MOD (3rd) | 1 d | New meds of oral terbinafine for tinea pedis | SubE, neu | DIF: IgA (linear) IIF: NR | NR | TC, SC | Resolved (20 d) | NR |
| Nahm 2023 [70] | US | 66 M | Trunk and limbs | MOD (3rd) | 5 d | No new meds | SubE, eos, neu | DIF: IgA/IgM (linear) IIF: IgA | BP180−/230− | TC, SC, Dapsone | Resolved (3 m) | NR |
| PV | ||||||||||||
| Solimani 2021 [71] | Asian | 40 F | Trunk, back, and oral mucosa | BNT (both) | 1st: 5 d 2nd: 3 d | No skin disease hx, no new meds | IntraE, lym, plasma cells | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | SC, AZA | Improved (NR) | Flare after both doses |
| Agharbi 2022 (2) [72] | Morocco | 72 F | Head, neck, trunk, limbs, and oral mucosa | BNT (2nd) | 7 d | No past hx, no new meds | SupraB, lym | DIF: C3/IgG (IC) IIF: positive | Dsg1+/3+ | SC, AZA | Resolved (3 w) | NR |
| Akoglu 2022 [6] | Turkey | 69 F | Mucocutaneous | SINV (2nd) | 7 d | No COVID-19 infection/exposure or meds | SupraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | TC, MTX | Resolved (12 w) | NR |
| Aryanian 2022 [73] | Iran | 43 M | Scalp, face, and oral mucosa | AZ (2nd) | 2 d | No past hx, no new meds | NR | NR | NR | SC, AZA | Improved (NR) | NR |
| Calabria 2022 [74] | Italy | 60 F | Oral mucosa | BNT (2nd) | 7 d | NR | SupraB, lym, eos | DIF: IgG (IC) IIF: NR | Dsg1−/3+ | SC, RIX | Improved (3 w) | NR |
| Corrá 2022 [75] | Italy | 61 F | Face and lower trunk | BNT (3rd) | 3 d | No past skin hx | SupraB | DIF: C3/IgG (IC) IIF: IgG (IC) | Dsg1+/3+ | SC | NR | NR |
| Italy | 73 F | Oral mucosa | BNT (3rd) | 28 d | No new meds | NR | DIF: C3/IgG (IC) IIF: IgG (IC) | Dsg1−/3+ | SC, RIX | NR | NR | |
| Italy | 63 F | Oral mucosa | AZ (both) | 1st: 28 d 2nd: 4 d | No past skin hx | IntraE | DIF: C3/IgG (IC) IIF: IgG (IC) | Dsg1+/3+ | SC, RIX | Improved (8 w) | Flare after both doses | |
| Das 2022 [76] | India | NR | NR | AZ (2nd) | 14 d | NR | NR | NR | NR | NR | NR | NR |
| Hali (1)2022 [41] | Morocco | 58 F | Face, trunk, lower limbs, oral and genital mucosa | BNT (1st) | 1 m | NR | IntraE, lym, eos | DIF: C3/IgG (IC) IIF: NR | NR | SC | Improved (NR) | NR |
| Hatami 2022 [77] | Iran | 34 M | Oral mucosa | AZ (NR) | days | No past hx | NR | NR | NR | SC, AZA | NR | NR |
| Knecht 2022 [78] | Switzerland | 89 M | Trunk, left arm, oral mucosa | BNT (2nd) | 30 d | Worsened post urology procedure under GA, no past hx | SupraB, lym, his | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | SC, RIX | Resolved (10 w) | NR |
| Koutlas 2022 [79] | US | 60 M | Oral mucosa | MOD (2nd) | 7 d | No past hx | SupraB | DIF: C3/IgG (IC) IIF: IgG (IC) | Dsg1−/3− | SC, RIX | Resolved (1 m) | NR |
| Norimatsu 2022 [80] | Japan | 86 M | Face, back, upper limbs | BNT (2nd) | 1 d | No new meds | SupraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | TC, SC | Improved (42 d) | NR |
| Saffarian 2022 [81] | US | 76 F | Scalp, upper trunk, oral and genital mucosa | SINP (2nd) | 30 d | No past skin hx, no new meds, no DPP4i use | SupraB, eos, lym | DIF: C3/IgG (IC) IIF: NR | Dsg1−/3− | SC, RIX | Improved (NR) | NR |
| Shakoei 2022 [51] | Iran | 30 F | Oral mucosa | SINP (1st) | 16 d | No past hx, no new meds | NR | NR | NR | SC, RIX | Improved (NR) | NR |
| Singh 2022 [82] | India | 44 M | Face, neck, trunk, oral mucosa | AZ (2nd) | 7 d | No past hx, no new meds | SupraB | NR | Dsg3+ | SC, AZA, IVIG | Improved (1 m) | NR |
| Thongprasom 2022 [83] | Thailand | 38 F | Oral mucosa | AZ (1st) | 7 d | No allergic hx | NR | NR | NR | TC, steroid mouthwash | Improved (1 w) | NR |
| Cowan 2023 [57] | Australia | 49 F | NR | BNT (3rd) | 92 d | NR | NR | NR | NR | NR | NR | NR |
| Hui 2023 [84] | China | 49 F | 1st: scalp 2nd: whole body, oral mucosa | SINV (both) | 1st: 2 d 2nd: NR | No past hx | IntraE, eos | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | SC, AZA, IVIG, MTX, RTX | Improved (8 w) | NR |
| Khalayli 2023 [85] | Syria | 50 F | Limbs, oral and genital mucosa | mRNA (2nd) | 10 d | No past hx, no family hx | SupraB | DIF: IgG IIF: NR | NR | TC, SC | Improved (3 w) | NR |
| Norimatsu 2023 [80] | Japan | 86 M | Lumbar region, left arm, face | BNT (2nd) | 1 d | Concurrent w/hypopharyngeal and gastric ca | IntraE | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | TC, SC | Improved (42 d) | NR |
| Diab 2024 [63] | Iran | 45 M | Oral mucosa | BIV1 (2nd) | 20 d | NR | NR | NR | NR | SC, RIX | Improved (60 d) | NR |
| PF | ||||||||||||
| Alami 2022 [86] | Morocco | 44 M | Face, trunk and limbs | SINP (both) | 1st: 7 d 2nd: NR | No past hx, no new meds | IntraE | DIF: IgG (IC) IIF: NR | Dsg1+/3−/ICSA+ | SC, AZA | NR | Flare after both doses |
| Corrá 2022 [75] | Italy | 80 M | Face and trunk | BNT (3rd) | 17 d | No past skin hx, no new meds | SubC, neu | DIF: Negative IIF: IgG (IC) | Dsg1+ | SC, RIX, MMF | NR | NR |
| Italy | 66 F | Trunk | BNT (2nd) | 28 d | No past skin hx | SubC, neu | DIF: IgG (IC) IIF: Negative | Negative | SC, MMF | NR | No flare | |
| Gui 2022 [87] | US | 67 F | Trunk | MOD (2nd) | 14 d | No past skin hx | IntraE | DIF: C3/IgG (IC) IIF: positive | Dsg1+/3− | TC, SC | Improved (2 m) | NR |
| Hali (1) 2022 [41] | Morocco | 50 F | Scalp and trunk | BNT (2nd) | 15 d | No past hx, no new meds | SubC, eos | DIF: C3/IgG (IC) IIF: positive | NR | SC | Resolved (3 w) | NR |
| Lua 2022 [88] | Singapore | 83 M | Scalp, face, trunk, and limbs | BNT (2nd) | 2 d | No past skin hx | SpD, eos, plasma cells | DIF: C3 (IC) IIF: IgG (IC) | Dsg1+/3− | SC | Improved (NR) | NR |
| Pourani 2022 [89] | Iran | 75 M | Face and trunk | SINP (3rd) | 14 d | No new meds, no hx of COVID-19 pneumonia | IntraE | DIF: C3/IgG (IC) IIF: NR | NR | TC, RIX | Improved (4 w) | NR |
| Reis 2022 [90] | Caucasian | 35 F | Scalp, upper trunk | BNT (2nd) | 2 w | No past hx | SubC | DIF: C3/IgG (IC) IIF: positive | Dsg1+/3− | TC, SC | Improved (8 m) | NR |
| Rouatbi 2022 [91] | Tunisia | 70 M | Scalp, trunk, and limbs | BNT (3rd) | 7 d | No past skin hx | IntraE | DIF: C3/IgG (IC) IIF: NR | Dsg1+/3− | TC, SC | Improved (3 w) | NR |
| Tunisia | 48 M | 1st: scalp 2nd: face, trunk | AZ (both) | 1st: 5 d 2nd: NR | No past hx, no new meds | IntraE | DIF: C3/IgG (IC) IIF: NR | Dsg1+/3− | TC, SC | Resolved (6 m) | Flare after both doses | |
| Yildirici 2022 [92] | Turkey | 65 M | 1st: scalp, trunk 2nd: neck and trunk | BNT (both) | 1st: 30 d 2nd: 14 d | Valsartan-hydrochlorothiazide started 4 m ago | IntraE, neu | DIF: C3/IgG (IC) IIF: NR | Dsg1+/3− | SC, AZA | Improved (2 w) | Flare after both doses |
| Almasi-Nasrabadi 2023 [93] | The UK | 62 F | Face, trunk, and limbs | AZ (both) | 1st: 7 d 2nd: 2 d | No past hx, no new meds | SubC, neu | DIF: IgG (IC) IIF: NR | NR | SC, MMF | Improved (NR) | Flare after both doses |
| Pham 2023 [94] | Vietnam | 53 F | Face, trunk, limbs | AZ (4th) | 3 w | HTN, no new meds, no family hx | SupraB, lym, neu | DIF: C3/IgG (IC) IIF: NR | NR | SC, RIX | Improved (1 m) | NR |
| Vietnam | 30 F | Face, neck, trunk | MOD (2nd) | 2 m | No family hx | SupraB | DIF: C3/IgG (IC) IIF: NR | NR | TC, SC, TCI | Resolved (4 m) | NR | |
| Weschawalit 2023 [95] | Thailand | NR | NR | AZ (NR) | NR | NR | SubC, neu, eos | DIF: C3/IgG (IC) IIF: NR | NR | NR | NR | NR |
| Diab 2024 [63] | Iran | 30 F | Trunk | SINP (2nd) | 14 d | NR | IntraE | NR | NR | RIX | Improved (30 d) | NR |
| PE | ||||||||||||
| Falcinelli 2022 [96] | Italy | 63 F | Scalp, face, and upper trunk | BNT (2nd) | 2 d | NR | SubC | DIF: IgG (IC) IIF: NR | NR | SC | NR | NR |
| PVeg | ||||||||||||
| Gui 2022 [87] | Asian | 25 M | Face, trunk, limbs, oral and genital mucosa | BNT (2nd) | 30 d | No past hx | SupraB, acan | DIF: C3/IgG (IC) IIF: IgG (IC) | Dsg1+/3+ | TC, ILOBTX, SC, MMF | Resolved (6 m) | NR |
| IgA pemphigus | ||||||||||||
| Lansang 2023 [97] | Canada | 64 M | Back, left leg | MOD (NR) | 20 d | No new meds | SpD, eos, acantholysis | DIF: C3/IgA/IgG (IC) IIF: NR | NR | TC, IMT | Improved (NR) | NR |
| Not specified | ||||||||||||
| Kianfar 2022 [98] | Iran | 5 pts | NR | NR (1st) (n = 3) NR (2nd) (n = 2) | NR | NR | NR | NR | NR | NR | NR | NR |
| Author, Year | Country | Age, Sex | Blister Sites | Vaccine (Dose) | Onset | Other Triggers | Pathology | DIF/IIF | ELISA | Prior tx | Tx after Flare | Outcome (Time) | Further Vaccine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP | |||||||||||||
| Damiani 2021 [18] | Italy | 63 F | Trunk | MOD (1st) | 1 d | NR | NR | NR | NR | SC | SC | NR | No flare |
| Italy | 84 M | Widespread, oral mucosa | MOD (both) | 14 d | NR | NR | NR | NR | SC, AZA | SC | NR | Flare after both doses | |
| Italy | 82 F | Arms, legs | BNT (1st) | 3 d | NR | NR | NR | NR | SC, MMF | SC | NR | No flare | |
| Tomayko 2021 [28] | US | 83 M | NR | BNT (1st) | 7 d | NR | NR | NR | NR | NR | TC, SC | Ongoing (45 d) | Not received |
| Afacan 2022 [14] | Turkey | 74 F | NR | SINV (1st) | 7 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | NR | TC, SC, DOX, MTX | Improved (NR) | NR |
| Turkey | 65 F | NR | SINV (2nd) | 7 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | NR | TC, MTX | Improved (NR) | NR | |
| Turkey | 71 M | NR | SINV (2nd) | 45 d | NR | SubE | DIF: C3/IgG (linear) IIF: NR | NR | NR | TC, SC, AZA | Improved (NR) | NR | |
| Bardazzi 2022 [32] | Italy | 57 F | Trunk, arms | MOD (3rd) | 7 d | NR | NR | NR | BP180+/230+ | NR | TC, SC, NAM | Resolved (1 m) | NR |
| Italy | 62 M | Trunk, arms | BNT (3rd) | 7 d | NR | NR | NR | BP180+/230+ | NR | TC, SC, NAM | Resolved (1 m) | NR | |
| Happaerts 2022 [99] | Caucasian | 75 M | Right arm and left buttock | AZ (1st) | 10 d | Intake of NSAID once, concomitant AHA, history of COVID-19 pneumonia | NR | NR | NR | SC, NAM, DOX | SC, EMI, rFVII, RIX | Died (15 d) | Not received |
| Juay 2022 [100] | Singapore | 70 F | NR | BNT (1st) | 14 d | No new meds, no infection | NR | NR | NR | SC | TC, SC | NR | NR |
| Martora 2022 [101] | Italy | 4 pts (60–80 *, 3M1F) | NR | BNT (2nd) (n = 3) MOD(1st) (n = 1) | 5–8 d * | NR | NR | NR | NR | SC+AZA (n = 2) AZA (n = 2) | SC±AZA | Improved (NR) | No flare |
| Massip 2022 [102] | France | 3 pts | NR | NR | 1.5–3 d * | NR | NR | NR | NR | NR | NR | NR | NR |
| Cowan 2023 [57] | Australia | 82 M | NR | AZ (NR) | 92 d | NR | NR | NR | NR | NR | NR | NR | NR |
| Australia | 83 M | NR | BNT (NR) | 90 d | NR | NR | NR | NR | NR | NR | NR | NR | |
| Australia | 86 F | NR | BNT (NR) | 91 d | NR | NR | NR | NR | NR | NR | NR | NR | |
| Rasner 2023 [103] | USA | 88 M | Trunk, limbs | BNT (2nd) | 1 d | No COVID-19 infection | NR | IIF: IgG | BP180-; BP230+ | TC, SC | SC | Improved (5 w) | NR |
| USA | 69 M | Limbs | MOD (2nd) | 14 d | Erythrodermic psoriasis, COVID-19 infection 4 m before | NR | NR | NR | CsA, ADA | TC, ADA | Resolved (6 w) | NR | |
| EBA | |||||||||||||
| Minakawa 2023 [104] | Japan | 20 F | Face, trunk, upper arms, lip | mRNA (1st) | 2 d | No medical hx | SubE, neu | DIF: C3/IgG/IgM (linear) IIF: IgG/IgM | BP180-/BP230-/type VII collagen- | SC | SC | Improved (1 w) | NR |
| PV | |||||||||||||
| Damiani 2021 [18] | Italy | 40 M | Back and upper limbs | MOD (1st) | 3 d | NR | NR | NR | NR | RIX | SC, MMF | NR | No flare |
| Italy | 80 M | Back | BNT (1st) | 3 d | NR | NR | NR | NR | SC, MMF | SC | NR | No flare | |
| Akoglu 2022 [6] | Turkey | 58 F | Mucocutaneous | SINV (both) | days | No COVID-19 infection/exposure or medical tx | SupraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | Multiple IMMs | SC, IVIG | Resolved (NR) | Flare after both doses |
| Turkey | 31 F | Scalp, genital and oral mucosa | BNT (1st) | 7 d | No COVID-19 infection/exposure or medical tx | SupraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | TC | SC | Resolved (8 w) | NR | |
| Avallone 2022 [105] | Italy | 46 M | Trunk, arms, oral mucosa | BNT (both) | 1st: 5 d 2nd: 5 d | NR | SupraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | SC, AZA | SC, RIX | Ongoing (NR) | Flare after both doses |
| Hatami 2022 [77] | Iran | 61 M | Scalp and trunk | AZ (NR) | 7 d | NR | NR | NR | NR | RIX | SC | NR | NR |
| Martora 2022 (2) [106] | Italy | 7 pts (55–71 *, 4M3F) | NR | BNT (1st) (n = 2) BNT (2nd) (n = 3) MOD (1st) (n = 2) | 5–11 d * | NR | NR | NR | NR | SC (n = 1), AZA(n = 6) | SC | NR | NR |
| Ong 2022 [107] | Asian | 46 F | Scalp, trunk, limbs, and oral mucosa | MOD (1st) | 7 d | NR | NR | NR | Dsg1+/3+ | RIX | SC | Improved (NR) | No flare |
| Saleh 2022 [108] | Egypt | 35 F | NR | SINP (2nd) | 5 d | NR | NR | NR | NR | SC | RIX | Improved (NR) | NR |
| Shakoei 2022 [51] | Iran | 28 F | Mucocutaneous | SINP (1st) | 14 d | No new meds | NR | NR | NR | SC | SC, RIX | Improved (NR) | NR |
| Chen 2023 [109] | Taiwan | 39 M | Trunk, limbs, oral mucosa | BNT (1st) | 7 d | NR | IntraE | DIF: IgG (IC) IIF: NR | NR | TC | SC, RIX, AZA | Improved (NR) | Not received |
| Cowan 2023 [57] | Australia | 32 F | NR | BNT (NR) | 6 d | NR | NR | NR | NR | NR | NR | NR | NR |
| Australia | 73 M | NR | BNT (NR) | 15 d | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ligrone 2023 [110] | Italy | 56 F | Generalized | MOD (3rd) | 5 d | NR | IntraE, supraB | DIF: IgG (IC) IIF: NR | Dsg1+/3+ | SC | SC, RIX | Improved (3 w) | NR |
| PF | |||||||||||||
| Salmi 2022 [111] | Oman | NR | NR | BNT (NR) | 2 d | NR | NR | NR | NR | NR | NR | NR | NR |
| Rasner 2023 [103] | USA | 50 F | NR | BNT (both) | 1st: 1 w | NR | NR | IIF: negative | Dsg1+ | Not received | TC, SC | Improved (10 w) | NR |
| Pemphigus | |||||||||||||
| Massip 2022 [102] | France | 2 pts | NR | NR | 18 d | NR | NR | NR | NR | NR | NR | NR | NR |
| Özgen 2022 [112] | Turkey | 18 pts | NR | SINV (n = 7) BNT (n = 11)/ 1st (n = 15) 2nd (n = 3) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Not specified | |||||||||||||
| Kasperkiewicz 2023 [113] | US | 84 pts | NR | NR (3rd) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kianfar 2022 [98] | Iran | 66 pts | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AIBD Type | Study (n) | Patient (n) | Country | Age * | Sex | Vaccine | Dose | Onset * | Outcome | Time to Improvement/Resolution * | Further Vaccine |
|---|---|---|---|---|---|---|---|---|---|---|---|
| New onset | |||||||||||
| BP | 47 | 174 | Asia 30 (17.24%) Africa 4 (2.30%) America 90 (51.72%) Europe 46 (26.44%) Oceania 4 (2.30%) | 11–97 | M 58 (57.43%) F 43 (42.57%) NR 73 | AZ 10 (8.93%) MOD 19 (16.96%) SINV 10 (8.93%) SINP 6 (5.36%) Inactivated 1 (0.89%) BNT 66 (58.93%) NR 62 | 1st 44 (44.00%) 2nd 32 (32.00%) 3rd 9 (9.00%) Both 15 (15.00%) NR 74 | 1 d–123 d | Died 1 (1.18%) Improved 44 (51.76%) Resolved 31 (36.47%) Ongoing 8 (9.41%) Other 1 (1.18%) NR 89 | 1 w–6 m | No flare (2nd) 2 (9.52%) Flare (both) 14 (66.67%) Not received 5 (23.81%) NR 153 |
| PGes | 1 | 1 | Europe 1 (100.00%) | 36 | F 1 (100.00%) | BNT 1 (100.00%) | 2nd 1 (100.00%) | 10 d | Resolved 1 (100.00%) | 7 m | NR 1 |
| MMP | 3 | 3 | Asia 1 (33.33%) Europe 2 (66.67%) | 72–74 | F 2 (100.00%) NR 1 | BNT 2 (100.00%) NR 1 | 1st 1 (50.00%) 3rd 1 (50.00%) NR 1 | 9 d–3 w | Improved 1 (50.00%) Resolved 1 (50.00%) NR 1 | 2 w–6 w | Not received 1 (100.00%) NR 2 |
| LABD | 4 | 4 | Africa 1 (25.00%) America 2 (50.00%) Europe 1 (25.00%) | 61–86 | M 3 (75.00%) F 1 (25.00%) | AZ 1 (25.00%) MOD 2 (50.00%) BNT 1 (25.00%) | 2nd 2 (50.00%) 3rd 2 (50.00%) | 1 d–5 d | Improved 1 (75.00%) Resolved 3 (25.00%) | 20 d–3 m | NR 4 |
| PV | 21 | 23 | Asia 13 (56.52%) Africa 2 (8.70%) America 2 (8.70%) Europe 5 (21.74%) Oceania 1 (4.35%) | 30–89 | M 8 (36.36%) F 14 (63.64%) NR 1 | AZ 6 (26.09%) MOD 1 (4.35%) SINV 2 (8.70%) SINP 2 (8.70%) BNT 10 (43.48%) BIV1 1 (4.35%) mRNA 1 (4.35%) | 1st 3 (13.64%) 2nd 13 (59.09%) 3rd 3 (13.64%) Both 3 (13.64%) NR 1 | 1 d–92 d | Improved 14 (77.78%) Resolved 4 (22.22%) NR 5 | 1 w–12 w | Flare (both) 2 (100.00%) NR 21 |
| PF | 13 | 16 | Asia 7 (43.75%) Africa 4 (25.00%) America 1 (6.25%) Europe 4 (25.00%) | 30–83 | M 7 (46.67%) F 8 (53.33%) NR 1 | AZ 4 (25.00%) MOD 2 (12.5%) SINP 3 (18.75%) BNT 7 (43.75%) | 2nd 7 (46.67%) 3rd 3 (20.00%) 4th 1 (6.67%) Both 4 (26.67%) NR 1 | 2 d–2 m | Improved 9 (75.00%) Resolved 3 (25.00%) NR 4 | 2 w–8 m | No flare (2nd) 1 (20.00%) Flare (both) 4 (80.00%) NR 11 |
| PE | 1 | 1 | Europe 1 (100%) | 63 | F 1 (100.00%) | BNT 1 (100.00%) | 2nd 1 (100.00%) | 2 d | NR 1 | NR | NR 1 |
| PVeg | 1 | 1 | Asia 1 (100%) | 25 | M 1 (100.00%) | BNT 1 (100.00%) | 2nd 1 (100.00%) | 30 d | Resolved 1 (100.00%) | 6 m | NR 1 |
| IgA pemphigus | 1 | 1 | America 1 (100.00%) | 64 | M 1 (100.00%) | MOD 1 (100.00%) | NR 1 | 20 d | Improved 1 (100.00%) | NR | NR 1 |
| Not specified | 1 | 5 | Asia 5 (100.00%) | NR | NR 5 | NR 5 | 1st 3 (60.00%) 2nd 2 (40.00%) | NR | NR 5 | NR | NR 5 |
| Total | 83 | 229 | Asia 57 (24.89%) Africa 11 (4.80%) America 96 (41.92%) Europe 60 (26.20%) Oceania 5 (2.18%) | 11–97 | M 78 (52.70%) F 70 (47.30%) NR 81 | AZ 21 (13.04%) MOD 25 (15.53%) SINV 12 (7.45%) SINP 11 (6.83%) Inactivated 1 (0.62%) BNT 89 (55.28%) BIV1 1 (0.62%) mRNA 1 (0.62%) NR 68 | 1st 51 (33.77%) 2nd 59 (39.07%) 3rd 18 (11.92%) 4th 1 (0.66%) Both 22 (14.57%) NR 78 | 1 d–123 d | Died 1 (0.81%) Improved 70 (56.45%) Ongoing 8 (6.45%) Other 1 (0.81%) Resolved 44 (35.48%) NR 105 | 1 w–8 m | No flare (2nd) 3 (10.34%) Flare (both) 20 (68.97%) Not received 6 (20.69%) NR 200 |
| Flare | |||||||||||
| BP | 10 | 23 | Asia 4 (17.39%) America 3 (13.04%%) Europe 13 (56.52%) Oceania 3 (13.04%) | 57–88 | M 12 (60.00%) F 8 (40.00%) NR 3 | AZ 2 (10.00%) MOD 5 (25.00%) SINV 3 (15.00%) BNT 10 (50.00%) NR 3 | 1st 7 (41.18%) 2nd 7 (41.18%) 3rd 2 (11.76%) Both 1 (5.88%) NR 6 | 1 d–92 d | Died 1 (7.69%) Improved 8 (61.54%) Ongoing 1 (7.69%) Resolved 3 (23.08%) NR 10 | 1 m–45 d | No flare (2nd) 6 (66.67%) Flare (both) 1 (11.11%) Not received 2 (22.22%) NR 14 |
| EBA | 1 | 1 | Asia 1 (100.00%) | 20 | F 1 (100.00%) | mRNA 1 (100.00%) | 1st 1 (100.00%) | 2 d | Improved 1 (100.00%) | 1 w | NR 1 |
| PV | 12 | 20 | Asia 6 (30.00%) Africa 1 (5.00%) Europe 11 (55.00%) Oceania 2 (10.00%) | 28–80 | M 10 (50.00%) F 10 (50.00%) | AZ 1 (5.00%) MOD 5 (25.00%) SINV 1 (5.00%) SINP 2 (10.00%) BNT 11 (55.00%) | 1st 10 (58.82%) 2nd 4 (23.53%) 3rd 1 (5.88%) Both 2 (11.76%) NR 3 | 3 d–15 d | Improved 5 (62.50%) Ongoing 1 (12.50%) Resolved 2 (25.00%) NR 12 | 3 w–8 w | No flare (2nd) 3 (50.00%) Flare (both) 2 (33.33%) Not received 1 (16.67%) NR 14 |
| PF | 2 | 2 | Asia 1 (50.00%) America 1 (50.00%) | 50 | F 1 (100.00%) NR 1 | BNT 2 (100.00%) | Both 1 (100.00%) NR 1 | 2 d–1 w | Improved 1 (100.00%) NR 1 | 10 w | NR 2 |
| Pemphigus | 2 | 20 | Asia 18 (90.00%) Europe 2 (10.00%) | NR | NR 20 | SINV 7 (38.89%) BNT 11 (61.11%) NR 2 | 1st 15 (83.33%) 2nd 3 (16.67%) NR 2 | 18 d | NR 20 | NR | NR 20 |
| Not specified | 2 | 150 | Asia 66 (44.00%) America 84 (56.00%) | NR | NR 150 | NR 150 | 3rd 84 (100.00%) NR 66 | NR | NR 150 | NR | NR 150 |
| Total | 24 | 216 | Asia 96 (44.44%) Africa 1 (0.46%) America 88 (40.74%) Europe 26 (12.04%) Oceania 5 (2.31%) | 20–88 | M 22 (52.38%) F 20 (47.62%) NR 174 | AZ 3 (4.92%) MOD 10 (16.39%) SINV 11 (18.03%) SINP 2 (3.28%) BNT 34 (55.74%) mRNA 1 (1.64%) NR 155 | 1st 33 (23.91%) 2nd 14 (10.14%) 3rd 87 (63.04%) Both 4 (2.90%) NR 78 | 1 d–92 d | Died 1 (4.35%) Improved 15 (65.22%) Ongoing 2 (8.70%) Resolved 5 (21.74%) NR 193 | 1 w–10 w | No flare (2nd) 9 (60.00%) Flare (both) 3 (20.00%) Not received 3 (20.00%) NR 201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-C.; Huang, I.-H.; Wang, C.-Y.; Chi, C.-C. New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review. Vaccines 2024, 12, 465. https://doi.org/10.3390/vaccines12050465
Wu P-C, Huang I-H, Wang C-Y, Chi C-C. New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review. Vaccines. 2024; 12(5):465. https://doi.org/10.3390/vaccines12050465
Chicago/Turabian StyleWu, Po-Chien, I-Hsin Huang, Ching-Ya Wang, and Ching-Chi Chi. 2024. "New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review" Vaccines 12, no. 5: 465. https://doi.org/10.3390/vaccines12050465
APA StyleWu, P.-C., Huang, I.-H., Wang, C.-Y., & Chi, C.-C. (2024). New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review. Vaccines, 12(5), 465. https://doi.org/10.3390/vaccines12050465






