The Effect of an Attenuated Live Vaccine against Salmonid Rickettsial Septicemia in Atlantic Salmon (Salmo salar) Is Highly Dependent on Water Temperature during Immunization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Trials, Study Sites, and Ethics
2.2. Vaccines and Vaccination
2.3. Challenge Material and Challenge
2.4. Trial 1—Vaccine Efficacy 15 Months after Vaccination
2.5. Trial 2—Vaccine Efficacy at Different Immunization Temperatures
2.6. Trials 3 and 4—Vaccination at Different Temperature Regimes and Sampling of Liver for Assessment of P. salmonis Growth In Vivo
2.7. In Vitro Growth and Growth Curves
2.8. RNA Extraction and Real-Time RT-PCR
2.9. Data Handling and Statistics
3. Results
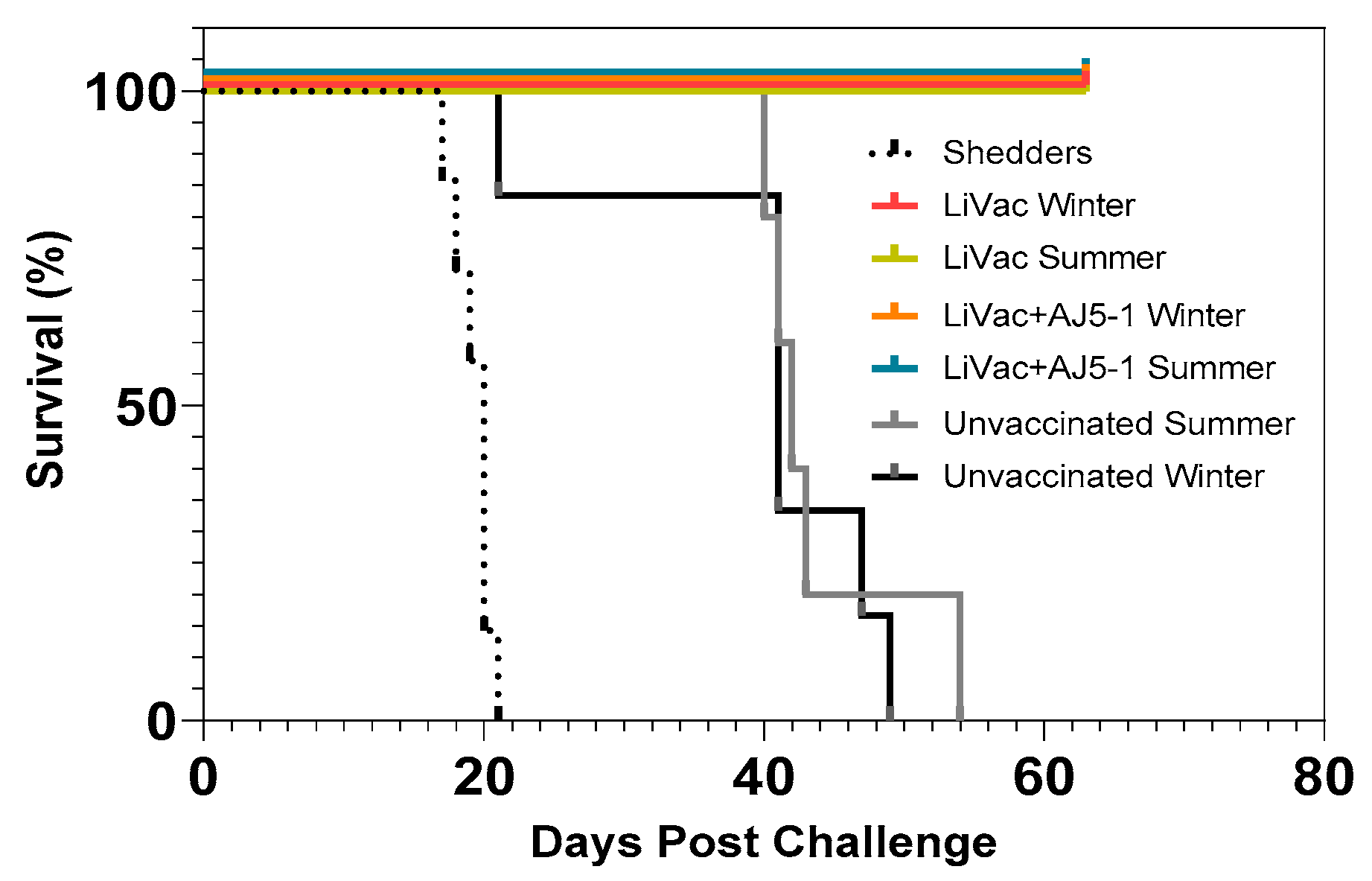
3.1. Vaccination of Atlantic Salmon with AJ LiVac SRS Is Highly Protective against Mortality Caused by Subsequent Challenge with P. salmonis for at Least 15 Months
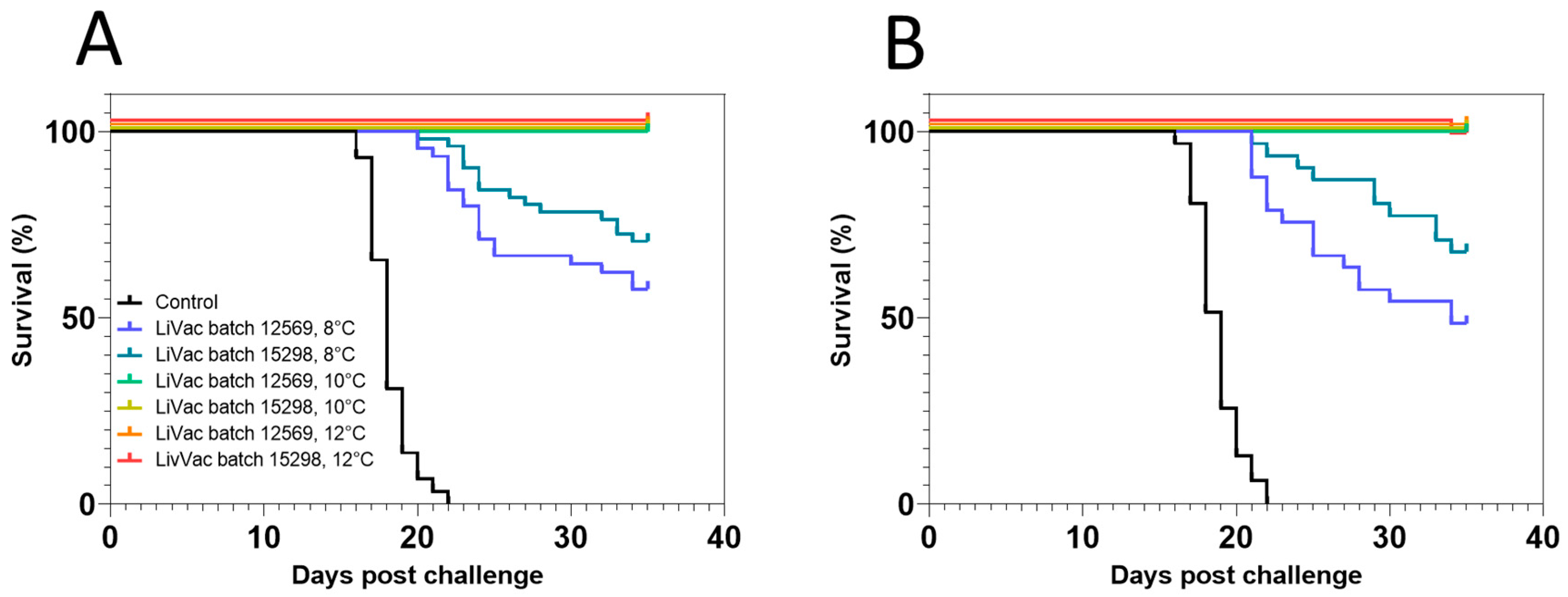
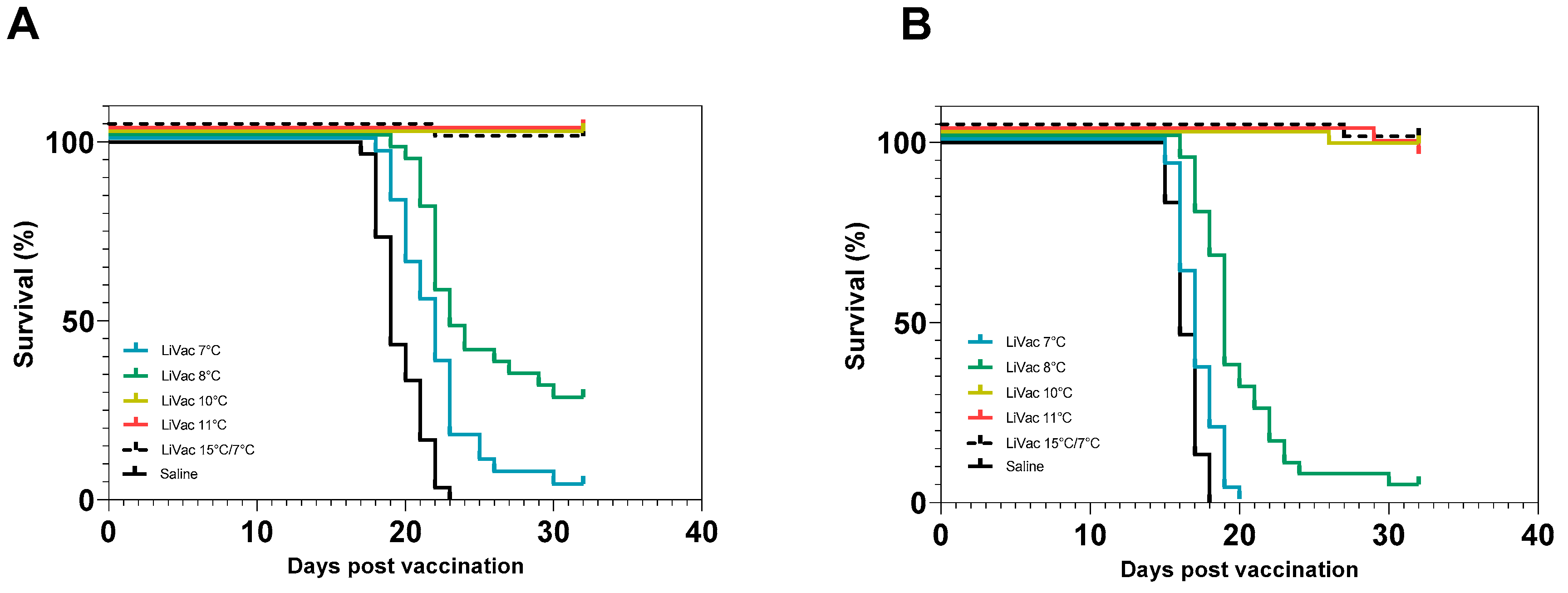
3.2. Vaccine Efficacy Is Dependent on Immunization Temperature
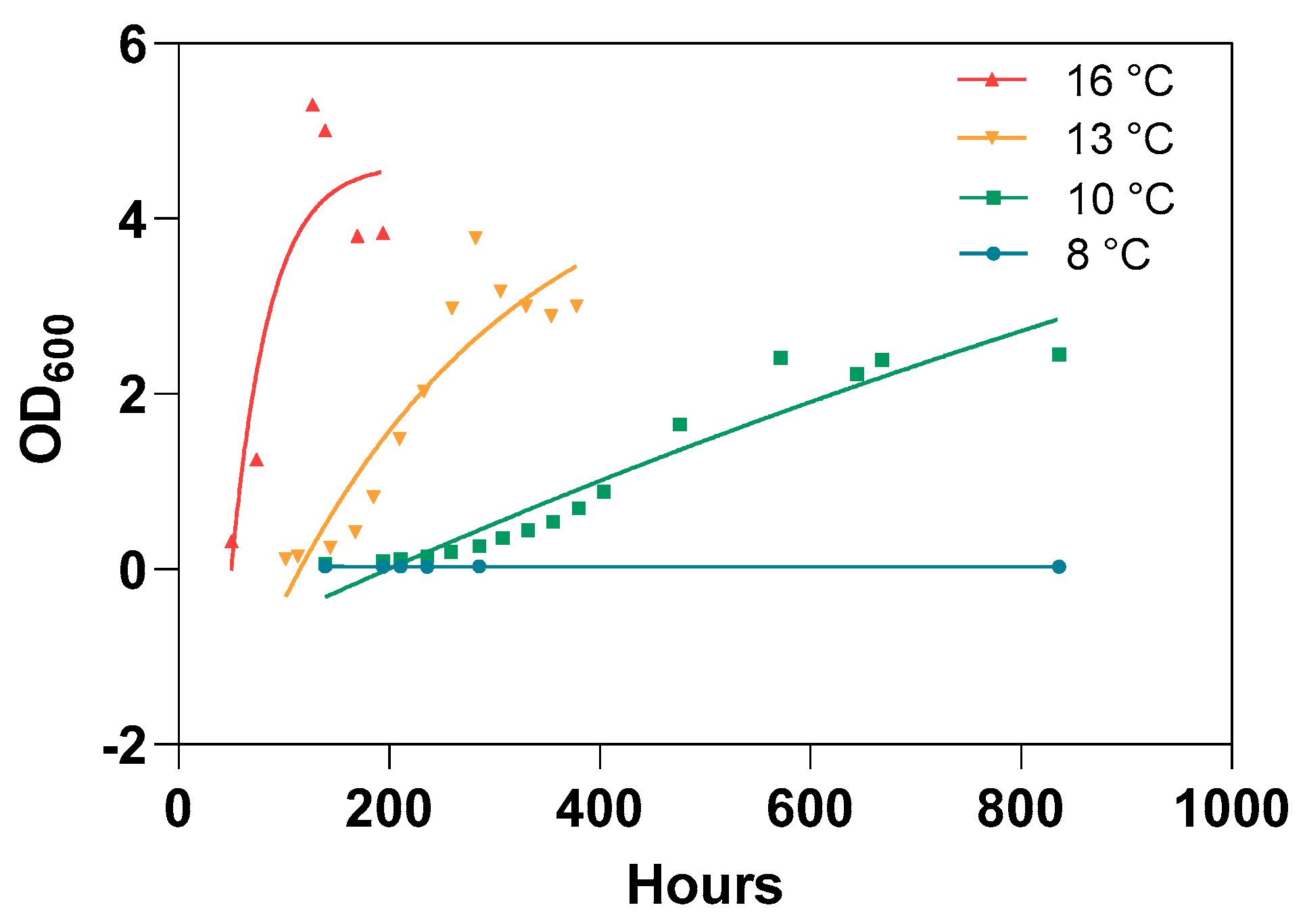
3.3. Lack of In Vitro Bacterial Growth at Low Temperature
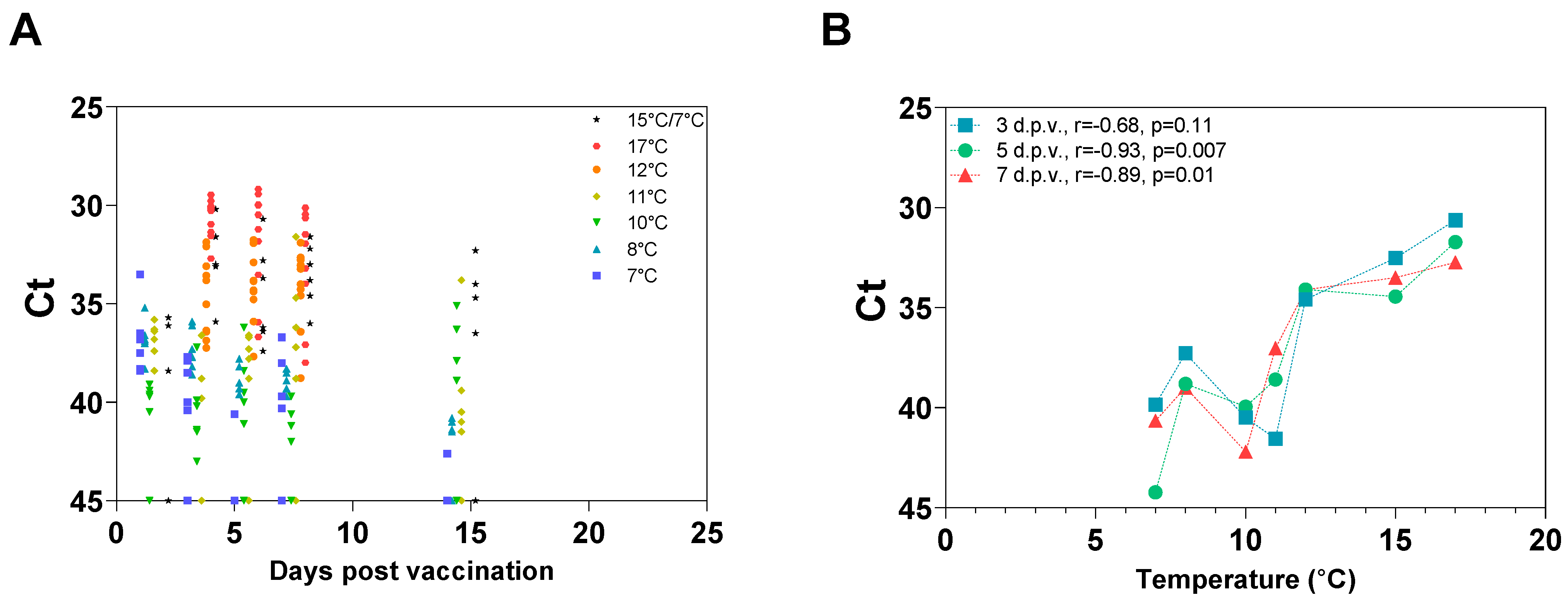
3.4. Temperature-Dependent Loss of Protection Is Associated with Reduced In Vivo Growth the First 5 Days Post-Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sernapesca. Informe Sanitario con Información Sanitaria de Agua Dulce y mar Año 2022; Servicio Nacional de Pesca y Acuicultura: Santiago, Chile, 2023; pp. 1–54. [Google Scholar]
- Bravo, S.; Campos, M. Coho salmon syndrome in Chile. FHS/AFS Newsl. 1989, 17, 3. [Google Scholar]
- Fryer, J.L.; Lannan, C.N.; Garcès, L.H.; Larenas, J.J.; Smith, P.A. Isolation of a rickettsiales-like organism from diseased coho salmon Oncorhynchus kisutch in Chile. Fish. Pathol. 1990, 25, 107–114. [Google Scholar] [CrossRef]
- Garces, L.H.; Larenas, J.J.; Smith, P.A.; Sandino, S.; Lannan, C.N.; Fryer, J.L. Infectivity of a rickettsia isolated from coho salmon Oncorhynchus kisutch. Dis. Aquat. Org. 1991, 11, 93–97. [Google Scholar] [CrossRef]
- Jones, S.R.M. Characterization of Piscirickettsia salmonis and Salmonid Rickettsial Septicaemia to Inform Pathogen Transfer Risk Assessments in British Columbia; Canadian Science Advisory Secretariat (CSAS) Research Document 2019/020; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2019; 21p. [Google Scholar]
- Price, D.; Stryhn, H.; Sánchez, J.; Ibarra, R.; Tello, A.; St-Hilaire, S. Retrospective analysis of antibiotic treatments against piscirickettsiosis in farmed Atlantic salmon Salmo salar in Chile. Dis. Aquat. Organ. 2016, 118, 227–235. [Google Scholar] [CrossRef]
- Bravo, S.; Midtlyng, P. The use of fish vaccines in the Chilean salmon industry 1999–2003. Aquaculture 2007, 270, 36–42. [Google Scholar] [CrossRef]
- Valenzuela-Aviles, P.; Torrealba, D.; Figueroa, C.; Mercado, L.; Dixon, B.; Conejeros, P.; Gallardo-Matus, J. Why vaccines fail against Piscirickettsiosis in farmed salmon and trout and how to avoid it: A review. Front. Immunol. 2022, 13, 1019404. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Torrealba, D.; Morales-Lange, B.; Mercado, L.; Dixon, B.; Conejeros, P.; Silva, G.; Soto, C.; Gallardo, J.A. Commercial Vaccines Do Not Confer Protection against Two Genogroups of Piscirickettsia salmonis, LF-89 and EM-90, in Atlantic Salmon. Biology 2022, 11, 993. [Google Scholar] [CrossRef]
- Soto, E.; Brown, N.; Gardenfors, Z.O.; Yount, S.; Revan, F.; Francis, S.; Kearney, M.T.; Camus, A. Effect of size and temperature at vaccination on immunization and protection conferred by a live attenuated Francisella noatunensis immersion vaccine in red hybrid tilapia. Fish. Shellfish. Immunol. 2014, 41, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, A.; Brevik, Ø.J.; Jensen, D.; Duesund, H.; Sommerset, I.; Frost, P.; Mendoza, J.; McKenzie, P.; Nylund, A.; Apablaza, P. Phenotypic and genetic characterization of Piscirickettsia salmonis from Chilean and Canadian salmonids. BMC Vet. Res. 2016, 12, 55. [Google Scholar] [CrossRef]
- Karlsen, M. Mort-O-matic, v0.2 (0.2.0); Zenodo: Geneva, Switzerland, 2023.
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Jenner, E. An Inquiry into the Causes and Effects of the Variolae Vaccinae, a Disease Discovered in Some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of the Cow Pox; Ashley & Brewer: Springfield, MA, USA, 1798. [Google Scholar]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish. Shellfish. Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Stolen, J.S.; Gahn, T.; Kasper, V.; Nagle, J.J. The effect of environmental temperature on the immune response of a marine teleost (Paralichthys dentatus). Dev. Comp. Immunol. 1984, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Control of Infectious Hematopoietic Necrosis Virus Disease by Elevating the Water Temperature. J. Fish. Res. Board Can. 1970, 27, 265–270. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Xing, J.; Sheng, X.; Chi, H.; Zhan, W. Vaccination with live Hirame novirhabdovirus (HIRRV) at temperature-controlled condition induced protective immunity in flounder (Paralichthys olivaceus). Microb. Pathog. 2021, 157, 104993. [Google Scholar] [CrossRef] [PubMed]
- Meza, K.; Inami, M.; Dalum, A.S.; Lund, H.; Bjelland, A.M.; Sørum, H.; Løvoll, M. Comparative evaluation of experimental challenge by intraperitoneal injection and cohabitation of Atlantic salmon (Salmo salar L) after vaccination against Piscirickettsia salmonis (EM90-like). J. Fish. Dis. 2019, 42, 1713–1730. [Google Scholar] [CrossRef] [PubMed]
- Ramstad, A.; Romstad, A.B.; Knappskog, D.H.; Midtlyng, P.J. Field validation of experimental challenge models for IPN vaccines. J. Fish. Dis. 2007, 30, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.; Tingbø, T.; Solbakk, I.T.; Evensen, Ø.; Furevik, A.; Aas-Eng, A. Efficacy and safety of an inactivated vaccine against Salmonid alphavirus (family Togaviridae). Vaccine 2012, 30, 5688–5694. [Google Scholar] [CrossRef] [PubMed]
- Happold, J.; Sadler, R.; Meyer, A.; Hillman, A.; Cowled, B.; Mackenzie, C.; Gallardo Lagno, A.L.; Cameron, A. Effectiveness of vaccination for the control of salmonid rickettsial septicaemia in commercial salmon and trout farms in Chile. Aquaculture 2020, 520, 734968. [Google Scholar] [CrossRef]
- Figueroa, C.; Veloso, P.; Espin, L.; Dixon, B.; Torrealba, D.; Elalfy, I.S.; Afonso, J.M.; Soto, C.; Conejeros, P.; Gallardo, J.A. Host genetic variation explains reduced protection of commercial vaccines against Piscirickettsia salmonis in Atlantic salmon. Sci. Rep. 2020, 10, 18252. [Google Scholar] [CrossRef]
- Figueroa, C.; Bustos, P.; Torrealba, D.; Dixon, B.; Soto, C.; Conejeros, P.; Gallardo, J.A. Coinfection takes its toll: Sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Sci. Rep. 2017, 7, 17817. [Google Scholar] [CrossRef]
- Saavedra, J.; Hernandez, N.; Osses, A.; Castillo, A.; Cancino, A.; Grothusen, H.; Navas, E.; Henriquez, P.; Bohle, H.; Bustamante, F.; et al. Prevalence, geographic distribution and phenotypic differences of Piscirickettsia salmonis EM-90-like isolates. J. Fish. Dis. 2017, 40, 1055–1063. [Google Scholar] [CrossRef] [PubMed]

| Tank | Vaccine Group | Tag | N | Winter | Challenge By |
|---|---|---|---|---|---|
| 3 | AJ5-1(−3 w)—LiVac | AF | 12 | Yes | Intraperitoneal injection |
| AJ5-1 + LiVac | RM | 19 | Yes | ||
| LiVac | LM | 27 | Yes | ||
| Saline | RM + AF | 12 | Yes | ||
| AJ5-1(−3 w)—LiVac—LiVac (Boost) | LM + AF | 12 | Yes | ||
| Unvaccinated | PIT | 12 | Yes | ||
| 5 | AJ5-1(−3 w)—LiVac | AF | 13 | No | Intraperitoneal injection |
| AJ5-1 + LiVac | RM | 18 | No | ||
| LiVac | LM | 26 | No | ||
| Saline | RM + AF | 12 | No | ||
| AJ5-1(−3 w)—LiVac—LiVac (Boost) | LM + AF | 13 | No | ||
| Unvaccinated | PIT | 15 | No | ||
| L23 | AJ5-1 + LiVac | RM | 5 | No | Cohabitation |
| LiVac | LM | 5 | No | ||
| Unvaccinated | PIT | 5 | No | ||
| AJ5-1 + LiVac | RM + AF | 4 | Yes | ||
| LiVac | LM + AF | 5 | Yes | ||
| Unvaccinated | PIT + AF | 6 | Yes | ||
| Shedders | None | 7 | No | Intraperitoneal injection |
| Group | Vaccine | N | Tag | Tanks | Immunization Temperature | Date of Vaccination |
|---|---|---|---|---|---|---|
| L1-8 | LiVac (12569) | 30 + 30 | RM | C6T1 * + C6T2 * | 8 °C | 13 February 2019 |
| L2-8 | LiVac (15298) | 30 + 30 | LM | C6T1 * + C6T2 * | ||
| Control-8 | 0.9% NaCl | 30 + 30 | AF | C6T1 * + C6T2 * | ||
| L1-10 | LiVac (12569) | 30 + 30 | RM + AF | C3T1 + C3T2 | 10 °C | 25 February 2019 |
| L2-10 | LiVac (15298) | 30 + 30 | LM + AF | C3T1 + C3T2 | ||
| L1-12 | LiVac (12569) | 30 + 30 | VIE Red | C3T3 + C3T4 | 12 °C | 4 March 2019 |
| L2-12 | LiVac (15298) | 30 + 30 | VIE Green | C3T3 + C3T4 |
| Trial | Vaccine Group | Immunization Temperature | Tag | N for Challenge | N for Liver Sampling | Sampling Days p.v. | Date for Vaccination |
|---|---|---|---|---|---|---|---|
| Trail 3 | LiVac | 7 °C | RM | 60 | 30 | 1, 3, 5, 7,1 4 | 3 March 2016 |
| LiVac | 8 °C | AF | 60 | 30 | 1, 3, 5, 7,1 4 | 29 March 2016 | |
| LiVac * | 10 °C | RM + AF | 60 | 30 | 1, 3, 5, 7,1 4 | 8 April 2016 | |
| LiVac | 11 °C | VIE Blue + RM | 60 | 30 | 1, 3, 5, 7,1 4 | 15 April 2016 | |
| LiVac | 15 °C /7 °C | VIE Red + RM | 60 | 30 | 1, 3, 5, 7,1 4 | 31 March 2016 | |
| 0.9% NaCl * | 10 °C | VIE Green | 60 | 0 | - | 8 April 2016 | |
| Trail 4 | LiVac | 12 °C | - | 0 | 30 | 3, 5, 7 | 11 March 2016 |
| LiVac | 17 °C | - | 0 | 30 | 3, 5, 7 | 11 March 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsen, R.H.; Finne-Fridell, F.; Bordevik, M.; Nygaard, A.; Rajan, B.; Karlsen, M. The Effect of an Attenuated Live Vaccine against Salmonid Rickettsial Septicemia in Atlantic Salmon (Salmo salar) Is Highly Dependent on Water Temperature during Immunization. Vaccines 2024, 12, 416. https://doi.org/10.3390/vaccines12040416
Olsen RH, Finne-Fridell F, Bordevik M, Nygaard A, Rajan B, Karlsen M. The Effect of an Attenuated Live Vaccine against Salmonid Rickettsial Septicemia in Atlantic Salmon (Salmo salar) Is Highly Dependent on Water Temperature during Immunization. Vaccines. 2024; 12(4):416. https://doi.org/10.3390/vaccines12040416
Chicago/Turabian StyleOlsen, Rolf Hetlelid, Frode Finne-Fridell, Marianne Bordevik, Anja Nygaard, Binoy Rajan, and Marius Karlsen. 2024. "The Effect of an Attenuated Live Vaccine against Salmonid Rickettsial Septicemia in Atlantic Salmon (Salmo salar) Is Highly Dependent on Water Temperature during Immunization" Vaccines 12, no. 4: 416. https://doi.org/10.3390/vaccines12040416
APA StyleOlsen, R. H., Finne-Fridell, F., Bordevik, M., Nygaard, A., Rajan, B., & Karlsen, M. (2024). The Effect of an Attenuated Live Vaccine against Salmonid Rickettsial Septicemia in Atlantic Salmon (Salmo salar) Is Highly Dependent on Water Temperature during Immunization. Vaccines, 12(4), 416. https://doi.org/10.3390/vaccines12040416




