Sex-Dependent Effects on Influenza-Specific Antibody Quantity and Neutralizing Activity following Vaccination of Newborn Non-Human Primates Is Determined by Adjuvants
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Approval
2.3. Influenza A/PR/8/34 (H1N1)
2.4. Vaccination and Sampling
2.5. ELISA for the Detection of Influenza Virus-Specific Antibody
2.6. Neutralization Assay
2.7. Statistical Analysis
3. Results
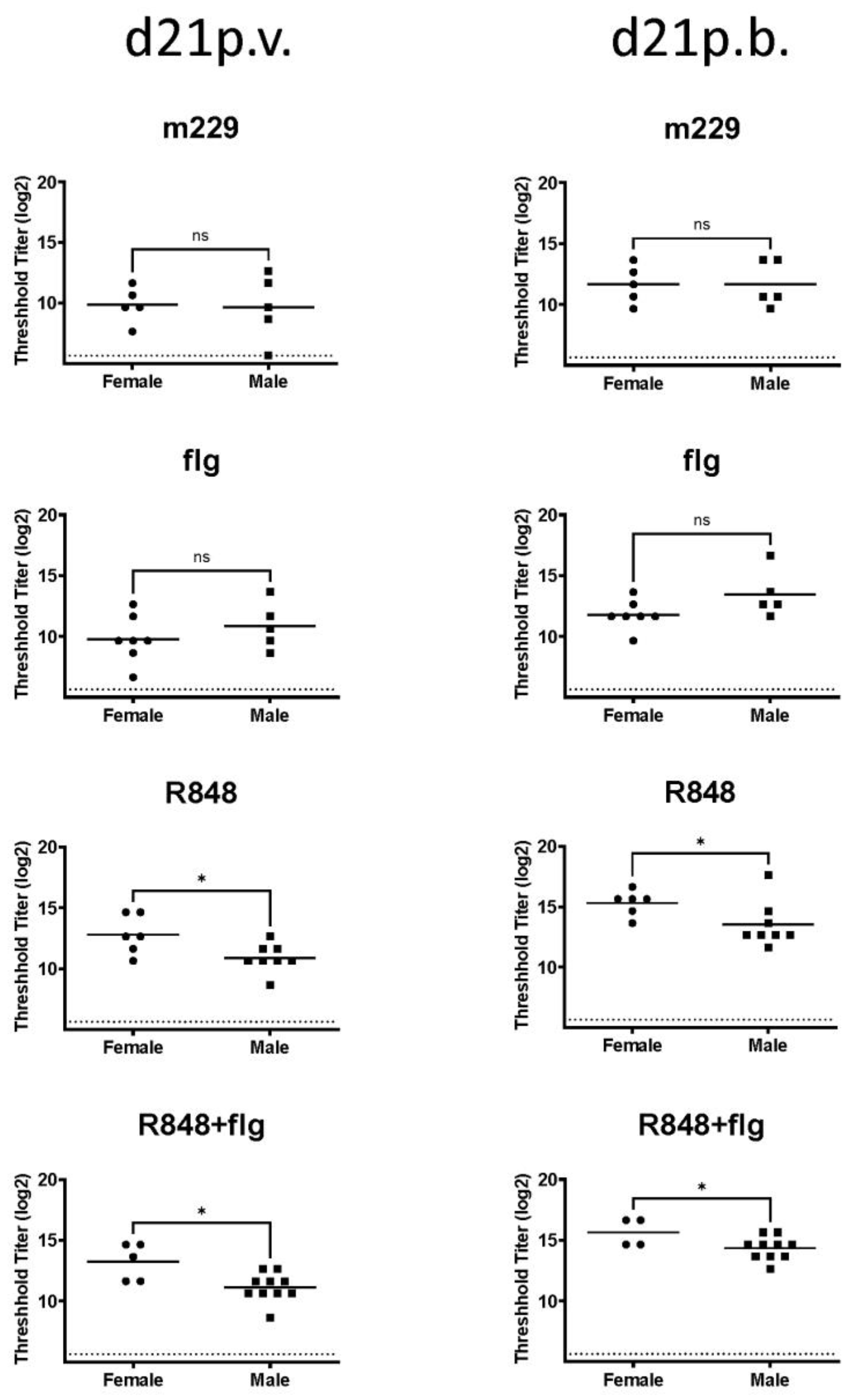
3.1. Sex-Dependent Differences Following the Influenza Vaccination of Newborn NHPs Were Apparent with a TLR7/8 Agonist but Not with a TLR5 Agonist or in the Absence of Adjuvants
3.2. The Sex-Dependent Differences in Anti-Influenza Virus IgG Were Not Observed in the HA Stem-Specific Response
3.3. The Combination of R848 and Flagellin Promotes a Higher Neutralizing Antibody in Females Compared to Male Newborn NHPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 2024. [Google Scholar] [CrossRef]
- Ghosh, S.; Klein, R.S. Sex drives dimorphic immune responses to viral infections. J. Immunol. 2017, 198, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Addo, M.M.; Dahlke, C. Sex differences in immunity: Implications for the development of novel vaccines against emerging pathogens. Front. Immunol. 2020, 11, 601170. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.F.; Barr, I.; Hartel, G.; Pond, D.; Hampson, A.W. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 2006, 24, 2395–2402. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex hormone signaling and regulation of immune function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef]
- Kuiri-Hanninen, T.; Haanpaa, M.; Turpeinen, U.; Hamalainen, E.; Seuri, R.; Tyrvainen, E.; Sankilampi, U.; Dunkel, L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.; Faiman, C.; Hobson, W.C.; Prasad, A.V.; Reyes, F.I. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J. Clin. Endocrinol. Metab. 1975, 40, 545–551. [Google Scholar] [CrossRef]
- Bouvattier, C.; Carel, J.C.; Lecointre, C.; David, A.; Sultan, C.; Bertrand, A.M.; Morel, Y.; Chaussain, J.L. Postnatal changes of T, LH, and FSH in 46,XY infants with mutations in the AR gene. J. Clin. Endocrinol. Metab. 2002, 87, 29–32. [Google Scholar] [CrossRef]
- Terasawa, E.; Garcia, J.P. Neuroendocrine mechanisms of puberty in non–human primates. Curr. Opin. Endocr. Metab. Res. 2020, 14, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmaier, F.; Wagner-Barnack, M.; Butenandt, O.; Knorr, D. Plasma estrogens in childhood and puberty under physiologic and pathologic conditions. Pediatr. Res. 1973, 7, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.; Hughes, I.A.; Reyes, F.I.; Faiman, C. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J. Clin. Endocrinol. Metab. 1976, 42, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.M.; Chellakooty, M.; Haavisto, A.M.; Boisen, K.A.; Damgaard, I.N.; Steendahl, U.; Toppari, J.; Skakkebaek, N.E.; Main, K.M. Gender difference in breast tissue size in infancy: Correlation with serum estradiol. Pediatr. Res. 2002, 52, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Peckham, H.; Radziszewska, A.; Menon, M.; Oliveri, P.; Simpson, F.; Deakin, C.T.; Lee, S.; Ciurtin, C.; Butler, G.; et al. Sex and pubertal differences in the Type 1 interferon pathwayassociate with both X chromosome number and serum sex hormone concentration. Front. Immunol. 2018, 9, 3167. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.G.; Nencioni, L.; Clemente, A.M.; Civitelli, L.; Celestino, I.; Limongi, D.; Fadigati, G.; Perissi, E.; Cozzolino, F.; Garaci, E.; et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS ONE 2012, 7, e39853. [Google Scholar] [CrossRef] [PubMed]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejia, J.E.; Guery, J.C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef] [PubMed]
- Youness, A.; Cenac, C.; Faz-Lopez, B.; Grunenwald, S.; Barrat, F.J.; Chaumeil, J.; Mejia, J.E.; Guery, J.C. TLR8 escapes X chromosome inactivation in human monocytes and CD4+ T cells. Biol. Sex Differ. 2023, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Maxfield Boumil, R.; Lee, J.T. Forty years of decoding the silence in X-chromosome inactivation. Hum. Mol. Genet. 2001, 10, 2225–2232. [Google Scholar] [CrossRef]
- Chassin, C.; Kocur, M.; Pott, J.; Duerr, C.U.; Gutle, D.; Lotz, M.; Hornef, M.W. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 2010, 8, 358–368. [Google Scholar] [CrossRef]
- Holbrook, B.C.; D’Agostino, R.B., Jr.; Parks, G.D.; Alexander-Miller, M.A. Adjuvanting an inactivated influenza vaccine with flagellin improves the function and quantity of the long-term antibody response in a nonhuman primate neonate model. Vaccine 2016, 34, 4712–4717. [Google Scholar] [CrossRef]
- Holbrook, B.C.; D’Agostino, R.B., Jr.; Tyler Aycock, S.; Jorgensen, M.J.; Hadimani, M.B.; Bruce King, S.; Alexander-Miller, M.A. Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6 months in a nonhuman primate neonate model. Vaccine 2017, 35, 6137–6142. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, B.C.; Kim, J.R.; Blevins, L.K.; Jorgensen, M.J.; Kock, N.D.; D’Agostino, R.B., Jr.; Aycock, S.T.; Hadimani, M.B.; King, S.B.; Parks, G.D.; et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J. Immunol. 2016, 197, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Holbrook, B.C.; Hayward, S.L.; Blevins, L.K.; Jorgensen, M.J.; Kock, N.D.; De Paris, K.; D’Agostino, R.B., Jr.; Aycock, S.T.; Mizel, S.B.; et al. Inclusion of flagellin during vaccination against influenza enhances recall responses in nonhuman primate neonates. J. Virol. 2015, 89, 7291–7303. [Google Scholar] [CrossRef] [PubMed]
- Clemens, E.A.; Alexander-Miller, M.A. Understanding antibody responses in early life: Baby steps towards developing an effective influenza vaccine. Viruses 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Clemens, E.A.; Holbrook, B.C.; McNeilly, B.; Kanekiyo, M.; Graham, B.S.; Alexander-Miller, M.A. TLR agonists induce sustained IgG to hemagglutinin stem and modulate T cells following newborn vaccination. NPJ Vaccines 2022, 7, 102. [Google Scholar] [CrossRef]
- McDermott, P.F.; Ciacci-Woolwine, F.; Snipes, J.A.; Mizel, S.B. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 2000, 68, 5525–5529. [Google Scholar] [CrossRef]
- Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Ruckwardt, T.J.; Crank, M.C.; Smatti, M.K.; Ledgerwood, J.E.; Graham, B.S. Use of hemagglutinin stem probes demonstrate prevalence of broadly reactive Group 1 influenza antibodies in human sera. Sci. Rep. 2018, 8, 8628. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, B.; Manicassamy, S.; Belicha-Villanueva, A.; Pisanelli, G.; Pulendran, B.; Garcia-Sastre, A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl. Acad. Sci. USA 2010, 107, 11531–11536. [Google Scholar] [CrossRef]
- Honko, A.N.; Sriranganathan, N.; Lees, C.J.; Mizel, S.B. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 2006, 74, 1113–1120. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, H.; Liu, G.D.; Xue, C.; Cao, Y. Targeting hemagglutinin: Approaches for broad protection against the influenza A virus. Viruses 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Garcia-Sastre, A.; Palese, P. Is it possible to develop a “universal” influenza virus vaccine? Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harb. Perspect. Biol. 2018, 10, a028845. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immunohorizons 2018, 2, 185–197. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-like Receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Jarrossay, D.; Napolitani, G.; Colonna, M.; Sallusto, F.; Lanzavecchia, A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 2001, 31, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Mansson, A.; Adner, M.; Cardell, L.O. Toll-like receptors in cellular subsets of human tonsil T cells: Altered expression during recurrent tonsillitis. Respir. Res. 2006, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P. Regulation of B-cell responses by Toll-like receptors. Immunology 2012, 136, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Guo, Z.; Kiniwa, Y.; Voo, K.S.; Peng, W.; Fu, T.; Wang, D.Y.; Li, Y.; Wang, H.Y.; Wang, R.F. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 2005, 309, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Crellin, N.K.; Garcia, R.V.; Hadisfar, O.; Allan, S.E.; Steiner, T.S.; Levings, M.K. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 2005, 175, 8051–8059. [Google Scholar] [CrossRef]
- Rock, F.L.; Hardiman, G.; Timans, J.C.; Kastelein, R.A.; Bazan, J.F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 1998, 95, 588–593. [Google Scholar] [CrossRef]
- Hagen, S.H.; Henseling, F.; Hennesen, J.; Savel, H.; Delahaye, S.; Richert, L.; Ziegler, S.M.; Altfeld, M. Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell Rep. 2020, 33, 108485. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Caramalho, I.; Demengeot, J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 2002, 14, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, B.C.; Aycock, S.T.; Machiele, E.; Clemens, E.; Gries, D.; Jorgensen, M.J.; Hadimani, M.B.; King, S.B.; Alexander-Miller, M.A. An R848 adjuvanted influenza vaccine promotes early activation of B cells in the draining lymph nodes of non-human primate neonates. Immunology 2018, 153, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Gazzinelli, R.T. Regulation of innate immune signaling by IRAK proteins. Front. Immunol. 2023, 14, 1133354. [Google Scholar] [CrossRef]
- Zivkovic, I.; Petrovic, R.; Arsenovic-Ranin, N.; Petrusic, V.; Minic, R.; Bufan, B.; Popovic, O.; Leposavic, G. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals 2018, 52, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, R.; Bufan, B.; Arsenovic-Ranin, N.; Zivkovic, I.; Minic, R.; Radojevic, K.; Leposavic, G. Mouse strain and sex as determinants of immune response to trivalent influenza vaccine. Life Sci. 2018, 207, 117–126. [Google Scholar] [CrossRef]
- Bates, J.T.; Honko, A.N.; Graff, A.H.; Kock, N.D.; Mizel, S.B. Mucosal adjuvant activity of flagellin in aged mice. Mech. Ageing Dev. 2008, 129, 271–281. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holbrook, B.C.; Clemens, E.A.; Alexander-Miller, M.A. Sex-Dependent Effects on Influenza-Specific Antibody Quantity and Neutralizing Activity following Vaccination of Newborn Non-Human Primates Is Determined by Adjuvants. Vaccines 2024, 12, 415. https://doi.org/10.3390/vaccines12040415
Holbrook BC, Clemens EA, Alexander-Miller MA. Sex-Dependent Effects on Influenza-Specific Antibody Quantity and Neutralizing Activity following Vaccination of Newborn Non-Human Primates Is Determined by Adjuvants. Vaccines. 2024; 12(4):415. https://doi.org/10.3390/vaccines12040415
Chicago/Turabian StyleHolbrook, Beth C., Elene A. Clemens, and Martha A. Alexander-Miller. 2024. "Sex-Dependent Effects on Influenza-Specific Antibody Quantity and Neutralizing Activity following Vaccination of Newborn Non-Human Primates Is Determined by Adjuvants" Vaccines 12, no. 4: 415. https://doi.org/10.3390/vaccines12040415
APA StyleHolbrook, B. C., Clemens, E. A., & Alexander-Miller, M. A. (2024). Sex-Dependent Effects on Influenza-Specific Antibody Quantity and Neutralizing Activity following Vaccination of Newborn Non-Human Primates Is Determined by Adjuvants. Vaccines, 12(4), 415. https://doi.org/10.3390/vaccines12040415





