A Streptococcus suis Strain Δcps/ssna-msly (P353L)-SC19 Provides Cross-Protection against Serotypes 2 and 9 Strain Challenges in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Ethics Statement
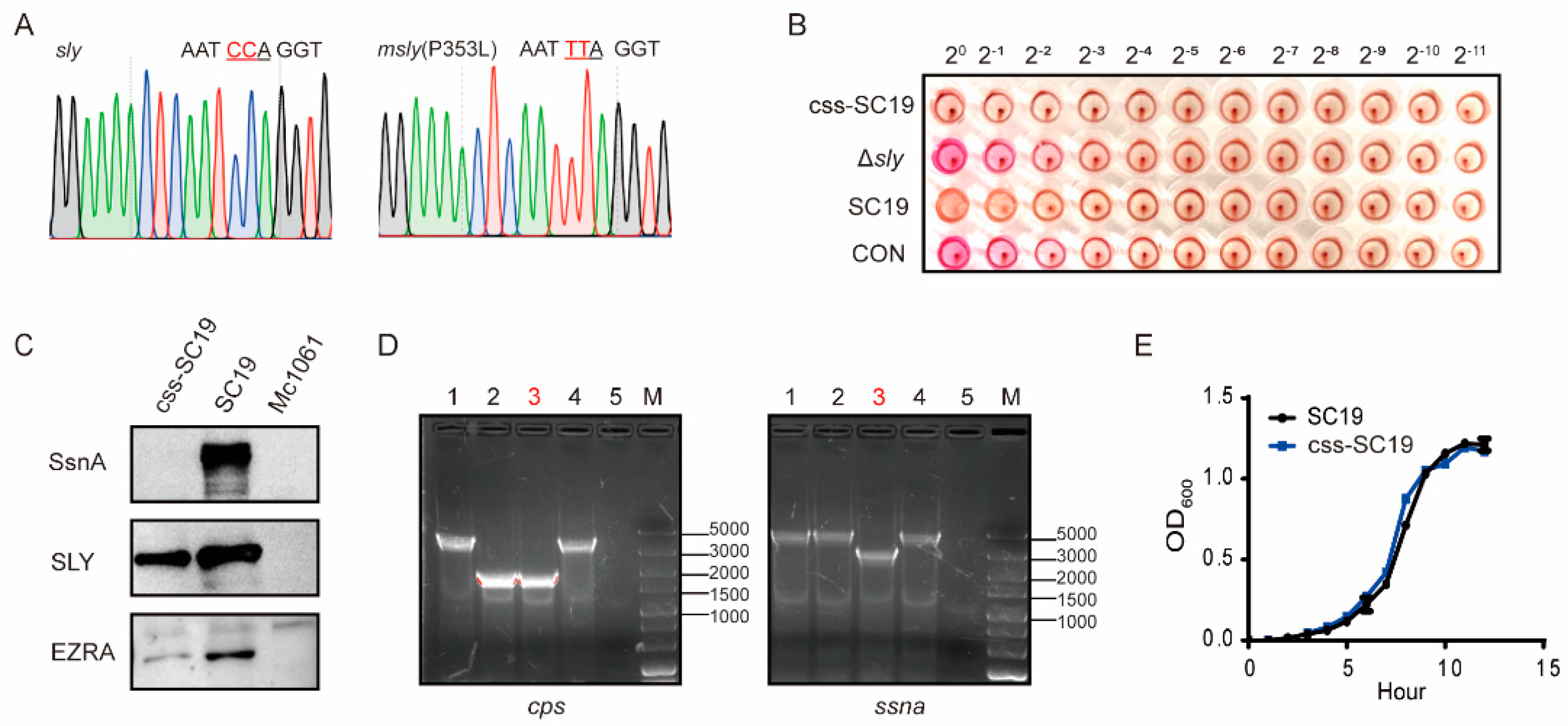
2.3. Construction of Δcps/ssna-msly (P353L)-SC19
2.4. In Vitro Virulence Test
2.5. Immunization and Experimental Infection of Mice
2.6. Cytokine Detection and Lymphocyte Proliferation In Vitro
2.7. Opsonic Killing Assay
2.8. Quantification of Antibody Responses
2.9. Evaluation of Blood–Brain Barrier Integrity
2.10. Statistical Analysis
3. Results
3.1. Construction of Δcps/ssna-msly (P353L)-SC19 and Its Biological Properties
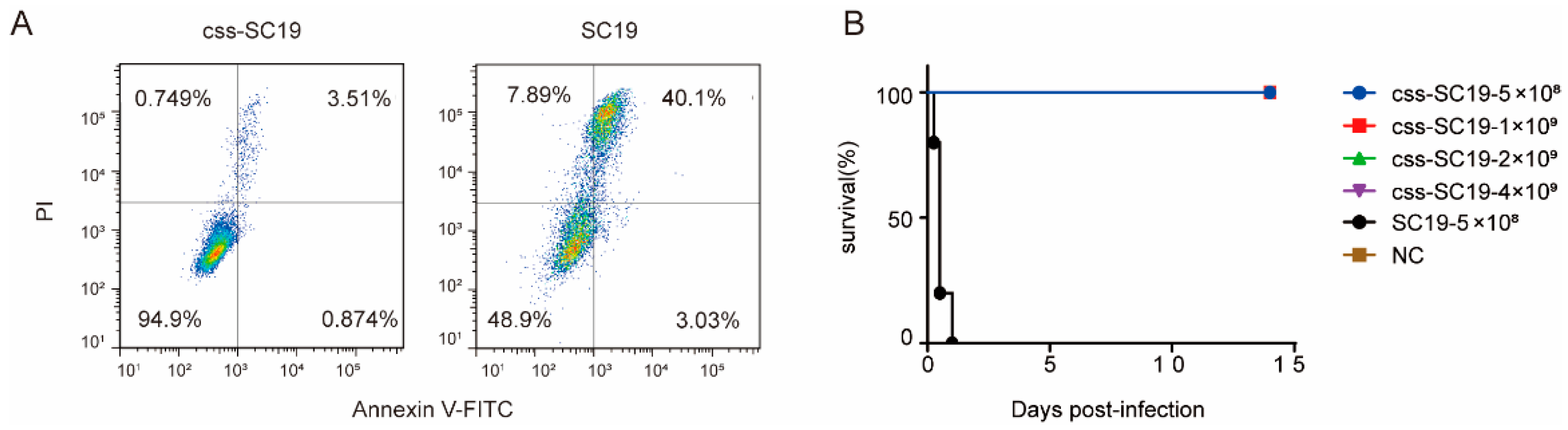
3.2. Δcps/ssna-msly (P353L)-SC19 Exhibited Avirulent Features Both In Vitro and In Vivo
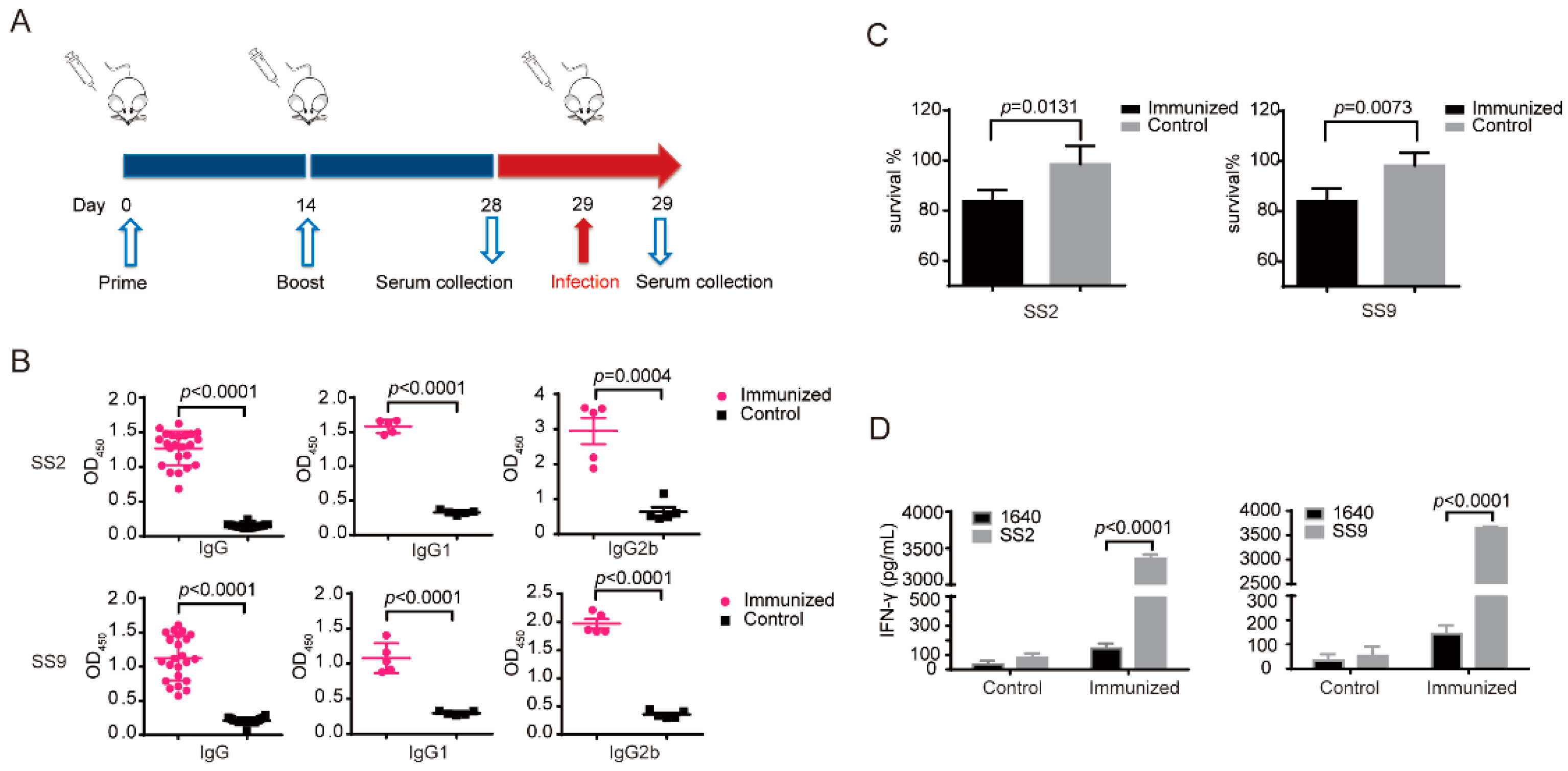
3.3. Δcps/ssna-msly (P353L)-SC19 Could Induce Significant Humoral and Cellular Immune Responses
3.4. Δcps/ssna-msly (P353L)-SC19 Confers Protection against Type 2 S. suis
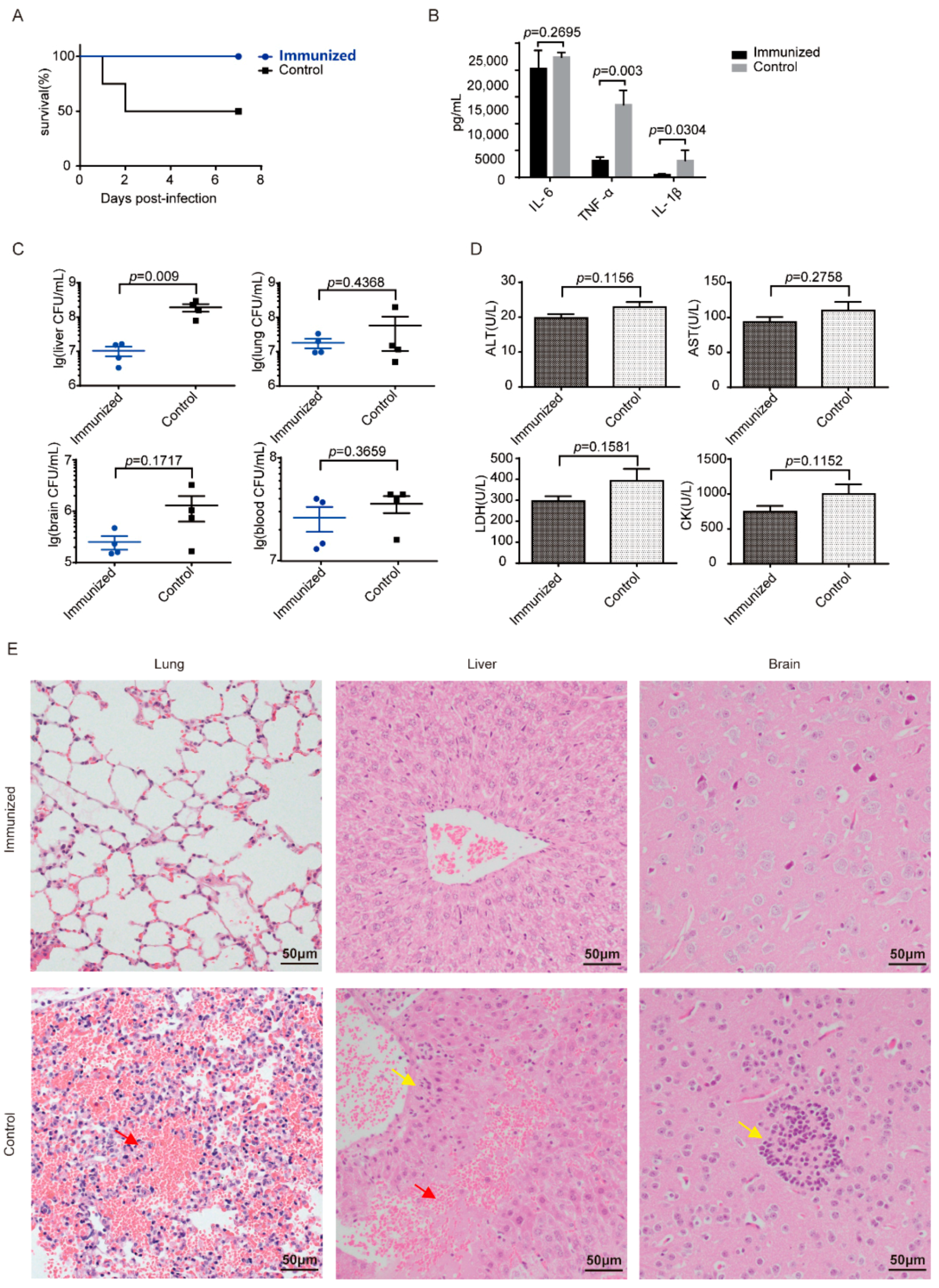
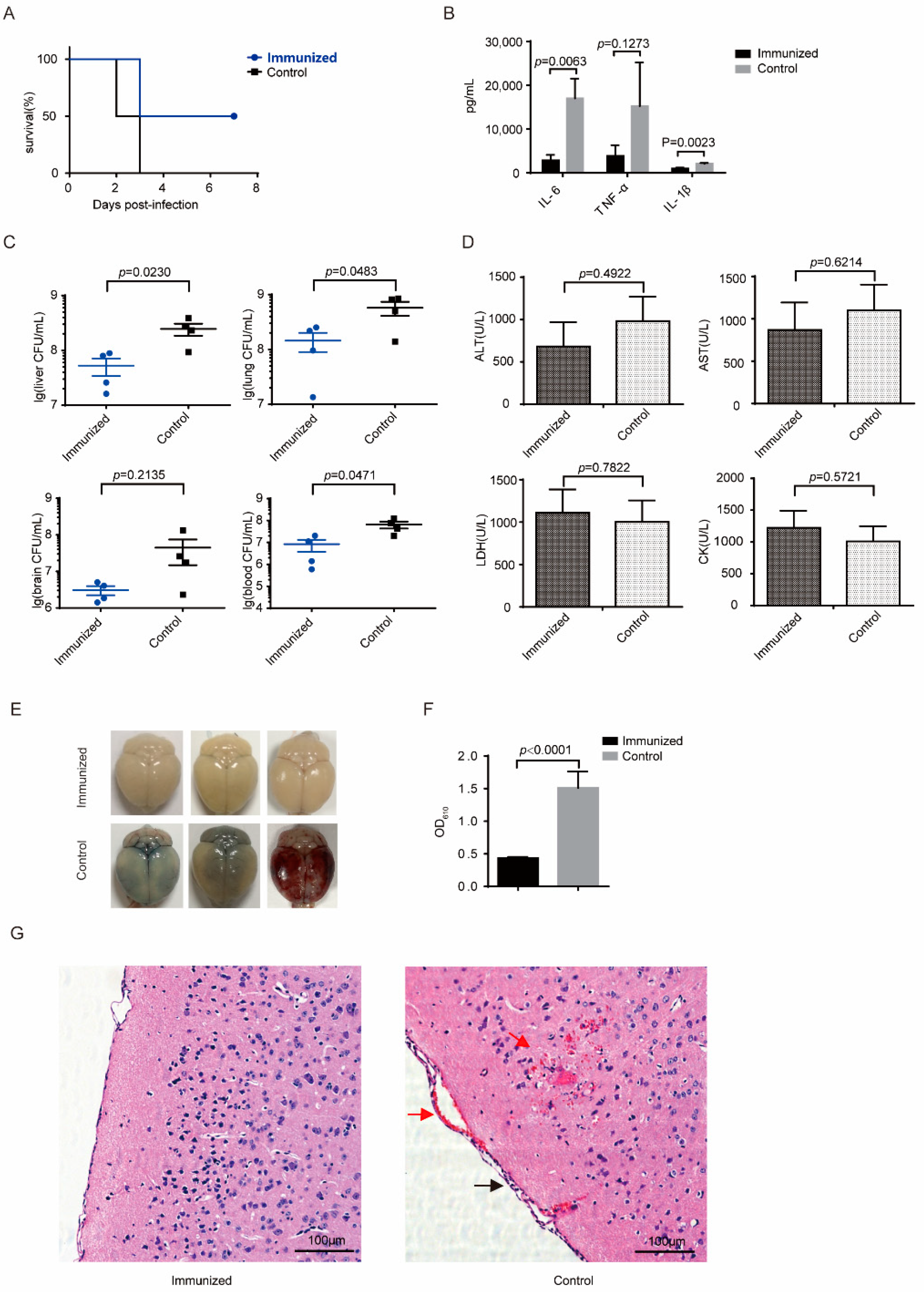
3.5. Δcps/ssna-msly (P353L)-SC19 Confers Protection against Type 9 Serotypes S. suis and Prevents Meningitis
3.6. Distinguish the Sera of S. suis-Infected Mice from Immunized Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottschalk, M.; Segura, M.; Xu, J. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.; Ha, N.; Huy, N.T.; Horby, P.; Nghia, H.D.; Thiem, V.D.; Zhu, X.; Hoa, N.T.; Hien, T.T.; Zamora, J.; et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014, 20, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Nghia, H.D.; Taylor, W.; Schultsz, C. Streptococcus suis: An emerging human pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef]
- Hatrongjit, R.; Fittipaldi, N.; Gottschalk, M.; Kerdsin, A. Tools for Molecular Epidemiology of Streptococcus suis. Pathogens 2020, 9, 81. [Google Scholar] [CrossRef]
- Hill, J.E.; Gottschalk, M.; Brousseau, R.; Harel, J.; Hemmingsen, S.M.; Goh, S.H. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 2005, 107, 63–69. [Google Scholar] [CrossRef]
- Lun, Z.R.; Wang, Q.P.; Chen, X.G.; Li, A.X.; Zhu, X.Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Bi, Y.; Li, J.; Yang, L.; Zhang, S.; Li, Y.; Jia, X.; Sun, L.; Yin, Y.; Qin, C.; Wang, B.; et al. Assessment of the pathogenesis of Streptococcus suis type 2 infection in piglets for understanding streptococcal toxic shock-like syndrome, meningitis, and sequelae. Vet. Microbiol. 2014, 173, 299–309. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Veldman, K.T.; Van den Eede, C.; Salmon, S.A.; Mevius, D.J. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licensed in veterinary medicine. Vet. Microbiol. 2006, 113, 73–82. [Google Scholar] [CrossRef]
- Segura, M.; Fittipaldi, N.; Calzas, C.; Gottschalk, M. Critical Streptococcus suis Virulence Factors: Are They All Really Critical? Trends Microbiol. 2017, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus suis vaccines: Candidate antigens and progress. Expert. Rev. Vaccines 2015, 14, 1587–1608. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Auger, J.P.; Segura, M.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Gottschalk, M. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015, 79, 141–146. [Google Scholar]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, D.; Akeda, Y.; Nakayama, T.; Kerdsin, A.; Sano, Y.; Kanda, T.; Hamada, S.; Dejsirilert, S.; Oishi, K. The contribution of suilysin to the pathogenesis of Streptococcus suis meningitis. J. Infect. Dis. 2014, 209, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.A.; Loeffen, P.L.; van den Berg, A.J.; Storm, P.K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 1994, 62, 1742–1748. [Google Scholar] [CrossRef]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 inflammasome-triggered cytokine storm contributes to Streptococcal toxic shock-like syndrome (STSLS). PLoS Pathog. 2019, 15, e1007795. [Google Scholar] [CrossRef]
- de Buhr, N.; Neumann, A.; Jerjomiceva, N.; von Kockritz-Blickwede, M.; Baums, C.G. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology 2014, 160, 385–395. [Google Scholar] [CrossRef]
- Li, M.; Cai, R.J.; Li, C.L.; Song, S.; Li, Y.; Jiang, Z.Y.; Yang, D.X. Deletion of ssnA Attenuates the Pathogenicity of Streptococcus suis and Confers Protection against Serovar 2 Strain Challenge. PLoS ONE 2017, 12, e0169791. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, S.; Lin, L.; Fu, L.; Yang, C.; Xu, Z.; Wei, Y.; Jin, M.; Zhang, A. Streptococcus suis serotype 2 strains can induce the formation of neutrophil extracellular traps and evade trapping. FEMS Microbiol. Lett. 2015, 362, fnv022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Chen, B.; Yuan, Z.; Li, R.; Liu, C.; Zhou, H.; Chen, H.; Jin, M. HP0197 contributes to CPS synthesis and the virulence of Streptococcus suis via CcpA. PLoS ONE 2012, 7, e50987. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Wang, L.; Zheng, D.Z.; Chen, S.; Shi, W.; Qiao, X.Y.; Jiang, Y.P.; Tang, L.J.; Xu, Y.G.; Li, Y.J. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018, 124, 368–378. [Google Scholar] [CrossRef]
- Luo, Y.; Dorf, M.E. Isolation of mouse neutrophils. Curr. Protoc. Immunol. 2001, 110, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mao, Y.; Ramirez, S.H.; Tuma, R.F.; Chabrashvili, T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 2010, 171, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.J.; Vecht, U.; Stockhofe-Zurwieden, N.; Smith, H.E. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet. Rec. 2001, 148, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Baums, C.G.; Bruggemann, C.; Kock, C.; Beineke, A.; Waldmann, K.H.; Valentin-Weigand, P. Immunogenicity of an autogenous Streptococcus suis bacterin in preparturient sows and their piglets in relation to protection after weaning. Clin. Vaccine Immunol. 2010, 17, 1589–1597. [Google Scholar] [CrossRef]
- Buttner, N.; Beineke, A.; de Buhr, N.; Lilienthal, S.; Merkel, J.; Waldmann, K.H.; Valentin-Weigand, P.; Baums, C.G. Streptococcus suis serotype 9 bacterin immunogenicity and protective efficacy. Vet. Immunol. Immunopathol. 2012, 146, 191–200. [Google Scholar] [CrossRef]
- Dekker, N.; Bouma, A.; Daemen, I.; Vernooij, H.; van Leengoed, L.; Wagenaar, J.A.; Stegeman, A. Effect of Simultaneous Exposure of Pigs to Streptococcus suis Serotypes 2 and 9 on Their Colonization and Transmission, and on Mortality. Pathogens 2017, 6, 46. [Google Scholar] [CrossRef]
- Seele, J.; Hillermann, L.M.; Beineke, A.; Seitz, M.; von Pawel-Rammingen, U.; Valentin-Weigand, P.; Baums, C.G. The immunoglobulin M-degrading enzyme of Streptococcus suis, IdeSsuis, is a highly protective antigen against serotype 2. Vaccine 2015, 33, 2207–2212. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Zhou, J.; Liu, H.; Liao, X.; Luo, J.; Li, X.; Fang, W. Peptidyl isomerase PrsA is surface-associated on Streptococcus suis and offers cross-protection against serotype 9 strain. FEMS Microbiol. Lett. 2019, 366, fnz002. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Serruto, D.; Safadi, M.A.; Klugman, K.P. Future challenges in the elimination of bacterial meningitis. Vaccine 2012, 30 (Suppl. 2), B78–B86. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Calzas, C.; Shiao, T.C.; Neubauer, A.; Kempker, J.; Roy, R.; Gottschalk, M.; Segura, M. Protection against Streptococcus suis Serotype 2 Infection Using a Capsular Polysaccharide Glycoconjugate Vaccine. Infect. Immun. 2016, 84, 2059–2075. [Google Scholar] [CrossRef]
- Li, Z.; Chang, P.; Xu, J.; Tan, C.; Wang, X.; Bei, W.; Li, J. A Streptococcus suis Live Vaccine Suppresses Streptococcal Toxic Shock-Like Syndrome and Provides Sequence Type-Independent Protection. J. Infect. Dis. 2019, 219, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, Y.; Zhu, L.; Gu, Y.; Shen, H.; Shan, Y.; Li, X.; Wu, J.; Fang, W. Live Streptococcus suis type 5 strain XS045 provides cross-protection against infection by strains of types 2 and 9. Vaccine 2016, 34, 6529–6538. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Stockhofe-Zurwieden, N.; Hilgers, L.A.; Smith, H.E. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet. Microbiol. 2002, 84, 155–168. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Perez-Casal, J.; Willson, P.J. Investigation of a novel DNase of Streptococcus suis serotype 2. Infect. Immun. 2004, 72, 774–781. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Xu, L.; Lin, L.; Zhou, L.; Dai, B.; Cui, S.; Zhang, A. A Streptococcus suis Strain Δcps/ssna-msly (P353L)-SC19 Provides Cross-Protection against Serotypes 2 and 9 Strain Challenges in a Mouse Model. Vaccines 2024, 12, 283. https://doi.org/10.3390/vaccines12030283
Lu X, Xu L, Lin L, Zhou L, Dai B, Cui S, Zhang A. A Streptococcus suis Strain Δcps/ssna-msly (P353L)-SC19 Provides Cross-Protection against Serotypes 2 and 9 Strain Challenges in a Mouse Model. Vaccines. 2024; 12(3):283. https://doi.org/10.3390/vaccines12030283
Chicago/Turabian StyleLu, Xi, Lei Xu, Lan Lin, Liting Zhou, Bingqian Dai, Shuyue Cui, and Anding Zhang. 2024. "A Streptococcus suis Strain Δcps/ssna-msly (P353L)-SC19 Provides Cross-Protection against Serotypes 2 and 9 Strain Challenges in a Mouse Model" Vaccines 12, no. 3: 283. https://doi.org/10.3390/vaccines12030283
APA StyleLu, X., Xu, L., Lin, L., Zhou, L., Dai, B., Cui, S., & Zhang, A. (2024). A Streptococcus suis Strain Δcps/ssna-msly (P353L)-SC19 Provides Cross-Protection against Serotypes 2 and 9 Strain Challenges in a Mouse Model. Vaccines, 12(3), 283. https://doi.org/10.3390/vaccines12030283





