Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccines
2.2. Mice and Immunization Regimens
2.3. Sample Processing
2.4. Overlapping Peptides Covering the Whole HTI Sequence
2.5. IFN-γ ELISPOT Assay
2.6. IFN-γ Response Mapping to Individual Peptides
2.7. Statistical Analysis
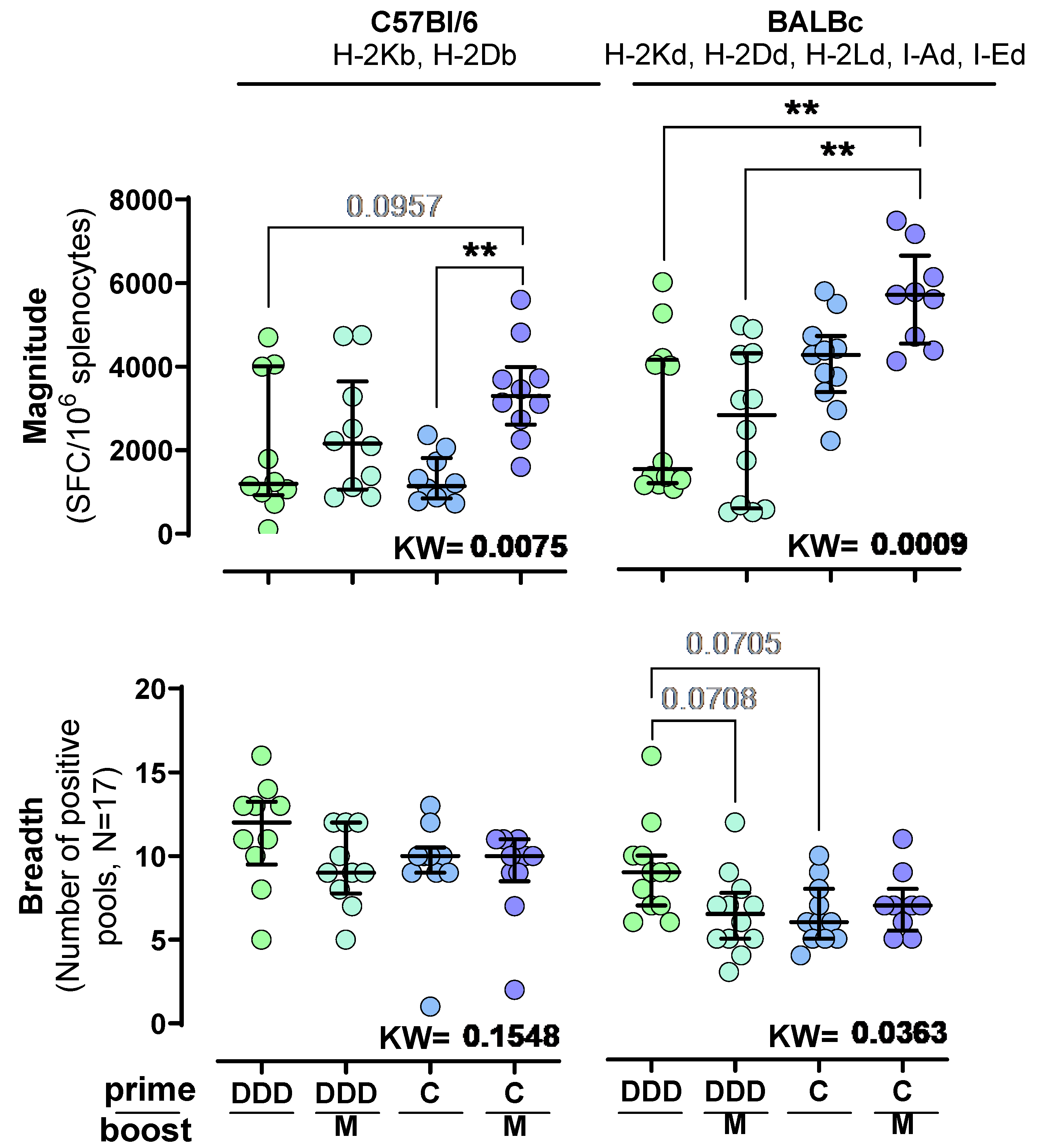
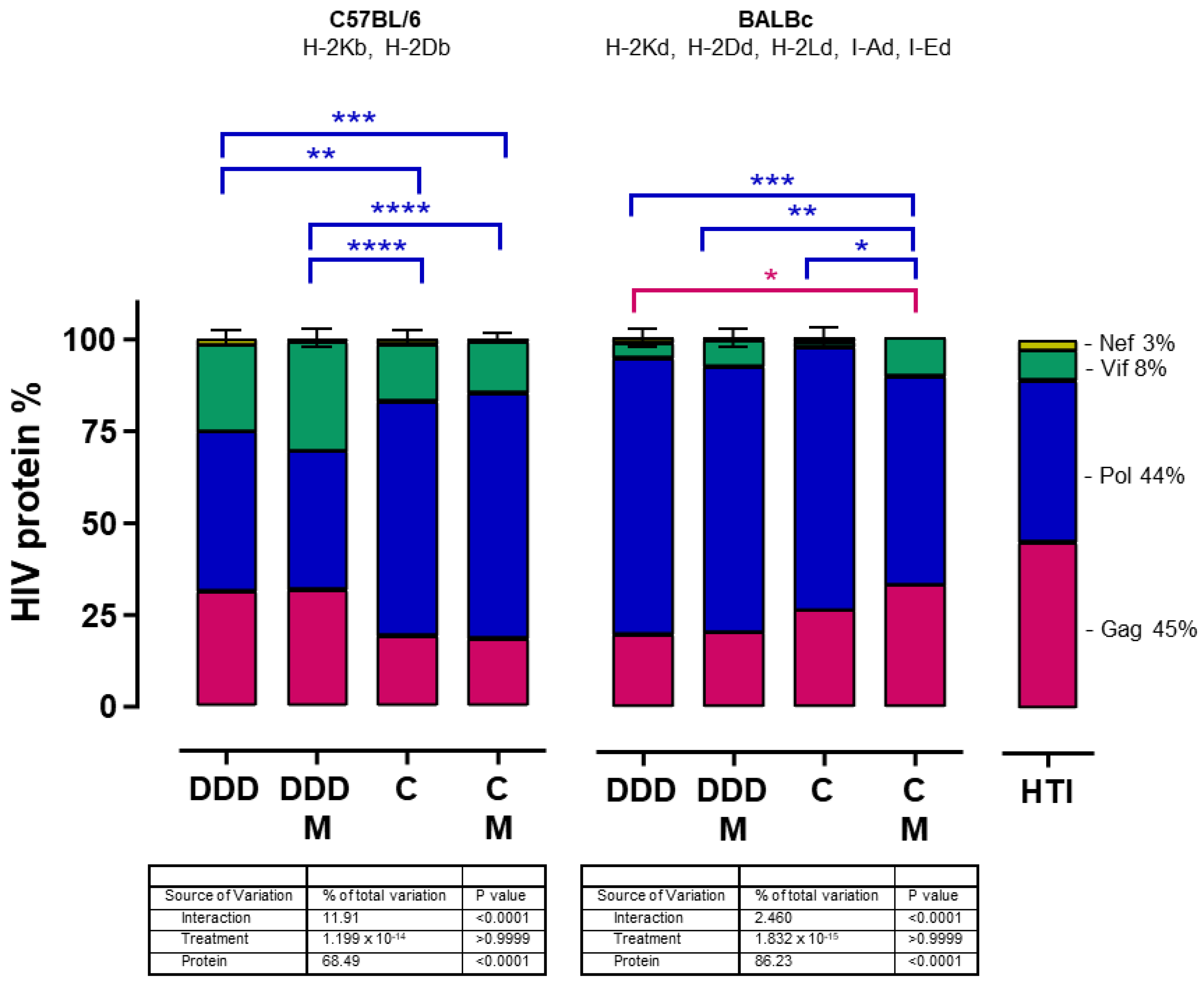
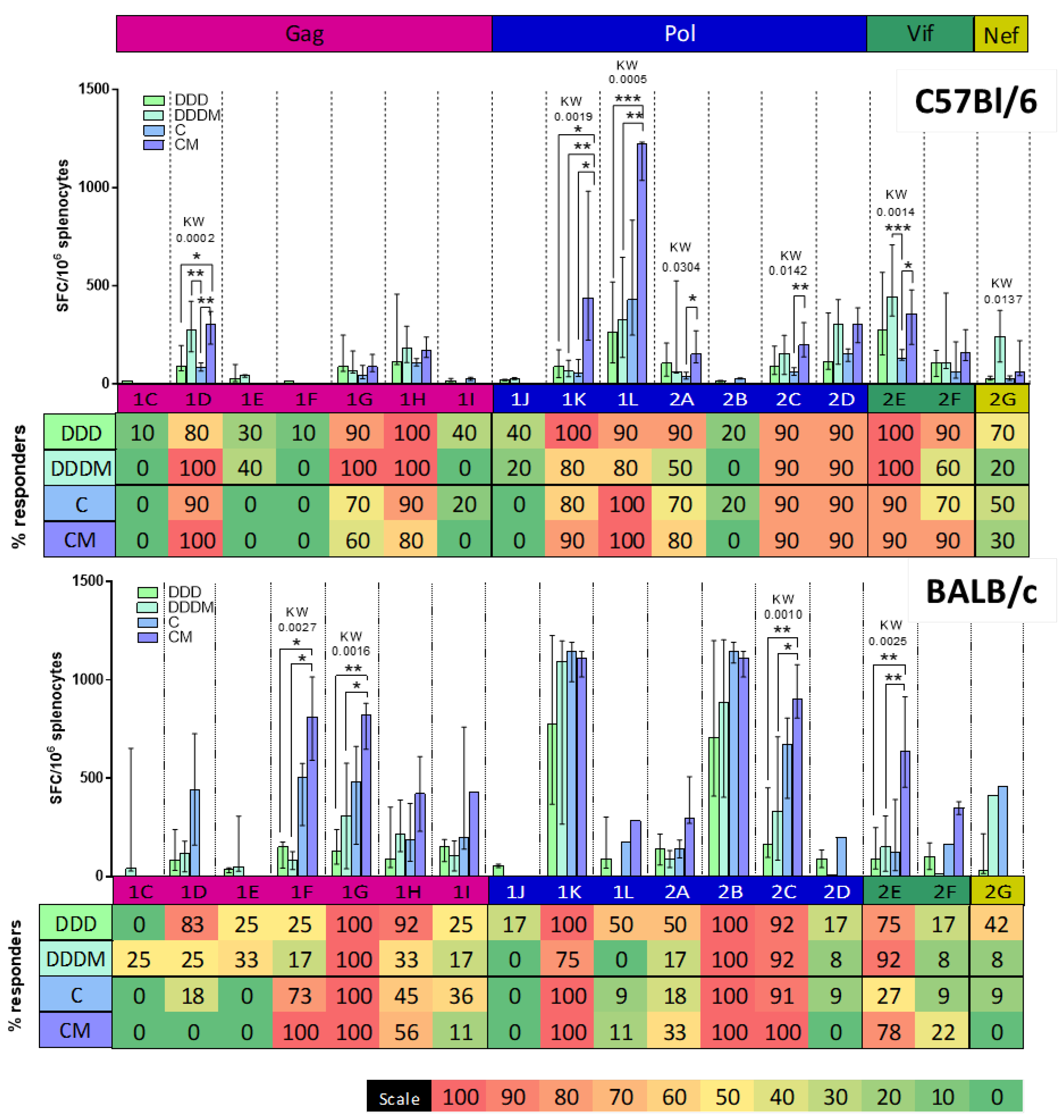
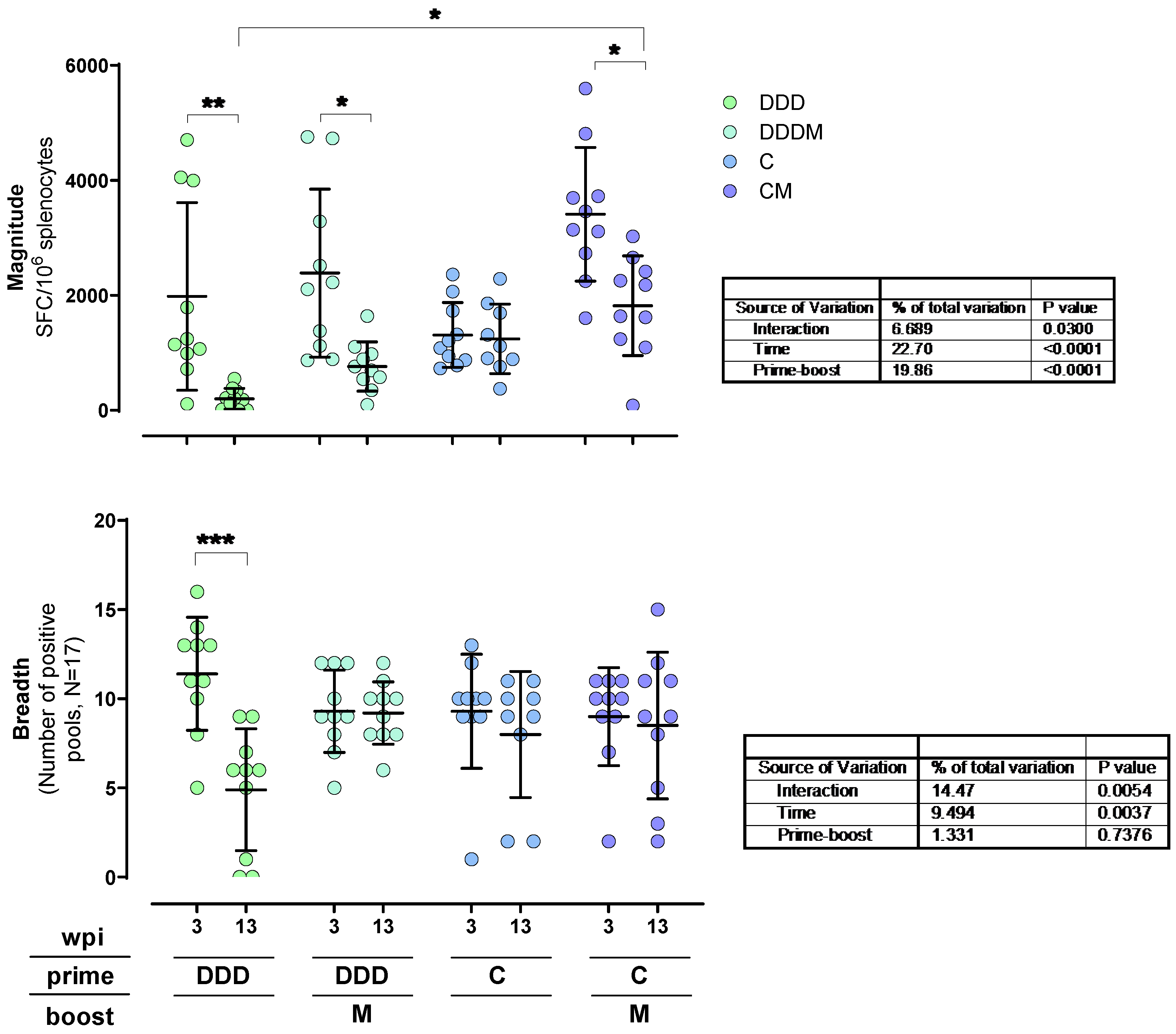
3. Results
3.1. ChAdOx1.HTI Is a Stronger T-Cell Prime Than Three DNA.HTI for an MVA.HTI Boost
3.2. ChAdOx1.HTI Prime Drives Different Immunodominance Compared to DNA.HTI Prime
3.3. T-Cell Responses to HTI Are Maintained over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andreano, E.; D’Oro, U.; Rappuoli, R.; Finco, O. Vaccine Evolution and Its Application to Fight Modern Threats. Front. Immunol. 2019, 10, 1722. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Barouch, D.H. Challenges in the Development of an HIV-1 Vaccine. Nature 2008, 455, 613–619. [Google Scholar] [CrossRef]
- Ng’uni, T.; Chasara, C.; Ndhlovu, Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020, 11, 590780. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct Viral Reservoirs in Individuals with Spontaneous Control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Turk, G.; Seiger, K.; Lian, X.; Sun, W.; Parsons, E.M.; Gao, C.; Rassadkina, Y.; Polo, M.L.; Czernikier, A.; Ghiglione, Y.; et al. A Possible Sterilizing Cure of HIV-1 Infection without Stem Cell Transplantation. Ann. Intern. Med. 2022, 175, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, J.F.; Lambotte, O. Epidemiology and Clinical Characteristics of Elite Controllers. Curr. Opin. HIV AIDS 2011, 6, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.G.; Olivo, A.; Harris, B.J.; Rodgers, M.A.; James, L.; Mampunza, S.; Niles, J.; Baer, F.; Yamaguchi, J.; Kaptue, L.; et al. A High Prevalence of Potential HIV Elite Controllers Identified over 30 Years in Democratic Republic of Congo. eBioMedicine 2021, 65, 103258. [Google Scholar] [CrossRef] [PubMed]
- Madec, Y.; Boufassa, F.; Porter, K.; Meyer, L. Spontaneous Control of Viral Load and CD4 Cell Count Progression among HIV-1 Seroconverters. AIDS 2005, 19, 2001–2007. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Ross, A.L.; Delfraissy, J.F. Past, Present and Future: 30 Years of HIV Research. Nat. Rev. Microbiol. 2013, 11, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.P.; Khoury, D.S.; Cromer, D.; Lewin, S.R.; Kelleher, A.D.; Kent, S.J. Functional Cure of HIV: The Scale of the Challenge. Nat. Rev. Immunol. 2019, 19, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Frahm, N.; Adams, S.; Kiepiela, P.; Linde, C.H.; Hewitt, H.S.; Lichterfeld, M.; Sango, K.; Brown, N.V.; Pae, E.; Wurcel, A.G.; et al. HLA-B63 Presents HLA-B57/B58-Restricted Cytotoxic T-Lymphocyte Epitopes and Is Associated with Low Human Immunodeficiency Virus Load. J. Virol. 2005, 79, 10218–10225. [Google Scholar] [CrossRef]
- Yang, O.O.; Kalams, S.A.; Trocha, A.; Cao, H.; Luster, A.; Johnson, R.P.; Walker, B.D. Suppression of Human Immunodeficiency Virus Type 1 Replication by CD8+ Cells: Evidence for HLA Class I-Restricted Triggering of Cytolytic and Noncytolytic Mechanisms. J. Virol. 1997, 71, 3120–3128. [Google Scholar] [CrossRef]
- Frahm, N.; Yusim, K.; Suscovich, T.J.; Adams, S.; Sidney, J.; Hraber, P.; Hewitt, H.S.; Linde, C.H.; Kavanagh, D.G.; Woodberry, T.; et al. Extensive HLA Class I Allele Promiscuity among Viral CTL Epitopes. Eur. J. Immunol. 2007, 37, 2419–2433. [Google Scholar] [CrossRef]
- McLaren, P.J.; Coulonges, C.; Bartha, I.; Lenz, T.L.; Deutsch, A.J.; Bashirova, A.; Buchbinder, S.; Carrington, M.N.; Cossarizza, A.; Dalmau, J.; et al. Polymorphisms of Large Effect Explain the Majority of the Host Genetic Contribution to Variation of HIV-1 Virus Load. Proc. Natl. Acad. Sci. USA 2015, 112, 14658–14663. [Google Scholar] [CrossRef]
- Henn, M.R.; Boutwell, C.L.; Charlebois, P.; Lennon, N.J.; Power, K.A.; Macalalad, A.R.; Berlin, A.M.; Malboeuf, C.M.; Ryan, E.M.; Gnerre, S.; et al. Whole Genome Deep Sequencing of HIV-1 Reveals the Impact of Early Minor Variants Upon Immune Recognition during Acute Infection. PLoS Pathog. 2012, 8, e1002529. [Google Scholar] [CrossRef]
- Brumme, Z.L.; John, M.; Carlson, J.M.; Brumme, C.J.; Chan, D.; Brockman, M.A.; Swenson, L.C.; Tao, I.; Szeto, S.; Rosato, P.; et al. HLA-Associated Immune Escape Pathways in HIV-1 Subtype B Gag, Pol and Nef Proteins. PLoS ONE 2009, 4, e6687. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.M.; Listgarten, J.; Pfeifer, N.; Tan, V.; Kadie, C.; Walker, B.D.; Ndung’u, T.; Shapiro, R.; Frater, J.; Brumme, Z.L.; et al. Widespread Impact of HLA Restriction on Immune Control and Escape Pathways of HIV-1. J. Virol. 2012, 86, 5230–5243. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, Z.M.; Kamya, P.; Mewalal, N.; Kløverpris, H.N.; Nkosi, T.; Pretorius, K.; Laher, F.; Ogunshola, F.; Chopera, D.; Shekhar, K.; et al. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impacts Viral Set Point. Immunity 2015, 43, 591. [Google Scholar] [CrossRef]
- McMichael, A.J. Is a Human CD8 T-Cell Vaccine Possible, and If So, What Would It Take? Could a CD8 + T-Cell Vaccine Prevent Persistent HIV Infection? Cold Spring Harb. Perspect. Biol. 2018, 10, a029124. [Google Scholar] [CrossRef]
- Boutwell, C.L.; Rolland, M.M.; Herbeck, J.T.; Mullins, J.I.; Allen, T.M. Viral Evolution and Escape during Acute HIV-1 Infection. J. Infect. Dis. 2010, 202, S309. [Google Scholar] [CrossRef]
- Kløverpris, H.N.; Leslie, A.; Goulder, P. Role of HLA Adaptation in HIV Evolution. Front. Immunol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Barton, J.P.; Goonetilleke, N.; Butler, T.C.; Walker, B.D.; McMichael, A.J.; Chakraborty, A.K. Relative Rate and Location of Intra-Host HIV Evolution to Evade Cellular Immunity Are Predictable. Nat. Commun. 2016, 7, 11660. [Google Scholar] [CrossRef]
- Nishimura, Y.; Gautam, R.; Chun, T.-W.; Sadjadpour, R.; Foulds, K.E.; Shingai, M.; Klein, F.; Gazumyan, A.; Golijanin, J.; Donaldson, M.; et al. Early Antibody Therapy Can Induce Long-Lasting Immunity to SHIV. Nature 2017, 543, 559–563. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W. T Cell-Based Strategies for HIV-1 Vaccines. Hum. Vaccin. Immunother. 2020, 16, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Manocheewa, S.; Swain, J.V.; Lanxon-Cookson, E.C.; Kim, M.; Westfall, D.H.; Larsen, B.B.; Gilbert, P.B.; Mullins, J.I. HIV-1 Conserved-Element Vaccines: Relationship between Sequence Conservation and Replicative Capacity. J. Virol. 2013, 87, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Valentin, A.; Bergamaschi, C.; Rosati, M.; Angel, M.; Burns, R.; Agarwal, M.; Gergen, J.; Petsch, B.; Oostvogels, L.; Loeliger, E.; et al. Comparative Immunogenicity of an MRNA/LNP and a DNA Vaccine Targeting HIV Gag Conserved Elements in Macaques. Front. Immunol. 2022, 13, 945706. [Google Scholar] [CrossRef] [PubMed]
- Dross, S.; Venkataraman, R.; Patel, S.; Huang, M.L.; Bollard, C.M.; Rosati, M.; Pavlakis, G.N.; Felber, B.K.; Bar, K.J.; Shaw, G.M.; et al. Efficient Ex Vivo Expansion of Conserved Element Vaccine-Specific CD8+ T-Cells from SHIV-Infected, ART-Suppressed Nonhuman Primates. Front. Immunol. 2023, 14, 1188018. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Rosás-Umbert, M.; Coll, P.; Manzardo, C.; Puertas, M.C.; Morón-López, S.; Llano, A.; Miranda, C.; Cedeño, S.; López, M.; et al. HIVconsv Vaccines and Romidepsin in Early-Treated HIV-1-Infected Individuals: Safety, Immunogenicity and Effect on the Viral Reservoir (Study BCN02). Front. Immunol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Manzardo, C.; Sanchez-Bernabeu, A.; Coll, P.; Morón-López, S.; Puertas, M.C.; Rosas-Umbert, M.; Cobarsi, P.; Escrig, R.; Perez-Alvarez, N.; et al. Therapeutic Vaccination Refocuses T-Cell Responses Towards Conserved Regions of HIV-1 in Early Treated Individuals (BCN 01 Study). EClinicalMedicine 2019, 11, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Beavis, A.C.; Wee, E.G.T.; Akis Yildirim, B.M.; Borthwick, N.; He, B.; Hanke, T. Combined Intranasal and Intramuscular Parainfluenza 5-, Simian Adenovirus ChAdOx1- and Poxvirus MVA-Vectored Vaccines Induce Synergistically HIV-1-Specific T Cells in the Mucosa. Front. Immunol. 2023, 14, 1186478. [Google Scholar] [CrossRef]
- Wee, E.G.; Moyo, N.; Hannoun, Z.; Giorgi, E.E.; Korber, B.; Hanke, T. Effect of Epitope Variant Co-Delivery on the Depth of CD8 T Cell Responses Induced by HIV-1 Conserved Mosaic Vaccines. Mol. Ther. Methods Clin. Dev. 2021, 21, 741–753. [Google Scholar] [CrossRef]
- Borthwick, N.; Silva-Arrieta, S.; Llano, A.; Takiguchi, M.; Brander, C.; Hanke, T. Novel Nested Peptide Epitopes Recognized by CD4+ T Cells Induced by HIV-1 Conserved-Region Vaccines. Vaccines 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.S.; Borthwick, N.J.; Moyo, N.; Murakoshi, H.; Akahoshi, T.; Siliquini, F.; Hannoun, Z.; Crook, A.; Hayes, P.; Fast, P.E.; et al. Specificity of CD8+ T-Cell Responses Following Vaccination with Conserved Regions of HIV-1 in Nairobi, Kenya. Vaccines 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Wee, E.G.; Moyo, N.A.; Saunders, K.O.; LaBranche, C.; Donati, F.; Capucci, S.; Parks, R.; Borthwick, N.; Hannoun, Z.; Montefiori, D.C.; et al. Parallel Induction of CH505 B Cell Ontogeny-Guided Neutralizing Antibodies and THIVconsvX Conserved Mosaic-Specific T Cells against HIV-1. Mol. Ther. Methods Clin. Dev. 2019, 14, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Moyo, N.; Wee, E.G.; Korber, B.; Bahl, K.; Falcone, S.; Himansu, S.; Wong, A.L.; Dey, A.K.; Feinberg, M.; Hanke, T. Tetravalent Immunogen Assembled from Conserved Regions of HIV-1 and Delivered as MRNA Demonstrates Potent Preclinical T-Cell Immunogenicity and Breadth. Vaccines 2020, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, A.; Saubi, N.; Guitart, N.; Moyo, N.; Wee, E.G.; Ravi, K.; Hanke, T.; Joseph, J. Priming with Recombinant BCG Expressing Novel HIV-1 Conserved Mosaic Immunogens and Boosting with Recombinant CHADOX1 Is Safe, Stable, and Elicits HIV-1specific T-Cell Responses in BALB/c Mice. Front. Immunol. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Hartnell, F.; Brown, A.; Capone, S.; Kopycinski, J.; Bliss, C.; Makvandi-Nejad, S.; Swadling, L.; Ghaffari, E.; Cicconi, P.; Del Sorbo, M.; et al. A Novel Vaccine Strategy Employing Serologically Different Chimpanzee Adenoviral Vectors for the Prevention of HIV-1 and HCV Coinfection. Front. Immunol. 2019, 10, 415786. [Google Scholar] [CrossRef] [PubMed]
- Moyo, N.; Vogel, A.B.; Buus, S.; Erbar, S.; Wee, E.G.; Sahin, U.; Hanke, T. Efficient Induction of T Cells against Conserved HIV-1 Regions by Mosaic Vaccines Delivered as Self-Amplifying MRNA. Mol. Ther. Methods Clin. Dev. 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Moyo, N.; Borthwick, N.J.; Wee, E.G.; Capucci, S.; Crook, A.; Dorrell, L.; Hanke, T. Long-Term Follow up of Human T-Cell Responses to Conserved HIV-1 Regions Elicited by DNA/Simian Adenovirus/MVA Vaccine Regimens. PLoS ONE 2017, 12, e0181382. [Google Scholar] [CrossRef]
- Hancock, G.; Morón-López, S.; Kopycinski, J.; Puertas, M.C.; Giannoulatou, E.; Rose, A.; Salgado, M.; Hayton, E.J.; Crook, A.; Morgan, C.; et al. Evaluation of the Immunogenicity and Impact on the Latent HIV-1 Reservoir of a Conserved Region Vaccine, MVA.HIVconsv, in Antiretroviral Therapy-Treated Subjects. J. Int. AIDS Soc. 2017, 20, 21171. [Google Scholar] [CrossRef]
- Borthwick, N.; Lin, Z.; Akahoshi, T.; Llano, A.; Silva-Arrieta, S.; Ahmed, T.; Dorrell, L.; Brander, C.; Murakoshi, H.; Takiguchi, M.; et al. Novel, in-Natural-Infection Subdominant HIV-1 CD8+ T-Cell Epitopes Revealed in Human Recipients of Conserved-Region T-Cell Vaccines. PLoS ONE 2017, 12, e0176418. [Google Scholar] [CrossRef]
- Wee, E.G.; Ondondo, B.; Berglund, P.; Archer, J.; McMichael, A.J.; Baltimore, D.; ter Meulen, J.H.; Hanke, T. HIV-1 Conserved Mosaics Delivered by Regimens with Integration-Deficient DC-Targeting Lentiviral Vector Induce Robust T Cells. Mol. Ther. 2017, 25, 494–503. [Google Scholar] [CrossRef]
- Abdul-Jawad, S.; Ondondo, B.; Van Hateren, A.; Gardner, A.; Elliott, T.; Korber, B.; Hanke, T. Increased Valency of Conserved-Mosaic Vaccines Enhances the Breadth and Depth of Epitope Recognition. Mol. Ther. 2016, 24, 375–384. [Google Scholar] [CrossRef]
- Hancock, G.; Yang, H.; Yorke, E.; Wainwright, E.; Bourne, V.; Frisbee, A.; Payne, T.L.; Berrong, M.; Ferrari, G.; Chopera, D.; et al. Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-Infection and Post-Vaccination Immune Responses. PLoS Pathog. 2015, 11, e1004658. [Google Scholar] [CrossRef]
- Ondondo, B.; Abdul-Jawad, S.; Bridgeman, A.; Hanke, T. Characterization of T-Cell Responses to Conserved Regions of the HIV-1 Proteome in BALB/c Mice. Clin. Vaccine Immunol. 2014, 21, 1565–1572. [Google Scholar] [CrossRef]
- Ondondo, B.; Murakoshi, H.; Clutton, G.; Abdul-Jawad, S.; Wee, E.G.T.; Gatanaga, H.; Oka, S.; McMichael, A.J.; Takiguchi, M.; Korber, B.; et al. Novel Conserved-Region T-Cell Mosaic Vaccine with High Global HIV-1 Coverage Is Recognized by Protective Responses in Untreated Infection. Mol. Ther. 2016, 24, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Hayton, E.J.; Rose, A.; Ibrahimsa, U.; Del Sorbo, M.; Capone, S.; Crook, A.; Black, A.P.; Dorrell, L.; Hanke, T. Safety and Tolerability of Conserved Region Vaccines Vectored by Plasmid DNA, Simian Adenovirus and Modified Vaccinia Virus Ankara Administered to Human Immunodeficiency Virus Type 1-Uninfected Adults in a Randomized, Single-Blind Phase I Trial. PLoS ONE 2014, 9, e101591. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, N.; Ahmed, T.; Ondondo, B.; Hayes, P.; Rose, A.; Ebrahimsa, U.; Hayton, E.J.; Black, A.; Bridgeman, A.; Rosario, M.; et al. Vaccine-Elicited Human T Cells Recognizing Conserved Protein Regions Inhibit HIV-1. Mol. Ther. 2014, 22, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Rosati, M.; Valentin, A.; Ganneru, B.; Singh, A.K.; Yan, J.; Rolland, M.; Alicea, C.; Beach, R.K.; Zhang, G.M.; et al. HIV-1 P24(Gag) Derived Conserved Element DNA Vaccine Increases the Breadth of Immune Response in Mice. PLoS ONE 2013, 8, e60245. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Valentin, A.; Rosati, M.; Rolland, M.; Mullins, J.I.; Pavlakis, G.N.; Felber, B.K. HIV-1 Conserved Elements P24CE DNA Vaccine Induces Humoral Immune Responses with Broad Epitope Recognition in Macaques. PLoS ONE 2014, 9, e111085. [Google Scholar] [CrossRef] [PubMed]
- Munson, P.; Liu, Y.; Bratt, D.; Fuller, J.T.; Hu, X.; Pavlakis, G.N.; Felber, B.K.; Mullins, J.I.; Fuller, D.H. Therapeutic Conserved Elements (CE) DNA Vaccine Induces Strong T-Cell Responses against Highly Conserved Viral Sequences during Simian-Human Immunodeficiency Virus Infection. Hum. Vaccin. Immunother. 2018, 14, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Valentin, A.; Cai, Y.; Dayton, F.; Rosati, M.; Ramírez-Salazar, E.G.; Kulkarni, V.; Broderick, K.E.; Sardesai, N.Y.; Wyatt, L.S.; et al. DNA Vaccine-Induced Long-Lasting Cytotoxic T Cells Targeting Conserved Elements of Human Immunodeficiency Virus Gag Are Boosted Upon DNA or Recombinant Modified Vaccinia Ankara Vaccination. Hum. Gene Ther. 2018, 29, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, Z.; Valentin, A.; Rosati, M.; Broderick, K.E.; Sardesai, N.Y.; Marx, P.A.; Mullins, J.I.; Pavlakis, G.N.; Felber, B.K. Gag and Env Conserved Element CE DNA Vaccines Elicit Broad Cytotoxic T Cell Responses Targeting Subdominant Epitopes of HIV and SIV Able to Recognize Virus-Infected Cells in Macaques. Hum. Vaccin. Immunother. 2018, 14, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Llano, A.; Ibarrondo, J.; Daniels, M.; Miranda, C.; Zamarreno, J.; Bach, V.; Zuniga, R.; Perez-Alvarez, S.; Berger, C.T.; et al. Definition of the Viral Targets of Protective HIV-1-Specific T Cell Responses. J. Transl. Med. 2011, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Hu, X.; Llano, A.; Rosati, M.; Olvera, A.; Kulkarni, V.; Valentin, A.; Alicea, C.; Pilkington, G.R.; Sardesai, N.Y.; et al. A Human Immune Data-Informed Vaccine Concept Elicits Strong and Broad T-Cell Specificities Associated with HIV-1 Control in Mice and Macaques. J. Transl. Med. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Llano, A.; Ibarrondo, J.; Zamarreño, J.; Schiaulini, M.; Miranda, C.; Ruiz-Riol, M.; Berger, C.T.; Herrero, M.J.; Palou, E.; et al. CTL Responses of High Functional Avidity and Broad Variant Cross-Reactivity Are Associated with HIV Control. PLoS ONE 2012, 7, e29717. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, A.; Saubi, N.; Guitart, N.; Olvera, A.; Hanke, T.; Brander, C.; Joseph, J. Recombinant BCG Expressing HTI Prime and Recombinant ChAdOx1 Boost Is Safe and Elicits HIV-1-Specific T-Cell Responses in BALB/c Mice. Vaccines 2019, 7, 78. [Google Scholar] [CrossRef]
- Saubi, N.; Kilpeläinen, A.; Eto, Y.; Chen, C.-W.; Olvera, À.; Hanke, T.; Brander, C.; Joseph-Munné, J. Priming with Recombinant BCG Expressing HTI Enhances the Magnitude and Breadth of the T-Cell Immune Responses Elicited by MVA.HTI in BALB/c Mice. Vaccines 2020, 8, 678. [Google Scholar] [CrossRef]
- Bailón, L.; Llano, A.; Cedeño, S.; Escribà, T.; Rosás-Umbert, M.; Parera, M.; Casadellà, M.; Lopez, M.; Pérez, F.; Oriol-Tordera, B.; et al. Safety, Immunogenicity and Effect on Viral Rebound of HTI Vaccines in Early Treated HIV-1 Infection: A Randomized, Placebo-Controlled Phase 1 Trial. Nat. Med. 2022, 28, 2611–2621. [Google Scholar] [CrossRef]
- Palgen, J.L.; Feraoun, Y.; Dzangué-Tchoupou, G.; Joly, C.; Martinon, F.; Le Grand, R.; Beignon, A.S. Optimize Prime/Boost Vaccine Strategies: Trained Immunity as a New Player in the Game. Front. Immunol. 2021, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.L.; Kim, J.H. Novel Prime-Boost Vaccine Strategies against HIV-1. Expert Rev. Vaccines 2019, 18, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Los Alamos HIV Databases PeptGen Peptide Generator. Available online: https://www.hiv.lanl.gov/content/sequence/PEPTGEN/peptgen.html (accessed on 23 December 2021).
- Bihl, F.; Frahm, N.; Di Giammarino, L.; Sidney, J.; John, M.; Yusim, K.; Woodberry, T.; Sango, K.; Hewitt, H.S.; Henry, L.; et al. Impact of HLA-B Alleles, Epitope Binding Affinity, Functional Avidity, and Viral Coinfection on the Immunodominance of Virus-Specific CTL Responses. J. Immunol. 2006, 176, 4094–4101. [Google Scholar] [CrossRef] [PubMed]
- Nolz, J.C.; Harty, J.T. Strategies and Implications for Prime-Boost Vaccination to Generate Memory CD8 T Cells. Adv. Exp. Med. Biol. 2011, 780, 69–83. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-Boost Vaccine Strategy against Viral Infections: Mechanisms and Benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Price, P.J.R.; Torres-Domínguez, L.E.; Brandmüller, C.; Sutter, G.; Lehmann, M.H. Modified Vaccinia Virus Ankara: Innate Immune Activation and Induction of Cellular Signalling. Vaccine 2013, 31, 4231–4234. [Google Scholar] [CrossRef]
- Hancock, G.; Blight, J.; Lopez-Camacho, C.; Kopycinski, J.; Pocock, M.; Byrne, W.; Price, M.J.; Kemlo, P.; Evans, R.I.; Bloss, A.; et al. A multi-genotype therapeutic human papillomavirus vaccine elicits potent T cell responses to conserved regions of early proteins. Sci. Rep. 2019, 9, 18713. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera, A.; Romero-Martin, L.; Oriol-Tordera, B.; Rosas-Umbert, M.; Escribà, T.; Mothe, B.; Brander, C. Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses. Vaccines 2024, 12, 279. https://doi.org/10.3390/vaccines12030279
Olvera A, Romero-Martin L, Oriol-Tordera B, Rosas-Umbert M, Escribà T, Mothe B, Brander C. Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses. Vaccines. 2024; 12(3):279. https://doi.org/10.3390/vaccines12030279
Chicago/Turabian StyleOlvera, Alex, Luis Romero-Martin, Bruna Oriol-Tordera, Miriam Rosas-Umbert, Tuixent Escribà, Beatriz Mothe, and Christian Brander. 2024. "Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses" Vaccines 12, no. 3: 279. https://doi.org/10.3390/vaccines12030279
APA StyleOlvera, A., Romero-Martin, L., Oriol-Tordera, B., Rosas-Umbert, M., Escribà, T., Mothe, B., & Brander, C. (2024). Impact of ChAdOx1 or DNA Prime Vaccination on Magnitude, Breadth, and Focus of MVA-Boosted Immunogen-Specific T Cell Responses. Vaccines, 12(3), 279. https://doi.org/10.3390/vaccines12030279





