Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Vaccine- and Infection-Related Characteristics
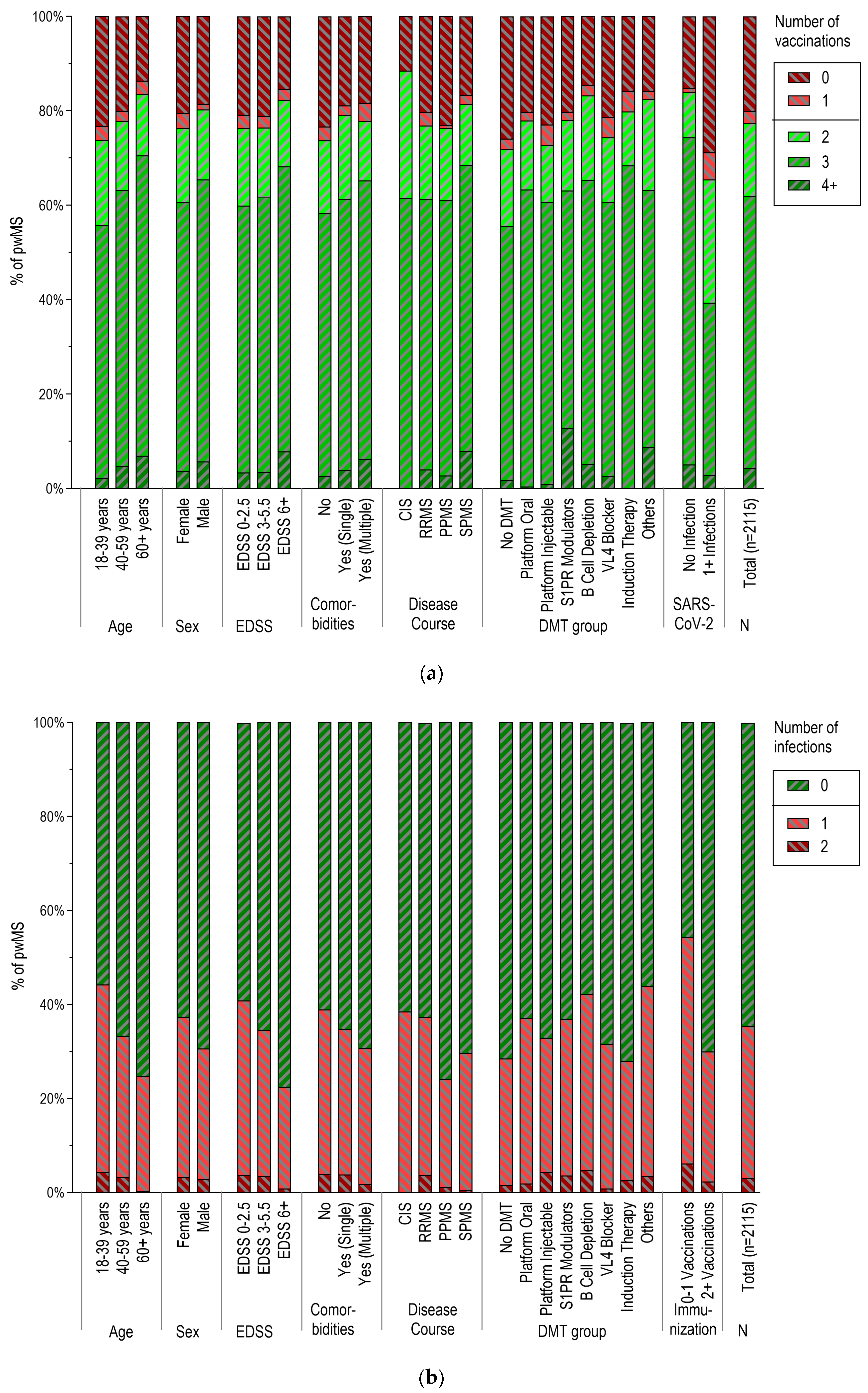
3.3. Vaccination Patterns According to Demographic and Clinical Characteristics
3.4. SARS-CoV-2 Infections According to Demographic and Clinical Characteristics
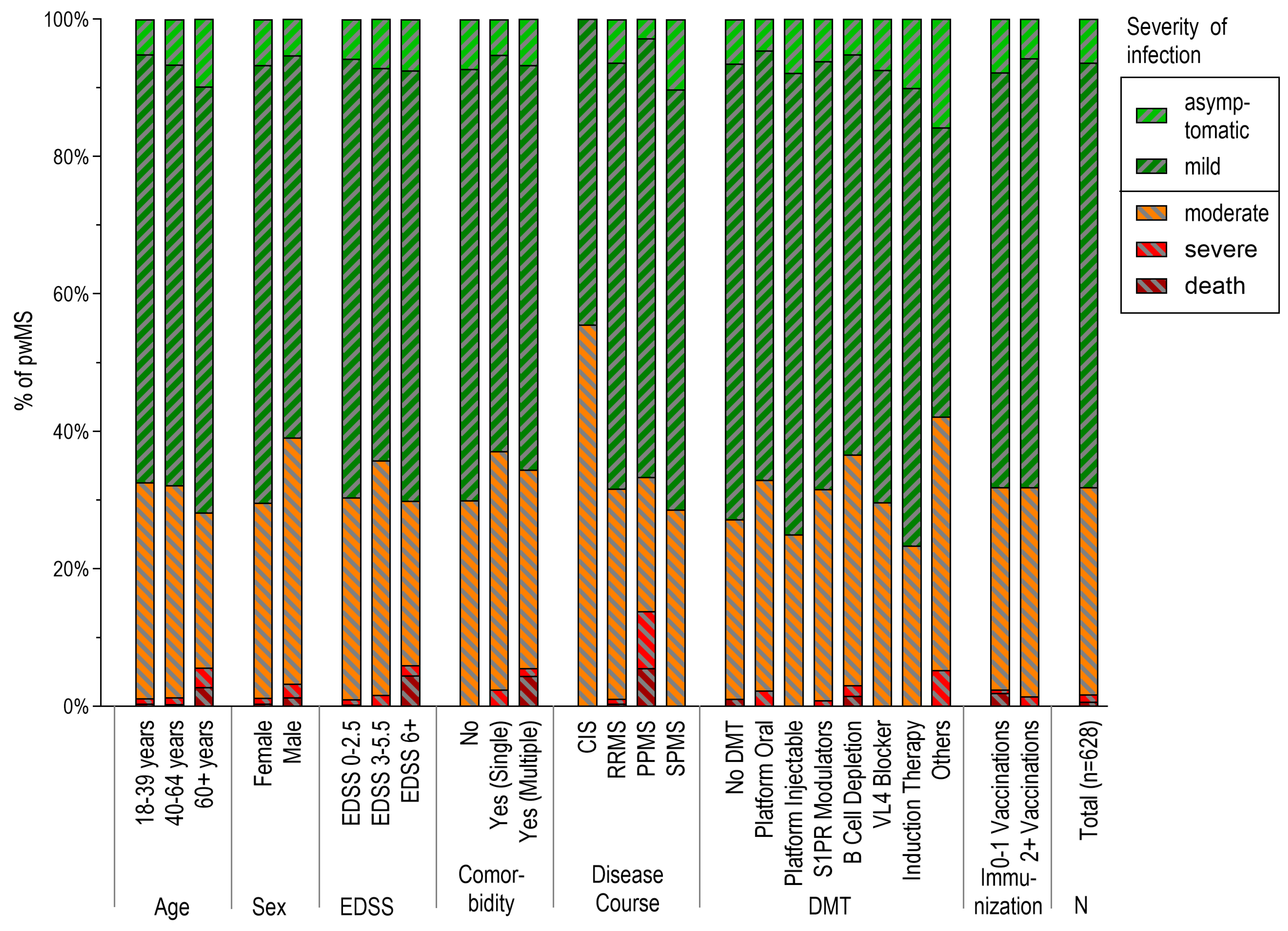
3.5. Severity of SARS-CoV-2 Infections
3.6. Temporal Distribution of Infections and Vaccinations across the Different Phases of the Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Barzegar, M.; Mirmosayyeb, O.; Gajarzadeh, M.; Afshari-Safavi, A.; Nehzat, N.; Vaheb, S.; Shaygannejad, V.; Maghzi, A.H. COVID-19 Among Patients with Multiple Sclerosis: A Systematic Review. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1001. [Google Scholar] [CrossRef]
- Fan, M.; Qiu, W.; Bu, B.; Xu, Y.; Yang, H.; Huang, D.; Lau, A.Y.; Guo, J.; Zhang, M.N.; Zhang, X.; et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e787. [Google Scholar] [CrossRef]
- Montgomery, S.; Hillert, J.; Bahmanyar, S. Hospital admission due to infections in multiple sclerosis patients. Eur. J. Neurol. 2013, 20, 1153–1160. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Llufriu, S.; Martínez-Hernández, E.; Català, M.; Artola, M.; Hernando, A.; Montejo, C.; Pulido-Valdeolivas, I.; Martínez-Heras, E.; Guasp, M.; et al. Incidence and Impact of COVID-19 in MS: A Survey From a Barcelona MS Unit. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e985. [Google Scholar] [CrossRef]
- Moreno-Torres, I.; Meca Lallana, V.; Costa-Frossard, L.; Oreja-Guevara, C.; Aguirre, C.; Alba Suárez, E.M.; Gómez Moreno, M.; Borrega Canelo, L.; Sabín Muñoz, J.; Aladro, Y.; et al. Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur. J. Neurol. 2021, 28, 3712–3721. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Faissner, S.; Bartig, D.; Tönges, L.; Hellwig, K.; Ayzenberg, I.; Krogias, C.; Gold, R. Multiple sclerosis is not associated with an increased risk for severe COVID-19: A nationwide retrospective cross-sectional study from Germany. Neurol. Res. Pract. 2021, 3, 42. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Manni, A.; Paolicelli, D.; Patti, F.; Capobianco, M.; Brescia Morra, V.; Sola, P.; Pesci, I.; Lus, G.; et al. Risk of Getting COVID-19 in People with Multiple Sclerosis: A Case-Control Study. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e1141. [Google Scholar] [CrossRef]
- Salter, A.; Fox, R.J.; Newsome, S.D.; Halper, J.; Li, D.K.B.; Kanellis, P.; Costello, K.; Bebo, B.; Rammohan, K.; Cutter, G.R.; et al. Outcomes and Risk Factors Associated with SARS-CoV-2 Infection in a North American Registry of Patients with Multiple Sclerosis. JAMA Neurol. 2021, 78, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Louapre, C.; Collongues, N.; Stankoff, B.; Giannesini, C.; Papeix, C.; Bensa, C.; Deschamps, R.; Créange, A.; Wahab, A.; Pelletier, J.; et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol 2020, 77, 1079–1088. [Google Scholar] [CrossRef]
- Xiang, X.M.; Hollen, C.; Yang, Q.; Brumbach, B.H.; Spain, R.I.; Wooliscroft, L. COVID-19 vaccination willingness among people with multiple sclerosis. Mult. Scler. J.—Exp. Transl. Clin. 2021, 7, 20552173211017159. [Google Scholar] [CrossRef] [PubMed]
- Nehal, K.R.; Steendam, L.M.; Campos Ponce, M.; van der Hoeven, M.; Smit, G.S.A. Worldwide Vaccination Willingness for COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.; Rechtman, A.; Zveik, O.; Haham, N.; Oiknine-Djian, E.; Wolf, D.G.; Levin, N.; Raposo, C.; Vaknin-Dembinsky, A. Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Ocrelizumab. JAMA Neurol. 2021, 78, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef] [PubMed]
- Woopen, C.; Dunsche, M.; Haase, R.; Raposo, C.; Pedotti, R.; Akgün, K.; Ziemssen, T. Timing of SARS-CoV-2 Vaccination Matters in People with Multiple Sclerosis on Pulsed Anti-CD20 Treatment. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e31. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.B.; Nam, H.K.; Lee, H. Which Group Should be Vaccinated First?: A Systematic Review. Infect. Chemother. 2021, 53, 261–270. [Google Scholar] [CrossRef]
- Word Health Organization. WHO Coronavirus (COVID-19) Dashboard, Germany. 2023. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 16 August 2023).
- Bsteh, G.; Gradl, C.; Heschl, B.; Hegen, H.; Di Pauli, F.; Assar, H.; Leutmezer, F.; Traxler, G.; Krajnc, N.; Zulehner, G.; et al. Impact of vaccination on COVID-19 outcome in multiple sclerosis. Eur. J. Neurol. 2022, 29, 3329–3336. [Google Scholar] [CrossRef]
- Bazylewicz, M.; Gudowska-Sawczuk, M.; Mroczko, B.; Kochanowicz, J.; Kułakowska, A. COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review. Int. J. Mol. Sci. 2023, 24, 9231. [Google Scholar] [CrossRef]
- Brunn, J.A.; Dunietz, G.L.; Romeo, A.R.; Braley, T.J. SARS-CoV-2 Infection and Vaccination Outcomes in Multiple Sclerosis. Neurol. Clin. Pract. 2022, 12, e14–e21. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Tolksdorf, K.; Loenenbach, A.; Buda, S. Dritte Aktualisierung der “Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland”. Epidemiol. Bull. 2022, 38, 3–6. Available online: https://edoc.rki.de/handle/176904/10260 (accessed on 16 August 2022).
- Tolksdorf, K.; Buda, S.; Schilling, J. Aktualisierung zur Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland. 2021. Available online: https://edoc.rki.de/handle/176904/7935 (accessed on 16 August 2022).
- Robert Koch Institute, R.K.I. Coronavirus Disease 2019 (COVID-19). Daily Situation Report. 2022. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Mai_2022/Archiv_Mai_2022.html?nn=13490888 (accessed on 16 August 2022).
- Craxì, L.; Casuccio, A.; Amodio, E.; Restivo, V. Who Should Get COVID-19 Vaccine First? A Survey to Evaluate Hospital Workers’ Opinion. Vaccines 2021, 9, 189. [Google Scholar] [CrossRef]
- Munzert, S.; Ramirez-Ruiz, S.; Çalı, B.; Stoetzer, L.F.; Gohdes, A.; Lowe, W. Prioritization preferences for COVID-19 vaccination are consistent across five countries. Humanit. Soc. Sci. Commun. 2022, 9, 439. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.-A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F. Evaluation of humoral and cellular responses in SARS-CoV-2 mRNA vaccinated immunocompromised patients. Front. Immunol. 2022, 13, 858399. [Google Scholar] [CrossRef] [PubMed]
- Doerre, A.; Doblhammer, G. The influence of gender on COVID-19 infections and mortality in Germany: Insights from age-and gender-specific modeling of contact rates, infections, and deaths in the early phase of the pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef] [PubMed]
- Mangia, C.; Russo, A.; Civitelli, S.; Gianicolo, E.A.L. Sex/gender differences in COVID-19 lethality: What the data say, and do not say. Epidemiol. Prev. 2020, 44, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Kihara, K.; Kinoshita, M.; Sugimoto, T.; Okazaki, S.; Murata, H.; Beppu, S.; Shiraishi, N.; Sugiyama, Y.; Koda, T.; Okuno, T. Humoral and cellular responses to SARS-CoV-2 vaccination in patients with autoantibody-mediated neuroimmunology. J. Neurol. Neurosurg. Psychiatry 2023, 94, 495–497. [Google Scholar] [CrossRef]
- Woopen, C.; Dunsche, M.; Al Rahbani, G.K.; Dillenseger, A.; Atta, Y.; Haase, R.; Raposo, C.; Pedotti, R.; Ziemssen, T.; Akgün, K. Long-Term Immune Response Profiles to SARS-CoV-2 Vaccination and Infection in People with Multiple Sclerosis on Anti-CD20 Therapy. Vaccines 2023, 11, 1464. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Assar, H.; Hegen, H.; Heschl, B.; Leutmezer, F.; Di Pauli, F.; Gradl, C.; Traxler, G.; Zulehner, G.; Rommer, P. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry. PLoS ONE 2021, 16, e0255316. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R.; Brandstadter, R.; Bar-Or, A. COVID-19 and MS disease-modifying therapies. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e761. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.M. Teriflunomide: A possible effective drug for the comprehensive treatment of COVID-19. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100055. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef]

| Phase | Description | Period | Comment (Pandemic-Related Events) |
|---|---|---|---|
| 1 | Sporadic infections | 27 January 2020–1 March 2020 |
|
| 2 | 1st SARS-CoV-2 wave | 2 March 2020–17 May 2020 |
|
| 3 | Summer plateau 2020 | 18 May 2020–27 September 2020 | |
| 4 | 2nd SARS-CoV-2 wave | 28 September 2020–28 February 2021 |
|
| 5 | 3rd SARS-CoV-2 wave | 1 March 2021–13 June 2021 |
|
| 6 | Summer plateau 2021 | 14 June 2021–1 August 2021 | |
| 7 | 4th SARS-CoV-2 wave | 2 August 2021–26 December 2021 |
|
| 8 | 5th SARS-CoV-2 wave | 27 December 2021–30 May 2022 |
|
| PwMS | |
|---|---|
| Sociodemographic characteristics: | |
| Age (years), mean (SD) | 46.5 (±12.9) |
| 18–39, n (%) | 702 (33.2%) |
| 40–59, n (%) | 1005 (49.8%) |
| 60+, n (%) | 360 (17.0%) |
| Sex | |
| Female, n (%) | 1533 (72.5%) |
| Body mass index (kg/m2), mean (SD) | 24.5 (5.3) |
| Underweight (<18.5), n (%) | 85 (4.0%) |
| Normal weight (18.5–< 25), n (%) | 1060 (50.5%) |
| Overweight (25–< 30), n (%) | 602 (28.7%) |
| Obesity (≥30), n (%) | 353 (16.8%) |
| MS disease-specific characteristics: | |
| Disease duration, years, median [IQR] | 10.0, [5–16] |
| <5 years, n (%) | 601 (28.5%) |
| 6–19 years, n (%) | 1157 (54.9%) |
| ≥20 years, n (%) | 349 (16.6%) |
| MS disease course, n (%) | |
| RRMS | 1691 (80.0%) |
| PPMS | 182 (8.6%) |
| SPMS | 216 (10.2%) |
| CIS | 26 (1.2%) |
| EDSS, median [IQR] | 2.5, [1.5–4.0] |
| 0–2.5, n (%) | 1094 (52.0%) |
| 3–5.5, n (%) | 624 (29.7%) |
| ≥6, n (%) | 384 (18.3%) |
| Comorbidities (n = 1927), mean (SD) | 0.89 (0.88) |
| No (0), n (%) | 723 (37.5%) |
| Single (1), n (%) | 816 (42.3%) |
| Multiple (2+), n (%) | 388 (20.1%) |
| DMT, n (%) | |
| No DMT | 456 (21.6%) |
| Platform injectables | 231 (10.9%) |
| Platform oral | 267 (12.6%) |
| S1PR modulation | 336 (15.9%) |
| B-cell depletion | 537 (25.4%) |
| Induction therapies | 114 (5.4%) |
| VCAM-1 blocker | 117 (5.5%) |
| Others | 57 (2.7%) |
| DMT treatment duration (years), mean (SD) | 4.26 (3.67) |
| PwMS | |
|---|---|
| Number of SARS-CoV-2 infections, mean (SD) | 0.39 (0.55) |
| 0, n (%) | 1365 (64.5%) |
| 1, n (%) | 684 (32.3%) |
| 2, n (%) | 66 (3.1%) |
| Severity of SARS-CoV-2 disease (n = 628), n (%) | |
| Asymptomatic | 40 (6.4%) |
| Mild | 388 (61.8%) |
| Moderate | 189 (30.1%) |
| Severe | 7 (1.1%) |
| Death | 4 (0.6%) |
| Number of vaccinations, mean (SD) | 2.25 (1.27) |
| 0, n (%) | 423 (20.0%) |
| 1, n (%) | 54 (2.6%) |
| 2, n (%) | 328 (15.5%) |
| 3, n (%) | 1220 (57.7%) |
| ≥4 (%) | 90 (4.3%) |
| Number of SARS-CoV-2 infections and/or vaccination, mean (SD) | 2.64 (1.24) |
| 0, n (%) | 207 (9.8%) |
| 1, n (%) | 205 (9.7%) |
| 2, n (%) | 190 (9.0%) |
| 3, n (%) | 1131 (53.5%) |
| ≥4, n (%) | 382 (18.1%) |
| (a) Vaccinations [≥2 vs. 0–1] | (b) SARS-CoV-2 Infections [≥1 vs. 0] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable * | Univariable | Multivariable * | ||||||
| OR | 95%-CI | OR | 95%-CI | OR | 95%-CI | OR | 95%-CI | ||
| Age | cont. (years) | 1.016 | 1.008–1.024 | 1.016 | 1.008–1.024 | 0.969 | (9.969–0.983) | 0.975 | 0.968–0.982 |
| Age | 18–39 years | Ref | Ref | Ref | Ref | ||||
| 40–59 years | 1.243 | 0.995–1.553 | 1.235 | 0.988–1.542 | 0.632 | 0.519–0.770 | 0.637 | 0.523–0.776 | |
| 60+ years | 1.812 | 1.308–2.510 | 1.815 | 1.310–2.515 | 0.415 | 0.313–0.551 | 0.414 | 0.312–0.549 | |
| Sex | Male | Ref. | Ref. | Ref. | Ref. | ||||
| Female | 0.797 | 0.629–1.008 | 0.793 | 0.626–1.005 | 1.351 | 1.101–1.657 | 1.366 | 1.111–1.680 | |
| EDSS | cont. (points) | 1.071 | 1.013–1.133 | 1.016 | 0.952–1.085 | 0.844 | 0.802–0.887 | 0.898 | 0.847–0.952 |
| EDSS | 0–2.5 | Ref | Ref | Ref | Ref | ||||
| 3–5.5 | 1.007 | 0.798–1.269 | 0.873 | 0.682–1.118 | 0.766 | 0.625–0.940 | 0.905 | 0.728–1.127 | |
| 6+ | 1.441 | 1.071–1.939 | 1.115 | 0.799–1.558 | 0.418 | 0.319–0.546 | 0.563 | 0.417–0.759 | |
| Comorbidity | No | Ref. | Ref. | Ref. | Ref. | ||||
| Single | 1.345 | 1.062–1.703 | 1.235 | 0.964–1.582 | 0.840 | 0.682–1.033 | 1.024 | 0.822–1.276 | |
| Multiple | 1.252 | 0.936–1.675 | 1.051 | 0.761–1.453 | 0.694 | 0.535–0.905 | 1.028 | 0.767–1.377 | |
| MS type | RRMS | Ref. | Ref. | Ref. | Ref. | ||||
| PPMS | 0.972 | 0.678–1.394 | 0.733 | 0.499–1.079 | 0.534 | 0.375–0.761 | 0.769 | 0.530–1.117 | |
| SPMS | 1.323 | 0.922–1.900 | 0.971 | 0.654–1.443 | 0.706 | 0.518–0.960 | 1.067 | 0.760–1.498 | |
| CIS | 2.306 | 0.689–7.721 | 2.352 | 0.701–7.894 | 1.047 | 0.472–2.322 | 1.026 | 0.460–2.288 | |
| Disease duration | cont. (years) | 1.010 | 0.998–1.023 | 0.999 | 0.985–1.013 | 0.981 | 0.971–0.992 | 0.998 | 0.986–1.011 |
| Disease duration | 0–5 years | Ref. | Ref. | Ref. | Ref. | ||||
| 6–19 years | 1.221 | 0.967–1.540 | 1.104 | 0.868–1.403 | 0.971 | 0.792–1.190 | 1.122 | 0.907–1.386 | |
| 20+ years | 1.145 | 0.838–1.566 | 0.872 | 0.615–1.237 | 0.603 | 0.451–0.805 | 0.879 | 0.639–1.210 | |
| DMT | No DMT | Ref. | Ref. | Ref. | Ref. | ||||
| Induction therapies | 1.972 | 1.181–3.293 | 1.544 | 0.936–2.548 | 0.979 | 0.620–1.544 | 0.754 | 0.472–1.205 | |
| Platform oral | 1.674 | 1.161–2.412 | 1.376 | 0.966–1.960 | 1.478 | 1.072–2.037 | 1.216 | 0.873–1.694 | |
| Platform injectables | 1.242 | 0.863–1.786 | 1.041 | 0.730–1.483 | 1.230 | 0.874–1.730 | 1.021 | 0.719–1.450 | |
| S1PR modulators | 1.601 | 1.140–2.250 | 1.382 | 0.994–1.920 | 1.467 | 1.086–1.981 | 1.299 | 0.952–1.772 | |
| B-cell depletion therapies | 2.357 | 1.711–3.247 | 1.938 | 1.429–2.629 | 1.836 | 1.408–2.395 | 1.552 | 1.175–2.052 | |
| VL4 blocker | 1.623 | 1.000–2.634 | 1.132 | 0.713–1.797 | 1.160 | 0.747–1.800 | 0.776 | 0.490–1.228 | |
| Others | 2.195 | 1.065–4.521 | 1.834 | 0.900–3.740 | 1.959 | 1.118–3.434 | 1.709 | 0.963–3.303 | |
| Vaccinations | 0–1 vaccination | Ref. | Ref. | ||||||
| 2+ vaccinations | 0.360 | 0.292–0.444 | 0.378 | 0.306–0.466 | |||||
| SARS-CoV-2 infections | 0 (no infection) | Ref. | Ref. | ||||||
| 1–2 | 0.360 | 0.292–0.444 | 0.378 | 0.306–0.467 | |||||
| Number of Infections | Number of Vaccinations | Age (years) | |
|---|---|---|---|
| Number of infections | 1 | −0.314 ** | −0.150 ** |
| Number of vaccinations | −0.314 ** | 1 | 0.127 ** |
| Age (years) | −0.150 ** | 0.127 ** | 1 |
| Comorbidities (number) | −0.067 * | 0.069 * | 0.448 ** |
| EDSS (points) | −0.135 ** | 0.082 ** | 0.547 ** |
| Disease duration (years) | −0.057 ** | 0.089 ** | 0.456 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inojosa, H.; Schriefer, D.; Atta, Y.; Dillenseger, A.; Proschmann, U.; Schleußner, K.; Woopen, C.; Ziemssen, T.; Akgün, K. Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort. Vaccines 2024, 12, 265. https://doi.org/10.3390/vaccines12030265
Inojosa H, Schriefer D, Atta Y, Dillenseger A, Proschmann U, Schleußner K, Woopen C, Ziemssen T, Akgün K. Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort. Vaccines. 2024; 12(3):265. https://doi.org/10.3390/vaccines12030265
Chicago/Turabian StyleInojosa, Hernan, Dirk Schriefer, Yassin Atta, Anja Dillenseger, Undine Proschmann, Katharina Schleußner, Christina Woopen, Tjalf Ziemssen, and Katja Akgün. 2024. "Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort" Vaccines 12, no. 3: 265. https://doi.org/10.3390/vaccines12030265
APA StyleInojosa, H., Schriefer, D., Atta, Y., Dillenseger, A., Proschmann, U., Schleußner, K., Woopen, C., Ziemssen, T., & Akgün, K. (2024). Insights from Real-World Practice: The Dynamics of SARS-CoV-2 Infections and Vaccinations in a Large German Multiple Sclerosis Cohort. Vaccines, 12(3), 265. https://doi.org/10.3390/vaccines12030265







