Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria and Definitions
2.3. Data Collection Process
2.4. Risk of Bias and Quality Assessment
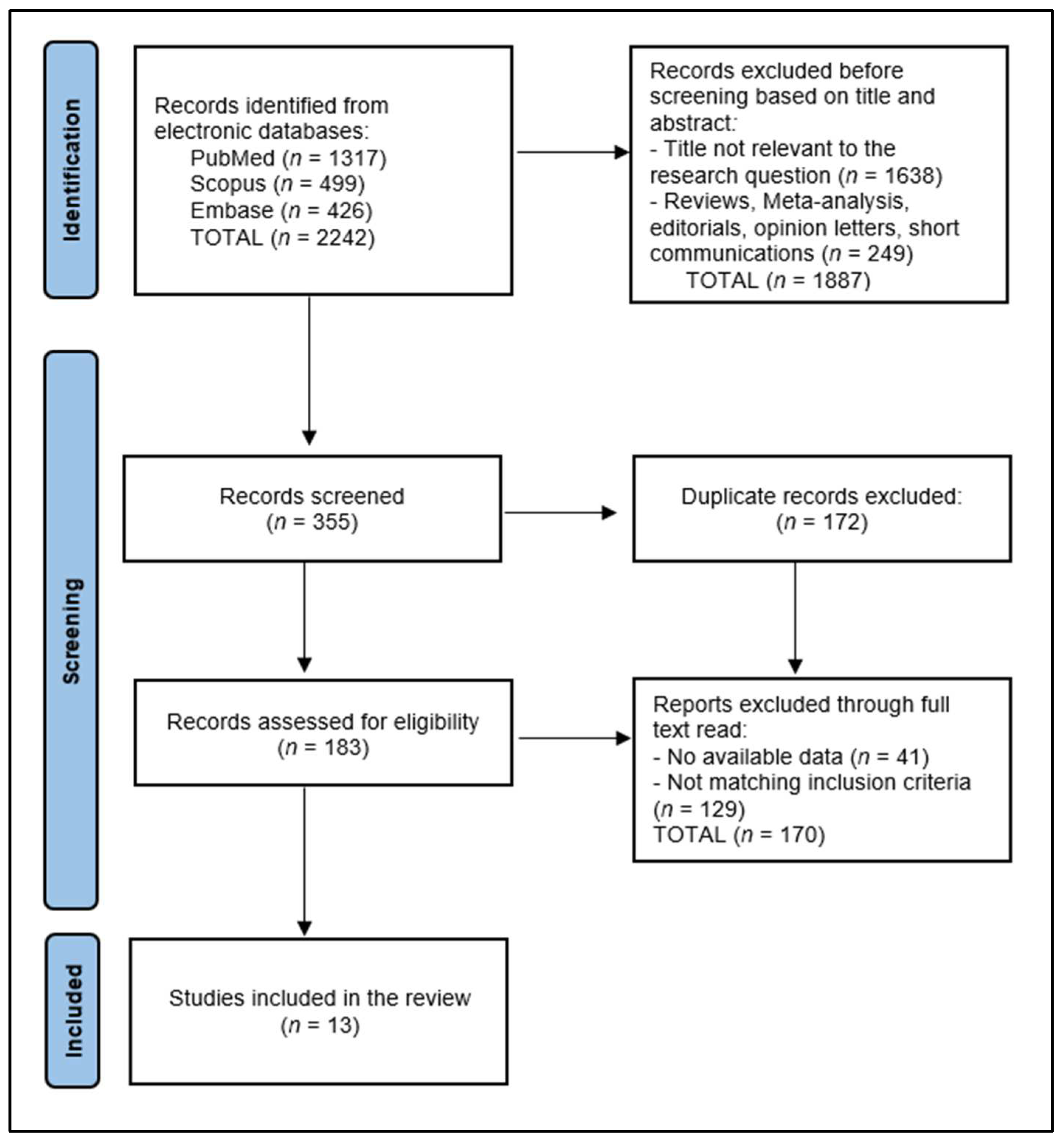
3. Results
3.1. Study Characteristics
3.2. Characteristics of Patients
3.3. COVID-19 Vaccination Characteristics
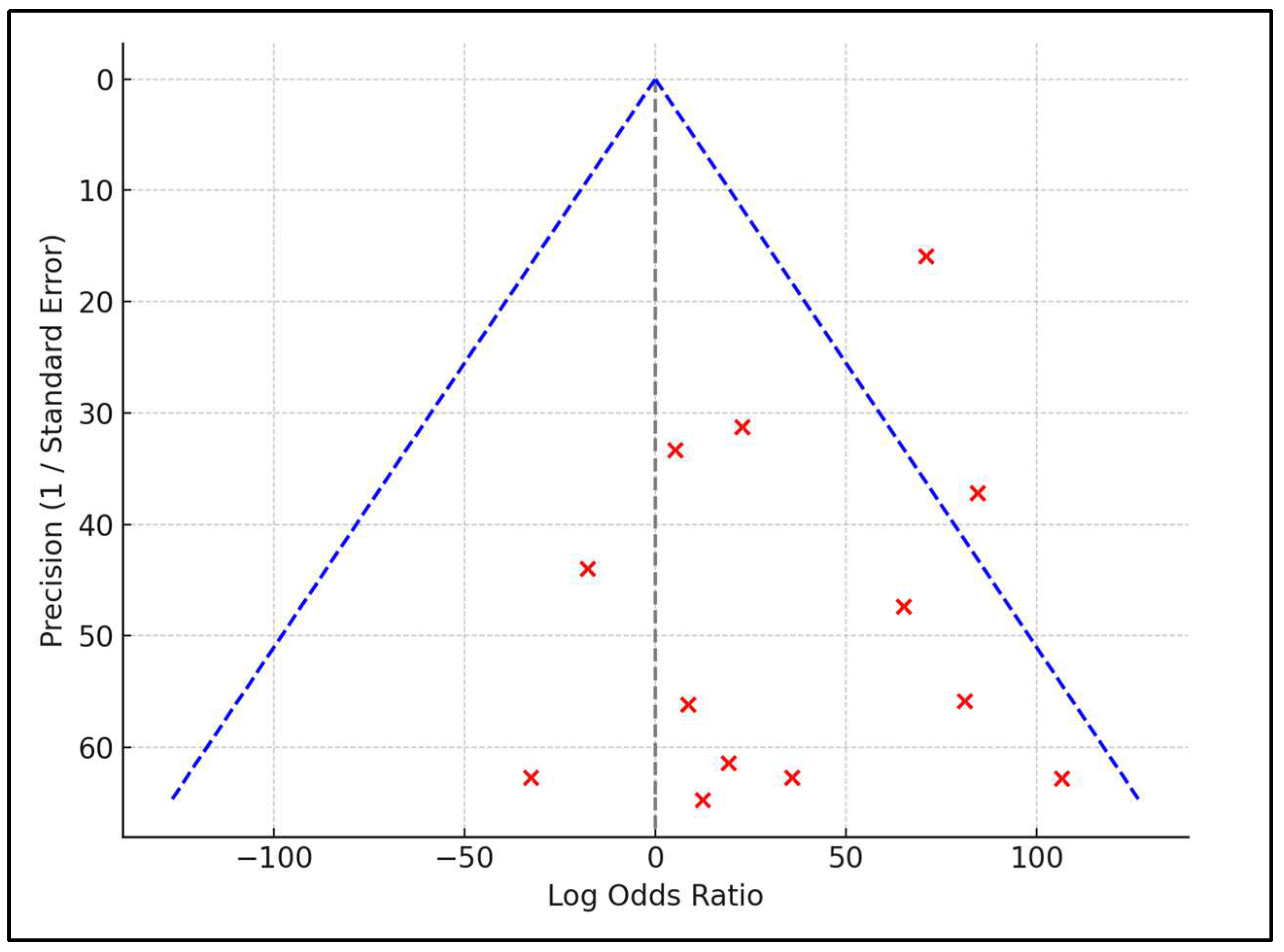
3.4. Analysis of Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sawicka, B.; Aslan, I.; Della Corte, V.; Periasamy, A.; Krishnamurthy, S.K.; Mohammed, A.; Tolba Said, M.M.; Saravanan, P.; Del Gaudio, G.; Adom, D.; et al. The coronavirus global pandemic and its impacts on society. Coronavirus Drug Discov. 2022, 1, 267–311. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Bardan, R.; Muntean, C.; Olariu, A.; Olariu, S. Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned. Medicina 2022, 58, 1322. [Google Scholar] [CrossRef] [PubMed]
- Haileamlak, A. The impact of COVID-19 on health and health systems. Ethiop. J. Health Sci. 2021, 31, 1073–1074. [Google Scholar] [PubMed]
- Toma, A.-O.; Prodan, M.; Reddyreddy, A.R.; Seclaman, E.; Crainiceanu, Z.; Bloanca, V.; Bratosin, F.; Dumitru, C.; Pilut, C.N.; Alambaram, S.; et al. The Epidemiology of Malignant Melanoma during the First Two Years of the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Fericean, R.M.; Rosca, O.; Citu, C.; Manolescu, D.; Bloanca, V.; Toma, A.-O.; Boeriu, E.; Dumitru, C.; Ravulapalli, M.; Barbos, V.; et al. COVID-19 Clinical Features and Outcomes in Elderly Patients during Six Pandemic Waves. J. Clin. Med. 2022, 11, 6803. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.; Lièvre, M.; Laurenson-Schaefer, H.; de Barros, S.; Jinnai, Y.; Andrews, S.; Stricker, T.; Formigo, J.P.; Schultz, C.; Perrocheau, A.; et al. The World Health Organization COVID-19 surveillance database. Int. J. Equity Health 2022, 21, 1–16. [Google Scholar] [CrossRef]
- Rahman, M.; Masum, H.U.; Wajed, S.; Talukder, A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. VirusDisease 2022, 33, 1–22. [Google Scholar] [CrossRef]
- Chirico, F.; da Silva, J.T.; Tsigaris, P.; Sharun, K. Safety & effectiveness of COVID-19 vaccines: A narrative review. Indian J. Med Res. 2022, 155, 91–104. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2021, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Tofarides, A.G.; Christaki, E.; Milionis, H.; Nikolopoulos, G.K. Effect of Vaccination against SARS-CoV-2 on Long COVID-19: A Narrative Review. Life 2022, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Regunath, H.; Goldstein, N.M.; Guntur, V.P. Long COVID: Where Are We in 2023? Mo. Med. 2023, 120, 102–105. [Google Scholar] [PubMed]
- Alghamdi, S.; Alfares, M.; Alsulami, R.; Alghamdi, A.F.; Almalawi, A.M.; Alghamdi, M.S.; Hazazi, H. Post-COVID-19 Syndrome: Incidence, Risk Factor, and the Most Common Persisting Symptoms. Cureus 2022, 14, e32058. [Google Scholar] [CrossRef] [PubMed]
- Lundberg-Morris, L.; Leach, S.; Xu, Y.; Martikainen, J.; Santosa, A.; Gisslén, M.; Li, H.; Nyberg, F.; Bygdell, M. Covid-19 vaccine effectiveness against post-covid-19 condition among 589 722 individuals in Sweden: Population based cohort study. BMJ 2023, 383, e076990. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Srikanth, S.; Boulos, J.R.; Dover, T.; Boccuto, L.; Dean, D. Identification and diagnosis of long COVID-19: A scoping review. Prog. Biophys. Mol. Biol. 2023, 182, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Yang, C.; Zhao, H.; Zhao, H.; Espín, E.; Espín, E.; Tebbutt, S.J.; Tebbutt, S.J. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir. Med. 2023, 11, 504–506. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.; Walker, A.S. Risk of Long COVID in People Infected With Severe Acute Respiratory Syndrome Coronavirus 2 After 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac464. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Brannock, M.D.; Chew, R.F.; Preiss, A.J.; Hadley, E.C.; Redfield, S.; McMurry, J.A.; Leese, P.J.; Girvin, A.T.; Crosskey, M.; Zhou, A.G.; et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ballouz, T.; Menges, D.; Kaufmann, M.; Amati, R.; Frei, A.; von Wyl, V.; Fehr, J.S.; Albanese, E.; Puhan, M.A. Post COVID-19 condition after Wildtype, Delta, and Omicron SARS-CoV-2 infection and prior vaccination: Pooled analysis of two population-based cohorts. PLoS ONE 2023, 18, e0281429. [Google Scholar] [CrossRef] [PubMed]
- Emecen, A.N.; Keskin, S.; Turunc, O.; Suner, A.F.; Siyve, N.; Sensoy, E.B.; Dinc, F.; Kilinc, O.; Oguz, V.A.; Bayrak, S.; et al. The presence of symptoms within 6 months after COVID-19: A single-center longitudinal study. Ir. J. Med Sci. 2022, 192, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Baraff, A.; Fox, A.; Shahoumian, T.; Hickok, A.; O’hare, A.M.; Bohnert, A.S.B.; Boyko, E.J.; Maciejewski, M.L.; Bowling, C.B.; et al. Rates and Factors Associated With Documentation of Diagnostic Codes for Long COVID in the National Veterans Affairs Health Care System. JAMA Netw. Open 2022, 5, e2224359. [Google Scholar] [CrossRef]
- Meza-Torres, B.; Delanerolle, G.; Okusi, C.; Mayor, N.; Anand, S.; Macartney, J.; Gatenby, P.; Glampson, B.; Chapman, M.; Curcin, V.; et al. Differences in Clinical Presentation With Long COVID After Community and Hospital Infection and Associations With All-Cause Mortality: English Sentinel Network Database Study. JMIR Public Health Surveill. 2022, 8, e37668. [Google Scholar] [CrossRef]
- Mohr, N.M.; Plumb, I.D.; Harland, K.K.; Pilishvili, T.; Fleming-Dutra, K.; Krishnadasan, A.; Hoth, K.F.; Saydah, S.H.; Mankoff, Z.; Haran, J.P.; et al. Presence of symptoms 6 weeks after COVID-19 among vaccinated and unvaccinated US healthcare personnel: A prospective cohort study. BMJ Open 2023, 13, e063141. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, T.; Mutubuki, E.N.; de Bruijn, S.; Leung, K.Y.; Knoop, H.; Slootweg, J.; Tulen, A.D.; Wong, A.; van Hoek, A.J.; Franz, E.; et al. Prevalence and Severity of Symptoms 3 Months After Infection With SARS-CoV-2 Compared to Test-Negative and Population Controls in the Netherlands. J. Infect. Dis. 2022, 227, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zisis, S.N.; Durieux, J.C.; Mouchati, C.; Perez, J.; McComsey, G. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study From a Large National Health Research Network. Open Forum Infect. Dis. 2022, 9, ofac228. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iran. J. Med. Sci. 2021, 46, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Babicki, M.; Kapusta, J.; Kałuzińska-Kołat, Z.; Kołat, D.; Jankowski, P.; Mastalerz-Migas,, A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses 2022, 14, 1755. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.V.A.; Garegnani, L.I.; Oltra, G.V.; Metzendorf, M.-I.; Trivisonno, L.F.; Sgarbossa, N.; Ducks, D.; Heldt, K.; Mumm, R.; Barnes, B.; et al. Long-Term Health Symptoms and Sequelae Following SARS-CoV-2 Infection: An Evidence Map. Int. J. Environ. Res. Public Health 2022, 19, 9915. [Google Scholar] [CrossRef]
- Lam, I.C.H.; Wong, C.K.H.; Zhang, R.; Chui, C.S.L.; Lai, F.T.T.; Li, X.; Chan, E.W.Y.; Luo, H.; Zhang, Q.; Man, K.K.C.; et al. Long-term post-acute sequelae of COVID-19 infection: A retrospective, multi-database cohort study in Hong Kong and the UK. EClinicalMedicine 2023, 60, 102000. [Google Scholar] [CrossRef]
- Mayor, N.; Meza-Torres, B.; Okusi, C.; Delanerolle, G.; Chapman, M.; Wang, W.; Anand, S.; Feher, M.; Macartney, J.; Byford, R.; et al. Developing a Long COVID Phenotype for Postacute COVID-19 in a National Primary Care Sentinel Cohort: Observational Retrospective Database Analysis. JMIR Public Health Surveill. 2022, 8, e36989. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of covid-19 vaccination on the symptoms of patients with long covid: A target trial emulation using data from the compare e-Cohort in France. SSRN J. 2021. [Google Scholar] [CrossRef]
- Wynberg, E.; Han, A.X.; Boyd, A.; van Willigen, H.D.; Verveen, A.; Lebbink, R.; van der Straten, K.; Kootstra, N.; van Gils, M.J.; Russell, C.; et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine 2022, 40, 4424–4431. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, U.; Man, K.K.C.; Chen, A.; Wong, I.C.K.; George, J.; Wilson, P.; Wei, L. Definition of Post–COVID-19 Condition Among Published Research Studies. JAMA Netw. Open 2023, 6, e235856. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after su ospital ospitalizationlisation with COVID-19: A longi-tudinal cohort study. Lancet Respir Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post Coronavirus Disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J. Infect Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Zadeh, F.H.; Wilson, D.R.; Agrawal, D.K. Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Finterer, J.; Scorza, F. A retrospective analysis of clinically confirmed long post-COVID vaccination syndrome. J. Clin. Transl. Res. 2022, 8, 506–508. [Google Scholar]
- Dhuli, K.; Medori, M.C.; Micheletti, C.; Donato, K.; Fioretti, F.; Calzoni, A.; Praderio, A.; De Angelis, M.G.; Arabia, G.; Cristoni, S.; et al. Presence of viral spike protein and vaccinal spike protein in the blood serum of patients with long-COVID syndrome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 13–19. [Google Scholar] [CrossRef]
| Reference Number and First Author | Country | Publication Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 [21] Al-Aly et al. | United States | 2022 | Retrospective cohort | Medium |
| 2 [22] Antonelli et al. | United Kingdom | 2022 | Prospective cohort | High |
| 3 [23] Ayoubkhani et al. | United Kingdom | 2022 | Retrospective cohort | Medium |
| 4 [24] Azzolini et al. | Italy | 2022 | Retrospective cohort | Medium |
| 5 [25] Brannock et al. | United States | 2023 | Retrospective cohort | High |
| 6 [26] Ballouz et al. | Switzerland | 2023 | Retrospective cohort | Medium |
| 7 [27] Emecen et al. | Turkey | 2023 | Prospective cohort | High |
| 8 [28] Ioannou et al. | United States | 2022 | Retrospective cohort | High |
| 9 [29] Meza-Torres et al. | United Kingdom | 2022 | Retrospective cohort | Medium |
| 10 [30] Mohr et al. | United States | 2023 | Prospective cohort | High |
| 11 [31] Taquet et al. | United Kingdom | 2022 | Retrospective cohort | Medium |
| 12 [32] van der Maaden et al. | Netherlands | 2023 | Prospective cohort | High |
| 13 [33] Zisis et al. | United States | 2022 | Retrospective cohort | Medium |
| Reference Number and First Author | Number of Patients | Sex/Gender | Age (Mean/Median) | Comparison Group |
|---|---|---|---|---|
| 1 [21] Al-Aly et al. | 33,940 vaccinated | 91.0% Male; 9.0% Female | 67.0 years (median) | Matched unvaccinated patients |
| 2 [22] Antonelli et al. | 1,240,009 (cases 1: first dose), 971,504 (cases 2: second dose) | Cases 1: 62.5% Female, 37.5% Male; Cases 2: 61.2% Female, 38.8% Male | Cases 1: 50.2 years (mean); Cases 2: 52.9 years (mean) | Controls matched by post-vaccination test, healthcare worker status, sex |
| 3 [23] Ayoubkhani et al. | 3333 double-vaccinated, 3090 matched unvaccinated | NR | Double-vaccinated: 49 years (mean); Unvaccinated: 47 years (mean) | Matched unvaccinated patients with COVID-19 |
| 4 [24] Azzolini et al. | 739 (29% of 2560 participants had COVID-19) | 32.7% Female, 26.1% Male (among COVID-19 cases) | 44.3 (mean) | Healthcare workers not requiring hospitalization for COVID-19 |
| 5 [25] Brannock et al. | Clinic-based: 47,404; Model-based: 198,514 | Clinic-based: 65.0% Female, 35.0% Male; Model-based: 64.6% Female, 35.4% Male | Clinic-based: 48.19 years (mean); Model-based: 47.23 years (mean) | Unvaccinated patients with COVID-19 |
| 6 [26] Ballouz et al. | 1350 vaccinated | 52.5% Female; 47.5% Male | 48 years (median) | Individuals infected with different SARS-CoV-2 variants (Wildtype, Delta, Omicron) |
| 7 [27] Emecen et al. | 5610 vaccinated | 51.8% Female, 48.2% Male | 43.1 years (mean) | NR |
| 8 [28] Ioannou et al. | 198,601 vaccinated | 89.1% Male, 10.9% Female | 60.4 years (mean) | Unvaccinated patients with COVID-19 |
| 9 [29] Meza-Torres et al. | 7,396,702 vaccinated | 55.89% Female, 44.11% Male | 44.5 years (median) | Unvaccinated patients with COVID-19 |
| 10 [30] Mohr et al. | 419 vaccinated | 84.0% Female, 15.3% Male | Age distribution: 21.5% (18–29 years), 39.9% (30–39 years), 20.3% (40–49 years), 18.4% (50–64 years) | Unvaccinated healthcare workers with COVID-19 |
| 11 [31] Taquet et al. | 10,024 vaccinated individuals matched to 9,479 controls | 59.4% Female, 50.6% Male | Vaccinated 57.0 years (mean), Unvaccinated 57.6 years (mean) | Unvaccinated (with influenza vaccine) patients with COVID-19 |
| 12 [32] van der Maaden et al. | 9166 cases, 1698 test-negative controls, 3708 population controls | NR | NR | Unvaccinated patients with COVID-19 |
| 13 [33] Zisis et al. | 1,578,719 COVID-19 patients (25,225 vaccinated) | 59.8 female, 50.2% male | Vaccine group: 54.82 years (mean); No-vaccine group: 42.91 years (mean) | Unvaccinated patients with COVID-19 |
| Reference Number and First Author | Vaccine Type * | Number of Doses | Time until Breakthrough Infection ** | Follow-Up |
|---|---|---|---|---|
| 1 [21] Al-Aly et al. | 1 Janssen or 2 Pfizer/Moderna | ≥2 | ≥14 days | 6 months |
| 2 [22] Antonelli et al. | BNT162b2, ChAdOx1 nCoV-19, mRNA-1273 | 2 | Cases 1: Mean 73 days (median 67 days); Cases 2: Mean 51 days (median 44 days) | 1 month |
| 3 [23] Ayoubkhani et al. | 74.0% received Oxford/AstraZeneca, 25.5% Pfizer/BioNTech, 0.5% Moderna | 2 | Median 96 days (IQR, 90–104) for double-vaccinated; Median 98 days (IQR, 89–109) for unvaccinated | ≥12 weeks |
| 4 [24] Azzolini et al. | BNT162b2 | 3 | ≥14 days | 1 month |
| 5 [25] Brannock et al. | 1 Janssen or 2 Pfizer/Moderna | ≥2 | ≥14 days | NR |
| 6 [26] Ballouz et al. | mRNA (BNT162b2 or mRNA-1273), Adenovirus vector (JNJ-78436735) | 1–3 doses | >6 months (77.6%)<6 months (22.4%) | 6 months |
| 7 [27] Emecen et al. | CoronaVac (inactivated virus), BNT162b2 (mRNA) | 1 dose (96.3%), ≥2 doses (3.7%) | NR | 1, 3, and 6 months |
| 8 [28] Ioannou et al. | Moderna and Pfizer | 1–2 doses | NR | 3–8 months |
| 9 [29] Meza-Torres et al. | NR | 1 dose (15,832), two doses (726) | ≥14 days | 1–6 months |
| 10 [30] Mohr et al. | mRNA COVID-19 vaccine | 2 | median of 24.1 weeks between the second vaccine dose and illness onset | 6 weeks |
| 11 [31] Taquet et al. | 1 Janssen or 2 Pfizer/Moderna | 1–2 doses | ≥14 days | 6 months |
| 12 [32] van der Maaden et al. | 1 Janssen or 2 Pfizer/Moderna | 1–2 doses | >2 months | 3 months |
| 13 [33] Zisis et al. | NR | NR | NR | 28 and 90 days post-COVID-19 diagnosis |
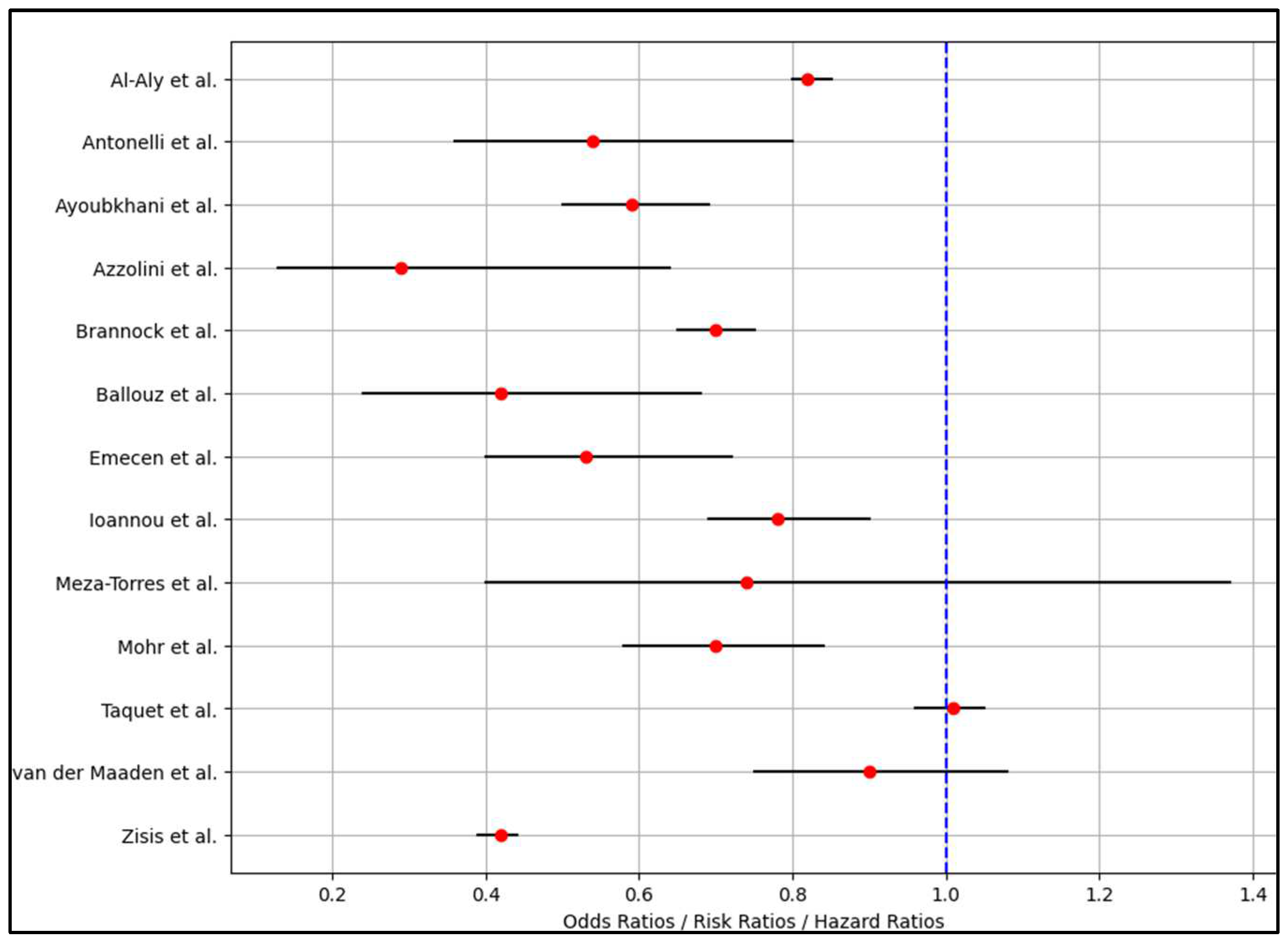
| Reference Number and First Author | Complications | Long COVID Risk (OR/RR/HR) | Other Risks (OR/RR/HR) |
|---|---|---|---|
| 1 [21] Al-Aly et al. | ICU admissions (2.4%) | 0.82 (0.80–0.85) | Mortality - 0.66 (0.58–0.74) |
| 2 [22] Antonelli et al. | Hospitalization in frail older adults post-first dose (23%) and post-second dose (6%) | 0.54 (0.36–0.80) | Frailty in older adults post-first dose (OR 1.93, 95% CI 1.50–2.48); Deprivation post-first dose (OR 1.11, 95% CI 1.01–1.23 for high deprivation) |
| 3 [23] Ayoubkhani et al. | Long COVID symptoms: 9.5% in double-vaccinated vs. 14.6% in unvaccinated; Activity-limiting symptoms: 5.5% in double-vaccinated vs. 8.7% in unvaccinated | 0.59 (0.50–0.69) | Activity-limiting symptoms: aOR 0.59 (95% CI, 0.48–0.73) |
| 4 [24] Azzolini et al. | Long COVID prevalence: Overall 31.0%, Wave 1: 48.1%, Wave 2: 35.9%, Wave 3: 16.5% | 0.29 (0.13–0.64) | Higher risk with older age (OR, 1.23), allergies (OR, 1.50), and more comorbidities (OR, 1.32) |
| 5 [25] Brannock et al. | NR | 0.70 (0.65–0.75) | Number of complications: 0.70 (0.60–0.81) |
| 6 [26] Ballouz et al. | Long-COVID: 25.3% after Wildtype infection, 17.2% after Delta infection, 13.1% after Omicron infection | 0.42 (0.24–0.68) | No clear pattern in long-COVID-related symptoms across variants; absolute risk reduction −10.6% |
| 7 [27] Emecen et al. | ICU admissions (52.0% at 1 month, 36.2% at 3 months, 28.3% at 6 months) | 0.53 (0.40–0.72) | ICU admission: 2.18 (1.51–3.14) |
| 8 [28] Ioannou et al. | Long-COVID (13.6%) | 0.78 (0.69–0.90) | Hospitalization (AOR 2.60: 2.51–2.69), mechanical ventilation (AOR 2.46: 2.26–2.69) |
| 9 [29] Meza-Torres et al. | ICU admissions (0.7%) | 0.74 (0.40–1.37) | OR 2.66 (CI 2.46–2.88) for community infection, OR 2.42 (CI 2.03–2.89) for hospital infection |
| 10 [30] Mohr et al. | NR | 0.70 (0.58–0.84) | Vaccinated patients returned to work sooner (HR 1.37, 1.04–1.79) |
| 11 [31] Taquet et al. | NR | 1.01 (0.96–1.05) | NR |
| 12 [32] van der Maaden et al. | NR | 0.90 (0.75–1.08) | Higher symptom prevalence in cases vs. controls (48.5% vs. 29.8% test-negative and 26.0% population) |
| 13 [33] Zisis et al. | NR | 0.42 (0.39–0.44) | Hypertension OR 0.33 (0.26–0.42), Respiratory symptoms OR 0.54 (0.50–0.57), Diarrhea and constipation OR 0.44 (0.40–0.49) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.-R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. https://doi.org/10.3390/vaccines12020189
Man MA, Rosca D, Bratosin F, Fira-Mladinescu O, Ilie AC, Burtic S-R, Fildan AP, Fizedean CM, Jianu AM, Negrean RA, et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines. 2024; 12(2):189. https://doi.org/10.3390/vaccines12020189
Chicago/Turabian StyleMan, Milena Adina, Daniela Rosca, Felix Bratosin, Ovidiu Fira-Mladinescu, Adrian Cosmin Ilie, Sonia-Roxana Burtic, Ariadna Petronela Fildan, Camelia Melania Fizedean, Adelina Maria Jianu, Rodica Anamaria Negrean, and et al. 2024. "Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis" Vaccines 12, no. 2: 189. https://doi.org/10.3390/vaccines12020189
APA StyleMan, M. A., Rosca, D., Bratosin, F., Fira-Mladinescu, O., Ilie, A. C., Burtic, S.-R., Fildan, A. P., Fizedean, C. M., Jianu, A. M., Negrean, R. A., & Marc, M. S. (2024). Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines, 12(2), 189. https://doi.org/10.3390/vaccines12020189








