Abstract
Introduction: The World Health Organization (WHO) recommends vaccination against hepatitis B as soon as possible following birth for all infants, regardless of prematurity. Hepatitis B vaccination at birth is clearly justified, represents a crucial step in the global control of perinatally acquired hepatitis B and there are no safety concerns in infants born at term. However, there is limited information on the safety of the hepatitis B vaccine in preterm infants, whose immune responses and morbidity risk differ from those in infants born at term. Objectives: The objectives of this paper are to systematically review the literature regarding the safety and risk of adverse events following immunisation (AEFIs) associated with the administration of the hepatitis B vaccine (monovalent or as part of a combination vaccine) to preterm infants. Methods: We performed a search for relevant papers published between 1 January 2002 and 30 March 2023 in the Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials and CINAHL Plus databases. Two authors independently reviewed and analysed each article to include in the systematic review. Narrative synthesis is presented. Results: Twenty-one relevant papers were identified and included in this systematic review. The vast majority of data pertained to multi-antigen (combination) vaccine preparations and vaccination episodes from 6 weeks of age onwards. We found no publications investigating the timing of the birth dose of the hepatitis B vaccine, and AEFI reporting was exclusively short-term (hours to days following administration). There was substantial variability in the reported rate of AEFIs between studies, ranging from 0% to 96%. Regardless of frequency, AEFIs were mostly minor and included injection site reactions, temperature instability and self-limiting cardiorespiratory events. Six studies reported serious adverse events (SAEs) such as the requirement for escalation of respiratory support. However, these occurred predominantly in high-risk infant populations and were rare (~1%). Using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach, the certainty of evidence was assessed as very low. Conclusions: Despite substantial variability between the relatively small number of published studies in terms of cohort selection, definitions, vaccine preparations and reporting, hepatitis B-containing vaccines (mostly as combination vaccines) appear to be relatively well tolerated in preterm infants from 6 weeks of age. Research focusing on the safety of hepatitis B vaccine in preterm infants specifically within 7 days of birth is lacking, particularly regarding long-term morbidity risk. Further research in this area is required.
1. Introduction
Hepatitis B is an important, vaccine-preventable viral infection affecting 257 million people living with chronic hepatitis B, and is responsible for 887,000 deaths a year [1]. This enveloped, partially double-stranded DNA virus can be transmitted in a variety of ways, including through contact with infected blood or bodily fluids [2]. The majority of hepatitis B transmission occurs vertically from a hepatitis B-positive mother, wherein the virus is transmitted from mother to child during pregnancy, delivery or breastfeeding via crackled nipples [3]. Infection in the newborn or during early childhood leads to chronic hepatitis in 95% of infections, whereas infection acquired during adulthood is less likely to lead to chronic disease, with less than 5% of patients in this category [4]. Childhood immunisation including a birth dose of the hepatitis B vaccine is included as two of the five core interventions in the WHO global control plan. As vertical transmission of hepatitis B can be prevented through appropriate vaccination of the infant at birth, timely vaccination is important.
The World Health Organization (WHO) recommends that all infants, regardless of prematurity, be vaccinated against hepatitis B as soon as possible after birth, preferably within 24 h, and an additional 2 or 3 doses of the vaccine are scheduled at least 4 weeks apart. The first dose of this vaccine is often given as a single-antigen product, whereas subsequent doses are most commonly given in combination, for example, with diphtheria, tetanus, pertussis (DTP), polio and Haemophilus influenzae type b (Hib) [5]. Notably, from a global perspective, adherence to vaccination as per national immunisation programs (NIPs) decreases with each scheduled dose. For example, the birth dose of the vaccine is usually given; however, subsequent vaccination rates decrease [6].
The preterm infant’s immune system is physiologically adjusted to life in the sterile intrauterine environment, and adaptation to dealing with extrauterine challenges takes time [7,8]. Moreover, depending on the degree of prematurity and the associated degree of maladaptation to the extrauterine life of other organ systems, preterm infants require substantial and prolonged medical support. Together, the unfit organs attempting to sustain life and adverse effects of such medical support commonly trigger local and systemic inflammation, which in turn contributes to multiple diseases of prematurity that, for instance, affect the brain, lung, heart, gut and eyes. It is important to consider how vaccination might interact with these circumstances [9]. Notably, the situation is different in term infants, who are more likely to be immunised on time (as compared to deferred vaccines in preterm infants), and who tend to tolerate the vaccines better.
We recently published that type 2-polarised inflammation (i.e., the dominance of a particular pathway of immune activation) is a major driver of bronchopulmonary dysplasia (BPD) and BPD-associated pulmonary hypertension (BPD-PH), both cardiopulmonary diseases that affect preterm infants [8]. Notably, we observed a strongly positive correlation between the signature cytokine of type 2 polarisation, IL-4, in T cells and hepatitis B vaccination when administered early, i.e., within the first few hours of life (e.g., odds ratio (OR) of 10.8 if hepatitis B vaccine was given within the first 24 h compared with no vaccination (95% confidence interval (CI) 2.9–4.0, p = 0.0004)) [8]. In contrast, vaccination at approximately day 7 of life was not associated with increased type 2 polarisation. Type 2-polarised inflammation was also strongly associated with BPD in the infants we studied, and we demonstrated the causality of this type of inflammation driving BPD in a murine model of disease (in which BPD was significantly prevented when type 2 inflammation was blocked) [8]. Therefore, these results raised an important question regarding the safety of early hepatitis B vaccination in preterm infants, especially those at risk of BPD.
In a previous systematic review [10], we noted that the timing of the first dose of the hepatitis B vaccine in preterm infants is highly variable around the world. Only 13% of the guidelines we identified and reviewed recommended a birth dose of the hepatitis B vaccine for all infants, regardless of prematurity. In 40% of the guidelines, the birth dose was only recommended for infants with a birth weight of more than 2000–2200 g; in 33% of the guidelines, there was no generalised birth dose recommended for all infants, and another 13% of guidelines had variable recommendations for this vaccine.
Hardly any research is published surrounding the safety of hepatitis B vaccination soon after birth in preterm babies. Adverse events following immunisation (AEFIs) are untoward events that occur following vaccination, irrespective of causality, while adverse effects are those where causality has been established. Based on our previous paper [10], it is clear that the timing of hepatitis B vaccination around the world is variable, and these circumstances call for a look at the literature for the safety of hepatitis B vaccination at different time points of administration. Accordingly, we aimed to assess the safety of the hepatitis B vaccine (as a monovalent or a combination vaccine) in infants. In doing so, we considered the AEFIs of the hepatitis B vaccine, specifically in association with the time point of administration in preterm infants at risk of BPD in addition to other long-term AEFIs of the vaccine.
2. Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [11].
2.1. Eligibility Criteria
Human studies of any study design investigating the safety of hepatitis B vaccination in preterm infants, defined as infants born less than 37 weeks of gestation, were included in this review. Studies of both term and preterm infants were included, provided the paper reported results of the preterm infant group. Studies investigating safety outcomes of the hepatitis B vaccination as a monovalent product or in combination with other vaccine products were eligible for this review. Relevant studies published after 1 January 2002 were included, even if participants were born prior to 1 January 2002. The exclusion criteria included protocols, literature reviews, conference abstracts, studies on animal models, studies not specific to preterm infants, studies investigating an intervention that did not include the hepatitis B vaccine, studies where the full text was not accessible in English and studies published prior to 1 January 2002.
2.2. Search Strategy
A search was initially performed broadly to identify articles related to the safety of vaccines given to preterm babies at any age. This search was subsequently narrowed to the safety of hepatitis B vaccines in preterm babies, and later further refined to the safety of hepatitis B vaccines in preterm babies given at or soon after birth. Relevant articles published between 1 January 2002 and 30 March 2023 were identified by searching the Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials and CINAHL Plus databases. The search strategy is outlined in Supplementary Table S1.
2.3. Study Selection
The search results from all databases were imported into the Covidence systematic review software (https://www.covidence.org/). At least two independent authors performed title and abstract screening to determine whether each article was to be included in the review, based on the inclusion and exclusion criteria. Any discrepancies in reviewer decisions were resolved via discussion with a third reviewer. This process was then applied to full-text screening.
2.4. Data Collection
Relevant articles identified were extracted and analysed by two independent reviewers. Information extracted from studies included study population, study design, methods, vaccine used, results and conclusions. This information was further summarised, with the primary outcome being the safety effects of the hepatitis B vaccine and a narrative synthesis of data was conducted. Definitions and terminology of AEFI and serious adverse events (SAEs) associated with vaccine administration were reported in accordance with The Definition and Application of Terms for Vaccine Pharmacovigilance, a report written by the Council for International Organizations of Medical Sciences and World Health Organization [12].
2.5. Quality Assessment
The risk of bias in included studies was performed using the Risk of Bias In Non-randomised Studies of Interventions (ROBINS-I) [13,14]. For each study, two independent reviewers assessed the risk of seven types of bias. These were bias due to confounding, bias in the selection of participants into the study, bias in classification of interventions, bias due to deviation from intended interventions, bias due to missing data, bias in measurement of outcomes and bias in selection of the reported results. Discrepancies between reviewer judgements were resolved through discussion with a third reviewer. The overall quality of evidence of included studies was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [15,16].
3. Results
3.1. Study Selection
Our search strategy identified a total of 4988 studies, of which 2993 duplicate articles were automatically removed and 9 were manually removed, resulting in 1986 articles being screened. After title and abstract screening, 1872 studies were excluded based on the predefined selection criteria, and an additional 9 articles were unable to be retrieved in full text. There were 105 articles that underwent full-text screening, and 84 studies were excluded for the following reasons: wrong population (n = 9), wrong intervention (n = 22), wrong outcome, specifically no safety data (n = 42), wrong study design (n = 14) and full text unavailable in English (n = 15). In total, 21 studies were deemed eligible for inclusion in this review (Figure 1).
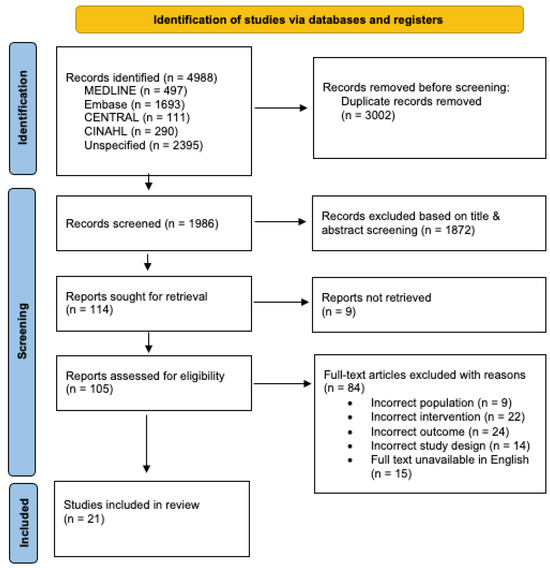
Figure 1.
PRISMA flow chart summarising the study selection and screening process.
3.2. Study Characteristics
The characteristics of included studies are outlined in Table 1 and Table 2. Out of the total 21 studies identified, the most commonly used vaccine was Infanrix Hexa (DTPa-HBV-IPV/Hib, GSK Vaccines, Middlesex, UK) (n = 12). All but 3 studies reported on multi-antigen vaccines such as the hexavalent DTPa-IPV-HepB-Hib administered from 6 weeks of age or later as opposed to the single-target hepatitis B vaccine. All of the 21 studies we included reported exclusively on short-term AEFIs, typically covering the first 24 h to 1 week at most. Additionally, the vast majority of vaccination episodes did not use a single-antigen vaccine, but antigen combinations instead.

Table 1.
Summary of safety outcomes of vaccines administered to preterm infants greater than 7 days post birth. Abbreviations used: AEFI, adverse event following immunisation; SAE, serious adverse event.

Table 2.
Summary of safety outcomes of vaccines containing hepatitis B given to preterm infants within 7 days of birth. Abbreviations used: AEFI, adverse event following immunisation; SAE, serious adverse event.
3.3. Safety of Hepatitis B Vaccine Administration in Preterm Infants
Seven studies reporting on a total number of 3655 infants showed a low rate of AEFIs (<10%, Table 1, Table 2 and Table 3). However, other studies reported somewhat different findings. For example, 9 other studies published AEFI rates greater than 10% in a total number of 1833 babies (Table 1 and Table 3), with one study observing non-cardiorespiratory AEFIs in up to 96% of vaccination episodes [32]. However, in each of these 15 studies, the reported AEFIs were mostly minor, including low-grade fever, local reactions such as rashes, local pain, erythema and swelling, and short-lived episodes of apnoea and bradycardia, which did not require escalation of support (total of 5488 babies; Table 1, Table 2 and Table 3).

Table 3.
Summary of AEFI rate seen in included studies. Abbreviations used: AEFI, adverse event following immunisation; SAE, serious adverse event.
In contrast, in certain patient groups, for example, infants who presented with apnoea following vaccination once [26] or infants who required in-hospital monitoring because of increased recurrence of cardiorespiratory events during the first immunisation [30], AEFIs were much more common (Table 1 and Table 3). In both studies reporting on such special cohorts, the recurrence of AEFIs was greater than 90%, and one infant in Clifford et al. developed a hypotonic hyporesponsive episode [26].
SAEs were reported to be associated with the vaccines containing the hepatitis B antigen in 6 studies [20,22,24,26,27,32] with a total number of 1051 infants (Table 1 and Table 3, SAEs highlighted in bold). Such SAEs comprised apnoea/bradycardia/desaturations (recurrent or at least requiring stimulation or intermittent positive pressure ventilation), which occurred in 8% to 18% of preterm infants following a multi-antigen vaccine containing hepatitis B. In 3 studies [22,26,27], escalation of respiratory support, including endotracheal intubation, use of CPAP or high flow, and admission to ICU was sometimes required. In [22], 9 of the 19 infants with a birth weight of less than 1500 g presenting with apnoea required endotracheal intubation and mechanical ventilation. Similarly, in a retrospective audit [27], of the 17 infants diagnosed with vaccine-related apnoea, 9 required re-admission to an intensive care unit for respiratory support with CPAP or high-flow nasal cannula. However, most of these SAEs were either reported in high-risk cohorts or in the studies that did identify them as relatively rare.
Besides our own study [8], which was not designed to address the question we are aiming to answer here, only two studies [35,36] reported on hepatitis B vaccination within 7 days of birth (Table 2). Neither of these studies made special mention of the birth dose, and AEFIs were either rare [36] or absent [35]. Hence, although 1666 infants who received a single-antigen hepatitis B vaccine at birth were included in studies we identified, there was no data to allow for any conclusions regarding the safety of hepatitis B vaccination at and around the time of birth in preterm infants.
Three studies explored the question of whether preterm birth predisposes infants to a higher risk of AEFIs in response to vaccinations ([19,32,34]; Table 1 and Table 3). In the 333 infants studied overall, this appeared not to be the case, as the occurrence of AEFIs was not significantly different between these groups. Similarly, this observation was also found in one study that reported on whether preterm birth predisposes infants to a higher risk of AEFIs when compared to term infants [32].
No studies reported on the specific impact of different timing strategies regarding hepatitis B vaccination in preterm infants upon the risk of BPD or on AEFIs following a first dose given in the first few hours of life. Moreover, we found no literature on long-term AEFIs, i.e., effects beyond hours to days. Further to this, there were no studies specifically focusing on the single-antigen hepatitis B vaccine on premature infants.
3.4. Quality Assessment
The risk of bias assessment of included studies is summarised in Supplementary Figure S1. Across the evaluated biases, most studies were judged as having low risk or unclear risk of bias due to a lack of information available. However, notably, 17 of a total of 21 studies were judged as having a moderate risk of bias in the measurement of outcomes. This judgement was based on the absence of investigator blinding across these studies. The GRADE system for rating the quality of evidence and strength of recommendation was used to assess the level of evidence of included studies. All studies began with an initial low quality of evidence (+2) due to their observational study designs. The certainty of evidence was downgraded (−1) due to the ‘serious’ inconsistency identified across the reported results of the 21 studies. Of note, the percentage of reported AEFIs varied from 0 to 90% across the 21 studies. In following the GRADE approach, the quality of evidence was not further downgraded as no serious risk of bias, indirectness, imprecision or publication bias was identified. The certainty of the evidence was not upgraded as no large effect, dose–response or all plausible confounding was identified. Thus, in following the GRADE approach, the overall quality of evidence included in this review was judged as very low.
4. Discussion
This systematic review was conducted to determine the safety of administering the hepatitis B vaccine (either monovalent or in combination vaccines) to preterm infants, especially soon after birth. This review was conducted on the background of our previous prospective immune profiling cohort study [8], raising the possibility that the birth dose of the single-antigen hepatitis B vaccine further increases the risk of BPD in preterm infants already at risk of developing this chronic cardiopulmonary disease. We did not find any of the literature investigating this specific question.
Birth dose hepatitis B vaccination policies for preterm infants are highly variable around the world [10]. Apart from the prevention of vertical transmission of hepatitis B—which of course is of critical importance, particularly in regions with high hepatitis B prevalence—there is no known added benefit of receiving a birth dose of the hepatitis B vaccine. For the development of immunogenicity in preterm infants, commencing hepatitis B vaccination at 6–8 weeks of life is sufficient, provided infants born <32 weeks of gestation or <2000 g birth weight receive an additional booster dose at 12 months of age [37]. Since our research has raised safety concerns in a specific patient group, i.e., preterm infants at risk for BPD, the question of the safety of the hepatitis B birth dose should be addressed as a matter of urgency.
The studies we identified were observational studies, with relatively small sample sizes, describing safety outcomes in the hours and days following hepatitis B vaccination. The hepatitis B antigen was almost always part of a multi-antigen (combination) vaccine preparation. Moreover, most studies did not include the birth dose, but commenced monitoring from the 6 week-time point onwards. We did not find any randomised studies or any research on long-term AEFIs. Therefore, it is difficult to determine the short-term safety of the hepatitis B vaccine at birth or in the first 6 weeks of life and is not possible to conclusively comment on its long-term safety. Thus, research in this field is required.
Overall, there were some consistent short-term temporal associations with vaccination administration, including minor local reactions and self-limiting cardiorespiratory events not requiring escalation of support after the 6-week to 2-month doses. Nonetheless, the published studies substantially diverge regarding the frequency of such minor AEFIs, ranging from 0 to 96%. The reported occurrence of SAEs, such as the requirement to escalate respiratory support, is similarly variable, with some studies reporting such SAEs and others not. These differences are likely due to cohort selection, duration of observation and variability in definitions and reporting. The importance of cohort selection in particular is demonstrated by studies such as Clifford et al. [26] and Bohnhorst et al. [30], which investigated higher-risk infants and consistently observed high AEFI rates. More definitive evidence in this regard would be useful, for example, to identify populations at higher risk of presenting with AEFIs, thus guiding monitoring requirements.
This review has several limitations. Almost all studies we reviewed reported on combination products including hepatitis B, typically with pertussis antigens, tetanus and diphtheria toxoids, Hib and polio. Therefore, it is nearly impossible to establish whether the AEFIs of these vaccines were related to the hepatitis B antigen/vaccine or other components of the preparation, possibly including adjuvants. Cardiorespiratory events similar to those identified in the studies included here have been described following pertussis-containing vaccines [38]. Furthermore, there are limitations regarding the implemented search strategy. In combining the search terms of ‘adverse event’, ‘safety’, ‘complication’, ‘surveillance’, ‘deterioration’ and ‘response’ with the Boolean operator ‘or’ studies that have used other terminology to describe safety outcomes may have been missed. Additionally, only studies where the full text was available in English were included in this study. In addressing such limitations, additional studies may have been eligible for this review and subsequently altered the overall conclusions made.
In summary, despite substantial variability between the relatively small number of published studies, hepatitis B-containing vaccines (mostly as a combination) appear to be relatively well tolerated in preterm infants from 6 weeks of age. There is minimal published information available on the safety of the birth dose of single-antigen (monovalent) hepatitis B vaccines in preterm infants, especially in terms of prematurity-related morbidities.
5. Conclusions
Due to the low number of published studies, low sample size, high variability in cohort selection, vaccine preparations and reporting, as well as very low certainty of evidence available, a conclusion is difficult to establish. Vaccination against hepatitis B at birth remains a critical component of global disease prevention, particularly in settings where maternal hepatitis B carriage is high and no safety concerns have been raised in infants born at term, who represent the vast majority of neonates worldwide. However, for the ~0.5% of neonates born prematurely who are at risk of BPD and for whom, therefore, hepatitis B vaccination at birth may carry an increased risk [8], there is a paucity of literature regarding the safety of this vaccine. Notably, guidelines in many regions with low hepatitis B prevalence and/or reliable antenatal screening programs and/or high vaccination rates in the first years of life already recommend not administering the birth dose to preterm infants (e.g., birth weight < 2000 g [10]). Given that the birth dose is not necessary for immunogenicity in preterm babies if a 12-month dose is used [31], the pragmatic approach already used in many regions appears sensible until reliable safety data are published.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12030261/s1, Figure S1: Risk of bias assessment of included clinical studies using. Abbreviations used: +, low risk of bias; ?, unclear risk of bias; −, moderate risk of bias; Table S1: Search strategy applied to (A) MEDLINE via Ovid 1946 to 30 March 2023; (B) Embase via Ovid 1947 to 30 March 2023; (C) Cochrane Central Register of Controlled Trials via 30 March 2023; (D) CINAHL Plus 30 March 2023.
Author Contributions
Concept and Design: M.F.N. and A.M.; Investigation: all authors; Data Collection: Q.W.T., R.O., J.B., C.A.N.-P. and E.P.; Data Analysis: Q.W.T., R.O., R.P., A.M. and E.P.; Validation: M.F.N. and A.M.; Manuscript Draft Q.W.T., R.O., M.F.N. and A.M.; Manuscript Review and Editing: all authors; Supervision: M.F.N. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was sought for this research. C.A.N.-P. is supported by an NHMRC Investigator Grant Leadership 1 (grant 1173584), M.F.N. by a Fielding Foundation Fellowship 2017, and NHMRC (Australia) supports A.M.’s research (grant 2008793).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article (and Supplementary Materials).
Conflicts of Interest
J.B. serves on a data safety monitoring board for GSK related to hepatitis B. He does not receive personal compensation but Murdoch Children’s Research Institute is compensated for his time. All other authors declare no conflicts of interest.
References
- Liu, J.; Liang, W.; Jing, W.; Liu, M. Countdown to 2030: Eliminating hepatitis B disease, China. Bull. World Health Organ. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Australian Government—Department of Health and Aged Care. Hepatitis B: Australian Government. 2022. Available online: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/hepatitis-b (accessed on 8 December 2023).
- Di Filippo Villa, D.; Navas, M.C. Vertical Transmission of Hepatitis B Virus—An Update. Microorganisms 2023, 11, 1140. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis B: World Health Organiation. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 8 December 2023).
- Australian Government—Department of Health and Aged Care. National Immunisation Program Schedule: Australian Government. 2023. Available online: https://www.health.gov.au/resources/publications/national-immunisation-program-schedule?language=en (accessed on 8 December 2023).
- Buttery, J.P.; Graham, S.M. Immunisation timing: The protective layer in vaccine coverage. Lancet 2009, 373, 1499–1500. [Google Scholar] [CrossRef]
- Di Simone, S.K.; Rudloff, I.; Nold-Petry, C.A.; Forster, S.C.; Nold, M.F. Understanding respiratory microbiome-immune system interactions in health and disease. Sci. Transl. Med. 2023, 15, eabq5126. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.C.; Bui, C.B.; Pang, M.A.; Cho, S.X.; Rudloff, I.; Elgass, K.; Schröder, J.; Maksimenko, A.; Mangan, N.E.; Starkey, M.R.; et al. Type 2 immune polarization is associated with cardiopulmonary disease in preterm infants. Sci. Transl. Med. 2022, 14, eaaz8454. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.M.; Moss, T.J. The immune consequences of preterm birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Miller, T.; Carr, J.; Buttery, J.; Nold-Petry, C.A.; Nold, M.F.; Malhotra, A. Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants. Vaccines 2022, 10, 1656. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. Surg. J. 2021, 88, 105906. [Google Scholar] [CrossRef]
- CIOMS; WHO. Definition and Application of Terms for Vaccine Pharmacovigilance; Council for International Organizations of Medical Sciences: Geneva, Switzerland; World Health Organization: Geneva, Switzerland, 2012; pp. 29–60. Available online: https://cioms.ch/wp-content/uploads/2017/01/report_working_group_on_vaccine_LR.pdf (accessed on 8 December 2023).
- Sterne, J.A.; Hernan, M.A.; Reevas, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, j4919. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Cuello, C.; Akl, E.A.; Mustafa, R.A.; Meerpohl, J.J.; Thayer, K.; Morgan, R.L.; Gartlehner, G.; Kunz, R.; Katikireddi, S.V.; et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 2019, 111, 105–114. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ellison, V.J.; Davis, P.G.; Doyle, L.W. Adverse reactions to immunization with newer vaccines in the very preterm infant. J. Paediatr. Child. Health 2005, 41, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, S.; Heininger, U.; Lücking-Famira, M.; Hubert, F. Apnoea and bradycardia in preterm infants following immunisation with pentavalent or hexavalent vaccines. Eur. J. Pediatr. 2005, 164, 432–435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omenaca, F.; Garcia-Sicilia, J.; Garcia-Corbeira, P.; Boceta, R.; Romero, A.; Lopez, G.; Dal-Ré, R. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: First experiences and solutions to a serious and sensitive issue. Pediatrics 2005, 116, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Pourcyrous, M.; Korones, S.B.; Arheart, K.L.; Bada, H.S. Primary immunization of premature infants with gestational age <35 weeks: Cardiorespiratory complications and C-reactive protein responses associated with administration of single and multiple separate vaccines simultaneously. J. Pediatr. 2007, 151, 167–172. [Google Scholar] [PubMed]
- Faldella, G.; Galletti, S.; Corvaglia, L.; Ancora, G.; Alessandroni, R. Safety of DTaP-IPV-HIb-HBV hexavalent vaccine in very premature infants. Vaccine 2007, 25, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.A.; Madhi, S.A.; Huebner, R.E.; Mbelle, N.; Karim, S.S.; Kleinschmidt, I.; Forrest, B.D.; Klugman, K.P. Apnea and its possible relationship to immunization in ex-premature infants. Vaccine 2008, 26, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Garcia, F.; Ruttimann, R.; Coconier, G.; Jacquet, J.M.; Schuerman, L. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr. 2008, 97, 1243–1249. [Google Scholar] [CrossRef]
- Hacking, D.F.; Davis, P.G.; Wong, E.; Wheeler, K.; McVernon, J. Frequency of respiratory deterioration after immunisation in preterm infants. J. Paediatr. Child Health 2010, 46, 742–748. [Google Scholar] [CrossRef]
- Furck, A.K.; Richter, J.W.; Kattner, E. Very low birth weight infants have only few adverse events after timely immunization. J. Perinatol. 2010, 30, 118–121. [Google Scholar] [CrossRef]
- Clifford, V.; Crawford, N.W.; Royle, J.; Lazzaro, T.; Danchin, M.; Perrett, K.P.; Lee, K.J. Recurrent apnoea post immunisation: Informing re-immunisation policy. Vaccine 2011, 29, 5681–5687. [Google Scholar] [CrossRef]
- Anderson, J.; Noori, K.; Morris, S.A. Apnoea after the 2-month immunisation in extremely preterm infants: What happens with the 4-month immunisation? J. Paediatr. Child Health 2013, 49, E217–E220. [Google Scholar] [CrossRef] [PubMed]
- Montague, E.C.; Hilinski, J.A.; Williams, H.O.; McCracken, C.E.; Giannopoulos, H.T.; Piazza, A.J. Respiratory Decompensation and Immunization of Preterm Infants. Pediatrics 2016, 137, e20154225. [Google Scholar] [CrossRef]
- Martinelli, D.; Fortunato, F.; Del Matto, G.; Iannelli, G.; Prato, R. Post-marketing surveillance study of the DTaP2-IPV-HB-Hib (Hexyon) vaccine administered in preterm infants in the Apulia region, Italy, in 2017. Vaccine 2020, 38, 5148–5153. [Google Scholar] [CrossRef]
- Bohnhorst, B.; Weidlich, C.; Peter, C.; Bohne, C.; Kattner, E.; Pirr, S. Cardiorespiratory Events Following the Second Routine Immunization in Preterm Infants: Risk Assessment and Monitoring Recommendations. Vaccines 2021, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, I.; Dammann, M.T.; Humberg, A.; Siller, B.; Stichtenoth, G.; Engels, G.; Marißen, J.; Faust, K.; Hanke, K.; Goedicke-Fritz, S.; et al. Five Year Follow up of Extremely Low Gestational Age Infants after Timely or Delayed Administration of Routine Vaccinations. Vaccines 2021, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Wilck, M.B.; Xu, Z.J.; Stek, J.E.; Lee, A.W. Safety and immunogenicity of a fully-liquid DTaP-IPV-Hib-HepB vaccine (Vaxelis) in premature infants. Hum. Vaccine Immunother. 2021, 17, 191–196. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, C.; Guo, X.; Zhang, L.; Mei, K.; Zhou, B.; Lu, J.; Lu, Y. Vaccination experiences of premature children in a retrospective hospital-based cohort in a Chinese metropolitan area. Hum. Vaccine Immunother. 2021, 17, 5235–5241. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.F.; Coblisan, P.; Capalna, L.; Panta, P.C.; Buzoianu, A.D.; Bocsan, I.C. Safety of Vaccination within First Year of Life-The Experience of One General Medicine Center. Children 2023, 10, 104. [Google Scholar] [CrossRef]
- Bhave, S.; Bhise, S.; Chavan, S.C.; Naik, S.S.; Pusapati, R.V.; Bavdekar, A.; Pandit, A. Hepatitis B vaccination in premature and low birth weight (LBW) babies. Indian Pediatr. 2002, 39, 625–631. [Google Scholar] [PubMed]
- Xu, Y.; Ji, C.; Liu, Y.; Li, M.; Yao, D.; Wang, X.; Du, J.; Chen, J. Vaccination recommendations, immunization status and safety of vaccination for premature infants in Zhejiang, China. Expert Rev. Vaccines 2020, 19, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Australian Government—Department of Health and Aged Care. Vaccination for Preterm Infants: Australian Government. 2023. Available online: https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-preterm-infants (accessed on 8 December 2023).
- Lee, J.; Robinson, J.L.; Spady, D.W. Frequency of apnea, bradycardia, and desaturations following first diphtheria-tetanus-pertussis-inactivated polio-Haemophilus influenzae type B immunization in hospitalized preterm infants. BMC Pediatr. 2006, 6, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).