Abstract
Background: In the Americas, deaths by diseases avoidable with vaccines are a significant contributor to child mortality. An essential means of reducing this is through broad vaccine coverage. The COVID-19 pandemic has posed a potential disruption to vaccine coverage due to its effects on the healthcare system. Objectives: this study aims to evaluate the impact of the COVID-19 pandemic on DTP3 vaccination coverage in the Americas, investigating trends from 2012 to 2022 to identify significant changes, regional disparities, and the overall effect of the pandemic on progress towards global immunization targets. Methods: This study used the coverage data for the third dose of the diphtheria, tetanus, and pertussis vaccine (DTP3) pulled from UNICEF databases spanning 2012 to 2022. We conducted a Joinpoint regression to identify points of significant trend changes. The annual percentage change (APC) and 95% confidence intervals (95% CIs) were calculated for America and its regions. We also used segmented regression analysis. Using the Chi-square test, we compared DTP3 vaccination coverage for each country between 2019 and 2022. Results: Overall, America saw a decrease in vaccine coverage during this period, with an APC of −1.4 (95% CI −1.8; −1.0). This trend varied across regions. In North America, the decrease was negligible (−0.1% APC). South America showed the steepest decrease, with an APC of −2.5%. Central America also declined, with an APC of −1.3%. Our findings suggest a concerning trend of declining DTP-vaccination rates in the Americas, exacerbated in certain regions, in the wake of the COVID-19 pandemic. The absolute decrease in vaccine coverage in the Americas was −4% between 2019 and 2022, with the most important drop being in Central America (−7%). However, six countries reported increased vaccination rates post-COVID-19, led by Brazil, with a 7% increase. Conversely, twenty-two countries registered a decline in DTP3 vaccine coverage, with the average decrease being −7.37%. This decline poses an important challenge to achieving the WHO’s target of 90% coverage for the third dose of DTP by 2030, as evidenced by the reduction in the number of countries meeting this target from 2019 to 2022. Conclusions: The COVID-19 pandemic has impacted vaccine coverage in America, leading to a decrease, especially across Central America.
1. Introduction
Immunization is a cornerstone in public health, pivotal in diminishing the prevalence and severity of infectious diseases and their effects on health outcomes [1,2,3]. Vaccine-preventable diseases remain a significant public health concern globally. Diphtheria, tetanus, and pertussis (DTP) vaccines prevent these life-threatening diseases, especially among children. The DTP vaccine’s broad coverage is imperative for public health protection. However, the COVID-19 pandemic, emerging in early 2020, posed significant challenges to healthcare systems globally [4], potentially impacting routine immunization services, including DTP vaccination. Programs emphasizing immunization, especially those incorporating the DTP vaccine, are central to reducing the worldwide incidence of diseases preventable by vaccines, thereby improving the lives of countless individuals [5,6,7,8]. The World Health Organization (WHO) launched, in response to this global health challenge, the Global Vaccine Action Plan (GVAP) for the period 2011–2020, initiated in 2012, to achieve widespread routine immunization for children across the globe [9].
Child mortality due to vaccine-preventable diseases is a significant concern, with pertussis alone accounting for over 300,000 deaths annually [10]. Factors influencing disparities in vaccination rates include geographic location (urban vs. rural), socioeconomic status, educational level [11,12], and the regularity of maternal prenatal care [13]. Parents in rural areas believe vaccine-preventable diseases (VPD) are not severe enough to justify vaccination [14]. The emergence of the COVID-19 pandemic has placed an extraordinary burden on global healthcare infrastructures, potentially impacting the continuity of standard immunization efforts [15,16,17,18,19,20]. Disruptions in the supply chain of vaccines, the redirection of healthcare resources and personnel to COVID-19 management [21], the logistic challenges of COVID-19 immunization [22], and heightened public reluctance to visit medical facilities due to infection risk have all been implicated in the observed decrease in vaccination rates [23,24,25,26,27,28]. Such a downturn in immunization coverage poses a severe danger to millions of children, leaving them vulnerable to diseases preventable by vaccines [29]. The pandemic has increased the likelihood of outbreaks of VPD [12]. Lower–middle-income regions with low vaccine coverage and circulating vaccine-derived viral strains, such as polio, bore an additional burden of zero-dose children. All over the world, there were 18.2 million zero-dose children in 2021 [30] that were more vulnerable to VPDs. [12] A precise understanding of the disruption’s impact is vital for tailoring public health policies and building resilience for future crises [31]. Research has shown a global decline of 7.7% in DTP3 coverage and of 7.9% in MCV1 (first dose of measles-containing vaccine) coverage up to December 2020 [32].
In assessing the success of DTP-vaccination programs, we focus on the proportion of infants receiving DTP3, a standard and widely recognized metric [33]. This indicator reflects the effectiveness of the immunization programs in reaching target demographics and completing the primary vaccination series, providing optimal protection against diphtheria, tetanus, and pertussis [34]. The analysis of both DTP1 and DTP3 coverages is essential, with DTP1 coverage indicating the initial reach and engagement of health services [35,36] and DTP3 demonstrating the success in administering the entire course of the vaccine [37]. For instance, a meta-analysis highlighted a significant dropout rate in vaccinations in Africa, with notable variations between countries [35,36]. Discrepancies between DTP1 and DTP3 coverages can shed light on challenges in patient retention and other barriers such as healthcare access, affordability, and education [37].
The Americas ranks as the second-worst region globally regarding vaccine coverage [38,39,40]. Two countries in America were included in the Immunization Agenda 2030 in 2021: Brazil was ranked seventh and Mexico in 15th. In 2022, Mexico disappeared from the list, and Brazil descended to the eighth position [41].
The pandemic’s disruption had widespread implications for DTP-vaccination coverage. Healthcare resources were reallocated to address the pandemic, leading to the neglect of routine vaccination programs. Social distancing and lockdowns are public health measures that may have hindered access to vaccination services. Moreover, the pandemic could have affected public perception and confidence in vaccines, further challenging vaccination efforts. In the United States, during the pandemic, there was a decrease in pediatric primary care visits [42]. Recent data points to a substantial impact of the COVID-19 pandemic on DTP-vaccination trends [43]. A significant decline in administered doses of DTP-containing vaccines was observed in the first half of 2020. This trend was not limited to specific regions but was a global phenomenon with varying degrees of impact across various parts of the world. In Africa, for instance, there was a notable decrease in DTP3 coverage post-2019 [44].
Similarly, a decline in vaccination coverage for multiple vaccines was observed in Latin America, with catch-up strategies implemented to address missed vaccinations [45]. Reports from WHO and UNICEF indicate a notable global decrease in child vaccinations, with DTP3 coverage falling by 5% between 2019 and 2021 [30]. The CDC’s 2021 data confirms this trend, showing the lowest global DTP3 coverage since 2008 [46]. From 2021 to 2022, there was a notable increase in global vaccination coverage for the first dose of the DTP vaccine, from 86% to 89%, and the measles-containing vaccine, from 81% to 84%. However, these levels did not return to the pre-pandemic coverage rates of 90% and 86%, respectively. Despite the challenges of the pandemic, there have been signs of recovery. By 2022, global DTP immunization coverage nearly returned to pre-pandemic levels, although millions of infants still lacked initial or complete vaccination [35,47]. This recovery highlights the resilience of health systems and the importance of ongoing assessment and the implementation of catch-up vaccination strategies, particularly for vulnerable populations, to ensure vaccine-coverage equity and health system resilience. This recovery in vaccination coverage was uneven across different regions and countries, with slower progress in low-income countries [48]. This data, part of the World Health Assembly’s endorsement of the Immunization Agenda 2030 (IA2030), highlights the ongoing challenge of restoring and improving global vaccination coverage after the COVID-19 pandemic, particularly among low- and lower–middle-income countries [49].
This study aims to analyze the trends in DTP3 vaccination coverage in America from 2012 to 2022, emphasizing the influence of the COVID-19 pandemic. Based on the evidence discussed, we posit that the pandemic has adversely affected vaccination rates [46,49].
Considering the available data, we observe a significant global impact of the COVID-19 pandemic on DTP-vaccination trends. Reports from WHO and UNICEF highlight a worldwide decline in child vaccinations during this period [50]. The risk for children in developing vaccine-preventable diseases is thus elevated. This study aims to assess the impact of the COVID-19 pandemic on DTP-vaccination coverage in the Americas. We hypothesize that the pandemic has led to declining vaccination rates, potentially reversing previous progress toward global immunization targets. This paper examines trends in DTP3-vaccine coverage across the Americas from 2012 to 2022, identifying significant changes and regional disparities considering the pandemic. The findings aim to provide a detailed understanding of the pandemic’s impact on DTP vaccination and guide public health strategies to address these challenges, ensuring continued progress towards global vaccination goals. We will focus primarily on the repercussions of the COVID-19 pandemic under the hypothesis that pandemic-related disruptions have affected vaccination programs [46].
2. Materials and Methods
Vaccine coverage data for individual countries were sourced from the United Nations Children’s Fund, in databases from 2012 through 2022 [51]. We omitted the following territories: French Guiana, Greenland, Alaska, Anguilla, Aruba, Bermuda, Bonaire, Curaçao, and Guadeloupe. We also acquired regional estimates from the United Nations Children’s Fund database [51]. The information regarding the annual count of newborns by country was gathered from the United Nations Children’s Fund database [52] and the World Bank [53].
2.1. Regional Analysis
Regional aggregated data for North America, Latin America, and the Caribbean were obtained from the United Nations Children’s Fund. Due to the absence of regional data from South America, Central America, and the Caribbean in the United Nations Children’s Fund data, we calculated these figures using birth-weighted vaccination rates. This method of calculating data, based on the births in each country, was essential for deriving accurate estimations of DTP3-vaccination coverage in these regions. The countries included in each region were as follows: in Central America, the countries were the Republic of Guatemala, the Republic of Panama, the Republic of Costa Rica, the Republic of Nicaragua, the Republic of El Salvador, the Republic of Honduras, and Belize. In North America, the countries assessed included the United States of America, the United Mexican States, and Canada. The Caribbean region’s analysis encompassed Jamaica, Saint Lucia, Grenada, the Bahamas, the Republic of Cuba, Saint Vincent and the Grenadines, Barbados, the Republic of Haiti, the Republic of Trinidad and Tobago, the Dominican Republic, the Federation of Saint Kitts and Nevis, Antigua and Barbuda, and the Commonwealth of Dominica. Lastly, in South America, the countries involved were the Federative Republic of Brazil, the Republic of Chile, the Bolivarian Republic of Venezuela, the Republic of Peru, the Argentine Republic, the Republic of Ecuador, the Plurinational State of Bolivia, the Republic of Colombia, Uruguay, Paraguay, Suriname, and Guyana.
2.2. Statistical Analysis
We utilized Joinpoint regression, a methodology that has previously been applied in the examination of vaccination trends [44]. We calculated the annual percentage change (APC) to gauge the extent of variation in each trend. In these statistical models, vaccine coverage was the dependent variable, while the year was the independent variable. The models assumed constant variance (homoscedasticity). We conducted a Durbin–Watson test to evaluate the presence of autocorrelation within the time series data [54]. First-order autocorrelation estimated from the data was computed in all the cases.
We employed an interrupted time series analysis method. This technique is considered the most effective quasi-experimental approach for assessing the impact of external events or interventions, such as the COVID-19 pandemic [55,56,57]. Our study analyzed eleven years, eight years pre-COVID (2012–2019) and three years, 2020–2022, post-COVID. The model of the analysis follows the equation below.
where “DTP3” is the number of doses administered, Year is the year of the calendar, and COVID-19 is a dummy variable that has value “1” for the pandemic years (2020–2022); “YearCOVID-19” is an interaction between Year and COVID-19.
DTP3t = Intercept + β1Year + β2COVID-19 + β3YearCOVID-19 + εt
Furthermore, we compared DTP3-vaccination coverage for each country in 2019 and its respective coverage data in 2022. Our examination of DTP3-vaccination coverage across American countries involved a comparison of coverage rates for 2019 and 2022. A 2 × 2 contingency table was constructed for each country, delineating the number of vaccinated and unvaccinated children based on the total number of births and the reported DTP3-vaccination percentages for each year. These tables facilitated the comparison of vaccination rates over the specified period, allowing for the assessment of the impact of COVID-19. Chi-square tests were employed to evaluate the significance of differences in DTP3-vaccination rates across various American countries between 2019 and 2022 in the statistical analysis of vaccination-rate changes.
2.3. Software
All Joinpoint analysis calculations were performed utilizing Joinpoint (Version 5.0.2. May 2023) [58,59]. The statistical comparison of rates between 2019 and 2022 was conducted using the IBM Statistical Package for the Social Sciences, version 27 (IBM Corp., Armonk, NY, USA). The interrupted time series analysis was computed using the “segmented” [60,61,62,63] package in R (version 4.3.1, 16 June 2023 ucrt) [64], under Rstudio (Version 1 September 2023 Build 494) [65]. This was complemented using the ‘ggplot2′ package for advanced graphical representations [66] and the ‘readxl’ package for seamlessly importing Excel data files [67]. We computed post hoc power calculations using the G*Power Software (Version 3.1.9.6) [68,69]. Maps were created with Mapchart (v 4.3.2.) [70]. The range of colors for the maps was elaborated with Colorbrewer (v.2.0) [71,72].
3. Results
Between 2012 and 2021 in the Americas, DTP3-vaccination rates displayed an overall APC of −1.4% with a 95% confidence interval (CI) ranging from −1.8 to −1.0 (p < 0.001). The Joinpoint analysis identified two distinct periods within this period. From 2012 to 2016, the APC was −0.7% (95% CI: −2.9 to 1.5; p = 0.464) during the first period, indicating a slight decrease. In contrast, a marked decline was observed in the second period, from 2016 to 2022, where the APC steepened to −1.8% (95% CI: −2.9 to −0.7), indicating a downward trend. These findings suggest a shift in the trajectory of vaccination rates over the decade, with a notable decline in the latter half of the period (Table 1, Figure 1).

Table 1.
Joinpoint analysis for the third doses of DTP-vaccination rates in the Americas, 2012–2022.
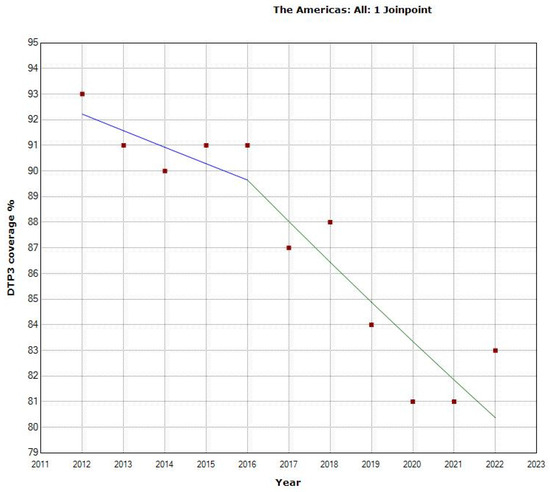
Figure 1.
Joinpoint graph of DTP3 in the Americas, 2012–2022 indicate joinpoints at the transitions between colored lines. (Statistical Power: 0.999).
The Joinpoint analysis for regional third-DTP-dose coverage in America from 2012–2022 reveals varied trends across regions. In North America, the overall APC for the total period was −0.1% (95% CI: −0.2 to 0), indicating a negligible decrease in vaccination rates. In Table 2, we can see the Joinpoint analysis for 2014. From 2012–2014, there was a slight increase; from then on, there was a decrease, as depicted in Figure 2.

Table 2.
DTP3 Joinpoint in American regions, 2012–2022.
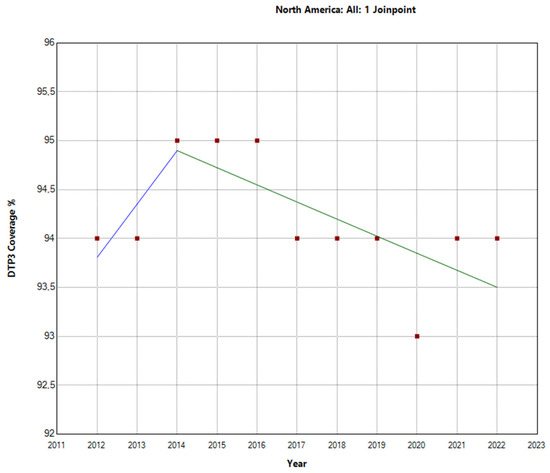
Figure 2.
Joinpoint graph of DTP3 in North America, 2012–2022 indicate joinpoints at the transitions between colored lines. (Power = 0.0303).
In contrast, Latin America and the Caribbean exhibited a more pronounced decline in vaccination rates. The total period APC was −2.1% (95% −2.7 to −1.5). Further analysis within this region revealed a Joinpoint in 2016. There were two distinct periods: the first period (2012–2016) showed an APC of −0.9% (95% CI: −4.4, 2.6, p = 0.531), indicating a stable trend. However, during the second period (2016–2022), the decline in vaccination rates was more substantial, with an APC of −2.7% (95% CI: −4.5, −0.9, p = 0.010), indicating a decrease in vaccination rates, as shown in Figure 3. These findings highlight substantial regional differences in third-DTP-dose-coverage trends within Latin America and the Caribbean, which are visually depicted in Figure 4, Figure 5 and Figure 6, illustrating the third-DTP-dose-vaccination-rate trends in Central America, the Caribbean, and South America, respectively, highlighting the Joinpoints.
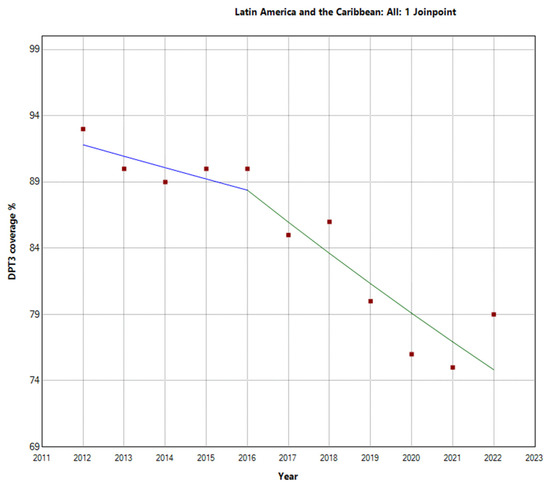
Figure 3.
Joinpoint graph of DTP3 in Latin America and the Caribbean, 2012–2022 indicate joinpoints at the transitions between colored lines. (Power = 0.9999).
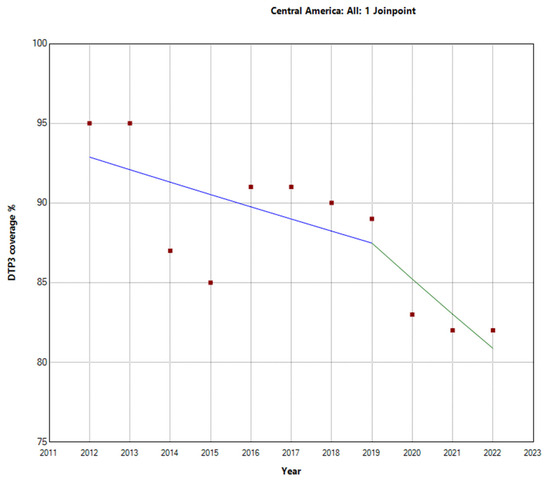
Figure 4.
Joinpoint analysis of DTP3-vaccination trends in Central America, 2012–2022 indicate joinpoints at the transitions between colored lines. (Statistical Power: 0.954).
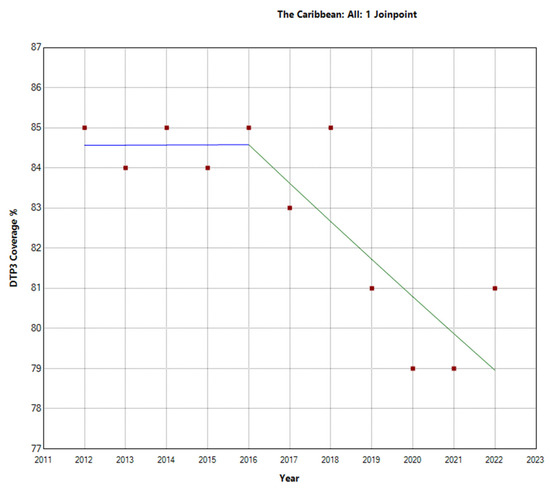
Figure 5.
Joinpoint graph of DTP3 in the Caribbean, 2012–2022 indicate joinpoints at the transitions between colored lines. (Statistical Power: 0.977).
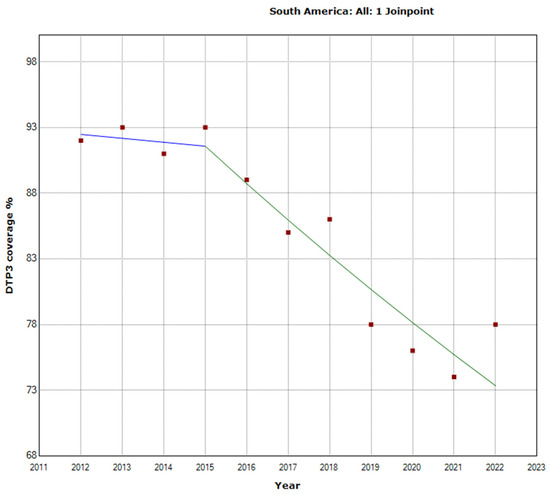
Figure 6.
Joinpoint graph of DTP3 in South America 2012–2022 indicate joinpoints at the transitions between colored lines. (Statistical Power: 0.999).
During the research period spanning from 2012 to 2022, there were notable variations in the vaccination rates for the third dose of DTP across different areas in Central America, the Caribbean, and South America. In Central America, the overall APC was −1.3% (95% CI −2.1, −0.4; p = 0.009. There was a Joinpoint in 2019. This region experienced a more pronounced decline in the latter period (2019–2022) with an APC of −2.6% (95% CI −8.6, 3.8; p = 0.354) (Figure 4).
The Caribbean region showed a different pattern, with an overall decrease in the vaccination rate with an APC of −0.7% (95% CI −1.1, −0.4; p = 0.001). There was a Joinpoint in 2016 (Figure 5). In the second period (2016–2022), there was a decrease with an APC of −1.1% (95% CI −2.1, −0.1; p = 0.031).
In South America, the total period saw a decline in vaccination rates with an APC of −2.5% (95% CI −3.1, −1.8; p < 0.001). There was a Joinpoint in 2015 (Figure 6). The trend intensified in the second period (2015–2022), with an APC of −3.1% (95% CI −4.4, −1.8; p = 0.001).
These findings suggest region-specific variations within Latin America and the Caribbean region, with South America experiencing the most impactful decline over the study period (Figure 7). The Americas experienced a 4% reduction in DTP3-vaccine coverage from 2019 to 2022. However, vaccine-coverage rates remained unchanged in North America, South America, and the Caribbean. In contrast, Central America witnessed a more substantial decline in vaccine coverage, with a decrease of 7% (Table 3).
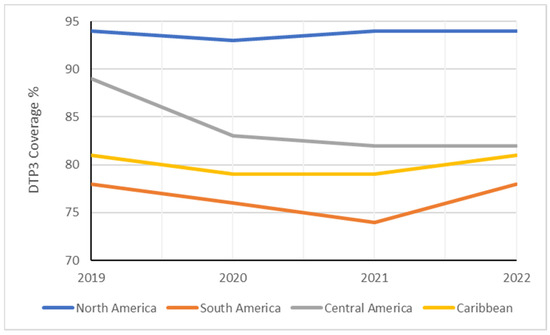
Figure 7.
Evolution of DTP3 rates (2019–2022) in South America by region.

Table 3.
Changes in DTP3 rate (%) in American regions between 2019 and 2022.
Table 4 displays the DTP3 rates for the years 2019 and 2022. On average, there was a reduction of −4.20% across countries, with a standard deviation of 6.08 (p < 0.001). In Figure 8, Figure 9 and Figure 10, we present the map of America indicating the absolute differences in vaccine coverage between 2012 and 2022. Between 2019 and 2022, the DTP3-vaccination rates showed a decline, as observed in the dataset, which revealed that twenty-two countries (61.1%) in America registered a decrease, while only six countries (16.7%) indicated an increase, and 8 (22.2%) remained unchanged.

Table 4.
Changes in DTP3 rates (%) across American countries in 2019 and 2022.
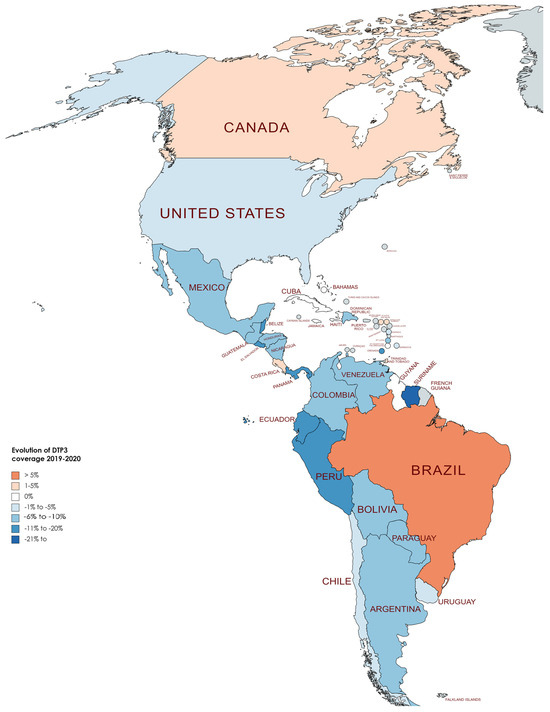
Figure 8.
Changes in DTP3 coverage (%) in America, 2019–2020.
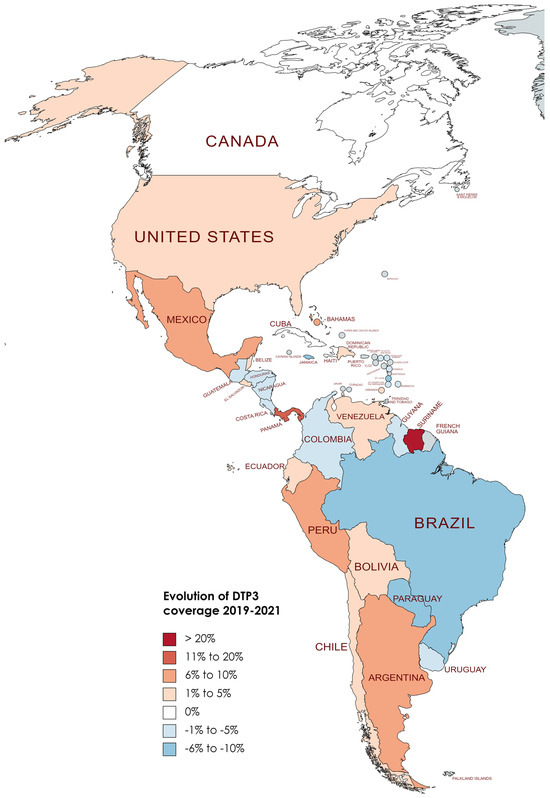
Figure 9.
Changes in DTP3 coverage (%) in America, 2019–2021 (Gray meaning no data or excluded).
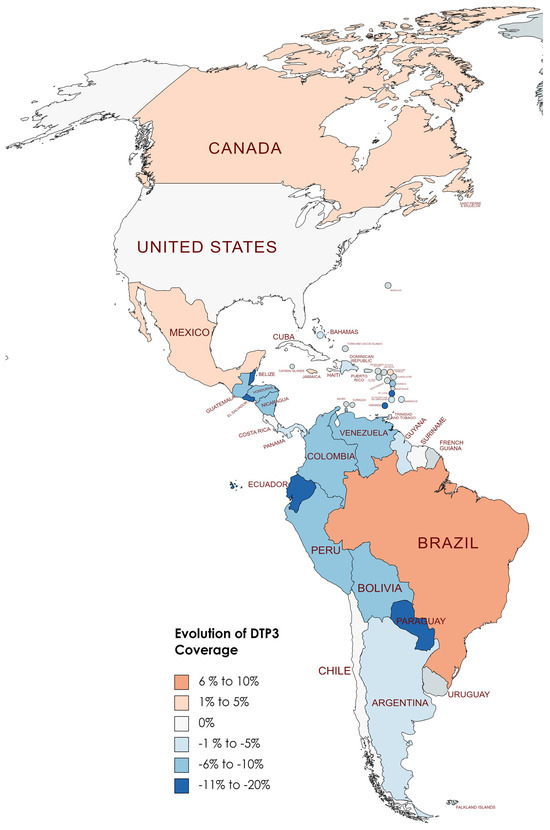
Figure 10.
Changes in DTP3 coverage (%) in America, 2019–2022.
Although COVID-19 impacted vaccine coverage, six countries had increased vaccination coverage after the COVID-19 pandemic. Brazil led this trend with a notable 7% increase in coverage. Following Brazil, Antigua and Barbuda saw a 4% rise, while Jamaica experienced a 2% increase. Canada and Mexico also reported modest gains of 1% each.
Conversely, eight countries demonstrated stability in their DTP3-vaccine coverage during the same period. Countries such as Chile, Costa Rica, Cuba, Haiti, Suriname, Trinidad and Tobago, the United States, and Uruguay maintained their coverage levels, with no percentage change observed. However, the study also identified 22 American countries where there was a decline in DTP3-vaccine coverage (Argentina, Bahamas, Barbados, Belize, Saint Kitts and Nevis, Bolivia, Colombia, Guyana, El Salvador, Nicaragua, Saint Lucia, Paraguay, Saint Vincent and the Grenadines, Ecuador, Honduras, Venezuela, Guatemala, Panama, Grenada, Peru, Dominica, and the Dominican Republic). The Dominican Republic, Guyana, Panama, and Saint Kitts and Nevis experienced a 1% decrease, while Argentina faced a 2% decline. In nations with declining vaccination coverage, the average absolute decrease was −7.37%, with a standard deviation of 5.39 (Table 4) (Figure 8, Figure 9 and Figure 10).
In North America, in two countries, Canada and Mexico, the DTP3-vaccine coverage slightly increased by 1% between 2019 and 2022, while in the United States, it remained the same. In South America, Brazil increased the coverage by 7%, and three countries, Chile, Suriname, and Uruguay, remained at the same level. In the other countries, the coverage decreased. In Central America, except for Costa Rica, whose rate remained equal, all the nations fell in their coverage. The same happened in the Caribbean. All the countries saw decreases; the only exceptions were Antigua, Barbuda, and Jamaica, which increased 4% and 2%, respectively, and Cuba, Haiti, and Trinidad and Tobago, which remained at the same level (Figure 10).
We found that in 2019, a total of 19 countries in the Americas, namely the United States, Canada, Chile, Uruguay, Trinidad and Tobago, Saint Vincent and the Grenadines, Saint Kitts and Nevis, Saint Lucia, Nicaragua, Jamaica, Guyana, Grenada, El Salvador, Dominica, Cuba, Costa Rica, Colombia, Belize, Barbados, and Antigua and Barbuda, had successfully achieved the World Health Organization’s goal of attaining at least 90% coverage for DTP3 vaccine by the year 2030. After the pandemic, by 2022, it was observed that six nations—Barbados, Belize, Colombia, El Salvador, Grenada, and Saint Lucia—no longer met the 90% coverage target for the DTP3 vaccine. This was a change from the list of countries that had achieved this goal in 2019 (Figure 11 and Figure 12).
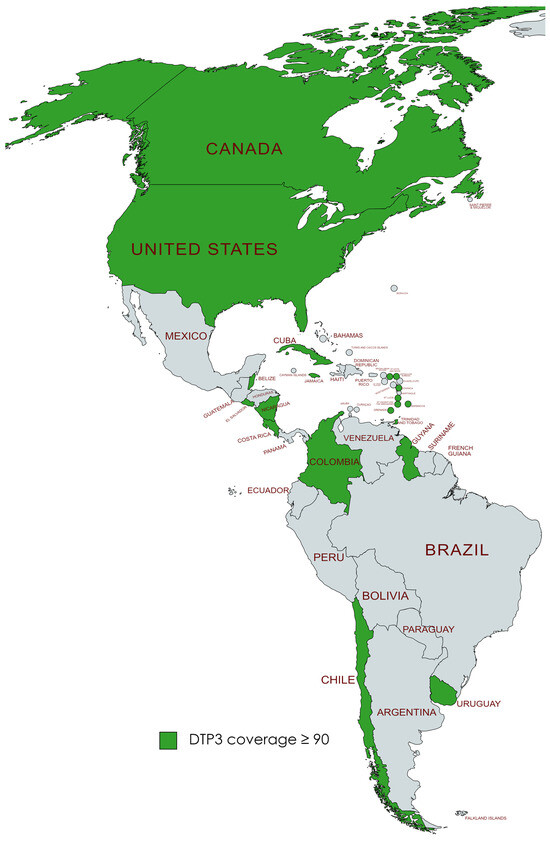
Figure 11.
American countries meeting the WHO 90% DTP3-vaccination-coverage target in 2019. (Gray color meaning not reaching the target).
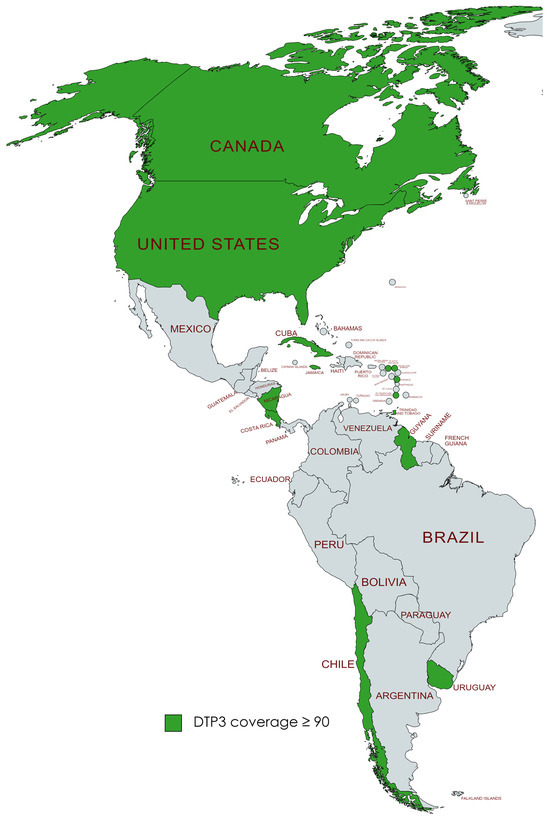
Figure 12.
American countries meeting the WHO 90% DTP3-vaccination-coverage target in 2022. (Gray color meaning not reaching the target).
Twenty countries had a Joinpoint close to 2019 (Table 5, Figures S1–S19). In five countries, Antigua and Barbuda, Costa Rica, Haiti, Peru, and Saint Vincent and the Grenadines, there was a Joinpoint in 2017 (95% IC 2014–2020). In five countries, Canada, Ecuador, Grenada, Jamaica, Nicaragua, and Paraguay, the Joinpoint was in 2018 (95% IC 2016–2019). In three countries, Belize, Colombia, and Saint Lucia, there was a Joinpoint in 2019 (95% IC 2017–2020). Finally, there were six countries, Bahamas, Dominica, Mexico, Saint Kitts and Nevis, Suriname, and Uruguay, with a Joinpoint in 2020 (95% IC 2014–2020). In all these countries, there was a decrease in the APC. The only exceptions were four countries, Canada, Bahamas, Mexico, and Suriname, which managed to achieve an increase in the period after the Joinpoint.

Table 5.
Joinpoint analysis DTP3 rates in America, 2012–2022.
Using interrupted time series analysis, we detected changes in all the regions of America. Table 6 presents the segmented regression analysis of DTP3-vaccine coverage parameters (Figure 13, Figure 14, Figure 15 and Figure 16).

Table 6.
Segmented regression analysis of regional DTP3-vaccine coverage.
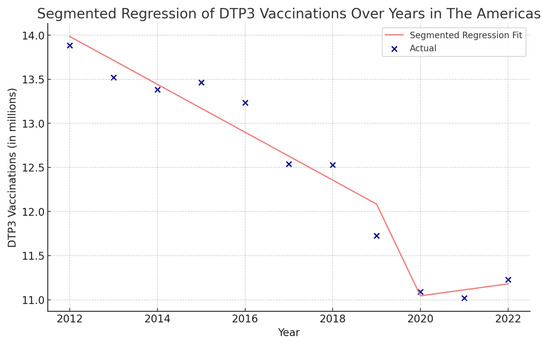
Figure 13.
Segmented regression analysis of DTP3 in the Americas, representing the actual DTP3-vaccination numbers (in dark blue) against the predicted values from the segmented regression (in light coral).
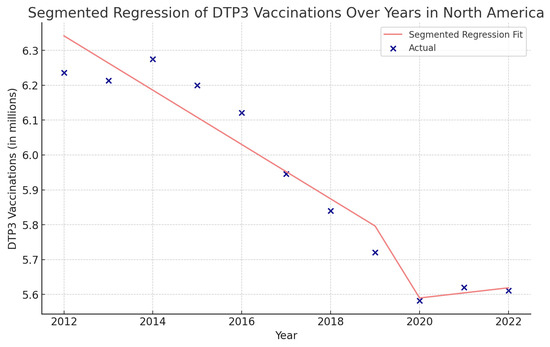
Figure 14.
Segmented regression analysis of DTP3 in North America, representing the actual DTP3-vaccination numbers (in dark blue) against the predicted values from the segmented regression (in light coral).
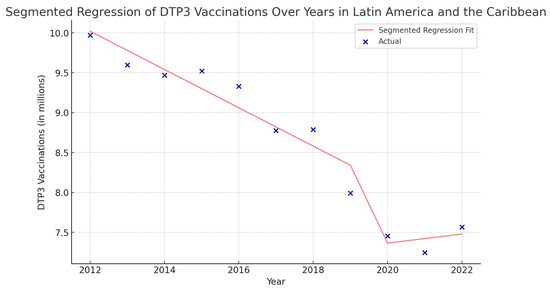
Figure 15.
Segmented regression analysis of DTP3 vaccination in Latin America and the Caribbean. The actual DTP3-vaccination numbers (in dark blue) against the predicted values from the segmented regression (in light coral).
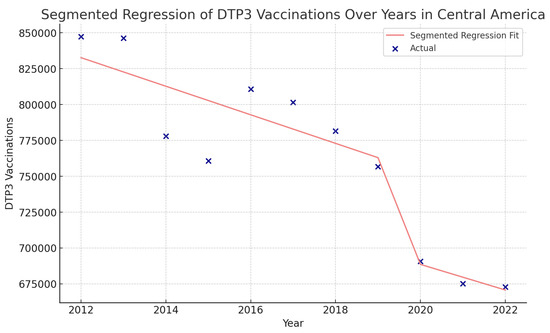
Figure 16.
Segmented regression analysis of DTP3 vaccination in Central America. The actual DTP3 (in dark blue) against the predicted values from the segmented regression (in light coral).
In the Americas (Figure 13), the year’s coefficient was −271,700 (p = 0.002), suggesting a decreasing trend in DTP3 vaccination over the years. The COVID-19 coefficient is negative, approximately −3,824,000, indicating a decrease during the pandemic.
In North America, the COVID-19 coefficient is negative, at approximately −960,900 (p = 0.140) (Figure 14).
In Latin America and the Caribbean, the coefficient of COVID-19 was −3,408,000 (p = 0.078), indicating a decrease during the pandemic (Figure 15).
In Central America, the coefficient of COVID-19 was −73,740 (p > 0.05) (Figure 16).
In the Caribbean, the COVID-19 coefficient was negative, −94,750 (p = 0.195) (Figure 17).
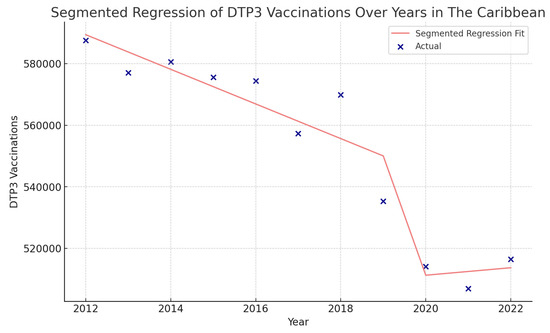
Figure 17.
Segmented regression analysis of DTP3 vaccination in the Caribbean. The actual DTP3 (in dark blue) against the predicted values from the segmented regression (in light coral).
In the Caribbean, the COVID-19 coefficient was negative, −2,071,000 (p > 0.05) (Figure 18). In the segmented regression, we also detected several American countries with fewer children vaccinated with DTP3 after the COVID-19 pandemic. Those countries were Belize, Grenada, Peru, Suriname, the United States, Mexico, Antigua and Barbuda, Argentina, Bahamas, Barbados, Bolivia, Brazil, Canada, Chile, Dominican Republic, Ecuador, El Salvador, Haiti, Honduras, Jamaica, Nicaragua, Panama, Saint Vincent and the Grenadines, Uruguay, and Venezuela (Table 7).
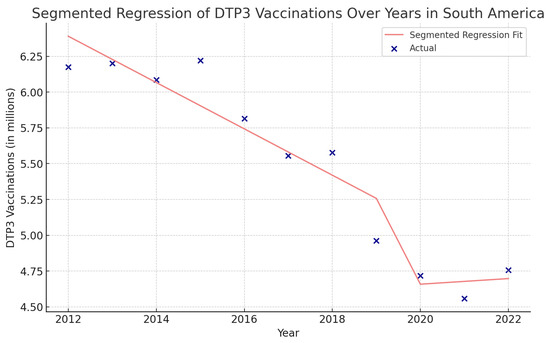
Figure 18.
Segmented regression analysis of DTP3 vaccination in South America. The actual DTP3 numbers (in dark blue) are represented against the predicted values from the segmented regression (in light coral).

Table 7.
Segmented regression of the number of DTP3 vaccinations in American nations.
In Belize, the segmented regression model explains a high proportion of the variance in the number of children who received DTP3 doses (Figure S21), with an R2 value of 0.937, suggesting a good fit. The COVID-19 variable had a coefficient of a −3833 (p < 0.05). This indicates that the COVID-19 pandemic is associated with a decrease in the number of DTP3 vaccinations. The Interaction term has a positive coefficient of roughly 240 (p < 0.05). For the data from Grenada (Figure S22), the segmented regression analysis has been conducted, yielding the following results: The R2 value of the model was 0.983, indicating that the model explains a remarkably high portion of the variance in the DTP3 vaccination numbers, suggesting a perfect fit. The coefficient for the COVID-19 variable has a value of −837.68 (p < 0.05), indicating that the start of the COVID-19 pandemic was associated with a substantial decrease in the number of DTP3 vaccinations. The Year variable has a negative coefficient of −12.09 (p = 0.067). This suggests a decline in vaccinations over the years. The Interaction term, which represents the interaction between the year and the occurrence of COVID-19, has a positive coefficient of 41.60 (p > 0.05).
The segmented regression model for Peru (Figure S23) has an R2 value of 0.803, which is considered a good fit, meaning that the model explains 80.3% of the variance in the DTP3-vaccination numbers. The coefficient for COVID-19 has a value of −372,300 (p < 0.05), indicating that the onset of the COVID-19 pandemic was associated with a high decrease in the number of DTP3 vaccinations. The Interaction term between the year and the occurrence of COVID-19 has a coefficient of 34,450 (p < 0.05). In the segmented regression analysis for Suriname (Figure S24), the model’s R2 value is 0.733, which explains much of the variance in the DTP3-vaccination numbers. The coefficient for COVID-19 is −15,210 (p < 0.05), which implies that the onset of the COVID-19 pandemic was associated with a decrease in the number of DTP3 vaccinations. The Interaction term has a coefficient of y 1420 (p < 0.05). In Mexico (Figure S25), the segmented regression model has a good fit, with an R2 value of 0.884. The coefficient for the Year was −63,130 (p < 0.05), indicating a decline in DTP3 vaccinations over the years. The COVID-19 coefficient is negative, with a value of approximately −1,379,000 (p = 0.098), suggesting a substantial decrease in vaccinations during the pandemic. The segmented regression analysis for the United States data (Figure S26) provides the following insights: The model has a good fit, with an R2 value of 0.848. The coefficient for the Year is −44,760 (p < 0.01, suggesting a declining trend in the number of DTP3 vaccinations over the years. The COVID-19 coefficient is negative, with a value of −1,093,000 (p = 0.092), which would indicate a substantial decrease in the number of vaccinations during the pandemic. The Interaction has a positive coefficient of 108,000 (p = 0.099), indicating a potential interaction effect between the point year and the occurrence of COVID-19. Table S1 summarizes all the main findings to allow for a global view.
4. Discussion
Our research focused on examining the effects of the COVID-19 pandemic on DTP-immunization patterns across the Americas. Our findings reveal that vaccination rates have been negatively impacted in various American countries, particularly Central America.
4.1. Overview of the Study and Its Context
4.1.1. Methodological Considerations and Data Limitations
Our methodologies included comparing country-specific vaccination coverage between 2019 and 2022 and analyzing trends using joint points and segmented regression during the period of 2011–2022, making our findings more robust.
While acknowledging the constraints of the available data and potential inconsistencies in reporting, the significance of the United Nations Children’s Fund’s dataset for this investigation remains paramount. Despite the United Nations Children’s Fund’s consistent methodology in data collection and reporting, variations in national healthcare infrastructures and reporting systems might lead to discrepancies in data quality and precision, potentially influencing the interpretations of our analysis. Furthermore, the data’s compilation at national and regional scales could mask local nuances in vaccination trends, especially in areas with healthcare-accessibility issues or socioeconomic disparities. Nevertheless, in the last 20 years, global immunization-coverage data quality improved [73]. As of the latest data update, in 2022, our study does not include real-time data from 2023 onwards. Thus, our findings might not fully capture the ongoing impact of the COVID-19 pandemic on DTP-vaccination rates in the Americas. Future research, with access to more current data, would be essential in providing a more comprehensive understanding of these trends.
Among the strengths of our study is that this study employs a robust methodological framework that includes joint points and segmented regression analysis. Using two different methods enhances the reliability and validity of the findings. Leveraging comprehensive data sets provided by the United Nations Children’s Fund, despite the acknowledged limitations, our approach benefits from consistent data collection and reporting methodologies. This is particularly valuable given the varied national healthcare infrastructures and reporting systems across the countries studied. Our analysis offers insightful revelations into regional disparities in vaccination trends, underlining the necessity for customized public health approaches. Such detailed examination allows for a nuanced understanding of vaccine-coverage trends across the Americas, providing a solid foundation for future policy formulation and implementation to mitigate the pandemic’s impact on essential vaccination programs.
Despite the methodological strengths, our study acknowledges certain limitations associated with the available data, potentially influencing our analysis’s robustness. The variance in data quality and precision due to differences in national healthcare infrastructures and reporting systems poses a challenge, necessitating a cautious approach to interpreting our findings. A broader comparative analysis incorporating other regions, such as Africa and Europe, could have enriched our understanding of global patterns and identified unique challenges and successful strategies in different contexts. Furthermore, the impact of the digital divide on data reporting accuracy and timeliness, especially in regions with limited access to technological resources, remains a critical area that warrants deeper exploration. Addressing these weaknesses would not only enhance the credibility of our study but also provide more comprehensive insights, supporting the development of more effective vaccination strategies and public health policies.
In addressing the methodological considerations of our study, it is important to highlight our dual-analytical approach, employing both Joinpoint regression and segmented regression analyses. This decision was underpinned by the objective to enhance the robustness of our findings, allowing for the identification of trend changes in DTP3-vaccination coverage without prior assumptions (Joinpoint regression) and the assessment of the direct impact of the COVID-19 pandemic with predefined intervention points (segmented regression). The complementary nature of these methods strengthens our analysis, as corroborated by the literature suggesting the value of utilizing multiple statistical approaches in public health research to ensure the validity of results [74]. However, it is crucial to acknowledge potential data limitations inherent in our study, such as the reliability of reported vaccination rates and the assumption that detected trend changes are solely attributable to the pandemic, without discounting other concurrent public health interventions or socioeconomic factors. These considerations underscore the complexity of interpreting trend data in the context of global health crises and the necessity of a cautious approach in attributing causality.
A possible methodological concern could be the statistical power of using Joinpoint regression analysis with only 11 data points. It is essential to highlight that, contrary to the assumption of insufficient data for robust analysis, this number of data points is quite suitable for detecting a single joinpoint. According to the statistical guidelines provided by the National Institutes of Health, for datasets comprising 7 to 11 data points, the default maximum number of joinpoints recommended is one [75]. To detect two Joinpoints, the number of data points should be between 12 and 16. Therefore, in the context of our study with 11 data points, applying a single joinpoint analysis falls well within these recommended parameters, providing a statistically sound approach for identifying trend changes in the data. This approach is further justified because our study aims to detect a single critical shift in the trend rather than multiple joinpoints, making our dataset adequate and optimal for the intended analysis.
4.1.2. Discrepancies in Vaccine-Coverage Data and Trend Analysis
There appears to be a disparity between the number of countries in the Americas that experienced a drop in immunization rates between 2019 and 2022, totaling 22, and the smaller subset of 16 countries within this group where a decrease in vaccination trends was observed. This variation can be attributed to the fact that, despite the reduction in vaccine coverage in particular countries over the observed years, the decrease is not yet significant enough to establish a trend shift in some of these nations due to the small number of births in some countries. Additionally, the discrepancy in results between Joinpoint and segmented regression may also be influenced by the methodologies employed: Joinpoint regression identifies year-significant trend changes but with a 95% confidence interval that spans a range of years, whereas segmented regression focuses on a precise time point. This difference in approach to determining trend changes at specific times versus a range of years can lead to varying interpretations in the vaccination-coverage data [76,77,78].
4.2. Analysis of DTP3-Vaccination Trends
The data analysis shows a declining trend in vaccination coverage throughout the Americas, possibly due to socioeconomic and cultural factors and vaccine hesitancy, which COVID-19 exacerbated [79,80].
In the context of DTP3-vaccine coverage between 2019 and 2022, several American countries exhibited an increase in vaccine coverage. Despite the challenging times of the COVID-19 pandemic, these enhancements in vaccine coverage highlight the effectiveness of public health interventions and the resilience of healthcare systems in these countries. Conversely, several countries demonstrated stability in their DTP3-vaccine coverage during the same period. Amidst the global health crisis, the stability in coverage underscores the strength and consistency of vaccination programs in these nations. It suggests that these countries successfully navigated the complexities introduced by the pandemic, ensuring uninterrupted vaccine delivery to their populations.
However, the study also identified countries with declining DTP3-vaccine coverage. These reductions highlight the challenges and disruptions caused by the COVID-19 pandemic, potentially reflecting resource reallocation, access issues, or public hesitancy toward vaccination. It underscores the need for focused efforts to strengthen and adapt vaccination programs in the face of such unprecedented global health challenges.
Our analysis revealed that DTP3 rates remained constant throughout the 2019–2022 pandemic in most North American nations, as shown in Table 4. In this period, Canada and Mexico exhibited a minor increase in DTP3 rates, whereas the United States maintained its existing level of coverage [4]. Some countries did not have their coverage affected at the end of the pandemic. These results could indicate the efficiency of the national vaccination programs and the resilience of healthcare systems in these countries, enabling them to sustain their immunization rates in the face of numerous challenges presented by the persistent COVID-19 pandemic globally.
Nevertheless, there are differences in subnational units. For example, In Haiti, a significant decrease in DPT3 service volume, amounting to 5% or more, was observed in 80% of the subnational regions during the third quarter of 2022 [81].
The reduction in coverage in some American nations might be ascribed to enduring issues in the country’s health system infrastructure, particularly shortcomings in the vaccine-distribution network, a situation potentially aggravated by the extra burden imposed by the COVID-19 pandemic [82,83,84,85,86,87].
Our study has revealed notable reductions in immunization rates across various American nations. Additionally, we observed a shift in the trend of vaccine coverage in Latin America and the Caribbean. This trend shift, marked by an accelerated decline in coverage, was evident in all subregions. Our findings are consistent with the literature indicating that DTP3 rates dropped by 5.06% in the Caribbean and Latin America between 2019 and 2021 [35].
4.3. Comparative Global and Regional Perspectives
The global decline in vaccine coverage, spurred by the COVID-19 pandemic, extends beyond DTP vaccines. From January to December 2020, approximately 30 million children missed DTP3 vaccinations, and 27.2 million missed MCV1 vaccinations [32]. It has been reported that there was also a reduction in HPV in some parts of the United States [79]. The worldwide coverage of DTP3 experienced a decline of 5.81% from 2019 to 2021, decreasing from 86% to 81% [51]. In considering the broader implications of our findings, it is crucial to assess the impact of the digital divide on data reporting. In regions of the Americas with limited access to technological resources, the accuracy and timeliness of data reporting may be compromised. This factor is crucial in understanding the regional disparities in vaccine coverage and the challenges in data collection.
The decrease in DTP coverage is a global problem. The global coverage of DTP3 decreased from 86% in 2019 to 83% in 2020 [51,88]. Similar declines had been found in Africa and Asia [44,89]. In contrast to other regions, Europe experienced a minor reduction in vaccination coverage. Specifically, the coverage for the third dose of the diphtheria–tetanus–pertussis (DTP3) vaccine saw a marginal decline of 1.05% from 2019 to 2021. This resulted in a slight dip in coverage rates, from 95% in 2019 to 94% in 2020. Furthermore, a comparative analysis with other regions, such as Africa or Europe, could offer valuable insights into global patterns and region-specific challenges in maintaining vaccination during the pandemic. Such a comparative perspective could help identify unique challenges and successful strategies in different regions, offering lessons for future healthcare planning and policy formulation.
4.4. Influential Factors and Challenges
Socioeconomic factors have been pivotal in influencing vaccine accessibility during the pandemic. Economic challenges exacerbated by the pandemic have widened existing disparities in healthcare access, further impacting vaccination rates. A detailed examination of these socioeconomic variables provides a more nuanced understanding of the vaccination landscape across different socio-demographic groups.
Numerous elements could account for the shift in vaccination coverage. Factors at the national level, like elevated fertility rates, combined with community-specific factors, such as widespread illiteracy, play a role in the higher incidence of unvaccinated children [33,90]. Research conducted in the US revealed that for all types of hepatitis vaccines, both adherence and completion rates are notably low, exhibiting considerable differences across various socio-demographic and clinical profiles. The likelihood of poor adherence and incomplete vaccination was typically linked to factors such as being male, belonging to a younger age group, identifying as Black or Hispanic, and having lower levels of education and household income [91].
In Africa, incomplete vaccinations are primarily due to caregivers’ time constraints, limited immunization knowledge, vaccine or staff shortages at health facilities, missed vaccination chances, concerns about side effects, poor access to services, and caregiver vaccination beliefs [92]. A recent study shows that 35.5% of African populations have incomplete immunization, with home births, rural residency, a lack of prenatal care, limited immunization awareness, and maternal illiteracy being the main risk factors [93].
Additionally, American countries’ varied public health policies and COVID-19 response strategies have potentially influenced DTP-vaccination rates [50]. Redirecting healthcare resources from routine vaccinations to COVID-19-response efforts in some regions may have contributed to the observed decline in vaccine coverage.
These reductions emphasize the significant influence of the COVID-19 pandemic on regular vaccination programs within the Americas, signaling a need for focused efforts to recover and enhance vaccine coverage. COVID-19 had an impact on children’s vaccination. The decline in vaccination rates can be linked to various factors associated with the COVID-19 pandemic, including the reallocation of healthcare resources to address the outbreak, the implementation of lockdowns, and the imposition of restrictions on movement that impeded access to vaccination services [94,95,96,97]. In a survey among parents of children aged 0–4 years across America, Europe, and Australia, 83% of respondents considered it crucial for their child to receive the recommended vaccines despite the COVID-19 pandemic. About half of the routine vaccine appointments were postponed or canceled due to the pandemic. However, 61% of parents indicated their desire to make up for missed vaccinations once COVID-19 restrictions were eased or lifted [98].
4.5. Strategies and Interventions for Enhancing Vaccine Coverage
Interestingly, despite the pandemic’s difficulties, some nations, including Canada, Mexico, Jamaica, Antigua and Barbuda, and Brazil, were able to increase their vaccination coverage. It is essential to highlight the situation in Brazil, where the DPT3 rate increased by 7% towards the conclusion of the COVID-19 pandemic. This rise could be credited to successful immunization strategies, focused interventions, or heightened governmental backing for vaccination initiatives during the crisis. Analyzing and drawing lessons from the experiences of these nations is essential for enhancing immunization services in future crises.
As there are many incomplete vaccinations and parents are willing to catch up, healthcare services should try to reach parents [4]. Strategies need to be developed to increase DTP3-vaccination coverage. One strategy that has been suggested is co-administration with other vaccines [99].
The disparities observed across different countries emphasize the importance of developing specific strategies to boost vaccination rates, particularly in nations experiencing a downturn in these trends. The persistent oversight of vaccination initiatives, addressing challenges within healthcare systems, and active community involvement are crucial for sustaining and improving immunization rates in these areas.
Our findings are consistent with previous studies showing that the worldwide COVID-19 pandemic has interrupted crucial health services, including vaccination efforts, and has been linked to growing inequities [100,101,102]. Moreover, concerns about contracting the virus and vaccine hesitancy might have contributed to parents’ hesitancy in seeking healthcare facilities for their children’s vaccinations [103,104]. Healthcare workers, especially community nurses, play an essential role [20].
Last, policies should enhance public awareness about the importance of routine vaccinations and combating misinformation. Community-based interventions and social media campaigns can play a crucial role in increasing vaccine uptake [14,105].
4.6. The Role of Healthcare Systems and Planning
The disruption caused by the COVID-19 pandemic has underscored the importance of having robust health systems capable of maintaining vital medical services during challenging times [106]. Research has shown statistically significant differences in monthly vaccination rates between rural and urban regions [107]. The differences in vaccination trends across regions emphasize the necessity for customized public health approaches, called precision public health [107,108]. Policies must be region-specific, considering each area’s unique challenges and resources.
Initiatives must be undertaken to fortify immunization programs and enhance vaccine coverage throughout the continent, especially in the areas most impacted by the decline in vaccination rates [109]. The decrease in vaccination rates, especially in areas with weaker healthcare infrastructures, underscores the need to strengthen health systems, ensure adequate funding, train healthcare workers, and improve vaccine supply chains.
4.7. Looking to the Future
While our study conclusively demonstrates the impact of the COVID-19 pandemic on vaccine coverage across America, it is important to acknowledge that it did not explicitly evaluate the effects of geographic location, socioeconomic factors, or the consistency of maternal prenatal care on these trends. Future research should aim to dissect these variables to provide a more nuanced understanding of their contributions to vaccine-coverage disparities observed during the pandemic period.
The decline in vaccination coverage has profound implications, such as the potential for outbreaks of diseases like diphtheria, tetanus, and pertussis, further straining healthcare systems amid the COVID-19 pandemic. Addressing the fall in immunization rates is critical, as the risks of postponing vaccinations are more significant than those associated with COVID-19 during routine immunizations [110].
Efforts to enhance immunization rates must consider factors like healthcare access, vaccine supply issues, and government commitment. The decrease in countries meeting WHO’s DTP targets, particularly in Latin America and the Caribbean, underscores the need for targeted interventions. Additionally, even with improved vaccination programs, the time required for catch-up immunizations could be extended, raising the risk of disease outbreaks [111,112]. Prompt and efficient vaccine distribution and the recovery of coverage levels are essential. Collaboration among governments, healthcare bodies, and international agencies is crucial to preserve vaccination progress and protect populations from vaccine-preventable diseases [113,114,115,116]. We should plan for the next pandemic, analyzing and replicating the strategies of countries that have successfully increased or maintained high vaccination rates post-pandemic, which could be beneficial. It is also essential to strengthen epidemiology services related to preventing vaccine-preventable diseases. The continuous monitoring and evaluation of vaccination programs are crucial. Identifying and addressing issues promptly can avoid further declines in vaccination rates.
Despite initial fears and logistical challenges like transportation restrictions, community commitment to vaccination during the pandemic remained strong in many countries. Systemic changes within healthcare, such as staff reallocations, caused temporary delays but were efficiently addressed. Notably, some community misconceptions about the pandemic existed but did not significantly deter vaccination efforts, reflecting a deep-rooted trust in the importance of vaccines [117]. It is crucial to monitor subnational units to avoid inequities [81].
5. Conclusions
This study comprehensively assessed the impact of the COVID-19 pandemic on DTP3 vaccination coverage in the Americas from 2012 to 2022. The findings indicate a significant and concerning decline in vaccination rates, particularly after 2019. The overall APC for the entire period was −1.4%, with an absolute decrease in vaccine coverage of −4% from 2019 to 2022. Notably, the most substantial relative decrease occurred in Central America, with a 7.87% reduction in coverage.
Interestingly, while the pandemic adversely affected vaccination coverage in most countries, some nations showed increased DTP3 vaccine coverage post-pandemic, and several countries maintained stable vaccination rates. The study’s results are alarming, considering the importance of maintaining high DTP-vaccination coverage to prevent outbreaks of vaccine-preventable diseases. The pandemic’s disruptive impact on public health initiatives is underscored by the decline in coverage rates, particularly in countries that had previously met the WHO target of 90% coverage. This decline in vaccination rates highlights the need for renewed efforts in vaccination campaigns and public health strategies to reverse the negative trends and ensure the well-being of populations across the Americas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12030238/s1. Figure S1: Joinpoint graph of DTP3 in Antigua and Barbuda (2012–2022); Figure S2: Joinpoint graph of DTP3 in the Bahamas (2012–2022); Figure S3: Joinpoint graph of DTP3 in Belize. (2012–2022); Figure S4: Joinpoint graph of DTP3 in Canada (2012–2022); Figure S5 Joinpoint graph of DTP3 in Colombia (2012–2022); Figure S6: Joinpoint graph of DTP3 in Costa Rica (2012–2022); Figure S7: Joinpoint graph of DTP3 in Dominica (2012–2022); Figure S8: Joinpoint graph of DTP3 in Ecuador (2012–2022); Figure S9: Joinpoint graph of DTP3 in Grenada (2012–2022); Figure S10: Joinpoint graph of DTP3 in Haiti (2012–2022); Figure S11: Joinpoint graph of DTP3 in Jamaica (2012–2022); Figure S12: Joinpoint graph of DTP3 in Mexico (2012–2022); Figure S13: Joinpoint graph of DTP3 in Nicaragua (2012–2022); Figure S14: Joinpoint graph of DTP3 in Paraguay (2012–2022); Figure S15: Joinpoint graph of DTP3 in Peru (2012–2022); Figure S16: Joinpoint graph of DTP3 in Saint Kitts and Nevis (2012–2022); Figure S17: Joinpoint graph of DTP3 in Saint Lucia (2012–2022); Figure S18: Joinpoint graph of DTP3 in Saint Vincent and the Grenadines (2012–2022); Figure S19: Joinpoint graph of DTP3 in Suriname (2012–2022); Figure S20: Joinpoint graph of DTP3 in Uruguay (2012–2022); Figure S21: Segmented regression analysis of DTP3 vaccination in Belize, representing the actual number of DTP3 vaccinations (in blue) and the fitted values from the segmented regression (in red); Figure S22: Segmented regression analysis of DTP3 vaccinations in Grenada represents the number of DTP3 vaccinations (in green) and the fitted values from the segmented regression (in orange). Figure S23: Segmented regression analysis of DTP3 vaccinations in Peru representing the actual DTP3 vaccination numbers (in purple) and the fitted values from the segmented regression (in gold); Figure S24: Segmented regression analysis of DTP3 vaccinations in Suriname representing the actual DTP3 vaccination numbers (in dark cyan) and the fitted values from the segmented regression (in magenta); Figure S25: Segmented regression analysis of DTP3 vaccinations in Mexico representing the actual DTP3 vaccination numbers (in brown) and the predicted values from the segmented regression (in teal); Figure S26: Segmented regression analysis of DTP3 vaccinations in the United States representing the actual DTP3 vaccination numbers in (dark red) against the predicted values from the segmented regression (in sky blue); Table S1: Summary table displaying Joinpoints close to 2020 and Chi-Square test and segmented regression analysis.
Author Contributions
Conceptualization, F.G.-G., E.A.-O., E.R.-V. and I.A.-O.; methodology, S.G.-A., L.G.-A., E.A.-O. and E.R.-V.; software, S.G.-A. and L.G.-A.; investigation, R.A.-B., E.A.-O. and E.R.-V.; writing—original draft preparation, F.G.-G., S.G.-A., L.G.-A., E.A.-O., I.A.-O., R.A.-B. and E.R.-V.; writing—review and editing, F.G.-G., S.G.-A., L.G.-A., I.A.-O., E.A.-O., R.A.-B. and E.R.-V.; visualization, S.G.-A. and L.G.-A.; supervision, F.G.-G. and I.A.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This is not applicable because the study was conducted using publicly available databases.
Informed Consent Statement
Not applicable because the study was conducted using public available database.
Data Availability Statement
Data are available from the United Nations Children’s Fund database.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ota, M.O.C.; de Moraes, J.C.; Vojtek, I.; Constenla, D.; Doherty, T.M.; Cintra, O.; Kirigia, J.M. Unveiling the Contributions of Immunization for Progressing towards Universal Health Coverage. Hum. Vaccines Immunother. 2022, 18, 2036048. [Google Scholar] [CrossRef]
- Suffel, A.M.; Ojo-Aromokudu, O.; Carreira, H.; Mounier-Jack, S.; Osborn, D.; Warren-Gash, C.; McDonald, H.I. Exploring the Impact of Mental Health Conditions on Vaccine Uptake in High-Income Countries: A Systematic Review. BMC Psychiatry 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N. A Survey on Hygienic Practices and Immunization Coverage among 12-59 Months Old Children in Urban Slum, Barishal, Bangladesh. Asian J. Med. Biol. Res. 2022, 8, 277–285. [Google Scholar] [CrossRef]
- Evans, A.; Mahar, A.L.; Deb, B.; Boblitz, A.; Brownell, M.; Guttmann, A.; Stukel, T.A.; Cohen, E.; Sarkar, J.; Eze, N.; et al. Gaps in Childhood Immunizations and Preventive Care Visits during the COVID-19 Pandemic: A Population-Based Cohort Study of Children in Ontario and Manitoba, Canada, 2016–2021. Can. J. Public Health 2023, 114, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A. Vaccination: A Public Health Intervention That Changed History & Is Changing with History. Am. Biol. Teach. 2011, 73, 513–519. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Cao, L.; Wang, Y.; Zhao, Q.; Tang, S.; Gong, W.; Guo, L.; Liu, Z.; Wen, Z.; et al. Impact of Immunization Programs on 11 Childhood Vaccine-Preventable Diseases in China: 1950–2018. Innovation 2021, 2, 100113. [Google Scholar] [CrossRef] [PubMed]
- Espinal, M.A. The Pan American Health Organization: 120 Years in the Americas Hemisphere. Lancet Reg. Health Am. 2023, 21, 100488. [Google Scholar] [CrossRef]
- Zulfan, G.P.; Sihombing, J.A.; Amin, D.M.; Widiantari, A.D.; Berti, M.P.E.; Murtiani, F. Clinical Manifestation of Childhood Diphtheria. J. Ilm. Kedokt. Wijaya Kusuma 2023, 12, 1. [Google Scholar] [CrossRef]
- WHO. Global Vaccine Action Plan 2011–2020; WHO Press: Geneva, Switzerland, 2013. [Google Scholar]
- Shahid, A.S.M.S.B.; Rahman, A.E.; Shahunja, K.M.; Afroze, F.; Sarmin, M.; Nuzhat, S.; Alam, T.; Chowdhury, F.; Sultana, M.S.; Ackhter, M.M.; et al. Vaccination Following the Expanded Programme on Immunization Schedule Could Help to Reduce Deaths in Children under Five Hospitalized for Pneumonia and Severe Pneumonia in a Developing Country. Front. Pediatr. 2023, 11, 1054335. [Google Scholar] [CrossRef]
- Atteraya, M.S.; Song, I.H.; Ebrahim, N.B.; Gnawali, S.; Kim, E.; Dhakal, T. Inequalities in Childhood Immunisation in South Asia. Int. J. Environ. Res. Public Health 2023, 20, 1755. [Google Scholar] [CrossRef]
- Basu, S.; Ashok, G.; Debroy, R.; Ramaiah, S.; Livingstone, P.; Anbarasu, A. Impact of the COVID-19 Pandemic on Routine Vaccine Landscape: A Global Perspective. Hum. Vaccines Immunother. 2023, 19, 2199656. [Google Scholar] [CrossRef]
- Budu, E.; Ahinkorah, B.O.; Guets, W.; Ameyaw, E.K.; Essuman, M.A.; Yaya, S. Socioeconomic and Residence-based Related Inequality in Childhood Vaccination in Sub-Saharan Africa: Evidence from Benin. Health Sci. Rep. 2023, 6, e1198. [Google Scholar] [CrossRef]
- Albers, A.N.; Wright, E.; Thaker, J.; Conway, K.; Daley, M.F.; Newcomer, S.R. Childhood Vaccination Practices and Parental Hesitancy Barriers in Rural and Urban Primary Care Settings. J. Community Health 2023, 48, 798–809. [Google Scholar] [CrossRef]
- Santoli, J.M.; Lindley, M.C.; DeSilva, M.B.; Kharbanda, E.O.; Daley, M.F.; Galloway, L.; Gee, J.; Glover, M.; Herring, B.; Kang, Y.; et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 591–593. [Google Scholar] [CrossRef]
- Zhong, Y.; Clapham, H.E.; Aishworiya, R.; Chua, Y.X.; Mathews, J.; Ong, M.; Wang, J.; Murugasu, B.; Chiang, W.C.; Lee, B.W.; et al. Childhood Vaccinations: Hidden Impact of COVID-19 on Children in Singapore. Vaccine 2021, 39, 780–785. [Google Scholar] [CrossRef]
- Tolera, S.T.; Kaweti, G.; Aboma, L.M. Article Review on Potential Impact of COVID-19 Pandemic on Socioeconomic of Ethiopia, Africa. Health Sci. 2022, 11, 19–30. [Google Scholar]
- Yang, J.; Vaghela, S.; Yarnoff, B.; De Boisvilliers, S.; Di Fusco, M.; Wiemken, T.L.; Kyaw, M.H.; McLaughlin, J.M.; Nguyen, J.L. Estimated Global Public Health and Economic Impact of COVID-19 Vaccines in the Pre-Omicron Era Using Real-World Empirical Data. Expert. Rev. Vaccines 2023, 22, 54–65. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Ju, J.; Sun, R.; Zhang, J. Impact of Vaccination on the COVID-19 Pandemic in US States. Sci. Rep. 2022, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, C. The ‘New Normal’: Delivering Childhood Immunisations during the COVID-19 Pandemic. Pract. Nurs. 2020. Available online: https://www.practicenursing.com/content/comment/the-new-normal-delivering-childhood-immunisations-during-the-covid-19-pandemic/ (accessed on 9 December 2023). [CrossRef]
- Athiyaman, A.; Ajayi, T.; Mutuku, F.; Luwaga, F.; Bryer, S.; Giwa, O.; Mngemane, S.; Edwige, N.N.; Berman, L. Recovering from the Unprecedented Backsliding in Immunization Coverage: Learnings from Country Programming in Five Countries through the Past Two Years of COVID-19 Pandemic Disruptions. Vaccines 2023, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Naor, M.; Pinto, G.D.; Davidov, P.; Abdrbo, L. Rapidly Establishing an Ultra-Cold Supply Chain of Vaccines in Israel: Evidence for the Efficacy of Inoculation to Mitigate the COVID-19 Pandemic. Vaccines 2023, 11, 349. [Google Scholar] [CrossRef]
- He, K.; Mack, W.J.; Neely, M.; Lewis, L.; Anand, V. Parental Perspectives on Immunizations: Impact of the COVID-19 Pandemic on Childhood Vaccine Hesitancy. J. Community Health 2022, 47, 39–52. [Google Scholar] [CrossRef]
- McDonald, H.I.; Tessier, E.; White, J.M.; Woodruff, M.; Knowles, C.; Bates, C.; Parry, J.; Walker, J.L.; Scott, J.A.; Smeeth, L.; et al. Early Impact of the Coronavirus Disease (COVID-19) Pandemic and Physical Distancing Measures on Routine Childhood Vaccinations in England, January to April 2020. Euro Surveill. 2020, 25, 2000848. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 988. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, M.; Alaqeel, A. Impact of the COVID-19 Pandemic on Routine Childhood Immunization in Saudi Arabia. Vaccines 2020, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Palo, S.K.; Dubey, S.; Negi, S.; Sahay, M.R.; Patel, K.; Swain, S.; Mishra, B.K.; Bhuyan, D.; Kanungo, S.; Som, M.; et al. Effective Interventions to Ensure MCH (Maternal and Child Health) Services during Pandemic Related Health Emergencies (Zika, Ebola, and COVID-19): A Systematic Review. PLoS ONE 2022, 17, e0268106. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Mansour, T.; Brousseau, N.; Rafferty, E.; Paudel, Y.R.; Sadarangani, M.; Svenson, L.W.; Robinson, J.L.; Gagneur, A.; Driedger, S.M.; et al. COVID-19 Pandemic Impact on Childhood Vaccination Coverage in Quebec, Canada. Hum. Vaccines Immunother. 2021, 18, 2007707. [Google Scholar] [CrossRef] [PubMed]
- Verrier, F.; de Lauzanne, A.; Diouf, J.-B.N.; Zo, A.Z.; Ramblière, L.; Herindrainy, P.; Sarr, F.D.; Sok, T.; Vray, M.; Collard, J.-M.; et al. Vaccination Coverage and Risk Factors Associated with Incomplete Vaccination Among Children in Cambodia, Madagascar, and Senegal. Open Forum Infect. Dis. 2023, 10, ofad136. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Gupta, A. Why Reaching Zero-Dose Children Holds the Key to Achieving the Sustainable Development Goals. Vaccines 2023, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Cabrera, M.; Moore, M.; Lomazzi, M. Driving Paediatric Vaccine Recovery in Europe. Vaccines 2023, 11, 184. [Google Scholar] [CrossRef]
- Causey, K.; Fullman, N.; Sorensen, R.J.D.; Galles, N.C.; Zheng, P.; Aravkin, A.; Danovaro-Holliday, M.C.; Martinez-Piedra, R.; Sodha, S.V.; Velandia-González, M.P.; et al. Estimating Global and Regional Disruptions to Routine Childhood Vaccine Coverage during the COVID-19 Pandemic in 2020: A Modelling Study. Lancet 2021, 398, 522–534. [Google Scholar] [CrossRef]
- Wiysonge, C.S.; Uthman, O.A.; Ndumbe, P.M.; Hussey, G.D. Individual and Contextual Factors Associated with Low Childhood Immunisation Coverage in Sub-Saharan Africa: A Multilevel Analysis. PLoS ONE 2012, 7, e37905. [Google Scholar] [CrossRef]
- WHO. Global Routine Immunization Strategies and Practices (Grisp)—A Companion Document to the Global Vaccine Action Plan (GVAP); WHO: Geneva, Switzerland, 2016. [Google Scholar]
- WHO. Immunization and Vaccine-Preventable Communicable Diseases. Available online: https://www.who.int/data/gho/data/themes/immunization (accessed on 12 November 2023).
- Immunization Coverage—Are We Losing Ground? Available online: https://data.unicef.org/resources/immunization-coverage-are-we-losing-ground/ (accessed on 29 April 2023).
- Cutts, F.T.; Izurieta, H.S.; Rhoda, D.A. Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys. PLoS Med. 2013, 10, e1001404. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J. Vaccination Week in the Americas Press Conference—Dr. Jarbas Barbosa, PAHO’s Director Remarks—20 April 2023—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/vaccination-week-americas-press-conference-dr-jarbas-barbosa-pahos-director-remarks-20 (accessed on 15 December 2023).
- PAHO. Fact Sheet—Vaccination Week in the Americas 2023—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/fact-sheet-vaccination-week-americas-2023 (accessed on 15 December 2023).
- PAHO. Risk of Vaccine-Preventable Disease Outbreaks at 30-Year High, PAHO Director Says. Available online: https://www.paho.org/en/news/20-4-2023-risk-vaccine-preventable-disease-outbreaks-30-year-high-paho-director-says (accessed on 15 December 2023).
- WHO/UNICEF. Progress and Challenges with Achieving Universal Immunization Coverage. 2022 WHO/UNICEF Estimates of National Immunization Coverage (WUENIC); WHO/UNICEF: Geneva, Switzerland, 2023. [Google Scholar]
- Brown, C.L.; Montez, K.; Amati, J.B.; Simeonsson, K.; Townsend, J.D.; Orr, C.J.; Palakshappa, D. Impact of COVID-19 on Pediatric Primary Care Visits at Four Academic Institutions in the Carolinas. Int. J. Environ. Res. Public Health 2021, 18, 5734. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.; Carr, K.; Danovaro-Holliday, M.C.; Sodha, S.V.; Prosperi, C.; Wunderlich, J.; Wonodi, C.; Reynolds, H.W.; Mirza, I.; Gacic-Dobo, M.; et al. Impact of the SARS-CoV-2 Pandemic on Routine Immunisation Services: Evidence of Disruption and Recovery from 170 Countries and Territories. Lancet Glob. Health 2022, 10, e186–e194. [Google Scholar] [CrossRef]
- Aguinaga-Ontoso, I.; Guillen-Aguinaga, S.; Guillen-Aguinaga, L.; Alas-Brun, R.; Onambele, L.; Aguina-ga-Ontoso, E.; Guillen-Grima, F. COVID-19 Impact on DTP Vaccination Trends in Africa: A Joinpoint Regression Analysis. Vaccines 2023, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Castrejon, M.M.; Leal, I.; de Jesus Pereira Pinto, T.; Guzmán-Holst, A. The Impact of COVID-19 and Catch-up Strategies on Routine Childhood Vaccine Coverage Trends in Latin America: A Systematic Literature Review and Database Analysis. Hum. Vaccines Immunother. 2022, 18, 2102353. [Google Scholar] [CrossRef]
- Munyangaju, I.; López-Varela, E.; Bassat, Q. Closing the Gap in Childhood Immunisation after the Pandemic. BMJ 2023, 380, 627. [Google Scholar] [CrossRef] [PubMed]
- The Global Health Observatory. Vaccine-Preventable Diseases. Available online: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/gho-immunization-vaccine-preventable-communicable-diseases (accessed on 29 November 2023).
- Kaur, G.; Danovaro-Holliday, M.C.; Mwinnyaa, G.; Gacic-Dobo, M.; Francis, L.; Grevendonk, J.; Sodha, S.V.; Sugerman, C.; Wallace, A. Routine Vaccination Coverage—Worldwide, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1155–1161. [Google Scholar] [CrossRef]
- World Health Assembly. Immunization Agenda 2030: A Global Strategy to Leave No One Behind; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- UNICEF. COVID-19 Pandemic Fuels Largest Continued Backslide in Vaccinations in Three Decades. Available online: https://www.unicef.org/press-releases/WUENIC2022release (accessed on 16 May 2023).
- Data Warehouse—Unicef Data. Available online: https://data.unicef.org/resources/data_explorer/unicef_f/?ag=UNICEF&df=IMMUNISATION&ver=1.0&dq=.IM_DTP3..&startPeriod=2000&endPeriod=2022 (accessed on 18 April 2023).
- UNICEF. Number of Births. Data Warehouse—UNICEF DATA. Available online: https://data.unicef.org/resources/data_explorer/unicef_f/?ag=UNICEF&df=GLOBAL_DATAFLOW&ver=1.0&dq=.DM_BRTS..&startPeriod=2019&endPeriod=2021 (accessed on 21 May 2023).
- World Bank. Population, Total|Data. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL (accessed on 21 May 2023).
- White, K.J. The Durbin-Watson Test for Autocorrelation in Nonlinear Models. Rev. Econ. Stat. 1992, 74, 370. [Google Scholar] [CrossRef]
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented Regression Analysis of Interrupted Time Series Studies in Medication Use Research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Cummins, S.; Gasparrini, A. Interrupted Time Series Regression for the Evaluation of Public Health Interventions: A Tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Slavova, S.; Rock, P.; Bush, H.M.; Quesinberry, D.; Walsh, S.L. Signal of Increased Opioid Overdose during COVID-19 from Emergency Medical Services Data. Drug Alcohol. Depend. 2020, 214, 108176. [Google Scholar] [CrossRef] [PubMed]
- Rockville, M. Joinpoint Regression Program, version 4.3.1.0; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute: Rockville, MA, USA, 2016.
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation Tests for Joinpoint Regression with Applications to Cancer Rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Muggeo, V.M.; Atkins, D.C.; Gallop, R.J.; Dimidjian, S. Segmented Mixed Models with Random Change-points: A Maximum Likelihood Approach with Application to Treatment for Depression Study. Stat. Model-Ling. 2014, 14, 293–313. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Interval Estimation for the Breakpoint in Segmented Regression: A Smoothed Score-based Approach. Aust. N. Z. J. Stat. 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Testing with a Nuisance Parameter Present Only under the Alternative: A Score-Based Approach with Application to Segmented Modelling. J. Stat. Comput. Simul. 2016, 86, 3059–3067. [Google Scholar] [CrossRef]
- Fasola, S.; Muggeo, V.M.R.; Küchenhoff, H. A Heuristic, Iterative Algorithm for Change-Point Detection in Abrupt Change Models. Comput. Stat. 2018, 33, 997–1015. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files_. R Package Version 1.4.3. 2023. Available online: https://cran.r-project.org/package=readxl (accessed on 17 December 2023).
- Mapchart Version 4.3.2. Mapchart. 30 November 2023. Available online: https://www.mapchart.net/ (accessed on 17 December 2023).
- Brewer, C.A.; Hatchard, G.W.; Harrower, M.A. ColorBrewer in Print: A Catalog of Color Schemes for Maps. Cart. Geogr. Inf. Sci. 2003, 30, 5–32. [Google Scholar] [CrossRef]
- Brewer, C.A.; Hatchard, G.W.; Harrower, M.A. COLORBREWER 2.0 Color Advice for Cartograph. 2013. Available online: https://colorbrewer2.org (accessed on 17 December 2023).
- Rau, C.; Lüdecke, D.; Dumolard, L.B.; Grevendonk, J.; Wiernik, B.M.; Kobbe, R.; Gacic-Dobo, M.; Danovaro-Holliday, M.C. Data Quality of Reported Child Immunization Coverage in 194 Countries between 2000 and 2019. PLoS Glob. Public Health 2022, 2, e0000140. [Google Scholar] [CrossRef]
- Maciak, M.; Mizera, I. Regularization Techniques in Joinpoint Regression. Stat. Pap. 2016, 57, 939–955. [Google Scholar] [CrossRef]
- Kontopantelis, E.; Doran, T.; Springate, D.A.; Buchan, I.; Reeves, D. Regression Based Quasi-Experimental Approach When Randomisation Is Not an Option: Interrupted Time Series Analysis. BMJ 2015, 350, h2750. [Google Scholar] [CrossRef]
- Taljaard, M.; McKenzie, J.E.; Ramsay, C.R.; Grimshaw, J.M. The Use of Segmented Regression in Analysing Interrupted Time Series Studies: An Example in Pre-Hospital Ambulance Care. Implement. Sci. 2014, 9, 77. [Google Scholar] [CrossRef]
- Bower, M.; Kothari, U.; Akerman, M.; Krilov, L.R.; Fiorito, T.M. Impact of COVID-19 on HPV Vaccination Rates in New York City and Long Island. Pediatr. Infect. Dis. J. 2023, 43, 84–87. [Google Scholar] [CrossRef]
- Urueña, A.; Machado, R.; Cunha, J.; López Colmano, C.; Rancaño, C.; Kfouri, R.; Pírez, C.; Bonvehí, P.; Calvo, M.; Cuadros, R.; et al. Opinions, Attitudes and Factors Related to SARS-CoV-2 Vaccine Uptake in Eight South American Countries. Vaccines 2023, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Mwinnyaa, G.; Peters, M.A.; Shapira, G.; Neill, R.; Sadat, H.; Yuma, S.; Akilimali, P.; Hossain, S.; Wendrad, N.; Atiwoto, W.K.; et al. Vaccination Utilization and Subnational Inequities during the COVID-19 Pandemic: An Interrupted Time-Series Analysis of Administrative Data across 12 Low- and Middle-Income Countries. Vaccines 2023, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Global Disparity in Access to Essential Vaccines, Says WHO Report|UN News. Available online: https://news.un.org/en/story/2022/11/1130402 (accessed on 17 December 2023).
- WHO. Vaccine Inequity Undermining Global Economic Recovery. Available online: https://www.who.int/news/item/22-07-2021-vaccine-inequity-undermining-global-economic-recovery (accessed on 17 December 2023).
- WHO. Global Vaccine Market Report 2022. Available online: https://www.who.int/publications/m/item/global-vaccine-market-report-2022 (accessed on 17 December 2023).
- Guglielmi, G. Pandemic Drives Largest Drop in Childhood Vaccinations in 30 Years. Nature 2022, 608, 253. [Google Scholar] [CrossRef] [PubMed]
- OECD. Access to COVID-19 Vaccines: Global Approaches in a Global Crisis—OECD. Available online: https://read.oecd-ilibrary.org/view/?ref=1069_1069384-ewmqrw9sx2&title=Access-to-COVID-19-vaccines-Global-approaches-in-a-global-crisis (accessed on 17 December 2023).
- Handfield, R. Challenges and Opportunities to Expand the COVID-19 Vaccine Supply Chain: A Global Summit|Supply Chain Resource Cooperative. Available online: https://scm.ncsu.edu/scm-articles/article/challenges-and-opportunities-to-expand-the-covid19-vaccine-supply-chain-a-global-summit (accessed on 17 December 2023).
- Muhoza, P.; Danovaro-Holliday, M.C.; Diallo, M.S.; Murphy, P.; Sodha, S.V.; Requejo, J.H.; Wallace, A.S. Routine Vaccination Coverage—Worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.; Keiser, O.; Kaiser, L.; Jombart, T. Analysis of Global Routine Immunisation Coverage Shows Disruption and Stagnation during the First Two-Years of the COVID-19 Pandemic with Tentative Recovery in 2022. Vaccine 2023, 15, 100383. [Google Scholar] [CrossRef] [PubMed]
- Rachlin, A.; Carolina Danovaro-Holliday, M.; Murphy, P.; Sodha, S.V.; Wallace, A.S. Report Routine Vaccination Coverage-Worldwide, 2021. Morb. Mortal. Wkly. 2021, 71, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- LaMori, J.; Feng, X.; Pericone, C.D.; Mesa-Frias, M.; Sogbetun, O.; Kulczycki, A. Hepatitis Vaccination Adherence and Completion Rates and Factors Associated with Low Compliance: A Claims-Based Analysis of US Adults. PLoS ONE 2022, 17, e0264062. [Google Scholar] [CrossRef] [PubMed]
- Périères, L.; Séror, V.; Boyer, S.; Sokhna, C.; Peretti-Watel, P. Reasons given for Non-Vaccination and under-Vaccination of Children and Adolescents in Sub-Saharan Africa: A Systematic Review. Hum. Vaccines Immunother. 2022, 18, 2076524. [Google Scholar] [CrossRef] [PubMed]
- Atnafu Gebeyehu, N.; Abebe Gelaw, K.; Asmare Adella, G.; Dagnaw Tegegne, K.; Adie Admass, B.; Mesele Gesese, M. Incomplete Immunization and Its Determinants among Children in Africa: Systematic Review and Meta-Analysis. Hum. Vaccines Immunother. 2023, 19, 2202125. [Google Scholar] [CrossRef] [PubMed]
- CDC CDC Strategy for Global Response to COVID-19 (2020–2023). 2022. Available online: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/global-covid-19/global-response-strategy.html (accessed on 29 November 2023).
- Dayaratna, K.; Tyrrell, P.; Vanderplas, A. A Comparative Analysis of Policy Approaches to COVID-19 around the World, with Recommendations for US Lawmakers|The Heritage Foundation. Available online: https://www.heritage.org/public-health/report/comparative-analysis-policy-approaches-covid-19-around-the-world (accessed on 17 December 2023).
- Bliss, K.E. Strengthening Routine Immunizations and Responding to COVID-19; Center at the Center for Strategic and International Studies: Washington, DC, USA, 2020. [Google Scholar]
- de Albuquerque Veloso Machado, M.; Roberts, B.; Wong, B.L.H.; van Kessel, R.; Mossialos, E. The Relationship Between the COVID-19 Pandemic and Vaccine Hesitancy: A Scoping Review of Literature Until August 2021. Front Public Health 2021, 9, 747787. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Safadi, M.A.P.; Horn, M.; Regojo Balboa, C.; Moya, E.; Schanbaum, J.; Pimenta, P.; Lambert, E.; Soumahoro, L.; Sohn, W.-Y.; et al. Pandemic’s Influence on Parents’ Attitudes and Behaviors toward Meningococcal Vaccination. Hum. Vaccines Immunother. 2023, 19, 2179840. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Steffen, R.; Schelling, J.; Balaisyte-Jazone, L.; Posiuniene, I.; Zatoński, M.; Van Damme, P. Vaccine Co-Administration in Adults: An Effective Way to Improve Vaccination Coverage. Hum. Vaccines Immunother. 2023, 19, 2195786. [Google Scholar] [CrossRef]
- Spencer, N.; Markham, W.; Johnson, S.; Arpin, E.; Nathawad, R.; Gunnlaugsson, G.; Homaira, N.; Rubio, M.L.M.; Trujillo, C.J. The Impact of COVID-19 Pandemic on Inequity in Routine Childhood Vaccination Coverage: A Systematic Review. Vaccines 2022, 10, 1013. [Google Scholar] [CrossRef]
- Ning, C.; Wang, H.; Wu, J.; Chen, Q.; Pei, H.; Gao, H. The COVID-19 Vaccination and Vaccine Inequity Worldwide: An Empirical Study Based on Global Data. Int. J. Environ. Res. Public Health 2022, 19, 5267. [Google Scholar] [CrossRef]
- Sabbatucci, M.; Odone, A.; Signorelli, C.; Siddu, A.; Silenzi, A.; Maraglino, F.P.; Rezza, G. Childhood Immunisation Coverage during the COVID-19 Epidemic in Italy. Vaccines 2022, 10, 120. [Google Scholar] [CrossRef]
- Olusanya, O.A.; Bednarczyk, R.A.; Davis, R.L.; Shaban-Nejad, A. Addressing Parental Vaccine Hesitancy and Other Barriers to Childhood/Adolescent Vaccination Uptake During the Coronavirus (COVID-19) Pandemic. Front. Immunol. 2021, 12, 663074. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.D.; Bingenheimer, J.B.; Long, M.; Ndiaye, K.; Donati, D.; Rao, N.M.; Akaba, S.; Nsofor, I.; Agha, S. Outcomes of a Social Media Campaign to Promote COVID-19 Vaccination in Nigeria. PLoS ONE 2023, 18, e0290757. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, D.; Northcote, N.; Röder, T.; Sauer-Sidor, K. Strategic Resilience during the COVID-19 Crisis|McKinsey. Available online: https://www.mckinsey.com/capabilities/strategy-and-corporate-finance/our-insights/strategic-resilience-during-the-covid-19-crisis (accessed on 29 April 2023).
- Soorapanth, S.; Cheung, R.; Zhang, X.; Mokdad, A.H.; Mensah, G.A. Rural–Urban Differences in Vaccination and Hesitancy Rates and Trust: US COVID-19 Trends and Impact Survey on a Social Media Platform, May 2021–April 2022. Am. J. Public Health 2023, 113, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Olstad, D.L.; McIntyre, L. Reconceptualising Precision Public Health. BMJ Open 2019, 9, e030279. [Google Scholar] [CrossRef]
- WHO; UNICEF. Warn of a Decline in Vaccinations during COVID-19. Available online: https://www.who.int/news/item/15-07-2020-who-and-unicef-warn-of-a-decline-in-vaccinations-during-covid-19 (accessed on 29 April 2023).
- Watts, E.; Mak, J.; Patenaude, B. Benefit-Cost Ratios of Continuing Routine Immunization During the COVID-19 Pandemic in Africa. J. Benefit Cost. Anal. 2022, 13, 91–106. [Google Scholar] [CrossRef]
- de Oliveira Roque e Lima, J.; Pagotto, V.; Rocha, B.S.; Scalize, P.S.; Guimarães, R.A.; de Lima, M.D.; da Silva, L.N.; da Silva Oliveira, M.D.; Moura, W.É.A.; Teles, S.A.; et al. Low Vaccine Coverage and Factors Associated with Incomplete Childhood Immunization in Racial/Ethnic Minorities and Rural Groups, Central Brazil. Vaccines 2023, 11, 838. [Google Scholar] [CrossRef]
- Tan, N.C.; Pang, J.; Koh, E. The Impact of a Revised National Childhood Immunization Schedule on Vaccination Defaulters. Vaccines 2023, 11, 859. [Google Scholar] [CrossRef]
- Saxena, K.; Marden, J.R.; Carias, C.; Bhatti, A.; Patterson-Lomba, O.; Gomez-Lievano, A.; Yao, L.; Chen, Y.-T. Impact of the COVID-19 Pandemic on Adolescent Vaccinations: Projected Time to Reverse Deficits in Rou-tine Adolescent Vaccination in the United States. Curr. Med. Res. Opin. 2021, 37, 2077–2087. [Google Scholar] [CrossRef]
- Patel Murthy, B.; Zell, E.; Kirtland, K.; Jones-Jack, N.; Harris, L.; Sprague, C.; Schultz, J.; Le, Q.; Bramer, C.A.; Kuramoto, S.; et al. Impact of the COVID-19 Pandemic on Administration of Selected Routine Childhood and Adolescent Vaccinations—10 US Jurisdictions, March–September 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 840–845. [Google Scholar] [CrossRef]
- Moraga-Llop, F.A.; Fernández-Prada, M.; Grande-Tejada, A.M.; Martínez-Alcorta, L.I.; Moreno-Pérez, D.; Pérez-Martín, J.J. Recovering Vaccine Coverage Lost Due to the COVID-19 Pandemic. Vacunas 2020, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- WHO. Scale-Up Routine Immunization along with COVID-19 Vaccination: WHO. Available online: https://www.who.int/southeastasia/news/detail/08-09-2021-scale-up-routine-immunization-along-with-covid-19-vaccination-who (accessed on 29 April 2023).
- Berhane, H.Y.; Worku, A.; Fawzi, W. Effect of COVID-19 on Routine Childhood Vaccination in Bahir Dar City, Northwestern, Ethiopia. Vaccines 2023, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Nadol, A. Get Childhood Immunizations Back on Track. Nature 2022, 608, 7–8. [Google Scholar]
- Oyo-Ita, A.; Oduwole, O.; Arikpo, D.; Effa, E.E.; Esu, E.B.; Balakrishna, Y.; Chibuzor, M.T.; Oringanje, C.M.; Nwachukwu, C.E.; Wiysonge, C.S.; et al. Interventions for Improving Coverage of Childhood Immunisation in Low-and Middle-Income Countries. Cochrane Database Syst. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi, C.; Eguchi, A.; Lwin, K.S.; Yoneoka, D.; Tanoue, Y.; Rauniyar, S.K.; Horiuchi, S.; Hashizume, M.; Nomura, S. Estimating Global Changes in Routine Childhood Vaccination Coverage During the COVID-19 Pandemic, 2020–2021. Vaccine 2023, 41, 4151–4157. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, V.; Wagner, A.L. Demographic Differences in Compliance with COVID-19 Vaccination Timing and Completion Guidelines in the United States. Vaccines 2023, 11, 369. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).