Abstract
Carditis in childhood is a rare disease with several etiologies. We report a case of infant death due to pericarditis and myocarditis after the mRNA vaccine against COVID-19 (COVIDmRNAV). A 7-year-old male child received the first dose of the COVIDmRNAV and presented with monoarthritis and a fever non-responsive to oral antibiotics. The laboratory investigation showed signs of infection (leukocytosis, high levels of c-reactive protein). His condition rapidly deteriorated, and the patient died. The autopsy identified pericardial fibrin deposits, hemorrhagic areas in the myocardium, and normal valves. A diffuse intermyocardial inflammatory infiltrate composed of T CD8+ lymphocytes and histiocytes was identified. An antistreptolysin O (ASO) dosage showed high titers. The presence of arthritis, elevated ASO, and carditis fulfills the criteria for rheumatic fever. However, valve disease and Aschoff’s nodules, present in 90% of rheumatic carditis cases, were absent in this case. The temporal correlation with mRNA vaccination prompted its inclusion as one of the etiologies. In cases of myocardial damage related to COVID-19mRNAV, it appears to be related to the expression of exosomes and lipid nanoparticles, leading to a cytokine storm. The potential effects of the COVID-19mRNAV must be considered in the pathogenesis of this disease, whether as an etiology or a contributing factor to a previously initiated myocardial injury.
1. Introduction
Carditis in childhood is a rare disease with a variable clinical presentation, sometimes non-specific and spontaneously resolving, with the possibility of developing sequelae, which are occasionally severe and fatal [1,2,3]. Precise etiological investigation is crucial for therapeutic management as different pathogenic mechanisms guide medication choice, with endomyocardial biopsy and histological evaluation being the current gold standard. [4].
The most common causes of myocarditis are bacterial and viral infection, systemic inflammatory disorders involving connective tissue, autoimmunity, and the effects of drugs and toxins [3]. Recently, an already-known pathogen has gained even more attention: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [5,6]. Conditions often related to multisystem inflammation have also been reported, albeit less frequently, following coronavirus disease 2019 (COVID-19) vaccination, primarily developed using viral mRNA [7,8,9,10,11,12].
We present a case of infant death from an unknown pathology that began after the use of an mRNA vaccine against COVID-19. The autopsy identified pericarditis and myocarditis, with extensive morphological overlap between the possible differential diagnoses and a difficult final etiological classification.
2. Case Report
A 7-year-old male child presented with myalgia and fever 3 days after receiving the first dose of the COVID-19 vaccine (BNT162b2). The parents denied previous contact with sick people or a history of symptoms of upper airway infection. After seven days, he presented with monoarthritis in his right ankle. Blood tests indicated leukocytosis, but a CT scan of the ankle showed no abnormalities. He was diagnosed with septic arthritis and discharged with empirical antibiotic therapy.
After 10 days, the patient was re-evaluated due to persistent symptoms. At this time, leukocytosis had improved, and O antistreptolysin (OAS) values were normal, leading to discharge with a new outpatient antibiotic regimen. Three weeks post-symptom onset, with ongoing joint pain and walking difficulty, new tests were performed. Elevated OAS levels prompted hospital admission for intravenous antibiotic treatment with oxacillin. The joint pain improved over nine days, but the patient developed mild gastrointestinal symptoms like vomiting with blood streaks and epigastric pain. His condition rapidly deteriorated upon diagnosis of upper gastrointestinal bleeding. Post-orotracheal intubation, active bleeding was observed from the tube, leading to an emergency department referral. Laboratory tests indicated leukocytosis, elevated C-reactive protein levels, and negative COVID-19 polymerase chain reaction (Table 1), alongside right upper and lower lung lobe consolidations and ground-glass opacities on chest CT. Unfortunately, the patient died.

Table 1.
Laboratory tests during the course of the disease.
On autopsy, external examination showed anasarca and increased abdominal volume. The internal organs showed diffuse edema, including the brain, with pleural, pericardial, and peritoneal cavitary effusion. The heart exhibited a granular, opaque, whitish external surface, akin to fibrin deposits on the pericardium (Figure 1).
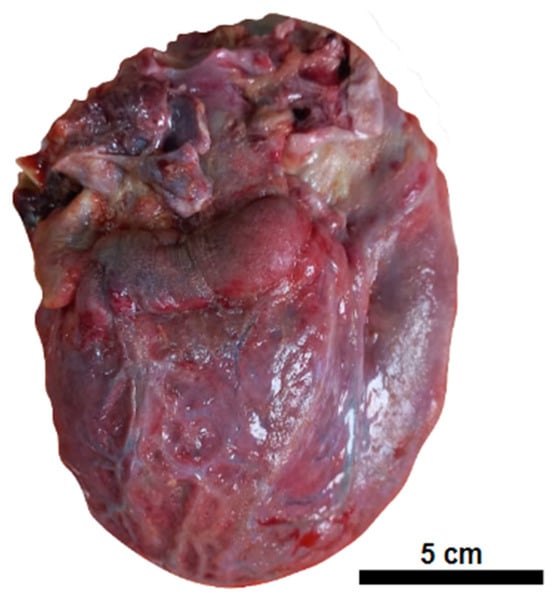
Figure 1.
Pericarditis. Globose heart with an opaque external surface covered in fine granulation and fibrinous debris.
The myocardium had a soft consistency, alternating between pale and hemorrhagic areas, while the valves remained preserved. Microscopy revealed disseminated vascular thromboembolism. The macroscopic and microscopic findings of the main organs are reported below (Table 2, Figure 2, Figure 3 and Figure 4).

Table 2.
Pathological findings of the autopsy procedure.
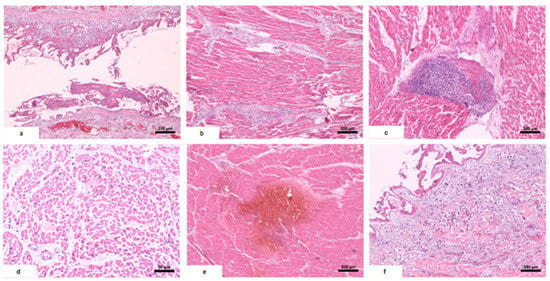
Figure 2.
Histological alterations in the heart. (a) Pericarditis: fibrin deposition in the pericardium and lymphocytic infiltrate. (b) Myocarditis: inflammatory infiltrate concentrated in intermyocardial fibrotic tracts, with focal extension to cardiac fibers. (c) Foci of mixed inflammatory aggregate in the myocardium: plasma cells, lymphocytes, and neutrophils. (d) Subendocardial necrosis: myocardial fibers with eosinophilic and vacuolized cytoplasm and absent nuclei. (e) Myocardial hemorrhage. (f) Focal endocarditis: discrete mixed inflammatory infiltrate in the endocardium with fibrin deposition.
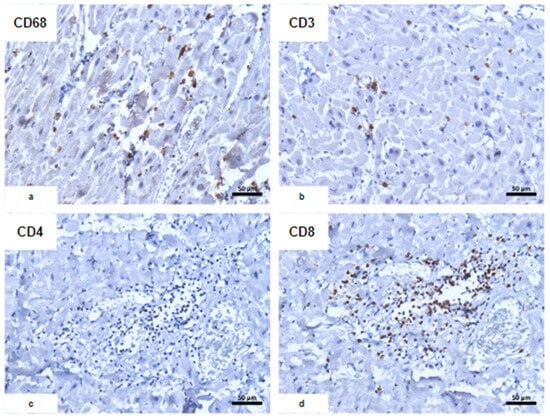
Figure 3.
Immunohistochemical characterization of the inflammatory infiltrate. (a) CD68; (b) CD3; (c) CD4; (d) CD8: predominance of CD8+ T lymphocytes associated with CD68+ macrophages with an usual morphological appearance.
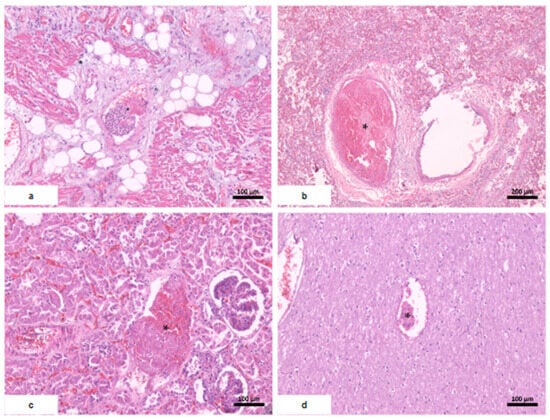
Figure 4.
Systemic thromboembolism. (a) Heart; (b) lungs; (c) kidney with severe acute tubular necrosis; (d) brain. * fibrin thrombi.
The condition was diagnosed as acute pericarditis and myocarditis, without valve involvement, with heart failure leading to pulmonary edema, complicated by acute tubular necrosis and ischemic hepatic necrosis.
3. Discussion
This case poses a diagnostic challenge due to overlapping risk factors, symptoms, and diverse histological findings, each with variable diagnostic specificity. The main differential diagnoses will be discussed.
3.1. Rheumatic Fever (RF) and Myocarditis
The previous occurrence of an upper airway infection associated with arthritis and elevated OSA levels would fulfill the modified Jones criteria for diagnosing RF, with the presence of one major and two minor criteria: carditis, fever, and elevated serum CRP levels, respectively. However, evidence of monoarthritis is not a diagnostic criteria, as only polyarthritis is considered in diagnosing a first outbreak [13].
Furthermore, the morphological presentation differs from that typically seen in cardiac involvement by rheumatic fever. Most rheumatic carditis involves the endocardium [14], with valve disease in up to 90% of symptomatic cases [13]. Associated pericarditis and myocarditis, when present, show morphological characteristics reflecting the pathogenic mechanisms involved.
Cross-immune activation through antigenic mimicry with streptococcal proteins leads to the systemic inflammatory damage characteristic of RF [14,15]. Other studies have shown integration between streptococcal proteins and type IV collagen in the extracellular matrix [16]. In the heart, this reaction is more accentuated at the endothelium, especially in the valves, with increased expression of VCAM-1, an adhesion molecule that helps in the migration of activated leukocytes [17]. Moreover, the inflammatory aggregates are arranged around the cardiac connective tissue, intermingling the muscle fibers, without pronounced myocardial necrosis [18], which, when present, is related to local cellular aggression caused by the inflammatory process. This presentation differs from virus-related carditis, where aggression primarily affecting the myocardium results in extensive necrosis and a corresponding rise in myocardial necrosis markers [3].
The most specific histopathological finding of rheumatic heart disease (RHD) is Aschoff’s nodules, perivascular histiocyte aggregates with characteristic nuclear changes [14]. Spina et al. identified the frequency of this finding in endomyocardial biopsies ranging from 19 to 67% (average: 41.8%). However, the studies failed to establish a consistent relationship with prognosis, corticoid use, or β-hemolytic Streptococcus prophylaxis [19].
3.2. Viral Carditis
Enteroviruses are classically associated with viral myocarditis. Over time, new entities have gained importance, such as parvovirus B19, influenza, adenovirus, cytomegalovirus, human immunodeficiency virus, and SARS-CoV-2 [4], accompanied by different pathogenic mechanisms. Adenoviruses and enteroviruses possess a cytolytic action profile that damages the myocardial cytoskeleton and is possibly linked to CCR5 receptor expression [20]. Parvovirus B19 exhibits vasculotropism and can remain quiescent in endothelial cells, causing damage to myocytes through inflammatory stimuli [4].
The expanding evidence on SARS-CoV-2 cardiotoxicity reveals various pathogenic mechanisms, including cardiomyotropism and cell damage via the angiotensin-converting enzyme receptor-binding protein, immune activation by the spike protein, and the production of antibodies that cross-react with cardiac cell antigens like α-myosin [21]. After a collaborative systematic review, Almamlouk found that 100% of studies show an association between cardiac infection with SARS-CoV-2 and myocardial necrosis, while there is no reference to signs of myocarditis, such as a pronounced inflammatory infiltrate [22]. The study failed to define a histological lesion pattern associated with COVID-19. Notably, a systematic review identified cardiomegaly, myocardial necrosis, an inflammatory infiltrate composed of CD3+ T lymphocytes, with prominent CD8+, and macrophages as the main cardiac signs identified. [5]. The absence of a clear relationship between viral load and cell damage, myocardial necrosis, and the low frequency of organized and pronounced inflammatory infiltrates make it less likely that the mechanism involved in COVID-19 is cytotoxic injury. The vasculitis caused by the virus, including arterial damage and occlusion, along with the systemic effects of the infection, such as adrenergic response and cathecolamin-induced cell stress, may be key contributors to its harmful effects [23,24,25,26].
3.3. Vaccination against COVID-19 and Myocarditis
The general population’s use of vaccines, following their safety confirmation in phase 3 studies, increases exposure and enables the identification of rarer side effects. This was also true for the COVID-19 vaccine, especially the viral mRNA-based one [27,28].
Vaccine-related myocarditis is one of these adverse effects. The Adverse Event Reporting System (VAERS) included 27,229 cases of myocarditis and pericarditis until June 2023 [29]. With an often favorable clinical course, several studies corroborate the higher frequency of this complication after the second dose in young males under 40 years of age [30], especially in the 18–25 age group, with a higher risk attributed to the mRNA-1273 vaccine than to the BNT162b2 [31]. However, studies show that the booster dose does not lead to a substantial increase in the risk of perimyocarditis [21].
Giannotta et al. described the mechanisms involved in cardiac injury stimulated by the mRNA vaccine. The stimulation of the expression of exosomes, containing both the spike viral protein and inflammatory mediators, associated with the expression of adhesion factors that dysfunctionally stimulate the endothelial cell, plays an important role in this mechanism [7,32]. The spike protein leads to activation of the TLR-4/NF-kB pathway and stimulation of the cell-mediated immune response, with inflammation directed at cardiomyocytes [33]. In addition to the effect related to the viral structure, the composition and quantity of lipid nanoparticles in the vaccine dose, which differ between manufacturers, can show toxic activity with a potent inflammatory response already in the first moments after application [34]. There is also evidence that the immune cells that absorb the lipid nanoparticles distribute them throughout the body with high levels of spike protein, inflicting a continuous immune response [29]. The immune reaction comprises CD8+ T lymphocytes, macrophages, and plasma cells, occasionally including an eosinophilic component without a characteristic morphological pattern [35].
Post-vaccination inflammatory activation is evidenced by a storm of inflammatory cytokines, such as high levels of IL-1, IL-1B, IL-6, and TNF-α. The circulation of these mediators might relate to the development of side effects and individual reactions after the first vaccine dose, but more frequently after the second dose, with varying clinical significance [35,36].
3.4. Multisystem Inflammatory Syndrome (MIS)
Multisystem inflammatory syndrome (MIS) is a condition related to COVID-19, with a predilection for children (MIS-C) [37]. The diagnostic criteria defined by the World Health Organization [38] include fever > 3 days, increased markers of inflammation, no evidence of other infections, and proof of COVID-19 infection, in addition to two of the following criteria: rash, non-purulent conjunctivitis, or mucocutaneous inflammation; hypotension or shock; myocardial dysfunction, pericarditis, or valvulitis; coagulopathy; and gastrointestinal symptoms. Diaz et al. identified a series of 35 children with defined criteria for a diagnosis of MIS-C, all with cardiac involvement. In another series of eight children with hyperinflammatory syndrome and probable COVID-19 infection, seven had gastrointestinal symptoms on initial presentation, as well as fever for 4 to 5 days [39].
Although rare, cases of MIS have also been reported after vaccination against COVID-19 (MIS-V) without evidence of concomitant virus infection [40]. Wassif et al. reported 10 cases of perimyocarditis related to COVID-19 vaccination, including 1 case associated with MIS, marked by a significant reduction in left ventricular function and requiring intensive treatment [41]. Ourdali identified 12 cases of MIS among over 4 million vaccinated children aged 12 to 17 with mRNA vaccines, with cardiac involvement in 83% of cases. Gastrointestinal symptoms (83%) and cytolytic hepatitis (50%) were also common [9].
3.5. Diagnostic Considerations
This is a case with complex clinical laboratory findings. Myocarditis and pericarditis, only suspected at the time of autopsy, developed in an indolent and nonspecific manner, which led to difficulty in raising this hypothesis for its appropriate investigation. This highlights the importance of investigating deaths with undefined causes. For comparison, Table 3 summarizes the histological findings of the diagnostic hypothesis.

Table 3.
Differential diagnosis of carditis.
The patient met the criteria defined for RHD. Nevertheless, some clinical and morphologic details raised suspicion for another causative or contributing factor since they differed from the classic presentation of rheumatic fever. The patient did not report a clinical history of streptococcal infection, despite the fact that Jones modified criteria acknowledge the possibility of subclinical infection if there is laboratorial evidence (i.e., high OSA levels). In addition, there were also criteria for MIS-C in the case, since myocardial disfunction, coagulopathy, and gastrointestinal symptoms developed related to fever > 3 days and an increase in inflammatory markers.
Previous exposure to streptococcal strains with immunogenic potential is an important risk factor for myocarditis. Still, the lack of typical findings in rheumatic heart disease, such as Aschoff nodules, even with extensive histological examination, makes the clinical pathological correlation difficult. A series of endomyocardial biopsies found a considerable prevalence of this finding, regardless of the limited material. In addition, the absence of valve disease is uncommon in RHD, reaching 10% of cases [19]. Unfortunately, the complementary methods for the detection of viral mRNA in the cardiac tissue were not feasible at the time of investigation.
4. Conclusions
It is reasonable to address the potential effects of the COVIDmRNAv in this setting. Temporal relationships must be evaluated carefully since they do not evoke a causal relationship. However, the growing evidence of the vaccine’s systemic immunological effects allows us to deduce the possibility of the contribution of the cytokine storm to the establishment of myocardial injury, already initiated by rheumatologic mechanisms. The systemic findings developed by the patient are similar to those of MIS, which can be frequently present in patients with post-COVID-19 vaccine myocarditis.
Author Contributions
Conceptualization, G.E.B.S., J.S.L., R.d.G.C.F.C. and P.M.B.d.S.; methodology, G.E.B.S.; validation, G.E.B.S., J.S.L., R.d.G.C.F.C. and M.A.G.C.; investigation, E.A.S., P.M.B.d.S. and G.E.B.S.; resources, J.S.L. and R.d.G.C.F.C.; data curation, G.E.B.S., R.d.G.C.F.C. and M.A.G.C.; writing—original draft preparation, P.M.B.d.S. and E.A.S.; writing—review and editing, G.E.B.S., P.M.B.d.S. and M.A.G.C.; supervision, G.E.B.S.; project administration, G.E.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University Hospital of the Federal University of Maranhão (protocol code 4.069.664; date of approval: 6 April 2020).
Informed Consent Statement
Informed consent was obtained from the parents of the patient.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Victor Eduardo Maulen Contreras and Ana Clea Feitosa Pestana, biologists at University Hospital, for their help in preparing the histological sections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tunuguntla, H.; Jeewa, A.; Denfield, S.W. Acute Myocarditis and Pericarditis in Children. Pediatr. Rev. 2019, 40, 14–25. [Google Scholar] [CrossRef]
- Durani, Y.; Giordano, K.; Goudie, B.W. Myocarditis and Pericarditis in Children. Pediatr. Clin. North Am. 2010, 57, 1281–1303. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The Spectrum of Myocarditis: From Pathology to the Clinics. Virchows Arch. 2019, 475, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Giannotta, G.; Murrone, A.; Giannotta, N. COVID-19 mRNA Vaccines: The Molecular Basis of Some Adverse Events. Vaccines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Oi, S.S.P.; Muniz, M.P.R.; Faria, I.M.; Filho, N.S.; de Brito, D.J.A.; Lages, J.S.; Lauande, L.P.; Oliveira, T.K.M.; de Araujo Cunha, K.; de Meneze Neves, P.D.M.; et al. Multisystemic Inflammatory Syndrome and Thrombotic Microangiopathy as Complications of COVID-19 in a Child: A Case Report. Front. Pediatr. 2021, 9, 659069. [Google Scholar] [CrossRef]
- Ouldali, N.; Bagheri, H.; Salvo, F.; Antona, D.; Pariente, A.; Leblanc, C.; Tebacher, M.; Micallef, J.; Levy, C.; Cohen, R.; et al. Hyper Inflammatory Syndrome Following COVID-19 mRNA Vaccine in Children: A National Post-Authorization Pharmacovigilance Study. Lancet Reg. Health Eur. 2022, 17, 100393. [Google Scholar] [CrossRef]
- Gill, J.R.; Tashjian, R.; Duncanson, E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch. Pathol. Lab. Med. 2022, 146, 925–929. [Google Scholar] [CrossRef]
- Hansen, T.; Titze, U.; Kulamadayil-Heidenreich, N.S.A.; Glombitza, S.; Tebbe, J.J.; Röcken, C.; Schulz, B.; Weise, M.; Wilkens, L. First Case of Postmortem Study in a Patient Vaccinated against SARS-CoV-2. Int. J. Infect. Dis. 2021, 107, 172–175. [Google Scholar] [CrossRef]
- Ilonze, O.J.; Guglin, M.E. Myocarditis Following COVID-19 Vaccination in Adolescents and Adults: A Cumulative Experience of 2021. Heart Fail. Rev. 2022, 27, 2033–2043. [Google Scholar] [CrossRef]
- De Faria Pereira, B.Á.; Belo, A.R.; Silva, N.A. da Febre reumática: Atualização dos critérios de Jones à luz da revisão da American Heart Association—2015. Rev. Bras. Reumatol. 2017, 57, 364–368. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute Rheumatic Fever and Rheumatic Heart Disease. Nat. Rev. Dis. Prim. 2016, 2, 15084. [Google Scholar] [CrossRef]
- Cunningham, M.W. Rheumatic Fever, Autoimmunity and Molecular Mimicry: The Streptococcal Connection. Int. Rev. Immunol. 2014, 33, 314–329. [Google Scholar] [CrossRef]
- Dinkla, K.; Rohde, M.; Jansen, W.T.M.; Kaplan, E.L.; Chhatwal, G.S.; Talay, S.R. Rheumatic Fever-Associated Streptococcus Pyogenes Isolates Aggregate Collagen. J. Clin. Invest. 2003, 111, 1905–1912. [Google Scholar] [CrossRef]
- Roberts, S.; Kosanke, S.; Dunn, S.T.; Jankelow, D.; Duran, C.M.G.; Cunningham, M.W. Pathogenic Mechanisms in Rheumatic Carditis: Focus on Valvular Endothelium. J. Infect. Dis. 2001, 183, 507–511. [Google Scholar] [CrossRef]
- Tandon, R.; Sharma, M.; Chandrashekhar, Y.; Kotb, M.; Yacoub, M.H.; Narula, J. Revisiting the Pathogenesis of Rheumatic Fever and Carditis. Nat. Rev. Cardiol. 2013, 10, 171–177. [Google Scholar] [CrossRef]
- Spina, G.S.; Sampaio, R.O.; Branco, C.E.; Miranda, G.B.; Rosa, V.E.E.; Tarasoutchi, F. Incidental Histological Diagnosis of Acute Rheumatic Myocarditis: Case Report and Review of the Literature. Front. Pediatr. 2014, 2, 126. [Google Scholar] [CrossRef][Green Version]
- Badorff, C.; Lee, G.H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral Protease 2A Cleaves Dystrophin: Evidence of Cytoskeletal Disruption in an Acquired Cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [CrossRef]
- Chen, C.; Fu, F.; Ding, L.; Fang, J.; Xiao, J. Booster Dose of COVID-19 mRNA Vaccine Does Not Increase Risks of Myocarditis and Pericarditis Compared with Primary Vaccination: New Insights from the Vaccine Adverse Event Reporting System. Front. Immunol. 2022, 13, 938322. [Google Scholar] [CrossRef]
- Almamlouk, R.; Kashour, T.; Obeidat, S.; Bois, M.C.; Maleszewski, J.J.; Omrani, O.A.; Tleyjeh, R.; Berbari, E.; Chakhachiro, Z.; Zein-Sabatto, B.; et al. COVID-19–Associated Cardiac Pathology at the Postmortem Evaluation: A Collaborative Systematic Review. Clin. Microbiol. Infect. 2022, 28, 1066–1075. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 Patients: Current Problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef]
- Freire, B.M.; de Melo, F.M.; Basso, A.S. Adrenergic Signaling Regulation of Macrophage Function: Do We Understand It Yet? Immunother. Adv. 2022, 2, ltac010. [Google Scholar] [CrossRef]
- Disruption of a Self-Amplifying Catecholamine Loop Reduces Cytokine Release Syndrome|Nature. Available online: https://www.nature.com/articles/s41586-018-0774-y (accessed on 18 September 2023).
- Yang, D.; Dai, X.; Xing, Y.; Tang, X.; Yang, G.; Wang, P.; Harrison, A.G.; Li, H.; Lv, X.; Yu, X.; et al. Intrinsic Cardiac Adrenergic Cells Contribute to Septic Cardiomyopathy. bioRxiv 2021, bioRxiv:2021.03.02.433552. [Google Scholar] [CrossRef]
- Le Vu, S.; Bertrand, M.; Jabagi, M.-J.; Botton, J.; Drouin, J.; Baricault, B.; Weill, A.; Dray-Spira, R.; Zureik, M. Age and Sex-Specific Risks of Myocarditis and Pericarditis Following Covid-19 Messenger RNA Vaccines. Nat. Commun. 2022, 13, 3633. [Google Scholar] [CrossRef]
- Lane, S.; Yeomans, A.; Shakir, S. Reports of Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: A Systematic Review of Spontaneously Reported Data from the UK, Europe and the USA and of the Scientific Literature. BMJ Open 2022, 12, e059223. [Google Scholar] [CrossRef]
- Hulscher, N.; Hodkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Wong, H.-L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beachler, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of Myocarditis and Pericarditis after the COVID-19 mRNA Vaccination in the USA: A Cohort Study in Claims Databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef]
- SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents|Vaccination|JAMA Cardiology|JAMA Network. Available online: https://jamanetwork.com/journals/jamacardiology/fullarticle/2791253 (accessed on 18 September 2023).
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The Spike Protein of SARS-CoV-2 Induces Endothelial Inflammation through Integrin A5β1 and NF-κB Signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The Emerging Role of Toll-like Receptor 4 in Myocardial Inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Giannotta, G.; Giannotta, N. Post-Vaccination Inflammatory Syndrome: A New Syndrome. Clin. Case Rep. Rev. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 Signature Associated with Effective Immune Response to SARS-CoV-2 in BNT162b2 mRNA Vaccine Recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults. JAMA Netw. Open 2021, 4, e2126456. [Google Scholar] [CrossRef]
- Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://www.who.int/news-room/commentaries/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 23 September 2023).
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210–1212. [Google Scholar] [CrossRef]
- Wassif, M.; Lo, P.; Satouris, P.; Swan, L.; Tardo, D.; Kovacic, J.C.; Muller, D.; Muthiah, K.; Kotlyar, E.; Bart, N.K. Acute Myocarditis and Pericarditis After mRNA COVID-19 Vaccinations—A Single-Centre Retrospective Analysis. Heart Lung Circ. 2023, 32, 467–479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).