Long-Term Dynamic Changes in Hybrid Immunity over Six Months after Inactivated and Adenoviral Vector Vaccination in Individuals with Previous SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Vaccines
2.3. Serological Testing
2.4. Surrogate Virus Neutralization Tests (sVNT) for Pre- and Omicron Variants
2.5. Focus Reduction Neutralization Test (FRNT50)
2.6. Quantification of Interferon-Gamma Response
2.7. Statistical Analysis
3. Results
3.1. Study Participants
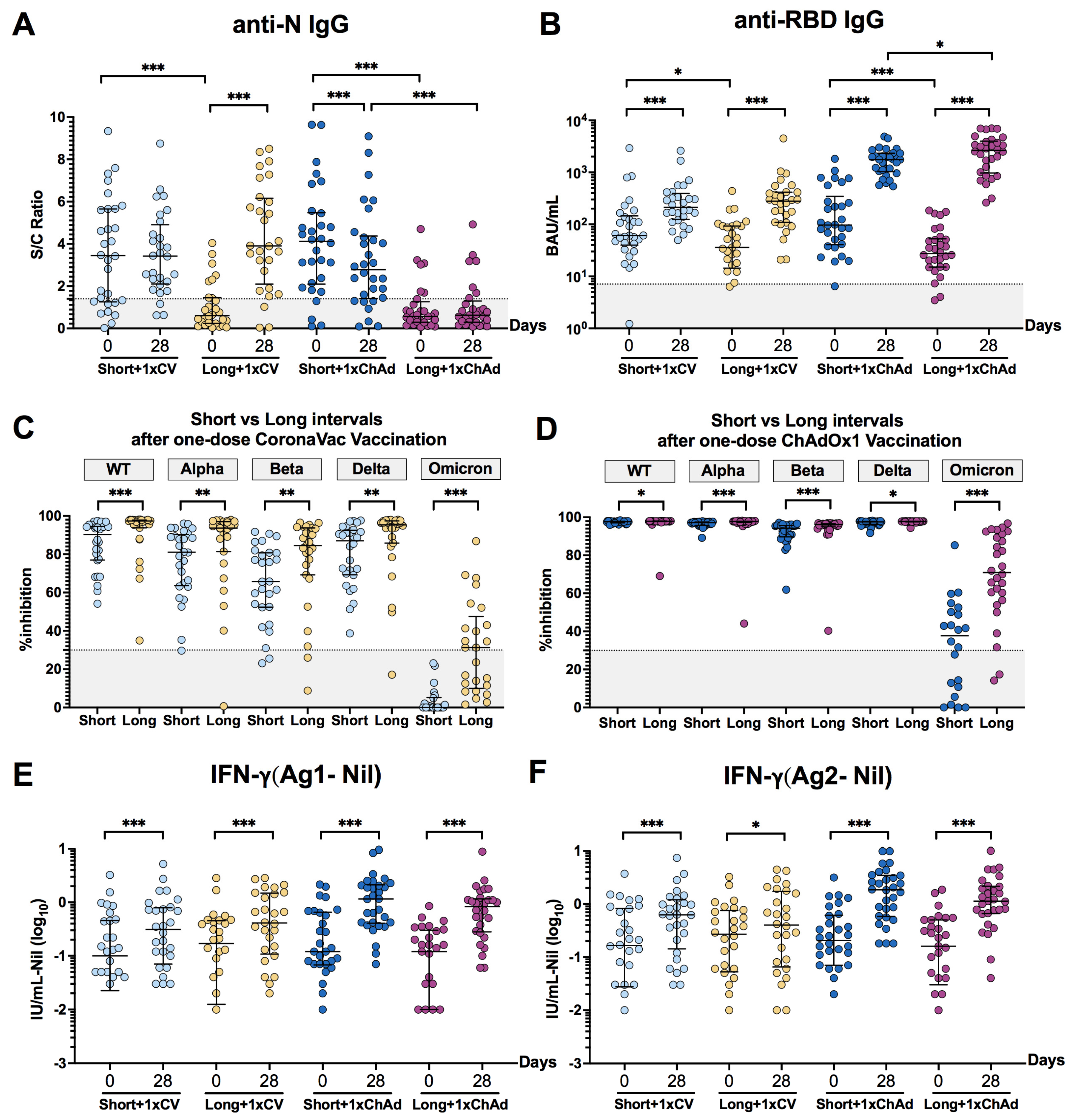
3.2. An Extended Interval between Infection and Vaccination Might Enhance the Antibody Response and Neutralizing Activities
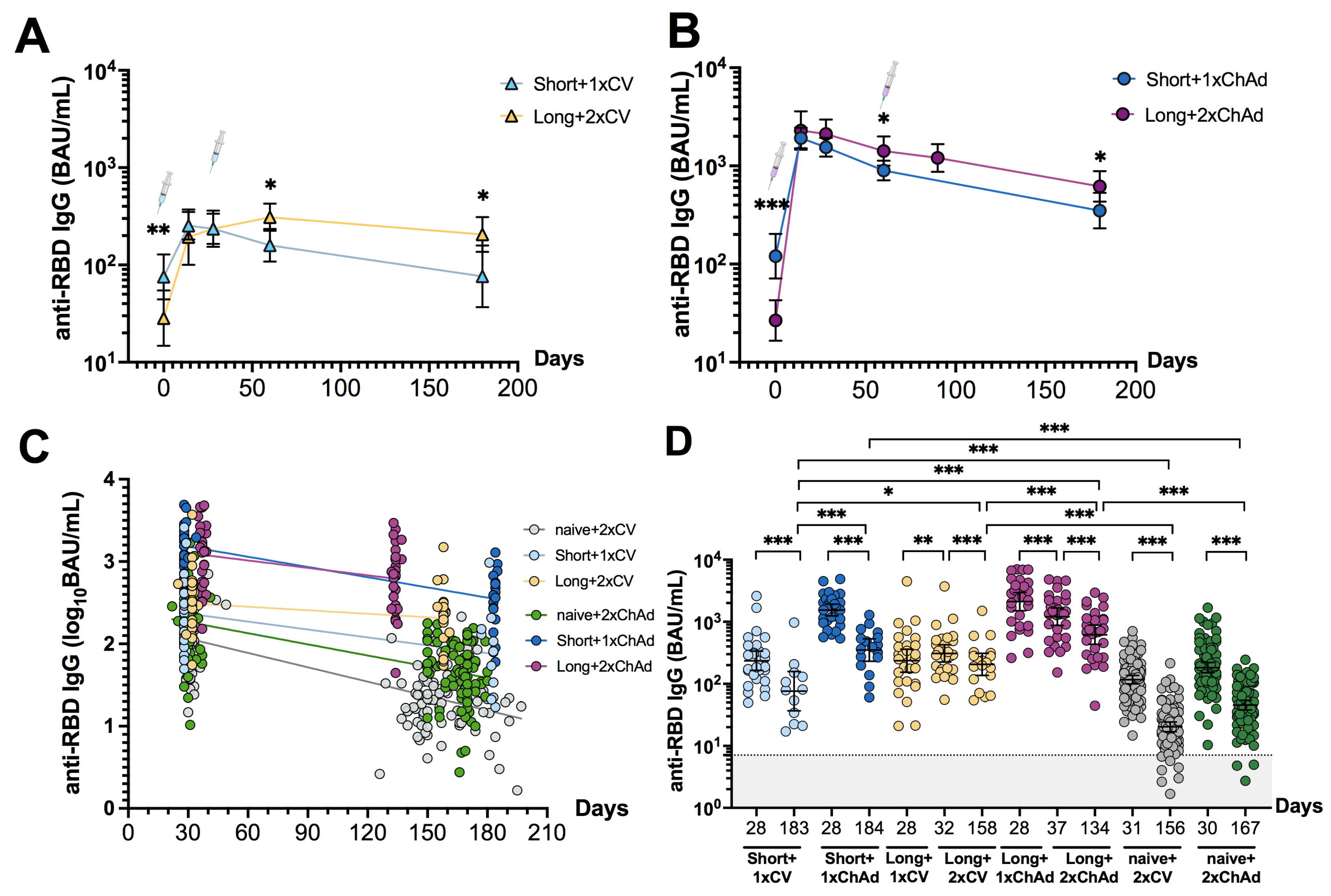
3.3. Dynamic Changes and Waning of Anti-RBD IgG following CoronaVac or ChAdOx1 Vaccination in Individuals with Prior Infection
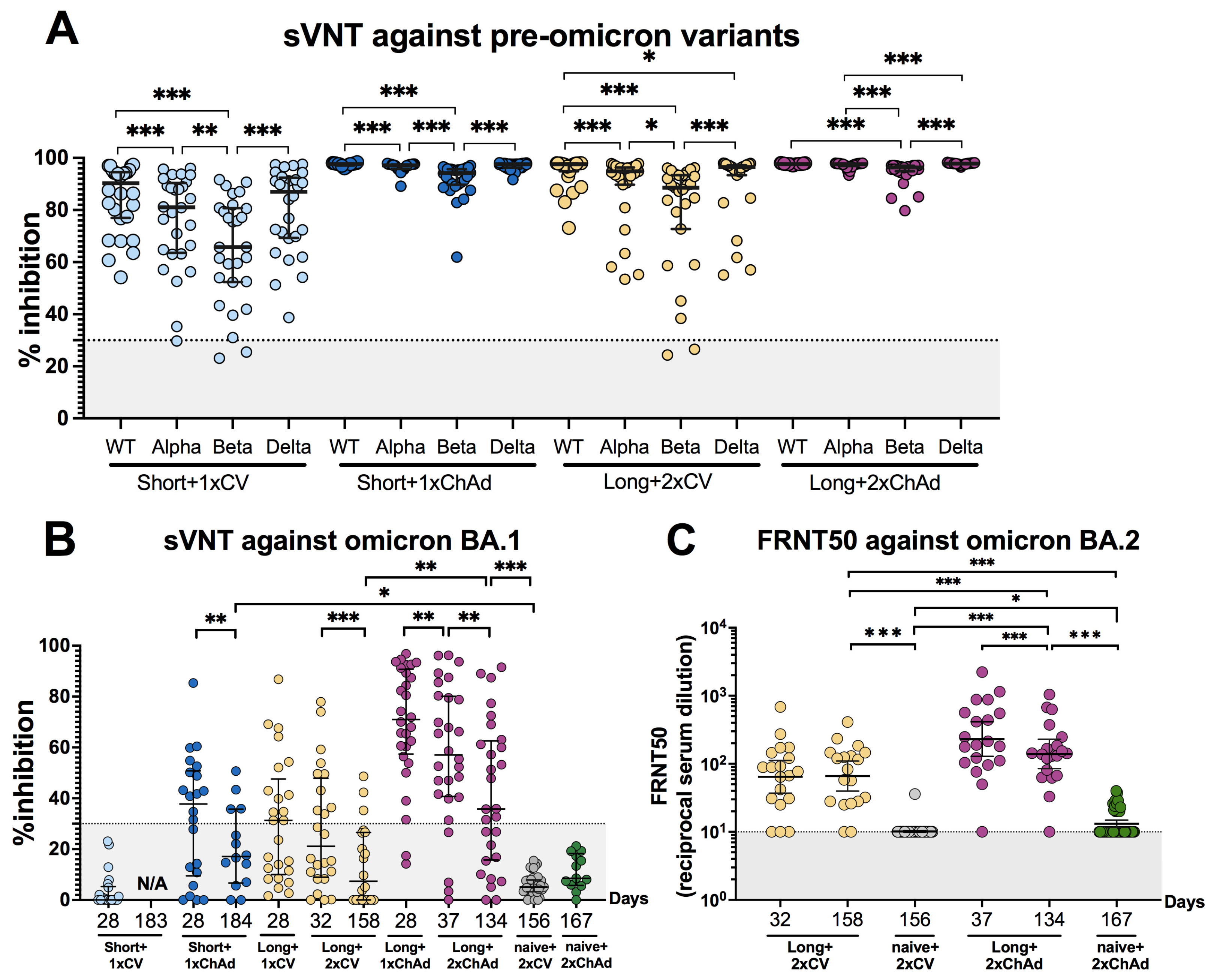
3.4. Hybrid Immunity Induces Broader Neutralizing Activities and Persists for at Least 6 Months after Vaccination Compared to Solely Receiving Vaccination
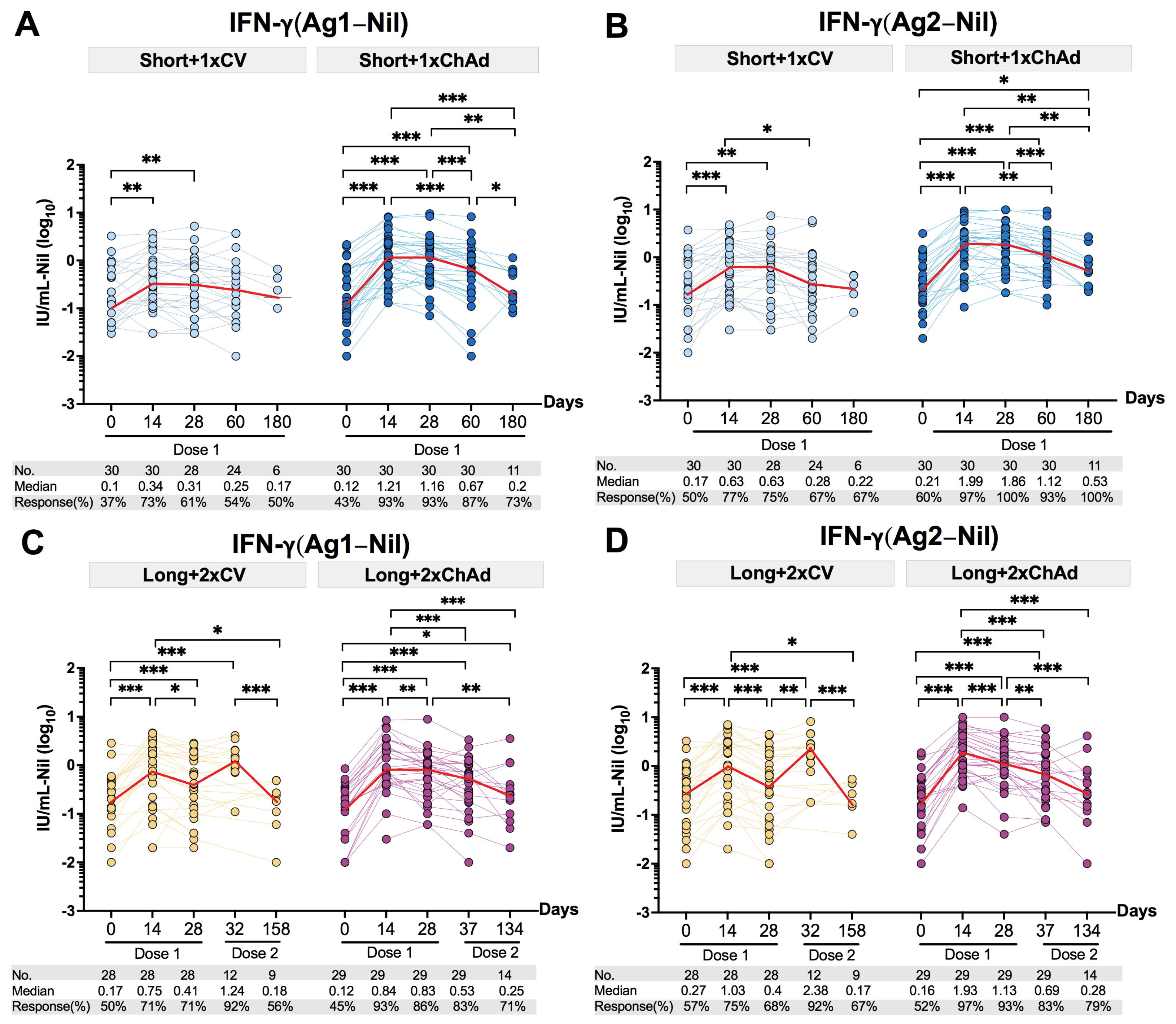
3.5. Decline of the Total T Cell IFN-γ Response after Vaccination in Individuals with Prior Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 20 November 2023).
- World Health Organization. Evidence Assessment: Sinovac/CoronaVac COVID-19 Vaccine. Available online: https://cdn.who.int/media/docs/default–source/immunization/sage/2021/april/5_sage29apr2021_critical–evidence_sinovac.pdf (accessed on 20 November 2023).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, A.M.; Spicer, K.B.; Thoroughman, D.; Glick, C.; Winter, K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira–Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; de Araújo Oliveira, V.; Paixão, E.S.; Júnior, J.B.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26. COV2. S among individuals with previous SARS-CoV-2 infection in Brazil: A test–negative, case–control study. Lancet Infect Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert–Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Tehrani, Z.R.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021, 325, 1467–1469. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Azamor, T.; Horbach, I.S.; Brito e Cunha, D.; Melgaço, J.G.; Silva, A.M.V.D.; Tubarão, L.N.; Azevedo, A.S.; Santos, R.T.; Alves, N.D.S.; Machado, T.L.; et al. Protective Immunity of COVID-19 Vaccination with ChAdOx1 nCoV-19 Following Previous SARS-CoV-2 Infection: A Humoral and Cellular Investigation. Viruses 2022, 14, 1916. [Google Scholar] [CrossRef]
- Dinc, H.O.; Saltoglu, N.; Can, G.; Balkan, I.I.; Budak, B.; Ozbey, D.; Caglar, B.; Karaali, R.; Mete, B.; Tuyji Tok, Y.; et al. Inactive SARS-CoV-2 vaccine generates high antibody responses in healthcare workers with and without prior infection. Vaccine 2022, 40, 52–58. [Google Scholar] [CrossRef]
- Hongjaisee, S.; Chawansuntati, K.; Sripan, P.; Rattanathammethee, K.; Sakkhachornphop, S.; Chaiwarith, R.; Sudjaritruk, T.; Supparatpinyo, K.; Wipasa, J. Comparison of antibody responses following natural infection with Severe Acute Respiratory Syndrome Coronavirus 2 or receipt of CoronaVac or ChAdOx1 (AZD1222) vaccination in Chiang Mai, Thailand. Vaccine X 2023, 14, 100305. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, and BA. 5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer–Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- GenScript. SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT). Available online: https://www.genscript.com/gsfiles/techfiles/Application–Note–SARS-CoV-2–Surrogate–Virus–Neutralization–Test–sVNT.pdf?21885562 (accessed on 29 October 2023).
- Supasa, P.; Zhou, D.; Dejnirattisai, W.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Nutalai, R.; Tuekprakhon, A.; et al. Reduced neutralization of SARS-CoV-2 B. 1.1. 7 variant by convalescent and vaccine sera. Cell 2021, 184, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Assawakosri, S.; Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; et al. Persistence of immunity against Omicron BA. 1 and BA. 2 variants following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-dose AZD1222 vaccination. Int. J. Infect. Dis. 2022, 122, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Assawakosri, S.; Kanokudom, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Neutralizing activities against the Omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J. Infect. Dis. 2022, 226, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Vichaiwattana, P.; Thongmee, T.; Auphimai, C.; Srimuan, D.; Thatsanatorn, T.; Assawakosri, S.; et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral–vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum. Vaccin Immunother. 2022, 18, 2029111. [Google Scholar] [CrossRef]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef] [PubMed]
- Mantus, G.; Nyhoff, L.E.; Edara, V.V.; Zarnitsyna, V.I.; Ciric, C.R.; Flowers, M.W.; Norwood, C.; Ellis, M.; Hussaini, L.; Manning, K.E.; et al. Pre–existing SARS-CoV-2 immunity influences potency, breadth, and durability of the humoral response to SARS-CoV-2 vaccination. Cell Rep. Med. 2022, 3, 100603. [Google Scholar] [CrossRef] [PubMed]
- Muena, N.A.; García–Salum, T.; Pardo–Roa, C.; Avendaño, M.J.; Serrano, E.F.; Levican, J.; Almonacid, L.I.; Valenzuela, G.; Poblete, E.; Strohmeier, S.; et al. Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals. eBioMedicine 2022, 78, 103972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer–Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Lu, Z.; Laing, E.D.; Pena DaMata, J.; Pohida, K.; Tso, M.S.; Samuels, E.C.; Epsi, N.J.; Dorjbal, B.; Lake, C.; Richard, S.A.; et al. Durability of SARS-CoV-2–specific T-cell responses at 12 months postinfection. J. Infect Dis. 2021, 224, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Barateau, V.; Peyrot, L.; Saade, C.; Pozzetto, B.; Brengel–Pesce, K.; Elsensohn, M.H.; Allatif, O.; Guibert, N.; Compagnon, C.; Mariano, N.; et al. Prior SARS-CoV-2 infection enhances and reshapes spike protein–specific memory induced by vaccination. Sci. Transl. Med. 2023, 15, eade0550. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville–West, J.E.; Cutino–Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef]
- Bi, X.; Takayama, T.; Tokoro, M.; Mizuno, T.; Hara, A.; Nakamura, H.; Oe, H.; Nagamatsu, S.; Kitano, Y.; Ichimura, H. Longer intervals before vaccination increase spike antibody titers in individuals previously infected with SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0023822. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; de Souza, T.F.G.; de Carvalho Araújo, F.M.; de Andrade, L.O.M. Dynamics of antibody response to CoronaVac vaccine. J. Med. Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Gelanew, T.; Mulu, A.; Abebe, M.; Bates, T.A.; Wassie, L.; Teferi, M.; Fentahun, D.; Alemu, A.; Tamiru, F.; Assefa, G.; et al. A single dose of ChAdOx1 nCoV-19 vaccine elicits high antibody responses in individuals with prior SARS-CoV-2 infection comparable to that of two-dose–vaccinated, SARS-CoV-2–infection-naïve Individuals: A longitudinal study in Ethiopian health workers. Vaccines 2022, 10, 859. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin–Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2–specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Cai, C.; Gao, Y.; Adamo, S.; Rivera–Ballesteros, O.; Hansson, L.; Österborg, A.; Bergman, P.; Sandberg, J.K.; Ljunggren, H.G.; Björkström, N.K.; et al. SARS-CoV-2 vaccination enhances the effector qualities of spike–specific T cells induced by COVID-19. Sci. Immunol. 2023, 8, eadh0687. [Google Scholar] [CrossRef]
- Borena, W.; Bánki, Z.; Bates, K.; Winner, H.; Riepler, L.; Rössler, A.; Pipperger, L.; Theurl, I.; Falkensammer, B.; Ulmer, H.; et al. Persistence of immunity to SARS-CoV-2 over time in the ski resort Ischgl. eBioMedicine 2021, 70, 103534. [Google Scholar] [CrossRef]
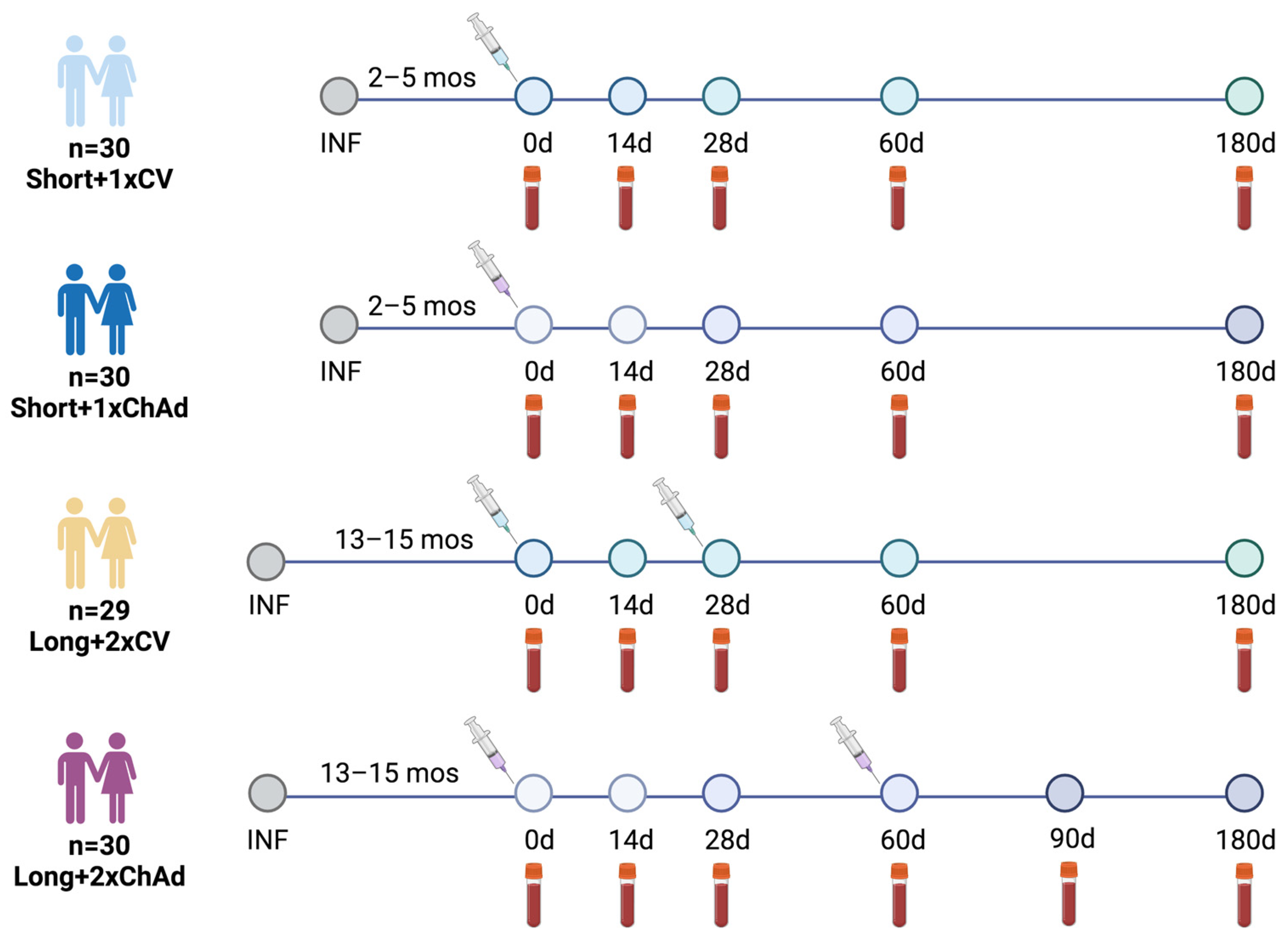
| Characteristics | Total (n = 119) | Short a+1xCV (n = 30) | Short a+1xChAd (n = 30) | Long b+2xCV (n = 29) | Long b+2xChAd (n = 30) | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 0.027 c | |||||
| Mean (SD) | 41.1 (10.1) | 37.6 (9.4) | 39.4 (8.9) | 43.1 (8.3) | 44.5 (12.2) | |
| Median (IQR) | 39 (34–49) | 35 (32–45) | 38.5 (34–44) | 43 (38–51) | 39.5 (35–54) | |
| Sex | 0.092 d | |||||
| Female, n (%) | 56 (47.1%) | 15 (50%) | 13 (43.3%) | 9 (31%) | 19 (63.3%) | |
| Male, n (%) | 63 (52.9%) | 15 (50%) | 17 (56.7%) | 20 (69%) | 11 (36.7%) | |
| BMI (kg m−2) | 0.301 d | |||||
| BMI < 30 | 105 (88.2%) | 26 (86.7%) | 26 (86.7%) | 24 (82.8%) | 29 (96.7%) | |
| BMI ≥ 30 | 14 (11.8%) | 4 (13.3%) | 4 (13.3%) | 5 (17.2%) | 1 (3.3%) | |
| Comorbidities c, n (%) | 17 (14.3%) | 2 (6.7%) | 3 (10%) | 6 (20.7%) | 6 (20%) | 0.282 d |
| Symptomatic | 0.075 d | |||||
| w pneumonia | 36 (30.3%) | 4 (13.3%) | 11 (36.7%) | 12 (41.4%) | 9 (30%) | |
| w/o pneumonia | 83 (69.7%) | 26 (86.7%) | 19 (63.3%) | 17 (58.6%) | 21 (70%) | |
| Interval between infection and first dose vaccination | ||||||
| Median (IQR) days | N/A | 74 (64–131) | 63 (62–151) | 425 (416–427) | 426 (417–434) | <0.001 c |
| Collection interval since first vaccination | ||||||
| 2nd visit (n) Median (IQR) days | (n = 119) 14 | (n = 30) 14 | (n = 30) 14 | (n = 29) 14 | (n = 30) 14 | 0.536 c |
| 3rd visit (n) Median (IQR) days | (n = 117) 28 | (n = 28) 28 | (n = 30) 28 | (n = 29) 28 | (n = 30) 28 (28–29) | 0.075 c |
| 4th visit (n) Median (IQR) days | (n = 116) 62 (60–64) | (n = 28) 64 (63–65) | (n = 30) 64 (60–64) | (n = 28) 60 | (n = 30) 62 | <0.001 c |
| 5th visit (n) Median (IQR) days | N/A | N/A | N/A | N/A | (n = 30) 99 (99–100) | |
| Timing of 6-month visit Median (IQR) days | (n = 77) 186 (184–196) | (n = 12) 183 (181–183) | (n = 17) 184 (183–184) | (n = 19) 186 | (n = 29) 196 (195–196) | <0.001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suntronwong, N.; Kanokudom, S.; Auphimai, C.; Thongmee, T.; Assawakosri, S.; Vichaiwattana, P.; Yorsaeng, R.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Long-Term Dynamic Changes in Hybrid Immunity over Six Months after Inactivated and Adenoviral Vector Vaccination in Individuals with Previous SARS-CoV-2 Infection. Vaccines 2024, 12, 180. https://doi.org/10.3390/vaccines12020180
Suntronwong N, Kanokudom S, Auphimai C, Thongmee T, Assawakosri S, Vichaiwattana P, Yorsaeng R, Duangchinda T, Chantima W, Pakchotanon P, et al. Long-Term Dynamic Changes in Hybrid Immunity over Six Months after Inactivated and Adenoviral Vector Vaccination in Individuals with Previous SARS-CoV-2 Infection. Vaccines. 2024; 12(2):180. https://doi.org/10.3390/vaccines12020180
Chicago/Turabian StyleSuntronwong, Nungruthai, Sitthichai Kanokudom, Chompoonut Auphimai, Thanunrat Thongmee, Suvichada Assawakosri, Preeyaporn Vichaiwattana, Ritthideach Yorsaeng, Thaneeya Duangchinda, Warangkana Chantima, Pattarakul Pakchotanon, and et al. 2024. "Long-Term Dynamic Changes in Hybrid Immunity over Six Months after Inactivated and Adenoviral Vector Vaccination in Individuals with Previous SARS-CoV-2 Infection" Vaccines 12, no. 2: 180. https://doi.org/10.3390/vaccines12020180
APA StyleSuntronwong, N., Kanokudom, S., Auphimai, C., Thongmee, T., Assawakosri, S., Vichaiwattana, P., Yorsaeng, R., Duangchinda, T., Chantima, W., Pakchotanon, P., Nilyanimit, P., Srimuan, D., Thatsanathorn, T., Sudhinaraset, N., Wanlapakorn, N., & Poovorawan, Y. (2024). Long-Term Dynamic Changes in Hybrid Immunity over Six Months after Inactivated and Adenoviral Vector Vaccination in Individuals with Previous SARS-CoV-2 Infection. Vaccines, 12(2), 180. https://doi.org/10.3390/vaccines12020180






