Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers
Abstract
1. ISG15: General Features and Signalling
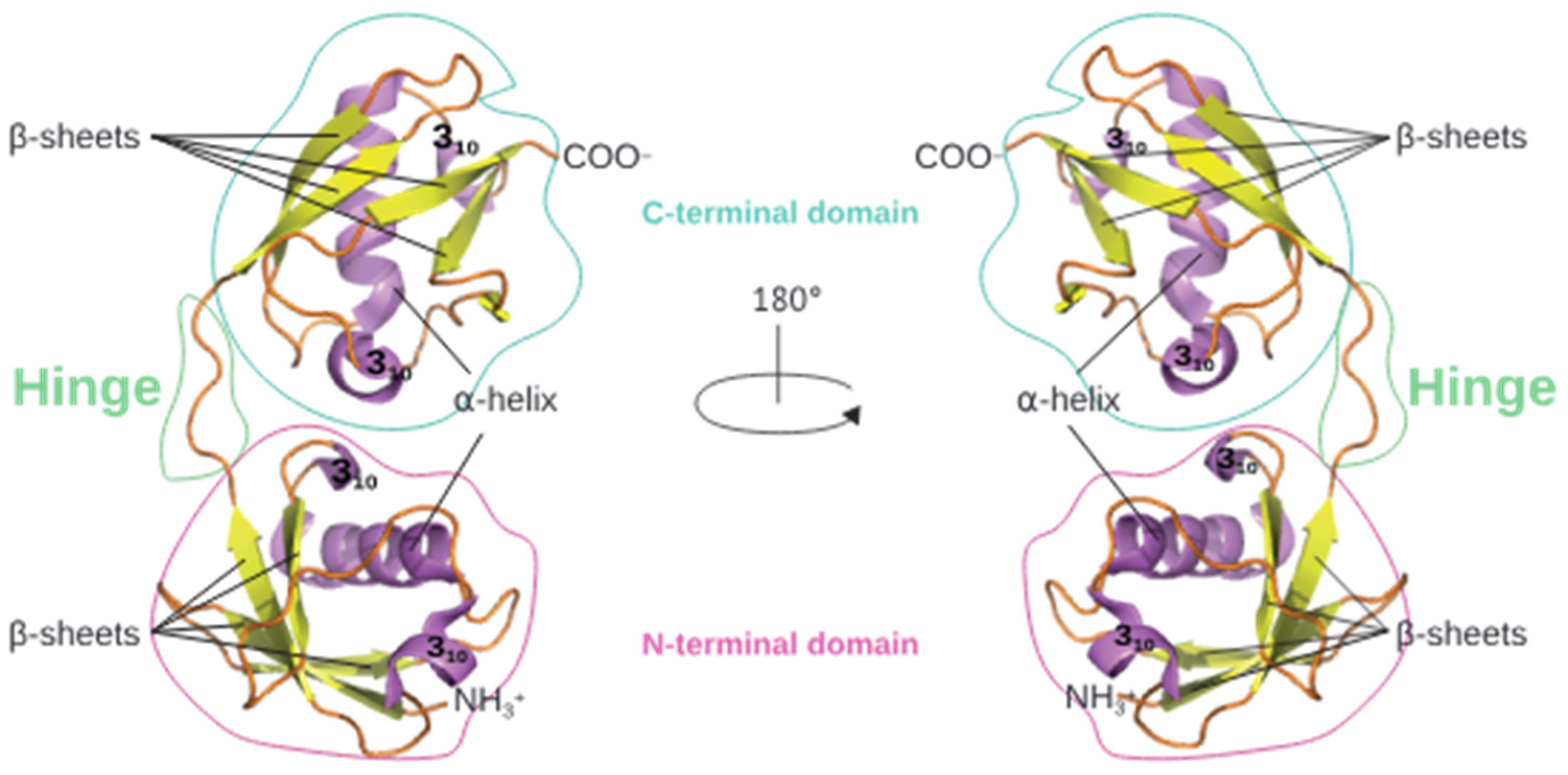
1.1. ISG15: General Features
1.2. ISGylation
- Antiviral effect of ISGylation
- The dual role of ISGylation in cancer
| Tumour Type | ISG15 Target | ISG15 Form | Effect | Ref. |
|---|---|---|---|---|
| Breast cancer | N.S. | Free intracellular | Antitumour | [57] |
| Breast cancer | N.S. | Conjugated | Protumour | [58] |
| HCC | p53 | Conjugated | Protumour | [53] |
| LUAD | YAP | Conjugated | Protumour | [51] |
| Lung adenocarcinoma | Glycosylated PD-L1 | Conjugated | Antitumour | [54] |
| Pancreatic cancer | ERK1/2 phosphorylation | Free extracellular | Protumour | [52] |
| PDAC | N.S. | Free intracellular/ extracellular | Protumour | [59] |
| Solid tumor | HIF1α | Conjugated/ Free intracellular | Antitumour | [60] |
1.3. Intracellular Free-ISG15 Functions
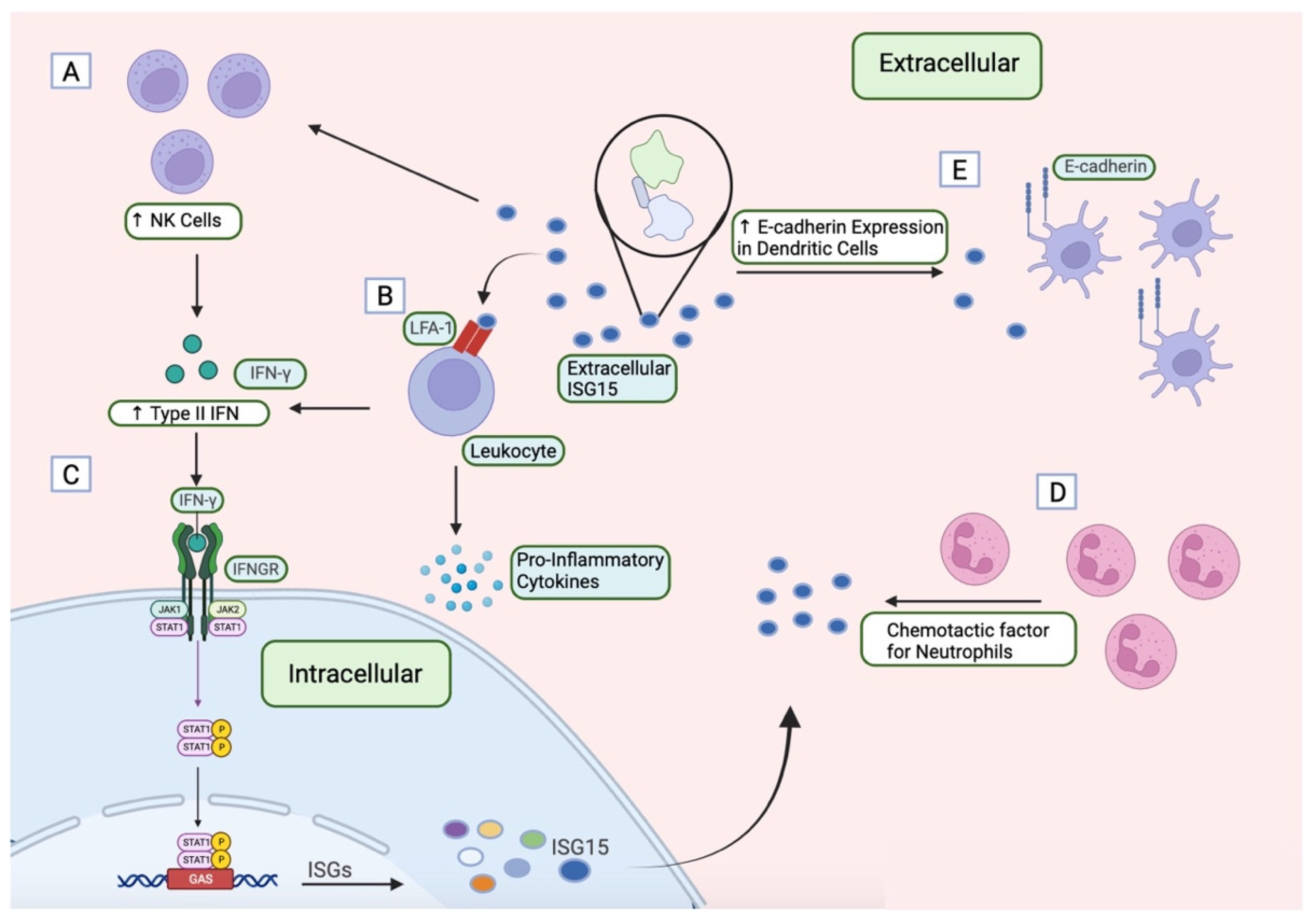
1.4. ISG15 as an Extracellular Cytokine
2. ISG15 as an Adjuvant in Vaccines
3. ISG15 as a Therapeutic Target in Cancer Treatment
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korant, B.D.; Blomstrom, D.C.; Jonak, G.J.; Knight, E. Interferon-Induced Proteins. Purification and Characterization of a 15,000-Dalton Protein from Human and Bovine Cells Induced by Interferon. J. Biol. Chem. 1984, 259, 14835–14839. [Google Scholar] [CrossRef]
- Haas, A.L.; Ahrens, P.; Bright, P.M.; Ankel, H. Interferon Induced a 15-Kilodalton Protein Exhibiting Marked Homology to Ubiquitin. J. Biol. Chem. 1987, 262, 11315–11323. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, J.; Wang, M.; Fu, Z.; Klein, J.M.; Haas, A.L.; Kim, J.P. Crystal Structure of the Interferon-Induced Ubiquitin-Like. J. Biol. Chem. 2005, 280, 27356–27365. [Google Scholar] [CrossRef]
- Dzimianski, J.V.; Scholte, F.E.M.; Bergeron, É.; Pegan, S.D. ISG15: It’s Complicated. J. Mol. Biol. 2019, 431, 4203–4216. [Google Scholar] [CrossRef]
- Liu, M.; Hummer, B.T.; Li, X.; Hassel, B.A. Camptothecin Induces the Ubiquitin-like Protein, ISG15, and Enhances ISG15 Conjugation in Response to Interferon. J. Interferon Cytokine Res. 2004, 24, 647–654. [Google Scholar] [CrossRef]
- Radoshevich, L.; Impens, F.; Ribet, D.; Quereda, J.J.; Tham, T.N.; Nahori, M.A.; Bierne, H.; Dussurget, O.; Pizarro-Cerdá, J.; Knobeloch, K.P.; et al. ISG15 Counteracts Listeria Monocytogenes Infection. Elife 2015, 4, e06848. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, O.; Malakhov, M.; Hetherington, C.; Zhang, D.E. Lipopolysaccharide Activates the Expression of ISG15-Specific Protease UBP43 via Interferon Regulatory Factor 3. J. Biol. Chem. 2002, 277, 14703–14711. [Google Scholar] [CrossRef] [PubMed]
- Pitha-Rowe, I.; Hassel, B.A.; Dmitrovsky, E. Involvement of UBE1L in ISG15 Conjugation during Retinoid-Induced Differentiation of Acute Promyelocytic Leukemia. J. Biol. Chem. 2004, 279, 18178–18187. [Google Scholar] [CrossRef]
- Hermann, M.; Bogunovic, D. ISG15: In Sickness and in Health. Trends Immunol. 2017, 38, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Speer, S.D.; Li, Z.; Buta, S.; Payelle-Brogard, B.; Qian, L.; Vigant, F.; Rubino, E.; Gardner, T.J.; Wedeking, T.; Hermann, M.; et al. ISG15 Deficiency and Increased Viral Resistance in Humans but Not Mice. Nat. Commun. 2016, 7, 11496. [Google Scholar] [CrossRef]
- Sandy, Z.; da Costa, I.C.; Schmidt, C.K. More than Meets the Isg15: Emerging Roles in the Dna Damage Response and Beyond. Biomolecules 2020, 10, 1557. [Google Scholar] [CrossRef]
- Raso, M.C.; Djoric, N.; Walser, F.; Hess, S.; Schmid, F.M.; Burger, S.; Knobeloch, K.P.; Penengo, L. Interferon-Stimulated Gene 15 Accelerates Replication Fork Progression Inducing Chromosomal Breakage. J. Cell Biol. 2020, 219, e202002175. [Google Scholar] [CrossRef]
- Wardlaw, C.P.; Petrini, J.H.J. ISG15 Conjugation to Proteins on Nascent DNA Mitigates DNA Replication Stress. Nat. Commun. 2022, 13, 5971. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernández-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation Controls Exosome Secretion by Promoting Lysosomal Degradation of MVB Proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef]
- Bécares, M.; Albert, M.; Tárrega, C.; Coloma, R.; Falqui, M.; Luhmann, E.K.; Radoshevich, L.; Guerra, S. ISG15 Is Required for the Dissemination of Vaccinia Virus Extracellular Virions. Microbiol. Spectr. 2023, 11, e0450822. [Google Scholar] [CrossRef]
- Bhushan, J.; Radke, J.B.; Perng, Y.C.; McAllaster, M.; Lenschow, D.J.; Virgin, H.W.; Sibley, L.D. Isg15 Connects Autophagy and Ifn-Dependent Control of Toxoplasma Gondii Infection in Human Cells. mBio 2020, 11, e00852-20. [Google Scholar] [CrossRef]
- Morales, D.J.; Lenschow, D.J. The Antiviral Activities of ISG15. J. Mol. Biol. 2013, 425, 4995–5008. [Google Scholar] [CrossRef]
- Han, H.G.; Moon, H.W.; Jeon, Y.J. ISG15 in Cancer: Beyond Ubiquitin-like Protein. Cancer Lett. 2018, 438, 52–62. [Google Scholar] [CrossRef]
- Perng, Y.C.; Lenschow, D.J. ISG15 in Antiviral Immunity and Beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef] [PubMed]
- González-Amor, M.; Dorado, B.; Andrés, V. Emerging Roles of Interferon-Stimulated Gene-15 in Age-Related Telomere Attrition, the DNA Damage Response, and Cardiovascular Disease. Front. Cell Dev. Biol. 2023, 11, 1128594. [Google Scholar] [CrossRef] [PubMed]
- Baldanta, S.; Fernández-Escobar, M.; Acín-Perez, R.; Albert, M.; Camafeita, E.; Jorge, I.; Vázquez, J.; Enríquez, J.A.; Guerra, S. ISG15 Governs Mitochondrial Function in Macrophages Following Vaccinia Virus Infection. PLoS Pathog. 2017, 13, e1006651. [Google Scholar] [CrossRef]
- Albert, M.; Bécares, M.; Falqui, M.; Fernández-Lozano, C.; Guerra, S. ISG15, a Small Molecule with Huge Implications: Regulation of Mitochondrial Homeostasis. Viruses 2018, 10, 629. [Google Scholar] [CrossRef]
- Albert, M.; Vázquez, J.; Falcón-Pérez, J.M.; Balboa, M.A.; Liesa, M.; Balsinde, J.; Guerra, S. ISG15 Is a Novel Regulator of Lipid Metabolism during Vaccinia Virus Infection. Microbiol. Spectr. 2022, 10, e03893-22. [Google Scholar] [CrossRef]
- Yángüez, E.; García-Culebras, A.; Frau, A.; Llompart, C.; Knobeloch, K.P.; Gutierrez-Erlandsson, S.; García-Sastre, A.; Esteban, M.; Nieto, A.; Guerra, S. ISG15 Regulates Peritoneal Macrophages Functionality against Viral Infection. PLoS Pathog. 2013, 9, e1003632. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, D.E. Interferon-Stimulated Gene 15 and the Protein ISGylation System. J. Interferon. Cytokine Res. 2011, 31, 119–130. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Yan, H.; Huang, J.; Wang, F.; Liu, T.; Zeng, L.; Zhou, F. ISGylation in Innate Antiviral Immunity and Pathogen Defense Responses: A Review. Front. Cell Dev. Biol. 2021, 9, 788410. [Google Scholar] [CrossRef]
- Potter, J.L.; Narasimhan, J.; Mende-Mueller, L.; Haas, A.L. Precursor Processing of Pro-ISG15/UCRP, an Interferon-β-Induced Ubiquitin-like Protein. J. Biol. Chem. 1999, 274, 25061–25068. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Giannakopoulos, N.V.; Gunn, L.J.; Johnston, C.; O’Guin, A.K.; Schmidt, R.E.; Levine, B.; Virgin, H.W. Identification of Interferon-Stimulated Gene 15 as an Antiviral Molecule during Sindbis Virus Infection In Vivo. J. Virol. 2005, 79, 13974–13983. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, J.; Potter, J.L.; Haas, A.L. Conjugation of the 15-KDa Interferon-Induced Ubiquitin Homolog Is Distinct from That of Ubiquitin. J. Biol. Chem. 1996, 271, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, N.A.; Paparisto, E.; Barr, S.D.; Spratt, D.E. HERC5 and the ISGylation Pathway: Critical Modulators of the Antiviral Immune Response. Viruses 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation—A Key to Lock the Cell Gates for Preventing the Spread of Threats. J. Cell Sci. 2017, 130, 2961–2969. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Malakhova, O.A.; Kim, K.I.; Ritchie, K.J.; Zhang, D. UBP43 (USP18) Specifically Removes ISG15 from Conjugated Proteins. J. Biol. Chem. 2002, 277, 9976–9981. [Google Scholar] [CrossRef]
- Gan, J.; Pinto-Fernández, A.; Flierman, D.; Akkermans, J.J.L.L.; O’Brien, D.P.; Greenwood, H.; Scott, H.C.; Fritz, G.; Knobeloch, K.-P.; Neefjes, J.; et al. USP16 Is an ISG15 Cross-Reactive Deubiquitinase That Targets pro-ISG15 and ISGylated Proteins Involved in Metabolism. Proc. Natl. Acad. Sci. USA 2023, 120, e2315163120. [Google Scholar] [CrossRef]
- Fan, J.B.; Arimoto, K.L.; Motamedchaboki, K.; Yan, M.; Wolf, D.A.; Zhang, D.E. Identification and Characterization of a Novel ISG15-Ubiquitin Mixed Chain and Its Role in Regulating Protein Homeostasis. Sci. Rep. 2015, 5, 12704. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.-L.; Hassel, B.A. Proteasomes Modulate Conjugation to the Ubiquitin-like Protein, ISG15. J. Biol. Chem. 2003, 278, 1594–1602. [Google Scholar] [CrossRef]
- Zhao, C.; Hsiang, T.; Kuo, R.; Krug, R.M. ISG15 Conjugation System Targets the Viral NS1 Protein in influenza A Virus–Infected Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2253–2258. [Google Scholar] [CrossRef]
- González-Sanz, R.; Mata, M.; Bermejo-Martín, J.; Álvarez, A.; Cortijo, J.; Melero, J.A.; Martínez, I. ISG15 Is Upregulated in Respiratory Syncytial Virus Infection and Reduces Virus Growth through Protein ISGylation. J. Virol. 2016, 90, 3428–3438. [Google Scholar] [CrossRef]
- Durfee, L.A.; Lyon, N.; Seo, K.; Huibregtse, J.M. The ISG15 Conjugation System Broadly Targets Newly Synthesized Proteins: Implications for the Antiviral Function of ISG15. Mol. Cell 2010, 38, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Okumura, A.; Pitha, P.M.; Harty, R.N. ISG15 Inhibits Ebola VP40 VLP Budding in an L-Domain-Dependent Manner by Blocking Nedd4 Ligase Activity. Proc. Natl. Acad. Sci. USA 2008, 105, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sridharan, H.; Chen, R.; Baker, D.P.; Wang, S.; Krug, R.M. Influenza B Virus Non-Structural Protein 1 Counteracts ISG15 Antiviral Activity by Sequestering ISGylated Viral Proteins. Nat. Commun. 2016, 7, 12754. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Bawono, R.G.; Abe, T.; Qu, M.; Kuroki, D.; Deng, L.; Matsui, C.; Ryo, A.; Suzuki, T.; Matsuura, Y.; Sugiyama, M.; et al. HERC5 E3 Ligase Mediates ISGylation of Hepatitis B Virus X Protein to Promote Viral Replication. J. Gen. Virol. 2021, 102, 001668. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Minami, N.; Bawono, R.G.; Matsui, C.; Deng, L.; Fukuhara, T.; Matsuura, Y.; Shoji, I. ISGylation of Hepatitis C Virus NS5A Protein Promotes Viral RNA Replication via Recruitment of Cyclophilin A. J. Virol. 2020, 94, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Thery, F.; Martina, L.; Asselman, C.; Zhang, Y.; Vessely, M.; Repo, H.; Sedeyn, K.; Moschonas, G.D.; Bredow, C.; Teo, Q.W.; et al. Ring Finger Protein 213 Assembles into a Sensor for ISGylated Proteins with Antimicrobial Activity. Nat. Commun. 2021, 12, 5772. [Google Scholar] [CrossRef]
- Malakhova, O.A.; Zhang, D.-E. ISG15 Inhibits Nedd4 Ubiquitin E3 Activity and Enhances the Innate Antiviral Response. J. Biol. Chem. 2008, 283, 8783–8787. [Google Scholar] [CrossRef]
- Lin, C.; Kuffour, E.O.; Fuchs, N.V.; Gertzen, C.G.W.; Kaiser, J.; Hirschenberger, M.; Tang, X.; Xu, H.C.; Michel, O.; Tao, R.; et al. Regulation of STING Activity in DNA Sensing by ISG15 Modification. Cell Rep. 2023, 42, 113277. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; He, W.; Chen, J.; Yang, G.; Chen, L.; Chang, H. Free ISG15 Inhibits Pseudorabies Virus Infection by Positively Regulating Type I IFN Signaling. PLoS Pathog. 2022, 18, e1010921. [Google Scholar] [CrossRef]
- Yuan, Y.; Qin, H.; Li, H.; Shi, W.; Bao, L.; Xu, S.; Yin, J.; Zheng, L. The Functional Roles of ISG15/ISGylation in Cancer. Molecules 2023, 28, 1337. [Google Scholar] [CrossRef]
- Desai, S.D.; Haas, A.L.; Wood, L.M.; Tsai, Y.C.; Pestka, S.; Rubin, E.H.; Saleem, A.; Nur-E-Kamal, A.; Liu, L.F. Elevated Expression of ISG15 in Tumor Cells Interferes with the Ubiquitin/26S Proteasome Pathway. Cancer Res. 2006, 66, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.; Reed, R.E.; Desai, S.D. ISGylation Governs the Oncogenic Function of Ki-Ras in Breast Cancer. Oncogene 2014, 33, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Tian, X.; Zhang, C.; Miao, Y.; Wang, Y.; Peng, Y.; Qiu, S.; Wang, H.; Cui, J.; Cao, L.; et al. YAP ISGylation Increases Its Stability and Promotes Its Positive Regulation on PPP by Stimulating 6PGL Transcription. Cell Death Discov. 2022, 8, 59. [Google Scholar] [CrossRef]
- Alcalá, S.; Sancho, P.; Martinelli, P.; Navarro, D.; Pedrero, C.; Martín-Hijano, L.; Valle, S.; Earl, J.; Rodríguez-Serrano, M.; Ruiz-Cañas, L.; et al. ISG15 and ISGylation Is Required for Pancreatic Cancer Stem Cell Mitophagy and Metabolic Plasticity. Nat. Commun. 2020, 11, 2682. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, J.; Ding, Z.; Zheng, M.; Qiu, L.; Tang, A.; Huang, D. NFE2L3 Drives Hepatocellular Carcinoma Cell Proliferation by Regulating the Proteasome-Dependent Degradation of ISGylated P53. Cancer Sci. 2023, 114, 3523–3536. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, W.; Yan, C.; Ren, D.; Wang, Y.; Guo, Y.; Guo, Q.; Wang, J.; Liu, L.; Han, L.; et al. ISG15 Targets Glycosylated PD-L1 and Promotes Its Degradation to Enhance Antitumor Immune Effects in Lung Adenocarcinoma. J. Transl. Med. 2023, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Mustachio, L.M.; Lu, Y.; Kawakami, M.; Roszik, J.; Freemantle, S.J.; Liu, X.; Dmitrovsky, E. Evidence for the ISG15-Specific Deubiquitinase USP18 as an Antineoplastic Target. Cancer Res. 2018, 78, 587–592. [Google Scholar] [CrossRef]
- Guo, Y.; Dolinko, A.V.; Chinyengetere, F.; Stanton, B.; Bomberger, J.M.; Demidenko, E.; Zhou, D.-C.; Gallagher, R.; Ma, T.; Galimberti, F.; et al. Blockade of the Ubiquitin Protease UBP43 Destabilizes Transcription Factor PML/RARα and Inhibits the Growth of Acute Promyelocytic Leukemia. Cancer Res. 2010, 70, 9875–9885. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.; Reed, R.E.; Desai, S.D. Free ISG15 Triggers an Antitumor Immune Response against Breast Cancer: A New Perspective. Oncotarget 2015, 6, 7221–7231. [Google Scholar] [CrossRef] [PubMed]
- Bolado-Carrancio, A.; Lee, M.; Ewing, A.; Muir, M.; Macleod, K.G.; Gallagher, W.M.; Nguyen, L.K.; Carragher, N.O.; Semple, C.A.; Brunton, V.G.; et al. ISGylation Drives Basal Breast Tumour Progression by Promoting EGFR Recycling and Akt Signalling. Oncogene 2021, 40, 6235. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, J.; Qiao, H.-Y.; Zhao, F.-Y.; Li, C.; Jiang, J.-Y.; Liu, B.-Q.; Meng, X.-N.; Wang, H.-Q. Loss of TRIM29 Suppresses Cancer Stem Cell-like Characteristics of PDACs via Accelerating ISG15 Degradation. Oncogene 2020, 39, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Yang, Y.-C.; Hsieh, M.-Y.; Yeh, Y.-C.; Li, T.-K. A Negative Feedback of the HIF-1α Pathway via Interferon-Stimulated Gene 15 and ISGylation. Clin. Cancer Res. 2013, 19, 5927–5939. [Google Scholar] [CrossRef]
- Zhang, X.; Bogunovic, D.; Payelle-Brogard, B.; Francois-Newton, V.; Speer, S.D.; Yuan, C.; Volpi, S.; Li, Z.; Sanal, O.; Mansouri, D.; et al. Human Intracellular ISG15 Prevents Interferon-α/β over-Amplification and Auto-Inflammation. Nature 2015, 517, 89–93. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hwang, S.-Y.; Imaizumi, T.; Yoo, J.-Y. Negative Feedback Regulation of RIG-I-Mediated Antiviral Signaling by Interferon-Induced ISG15 Conjugation. J. Virol. 2008, 82, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Nguyen, T.; Goins, W.F.; Chiocca, E.A. Interferon-Stimulated Gene 15 (ISG15) and ISG15-Linked Proteins Can Associate with Members of the Selective Autophagic Process, Histone Deacetylase 6 (HDAC6) and SQSTM1/P62. J. Biol. Chem. 2015, 290, 1485–1495. [Google Scholar] [CrossRef]
- Okumura, F.; Lenschow, D.J.; Zhang, D.-E. Nitrosylation of ISG15 Prevents the Disulfide Bond-Mediated Dimerization of ISG15 and Contributes to Effective ISGylation. J. Biol. Chem. 2008, 283, 24484–24488. [Google Scholar] [CrossRef] [PubMed]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Kariri, Y.A.; Alsaleem, M.; Joseph, C.; Alsaeed, S.; Aljohani, A.; Shiino, S.; Mohammed, O.J.; Toss, M.S.; Green, A.R.; Rakha, E.A. The Prognostic Significance of Interferon-Stimulated Gene 15 (ISG15) in Invasive Breast Cancer. Breast Cancer Res. Treat. 2021, 185, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.J.; Cordova, B. IFN-Induced 15-KDa Protein Is Released from Human Lymphocytes and Monocytes. J. Immunol. 1991, 146, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Zhou, Y.; Zhou, R.-H.; Ho, W.-Z.; Li, J.-L. Exosomes Contribute to the Transmission of Anti-HIV Activity from TLR3-Activated Brain Microvascular Endothelial Cells to Macrophages. Antivir. Res. 2016, 134, 167–171. [Google Scholar] [CrossRef]
- Dos Santos, P.F.; Mansur, D.S. Beyond ISGlylation: Functions of Free Intracellular and Extracellular ISG15. J. Interferon Cytokine Res. 2017, 37, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Swaim, C.D.; Scott, A.F.; Canadeo, L.A.; Huibregtse, J.M. Extracellular ISG15 Signals Cytokine Secretion through the LFA-1 Integrin Receptor. Mol. Cell 2017, 68, 581–590.e5. [Google Scholar] [CrossRef]
- Binnerts, M.E.; van Kooyk, Y.; Simmons, D.L.; Figdor, C.G. Distinct Binding of T Lymphocytes to ICAM-1, -2 or -3 upon Activation of LFA-1. Eur. J. Immunol. 1994, 24, 2155–2160. [Google Scholar] [CrossRef]
- Shimaoka, M.; Xiao, T.; Liu, J.-H.; Yang, Y.; Dong, Y.; Jun, C.-D.; McCormack, A.; Zhang, R.; Joachimiak, A.; Takagi, J.; et al. Structures of the Alpha L I Domain and Its Complex with ICAM-1 Reveal a Shape-Shifting Pathway for Integrin Regulation. Cell 2003, 112, 99–111. [Google Scholar] [CrossRef]
- Giagulli, C.; Ottoboni, L.; Caveggion, E.; Rossi, B.; Lowell, C.; Constantin, G.; Laudanna, C.; Berton, G. The Src Family Kinases Hck and Fgr Are Dispensable for Inside-out, Chemoattractant-Induced Signaling Regulating Beta 2 Integrin Affinity and Valency in Neutrophils, but Are Required for Beta 2 Integrin-Mediated Outside-in Signaling Involved in Sustained. J. Immunol. 2006, 177, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Stow, J.L.; Murray, R.Z. Intracellular Trafficking and Secretion of Inflammatory Cytokines. Cytokine Growth Factor Rev. 2013, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Østvik, A.E.; Svendsen, T.D.; Granlund, A.v.B.; Doseth, B.; Skovdahl, H.K.; Bakke, I.; Thorsvik, S.; Afroz, W.; Walaas, G.A.; Mollnes, T.E.; et al. Intestinal Epithelial Cells Express Immunomodulatory ISG15 During Active Ulcerative Colitis and Crohn’s Disease. J. Crohns Colitis 2020, 14, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Owhashi, M.; Taoka, Y.; Ishii, K.; Nakazawa, S.; Uemura, H.; Kambara, H. Identification of a Ubiquitin Family Protein as a Novel Neutrophil Chemotactic Factor. Biochem. Biophys. Res. Commun. 2003, 309, 533–539. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, J.; Knight, E.J.; Haas, A.L.; Truitt, R.L.; Borden, E.C. Immunoregulatory Properties of ISG15, an Interferon-Induced Cytokine. Proc. Natl. Acad. Sci. USA 1996, 93, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Padovan, E.; Terracciano, L.; Certa, U.; Jacobs, B.; Reschner, A.; Bolli, M.; Spagnoli, G.C.; Borden, E.C.; Heberer, M. Interferon Stimulated Gene 15 Constitutively Produced by Melanoma Cells Induces E-Cadherin Expression on Human Dendritic Cells. Cancer Res. 2002, 62, 3453–3458. [Google Scholar]
- Zhou, M.-J.; Chen, F.-Z.; Chen, H.-C.; Wan, X.-X.; Zhou, X.; Fang, Q.; Zhang, D.-Z. ISG15 Inhibits Cancer Cell Growth and Promotes Apoptosis. Int. J. Mol. Med. 2017, 39, 446–452. [Google Scholar] [CrossRef]
- Huggins, D.N.; LaRue, R.S.; Wang, Y.; Knutson, T.P.; Xu, Y.; Williams, J.W.; Schwertfeger, K.L. Characterizing Macrophage Diversity in Metastasis-Bearing Lungs Reveals a Lipid-Associated Macrophage Subset. Cancer Res. 2021, 81, 5284–5295. [Google Scholar] [CrossRef]
- Villarreal, D.O.; Wise, M.C.; Siefert, R.J.; Yan, J.; Wood, L.M.; Weiner, D.B. Ubiquitin-like Molecule ISG15 Acts as an Immune Adjuvant to Enhance Antigen-Specific CD8 T-Cell Tumor Immunity. Mol. Ther. 2015, 23, 1653–1662. [Google Scholar] [CrossRef]
- Iglesias-Guimarais, V.; Ahrends, T.; de Vries, E.; Knobeloch, K.-P.; Volkov, A.; Borst, J. IFN-Stimulated Gene 15 Is an Alarmin That Boosts the CTL Response via an Innate, NK Cell-Dependent Route. J. Immunol. 2020, 204, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.E.; Perdiguero, B.; Falqui, M.; Marín, M.Q.; Bécares, M.; Sorzano, C.Ó.S.; García-Arriaza, J.; Esteban, M.; Guerra, S. Enhancement of HIV-1 Env-Specific CD8 T Cell Responses Using Interferon-Stimulated Gene 15 as an Immune Adjuvant. J. Virol. 2020, 95, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Falqui, M.; Perdiguero, B.; Coloma, R.; Albert, M.; Marcos-Villar, L.; McGrail, J.P.; Sorzano, C.Ó.S.; Esteban, M.; Gómez, C.E.; Guerra, S. An MVA-Based Vector Expressing Cell-Free ISG15 Increases IFN-I Production and Improves HIV-1-Specific CD8 T Cell Immune Responses. Front. Cell Infect. Microbiol. 2023, 13, 1187193. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T.; Osiak, A.; Levine, B.; Schmidt, R.E.; García-Sastre, A.; et al. IFN-Stimulated Gene 15 Functions as a Critical Antiviral Molecule against Influenza, Herpes, and Sindbis Viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Osiak, A.; Utermöhlen, O.; Niendorf, S.; Horak, I.; Knobeloch, K.-P. ISG15, an Interferon-Stimulated Ubiquitin-like Protein, Is Not Essential for STAT1 Signaling and Responses against Vesicular Stomatitis and Lymphocytic Choriomeningitis Virus. Mol. Cell. Biol. 2005, 25, 6338–6345. [Google Scholar] [CrossRef] [PubMed]
- Werneke, S.W.; Schilte, C.; Rohatgi, A.; Monte, K.J.; Michault, A.; Arenzana-Seisdedos, F.; Vanlandingham, D.L.; Higgs, S.; Fontanet, A.; Albert, M.L.; et al. ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation. PLoS Pathog. 2011, 7, e1002322. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Cáceres, A.; Knobeloch, K.P.; Horak, I.; Esteban, M. Vaccinia Virus E3 Protein Prevents the Antiviral Action of ISG15. PLoS Pathog. 2008, 4, e1000096. [Google Scholar] [CrossRef]
- Bektas, N.; Noetzel, E.; Veeck, J.; Press, M.F.; Kristiansen, G.; Naami, A.; Hartmann, A.; Dimmler, A.; Beckmann, M.W.; Knüchel, R.; et al. The Ubiquitin-like Molecule Interferon-Stimulated Gene 15 (ISG15) Is a Potential Prognostic Marker in Human Breast Cancer. Breast Cancer Res. 2008, 10, R58. [Google Scholar] [CrossRef]
- Sainz, B.; Martín, B.; Tatari, M.; Heeschen, C.; Guerra, S. ISG15 Is a Critical Microenvironmental Factor for Pancreatic Cancer Stem Cells. Cancer Res. 2014, 74, 7309–7320. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, W.; Yang, Y.; Xue, L.; Liu, L.; Song, Y. ISG15 Promotes Esophageal Squamous Cell Carcinoma Tumorigenesis via C-MET/Fyn/β-Catenin Signaling Pathway. Exp. Cell Res. 2018, 367, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Zhang, H.; Zhu, M.; Chen, F.; Hu, Y.; Liu, H.; Zhu, H. Interferon-Stimulated Gene 15 (ISG15) Is a Trigger for Tumorigenesis and Metastasis of Hepatocellular Carcinoma. Oncotarget 2014, 5, 8429. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Reed, R.E.; Burks, J.; Wood, L.M.; Pullikuth, A.K.; Haas, A.L.; Liu, L.F.; Breslin, J.W.; Meiners, S.; Sankar, S. ISG15 Disrupts Cytoskeletal Architecture and Promotes Motility in Human Breast Cancer Cells. Exp. Biol. Med. 2012, 237, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D. ISG15: A Double-Edged Sword in Cancer. Oncoimmunology 2015, 4, e1052935. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.M.; Pan, Z.-K.; Seavey, M.M.; Muthukumaran, G.; Paterson, Y. The Ubiquitin-like Protein, ISG15, Is a Novel Tumor-Associated Antigen for Cancer Immunotherapy. Cancer Immunol. Immunother. 2012, 61, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-M.; Oladejo, M.; Paulishak, W.; Wood, L.M. A Listeria-Based Vaccine Targeting ISG15 Exerts Anti-Tumor Efficacy in Renal Cell Carcinoma. Cancer Immunol. Immunother. 2023, 72, 2889–2903. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Gaikwad, S.; Oladejo, M.; Paulishak, W.; Wood, L.M. Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer. Cancers 2023, 15, 1237. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, W.; Qi, L.; Cao, L.; Liu, C.; Huang, Q.; Li, G.; Li, L.; Wang, Y.; Guo, Q.; et al. ISG15 Induces ESRP1 to Inhibit Lung Adenocarcinoma Progression. Cell Death Dis. 2020, 11, 511. [Google Scholar] [CrossRef]

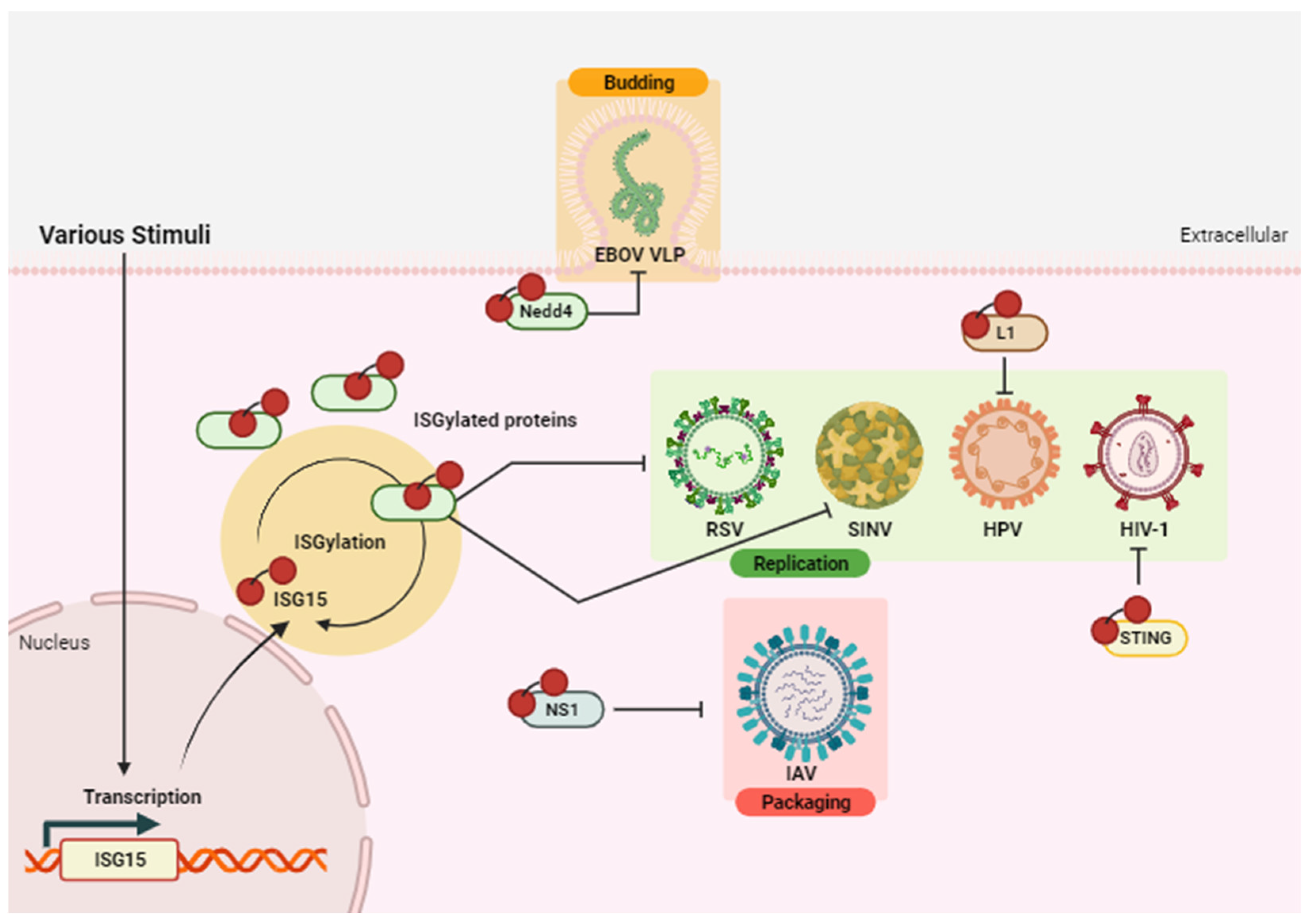


| Virus | ISG15 Target | ISG15 Form | Effect | Study Type | Refs. |
|---|---|---|---|---|---|
| EBOV | Nedd4 (Host) | Conjugated/ Free intracellular | Antiviral | In vitro | [39,45] |
| HBV | HBx (Viral) | Conjugated | Proviral | In vitro | [42] |
| HCV | NS5A (Viral) | Conjugated | Proviral | In vitro | [43] |
| HIV-1 | STING (Host) | Conjugated | Antiviral | In vitro | [46] |
| HPV16 | L1 (Viral) | Conjugated | Antiviral | In vitro | [38] |
| IAV | NS1 (Viral) | Conjugated | Antiviral | In vitro | [36] |
| PRV | STAT2 (Host) | Free intracellular | Antiviral | In vitro | [47] |
| RSV | N.S. | Conjugated | Antiviral | In vitro, ex vivo | [37] |
| SINV | N.S. | Conjugated | Antiviral | In vivo | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, E.; Falqui, M.; Sin, L.; McGrail, J.P.; Perdiguero, B.; Coloma, R.; Marcos-Villar, L.; Tárrega, C.; Esteban, M.; Gómez, C.E.; et al. Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers. Vaccines 2024, 12, 153. https://doi.org/10.3390/vaccines12020153
Álvarez E, Falqui M, Sin L, McGrail JP, Perdiguero B, Coloma R, Marcos-Villar L, Tárrega C, Esteban M, Gómez CE, et al. Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers. Vaccines. 2024; 12(2):153. https://doi.org/10.3390/vaccines12020153
Chicago/Turabian StyleÁlvarez, Enrique, Michela Falqui, Laura Sin, Joseph Patrick McGrail, Beatriz Perdiguero, Rocío Coloma, Laura Marcos-Villar, Céline Tárrega, Mariano Esteban, Carmen Elena Gómez, and et al. 2024. "Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers" Vaccines 12, no. 2: 153. https://doi.org/10.3390/vaccines12020153
APA StyleÁlvarez, E., Falqui, M., Sin, L., McGrail, J. P., Perdiguero, B., Coloma, R., Marcos-Villar, L., Tárrega, C., Esteban, M., Gómez, C. E., & Guerra, S. (2024). Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers. Vaccines, 12(2), 153. https://doi.org/10.3390/vaccines12020153







