Vitamin D Supplementation and Prior Oral Poliovirus Vaccination Decrease Odds of COVID-19 Outcomes among Adults Recently Inoculated with Inactivated Poliovirus Vaccine
Abstract
1. Introduction
1.1. Age
1.2. Underlying Medical Conditions
1.3. Vitamin D
1.4. Biological Sex
1.5. Education
1.6. Economic Stability and Employment
1.7. Race/Ethnicity
1.8. Geographic Location
2. Materials and Methods
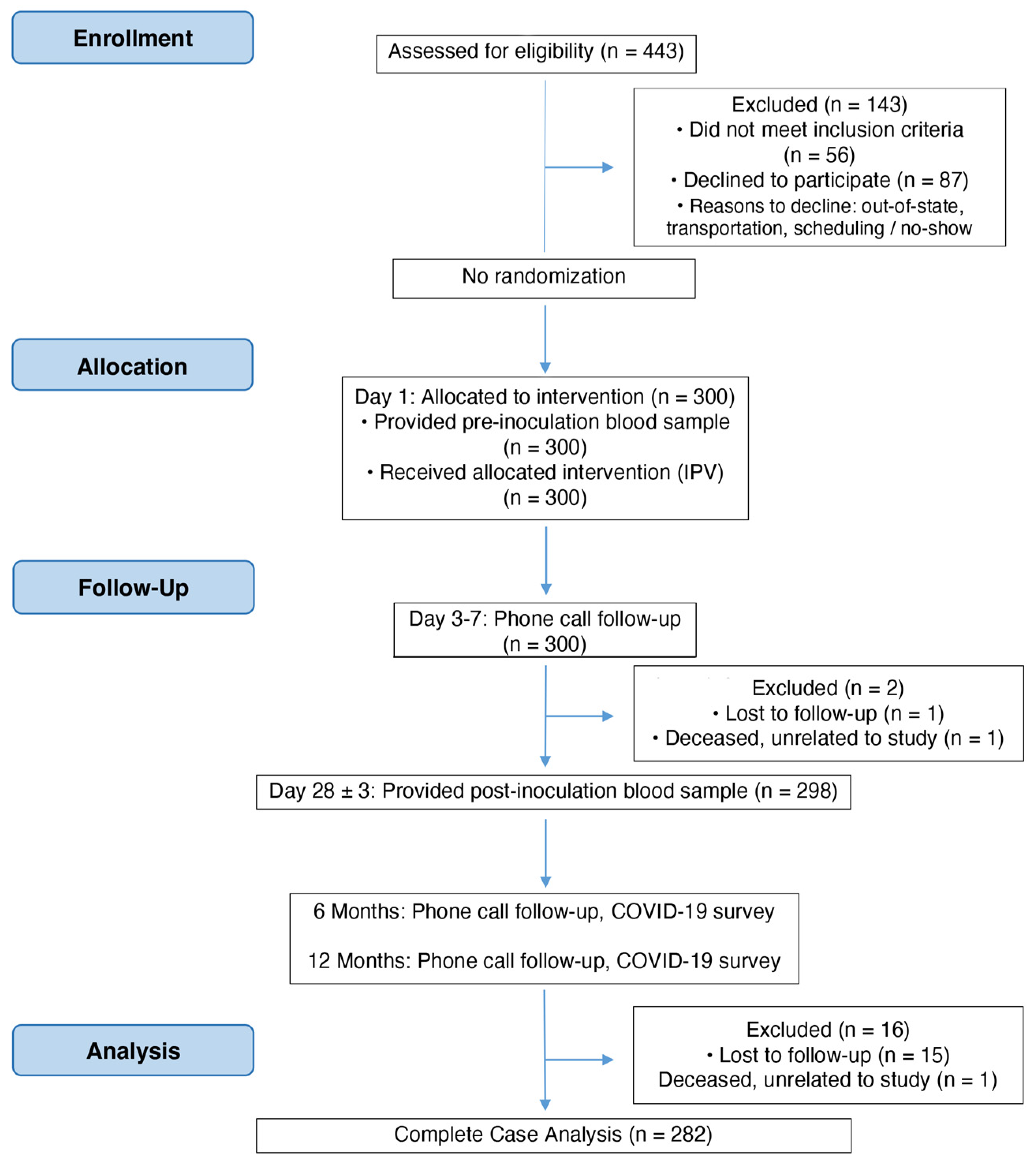
2.1. Clinical Trial Design
2.2. Data Source
2.3. Ethics
2.4. Measures/Variables
2.4.1. Independent Variables
2.4.2. Dependent Variables
2.5. Missing Data
2.6. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Main Effects of Potential Contributory Factors
3.2.1. Bivariate Analysis
3.2.2. Final Logistic Regression Models for Dichotomous Outcomes of Interest: Tested Positive for SARS-CoV-2 (Aim 1) and Experienced COVID-19 Symptoms (Aim 2)
3.2.3. Final Linear Regression Model for Continuous Outcome of Interest: Days Experienced with COVID-19 Symptoms (Aim 3)
4. Discussion
4.1. Synthesis
4.2. Strengths and Limitations
5. Conclusions and Implications for Research and Practice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipsitch, M.; Swerdlow, D.L.; Finelli, L. Defining the Epidemiology of COVID-19—Studies Needed. N. Engl. J. Med. 2020, 382, 1194–1196. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease 2019 Situtation Report—72. Updated 1 April 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331685/nCoVsitrep01Apr2020-eng.pdf (accessed on 12 December 2023).
- CDC COVID-19 Response Team; Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; et al. Severe Outcomes among Patients with Coronavirus Disease 2019 (COVID-19)-United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Chae, C.U.; Lee, R.T.; Rifai, N.; Ridker, P.M. Blood Pressure and Inflammation in Apparently Healthy Men. Hypertension 2001, 38, 399–403. [Google Scholar] [CrossRef]
- Javanmardi, F.; Keshavarzi, A.; Akbari, A.; Emami, A.; Pirbonyeh, N. Prevalence of underlying diseases in died cases of COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241265. [Google Scholar] [CrossRef]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Alinaghi, S.A.S.; Jahanfar, S. Predictors of mortality in patients with COVID-19–a systematic review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef]
- Rezende, L.F.M.; Thome, B.; Schveitzer, M.C.; de Souza-Júnior, P.R.B.; Szwarcwald, C.L. Adults at high-risk of severe coronavirus disease-2019 (COVID-19) in Brazil. Rev. Saude Publica 2020, 54, 50. [Google Scholar] [CrossRef]
- Paul, R.; Arif, A.; Pokhrel, K.; Ghosh, S. The Association of Social Determinants of Health with COVID-19 Mortality in Rural and Urban Counties. J. Rural Health 2021, 37, 278–286. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Underlying Medical Conditions. Updated 25 June 2020. Available online: https://covid.cdc.gov/covid-data-tracker/#underlying-med-conditions (accessed on 12 December 2023).
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Aygun, H. Vitamin D may protect against multiple organ damage caused by COVID-19. Bratisl Lek Listy. 2020, 121, 870–877. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020, 32, 2141–2158. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Ward, H.; Whitaker, M.; Flower, B.; Tang, S.N.; Atchison, C.; Darzi, A.; Donnelly, C.A.; Cann, A.; Diggle, P.J.; Ashby, D.; et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat. Commun. 2022, 13, 907. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, T.; Liang, B.; Deng, H.; Wang, H.; Feng, X.; Quan, X.; Wang, X.; Li, S.; Lu, S.; et al. Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting. Front. Immunol. 2021, 12, 802858. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Moulin, T.C.; Schiöth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Pan American Health Organization. Social Determinants of Health. Updated 2021. Available online: https://www.paho.org/en/topics/social-determinants-health (accessed on 12 December 2023).
- Morante-García, W.; Zapata-Boluda, R.M.; García-González, J.; Campuzano-Cuadrado, P.; Calvillo, C.; Alarcón-Rodríguez, R. Influence of Social Determinants of Health on COVID-19 Infection in Socially Vulnerable Groups. Int. J. Environ. Res. Public Health 2022, 19, 1294. [Google Scholar] [CrossRef]
- Barr, D.A. Health Disparities in the United States: Social Class, Race, Ethnicity, and Social Determinants Of Health, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Mackenbach, J.P.; Kulhánová, I.; Bopp, M.; Deboosere, P.; Eikemo, T.A.; Hoffmann, R.; Kulik, M.C.; Leinsalu, M.; Martikainen, P.; Menvielle, G.; et al. Variations in the relation between education and cause-specific mortality in 19 European populations: A test of the “fundamental causes” theory of social inequalities in health. Soc. Sci. Med. 2015, 127, 51–62. [Google Scholar] [CrossRef]
- Saydah, S.H.; Imperatore, G.; Beckles, G.L. Socioeconomic status and mortality: Contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care 2013, 36, 49–55. [Google Scholar] [CrossRef]
- Singu, S.; Acharya, A.; Challagundla, K.; Byrareddy, S.N. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front. Public Health 2020, 8, 406. [Google Scholar] [CrossRef]
- McMaughan, D.J.; Oloruntoba, O.; Smith, M.L. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front. Public Health 2020, 8, 231. [Google Scholar] [CrossRef]
- Hawkins, D. Disparities in Access to Paid Sick Leave During the First Year of the COVID-19 Pandemic. J. Occup. Environ. Med. 2023, 65, 370–377. [Google Scholar] [CrossRef]
- Palacio, A.; Tamariz, L. Social Determinants of Health Mediate COVID-19 Disparities in South Florida. J. Gen. Intern. Med. 2021, 36, 472–477. [Google Scholar] [CrossRef]
- Avdiu, B.; Nayyar, G. When face-to-face interactions become an occupational hazard: Jobs in the time of COVID-19. Econ. Lett. 2020, 197, 109648. [Google Scholar] [CrossRef]
- Dütsch, M. COVID-19 and the labour market: What are the working conditions in critical jobs? J. Labour Mark. Res. 2022, 56, 10. [Google Scholar] [CrossRef]
- Gould, E.; Shierholz, H. Not Everybody Can Work from Home: Black and Hispanic Workers are Much Less Likely to be Able to Telework. Economic Policy Institute. Updated 19 March 2020. Available online: https://www.epi.org/blog/black-and-hispanic-workers-are-much-less-likely-to-be-able-to-work-from-home/ (accessed on 12 December 2023).
- Dingel, J.I.; Neiman, B. How many jobs can be done at home? J. Public Econ. 2020, 189, 104235. [Google Scholar] [CrossRef]
- Angelucci, M.; Angrisani, M.; Bennett, D.; Kapteyn, A.; Schaner, S. Remote Work and the Heterogeneous Impact of COVID-19 on Employment and Health. 2020. Available online: http://www.nber.org/papers/w27749 (accessed on 18 January 2024).
- Hawkins, D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am. J. Ind. Med. 2020, 63, 817–820. [Google Scholar] [CrossRef]
- Carlsten, C.; Gulati, M.; Hines, S.; Rose, C.; Scott, K.; Tarlo, S.M.; Torén, K.; Sood, A.; de la Hoz, R.E. COVID-19 as an occupational disease. Am. J. Ind. Med. 2021, 64, 227–237. [Google Scholar] [CrossRef]
- Apelberg, B.J.; Buckley, T.J.; White, R.H. Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ. Health Perspect. 2005, 113, 693–699. [Google Scholar] [CrossRef]
- Nardone, A.; A Casey, J.; Morello-Frosch, R.; Mujahid, M.; Balmes, J.R.; Thakur, N. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: An ecological study. Lancet Planet. Health 2020, 4, e24–e31. [Google Scholar] [CrossRef]
- American Lung Association. Disparities in the Impact of Air Pollution. Updated 17 November 2022. Available online: https://www.lung.org/clean-air/outdoors/who-is-at-risk/disparities (accessed on 12 December 2023).
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef]
- Abedi, V.; Olulana, O.; Avula, V.; Chaudhary, D.; Khan, A.; Shahjouei, S.; Li, J.; Zand, R. Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. J. Racial Ethn. Health Disparit. 2021, 8, 732–742. [Google Scholar] [CrossRef]
- Figueroa, J.F.; Wadhera, R.K.; Lee, D.; Yeh, R.W.; Sommers, B.D. Community-Level Factors Associated with Racial And Ethnic Disparities In COVID-19 Rates In Massachusetts. Health Aff. 2020, 39, 1984–1992. [Google Scholar] [CrossRef]
- Azar, K.M.J.; Shen, Z.; Romanelli, R.J.; Lockhart, S.H.; Smits, K.; Robinson, S.; Brown, S.; Pressman, A.R. Disparities in Outcomes among COVID-19 Patients in a Large Health Care System in California. Health Aff. 2020, 39, 1253–1262. [Google Scholar] [CrossRef]
- Acosta, A.M.; Garg, S.; Pham, H.; Whitaker, M.; Anglin, O.; O’halloran, A.; Milucky, J.; Patel, K.; Taylor, C.; Wortham, J.; et al. Racial and Ethnic Disparities in Rates of COVID-19–Associated Hospitalization, Intensive Care Unit Admission, and in-Hospital Death in the United States from March 2020 to February 2021. JAMA Netw. Open 2021, 4, e2130479. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Shah, A.; Doubeni, C.A.; Sia, I.G.; Wieland, M.L. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin. Infect. Dis. 2021, 72, 703–706. [Google Scholar] [CrossRef]
- Gangopadhyaya, A.; Karpman, M.; Aarons, J. As the COVID-19 Recession Extended into the Summer of 2020, More Than 3 Million Adults Lost Employer-Sponsored Health Insurance Coverage and 2 Million Became Uninsured. 2020. Available online: https://www.rwjf.org/content/dam/farm/reports/issue_briefs/2020/rwjf462707 (accessed on 18 January 2024).
- Castañeda, H.; Holmes, S.M.; Madrigal, D.S.; Young, M.-E.D.; Beyeler, N.; Quesada, J. Immigration as a social determinant of health. Annu. Rev. Public Health 2015, 36, 375–392. [Google Scholar] [CrossRef]
- Bhuiyan, M.T.H.; Khan, I.M.; Jony, S.S.R.; Robinson, R.; Nguyen, U.-S.D.T.; Keellings, D.; Rahman, M.S.; Haque, U. The Disproportionate Impact of COVID-19 among Undocumented Immigrants and Racial Minorities in the US. Int. J. Environ. Res. Public Health 2021, 18, 12708. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Q.; Wei, X.; Cao, Z.; Yuan, H.-Y.; Zeng, D.D. Equitable access to COVID-19 vaccines makes a life-saving difference to all countries. Nat. Hum. Behav. 2022, 6, 207–216. [Google Scholar] [CrossRef]
- Hayawi, K.; Shahriar, S.; Serhani, M.A.; Alashwal, H.; Masud, M.M. Vaccine versus Variants (3Vs): Are the COVID-19 Vaccines Effective against the Variants? A Systematic Review. Vaccines 2021, 9, 1305. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2023, 385, 585–594, Erratum in N. Engl. J. Med. 2023, 388, 672. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764–766. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker. Approved Vaccines. 2023. Available online: https://covid19.trackvaccines.org/vaccines/approved/ (accessed on 18 January 2024).
- Comunale, B.A.; Engineer, L.; Jiang, Y.; Andrews, J.C.; Liu, Q.; Ji, L.; Yurkovich, J.T.; Comunale, R.A.; Xie, Q. Poliovirus Vaccination Induces a Humoral Immune Response That Cross Reacts with SARS-CoV-2. Front. Med. 2021, 8, 1285. [Google Scholar] [CrossRef]
- Zepp, F. Principles of vaccine design—Lessons from nature. Vaccine 2010, 28 (Suppl. 3), C14–C24. [Google Scholar] [CrossRef]
- Comunale, B.A.; Jackson-Ward, E.; Jiang, Y.; Ward, L.P.; Liu, Q.; Ji, L.; Lai, M.; Engineer, L.; Comunale, R.A.; Xie, Q. Inactivated Poliovirus Vaccine Induces Antibodies that Inhibit RNA Synthesis of SARS-CoV-2: An open-label, pre-post vaccine clinical trial. medRxiv 2021. medRxiv:2021.10.05.21264598. [Google Scholar] [CrossRef]
- Comunale, B.A.; Larson, R.J.; Hsu, Y.-J.; Jackson-Ward, E.; Azodoh, C.; Singh, A.; Engineer, L. Prior Oral Poliovirus Vaccination Decreases Odds of COVID-19 Outcomes. Ph.D. Dissertation, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA, 2023. [Google Scholar]
- Miller, M. Dyslipidemia and cardiovascular risk: The importance of early prevention. QJM 2009, 102, 657–667. [Google Scholar] [CrossRef]
- Pol, T.; Held, C.; Westerbergh, J.; Lindbäck, J.; Alexander, J.H.; Alings, M.; Erol, C.; Goto, S.; Halvorsen, S.; Huber, K.; et al. Dyslipidemia and Risk of Cardiovascular Events in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Therapy: Insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) Trial. J. Am. Heart Assoc. 2018, 7, e007444. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Mortensen, L.H.; Denwood, M.J.; Christiansen, L.E.; Møller, C.H.; Skov, R.L.; Spiess, K.; Fomsgaard, A.; Lassaunière, R.; Rasmussen, M.; et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat. Commun. 2022, 13, 5573. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, B.; Son, N.-H.; Heo, N.; Nam, Y.; Shin, A.; Yang, A.J.; Kim, M.H.; Kyong, T.; Kang, E.; et al. Vaccine Effect on Household Transmission of Omicron and Delta SARS-CoV-2 Variants. J. Korean Med. Sci. 2023, 38, e9. [Google Scholar] [CrossRef]
- California Department of Public Health. Variants in California. Updated 7 July 2023. Available online: https://covid19.ca.gov/variants/#in-california (accessed on 12 December 2023).
- Márquez, E.J.; Chung, C.-H.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349, Erratum in Lancet Infect. Dis. 2010, 10, 740. [Google Scholar] [CrossRef]
- Morgan, R.; Klein, S.L. The intersection of sex and gender in the treatment of influenza. Curr. Opin. Virol. 2019, 35, 35–41. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Hagan, T.; Duraisingham, S.S.; Lee, E.K.; Kwissa, M.; Rouphael, N.; Frasca, D.; Gersten, M.; Mehta, A.K.; Gaujoux, R.; et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 2015, 43, 1186–1198. [Google Scholar] [CrossRef]
- Höpping, A.M.; McElhaney, J.; Fonville, J.M.; Powers, D.C.; Beyer, W.E.; Smith, D.J. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine 2016, 34, 540–546. [Google Scholar] [CrossRef]
- Ghosh, A.; Venkatraman, S.; Soroka, O.; Reshetnyak, E.; Rajan, M.; An, A.; Chae, J.; Gonzalez, C.; Prince, J.; DiMaggio, C.; et al. Association between overcrowded households, multigenerational households, and COVID-19: A cohort study. Public Health 2021, 198, 273–279. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Lips, P.; de Jongh, R.T.; van Schoor, N.M. Trends in Vitamin D Status Around the World. JBMR Plus 2021, 5, e10585. [Google Scholar] [CrossRef]
- Rucker, D.; Allan, J.A.; Fick, G.H.; Hanley, D.A. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ 2002, 166, 1517–1524, Erratum in CMAJ 2002, 167, 850. [Google Scholar]
- Roth, D.E.; Martz, P.; Yeo, R.; Prosser, C.; Bell, M.; Jones, A.B. Are national vitamin D guidelines sufficient to maintain adequate blood levels in children? Can. J. Public Health 2005, 96, 443–449. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Terenzi, U.; Nannipieri, F.; Locatelli, M.; Ciceri, F.; Giustina, A. Lack of vitamin D predicts impaired long-term immune response to COVID-19 vaccination. Endocrine 2023, 82, 536–541. [Google Scholar] [CrossRef]
- Griffith, P.R.; Innes, F.C. The relationship of socio-economic factors to the use of vitamin supplements in the city of windsor. Nutr. Res. 1983, 3, 445–455. [Google Scholar] [CrossRef]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Herremans, T.M.P.T.; Reimerink, J.H.J.; Buisman, A.M.; Kimman, T.G.; Koopmans, M.P.G. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 1999, 162, 5011–5018. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- John, J.; Giri, S.; Karthikeyan, A.S.; Iturriza-Gomara, M.; Muliyil, J.; Abraham, A.; Grassly, N.C.; Kang, G. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: An open-label, randomised controlled trial. Lancet 2014, 384, 1505–1512. [Google Scholar] [CrossRef]
- Sekulic, M.; Stajic, D.; Skevin, A.J.; Kocovic, A.; Zaric, R.Z.; Djonovic, N.; Vasiljevic, D.; Radmanovic, B.; Spasic, M.; Janicijevic, K.; et al. Lifestyle, Physical Activity, Eating and Hygiene Habits: A Comparative Analysis Before and During the COVID-19 Pandemic in Student Population. Front. Public Health 2022, 10, 862816. [Google Scholar] [CrossRef]
- Thiab, S.H.; Nassar, R.I.; Thiab, S.; Basheti, I.A. Medications and natural products used in Jordan for prevention or treatment of COVID-19 infection during the second wave of the pandemic: A cross-sectional online survey. Saudi Pharm. J. 2022, 30, 856–862. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.T.; Gao, Y.; Xue, X.; Pang, H.; Hao, W.; Xia, Y.; Wang, S.; Su, X.; Zhao, L.; et al. Analysis of Dietary Supplement Use and Influencing Factors in the Mongolian Population. BioMed Res. Int. 2022, 2022, 4064588. [Google Scholar] [CrossRef]
- Foote, J.A.; Murphy, S.P.; Wilkens, L.R.; Hankin, J.H.; Henderson, B.E.; Kolonel, L.N. Factors associated with dietary supplement use among healthy adults of five ethnicities: The Multiethnic Cohort Study. Am. J. Epidemiol. 2003, 157, 888–897. [Google Scholar] [CrossRef]
| Variable | Factor Type | Variable Type | Levels |
|---|---|---|---|
| Age | Biological | Continuous | N/A (continuous, 18–80) |
| Age (categories) | Biological | Ordinal | 3 (18–50; 51–64; 65–80) |
| Biological Sex | Biological | Dichotomous | 2 (Male; Female) |
| Education Completed | SDOH | Ordinal | 4 (High School or Less; Bachelor’s or Associate’s; Master’s Degree; Doctoral Degree) |
| Employment Status | SDOH | Ordinal | 3 (Not Employed; Part-Time; Full-Time) |
| Previously Received OPV | SDOH | Dichotomous | 2 (Yes; No) |
| Received COVID-19 Vaccine | SDOH | Dichotomous | 2 (Yes; No) |
| Currently Taking Vitamin D Supplementation | SDOH | Dichotomous | 2 (Yes; No) |
| Underlying Medical Conditions | SDOH | Dichotomous | 2 (Yes; No) |
| Diabetes | SDOH | Dichotomous | 2 (Yes; No) |
| Hypertension | SDOH | Dichotomous | 2 (Yes; No) |
| Dyslipidemia | SDOH | Dichotomous | 2 (Yes; No) |
| Race/Ethnicity | SDOH | Categorical | 4 (white; Hispanic/Latinx; Asian; Other) |
| Health Insurance Types | SDOH | Categorical | 4 (Private; Medicare; Medicaid; No Insurance) |
| Exposed to Omicron or Delta SARS-CoV-2 Strain | Other-Potential Confounder | Dichotomous | 2 (Delta; Omicron) |
| Independent Variable | Adjusted Odds Ratio | 95% Confidence Interval | p-Value | Adjusted Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|
| Underlying Medical Conditions | ||||||
| No | 1.0 | --- | --- | 1.0 | --- | --- |
| Yes | 2.36 | 1.24–4.57 | <0.01 | 4.50 | 2.25–9.33 | <0.001 |
| Employment Status | ||||||
| Full-Time | 1.0 | --- | --- | 1.0 | --- | --- |
| Not Employed | 0.29 | 0.15–0.58 | <0.001 | 0.27 | 0.13–0.56 | <0.001 |
| Part-Time | 0.28 | 0.11–0.69 | <0.01 | 0.22 | 0.08–0.58 | <0.01 |
| Vitamin D Supplementation | ||||||
| No | 1.0 | --- | --- | 1.0 | --- | --- |
| Yes | 0.12 | 0.03–0.39 | <0.001 | 0.09 | 0.02–0.31 | <0.001 |
| Education Completed | ||||||
| Bachelor’s/Associate’s Degree | 1.0 | --- | --- | 1.0 | --- | --- |
| Graduate Degree | 0.30 | 0.10–0.83 | <0.05 | 0.42 | 0.14–1.20 | 0.11 |
| High school or less | 0.86 | 0.41–1.79 | 0.69 | 0.67 | 0.30–1.47 | 0.32 |
| Received COVID-19 Vaccine | ||||||
| No | 1.0 | --- | --- | 1.0 | --- | --- |
| Yes | 0.56 | 0.30–1.04 | 0.07 | 0.57 | 0.30–1.09 | 0.09 |
| Exposed to Omicron or Delta Strain | ||||||
| Delta | 1.0 | --- | --- | 1.0 | --- | --- |
| Omicron | 0.55 | 0.30–1.01 | 0.05 | 0.27 | 0.14–0.52 | <0.001 |
| Previously Received Oral Polio Vaccine (OPV) | ||||||
| Yes | 1.0 | --- | --- | 1.0 | --- | --- |
| No | 4.36 | 2.23–8.79 | <0.001 | 6.95 | 3.25–15.83 | <0.001 |
| Vitamin D × Education Completed (Yes × Graduate Degree) | 8.10 | 1.13–60.40 | <0.05 | 8.06 | 1.10–63.75 | <0.05 |
| Vitamin D × Education Completed (Yes × High School or Less) | 1.12 | 0.19–6.52 | 0.90 | 0.77 | 0.10–5.43 | 0.80 |
| Independent Variable | Regression Coefficient (ß) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Hypertension (Yes) | 1.99 | −0.29–4.27 | 0.08 |
| Vitamin D Supplementation (Yes) | −3.45 | −5.81–−1.09 | <0.01 |
| Received COVID-19 Vaccine (Yes) | −2.17 | −4.35–0.03 | 0.06 |
| Exposed to Omicron or Delta Strain (Omicron) | −2.04 | −4.24–0.16 | 0.07 |
| Previously Received Oral Polio Vaccine (No) | 5.81 | 3.35–8.28 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comunale, B.A.; Hsu, Y.-J.; Larson, R.J.; Singh, A.; Jackson-Ward, E.; Engineer, L.D. Vitamin D Supplementation and Prior Oral Poliovirus Vaccination Decrease Odds of COVID-19 Outcomes among Adults Recently Inoculated with Inactivated Poliovirus Vaccine. Vaccines 2024, 12, 121. https://doi.org/10.3390/vaccines12020121
Comunale BA, Hsu Y-J, Larson RJ, Singh A, Jackson-Ward E, Engineer LD. Vitamin D Supplementation and Prior Oral Poliovirus Vaccination Decrease Odds of COVID-19 Outcomes among Adults Recently Inoculated with Inactivated Poliovirus Vaccine. Vaccines. 2024; 12(2):121. https://doi.org/10.3390/vaccines12020121
Chicago/Turabian StyleComunale, Brittany A., Yea-Jen Hsu, Robin J. Larson, Aditi Singh, Erin Jackson-Ward, and Lilly D. Engineer. 2024. "Vitamin D Supplementation and Prior Oral Poliovirus Vaccination Decrease Odds of COVID-19 Outcomes among Adults Recently Inoculated with Inactivated Poliovirus Vaccine" Vaccines 12, no. 2: 121. https://doi.org/10.3390/vaccines12020121
APA StyleComunale, B. A., Hsu, Y.-J., Larson, R. J., Singh, A., Jackson-Ward, E., & Engineer, L. D. (2024). Vitamin D Supplementation and Prior Oral Poliovirus Vaccination Decrease Odds of COVID-19 Outcomes among Adults Recently Inoculated with Inactivated Poliovirus Vaccine. Vaccines, 12(2), 121. https://doi.org/10.3390/vaccines12020121






