Combination Adjuvants Enhance Recombinant H5 Hemagglutinin Vaccine Protection Against High-Dose Viral Challenge in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Recombinant Antigen and Adjuvants
2.3. Immuization and Viral Challenge Protection Trials
2.4. Hemagglutination Inhibition Assay
2.5. Virus Neutralization Assay
2.6. Isolation of Chicken PBMCs and Detection of Antigen Stimulated Cytokines
2.7. Statistical Analysis
3. Results
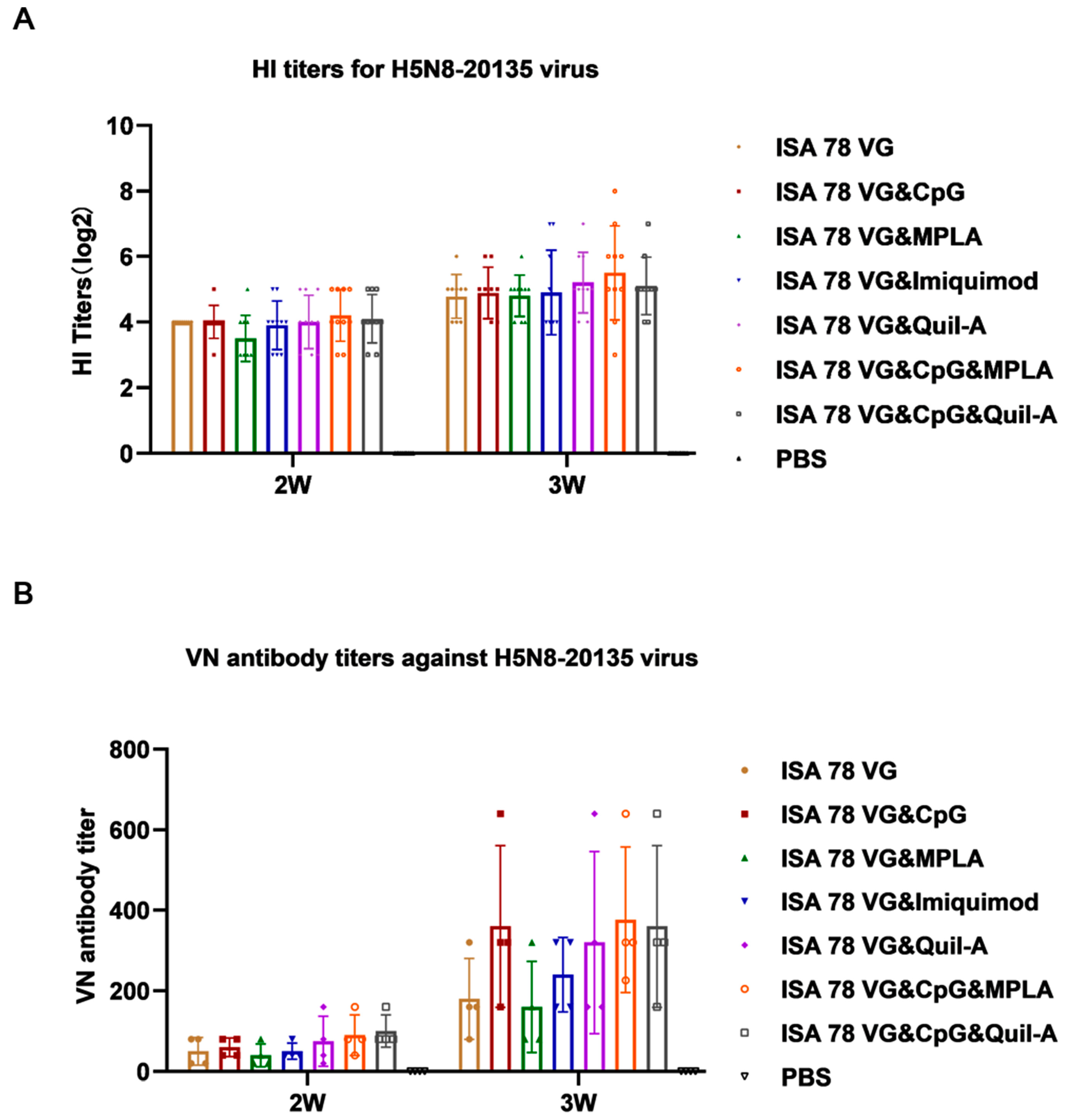
3.1. Enhanced Antibody Response to H5N6-20053 rHA Vaccine with Combination Adjuvants
3.2. Combination Adjuvants Enhanced Cytokine Production of H5N6-20053 rHA Vaccine
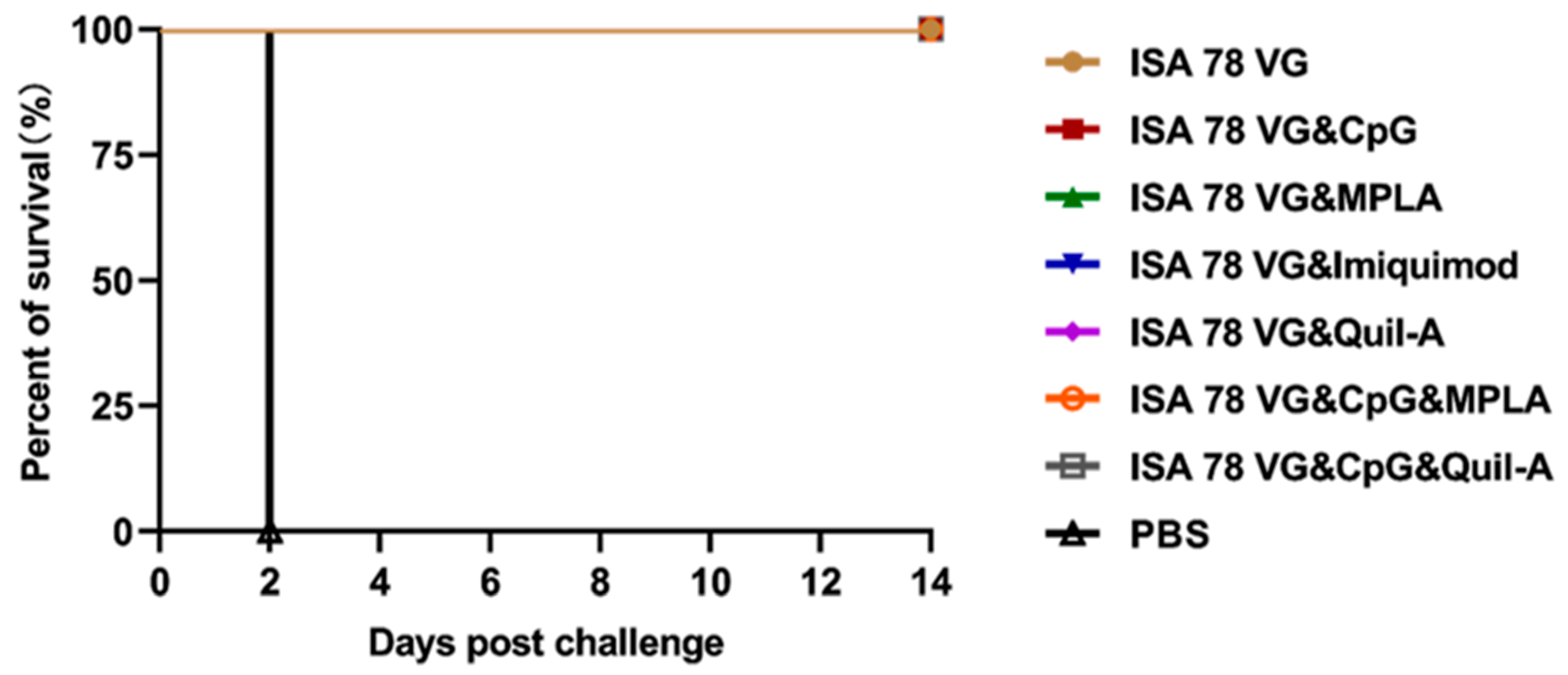
3.3. Efficacy of Combination Adjuvants in Inducing Protection Against H5N6-20053 Virus Challenge
3.4. Combination Adjuvant Vaccines Reduce Viral Shedding and Replication in Chickens
3.5. Rapid Antibody Response to ISA78VG&Quil-A Adjuvanted Vaccine in Immunized Chickens
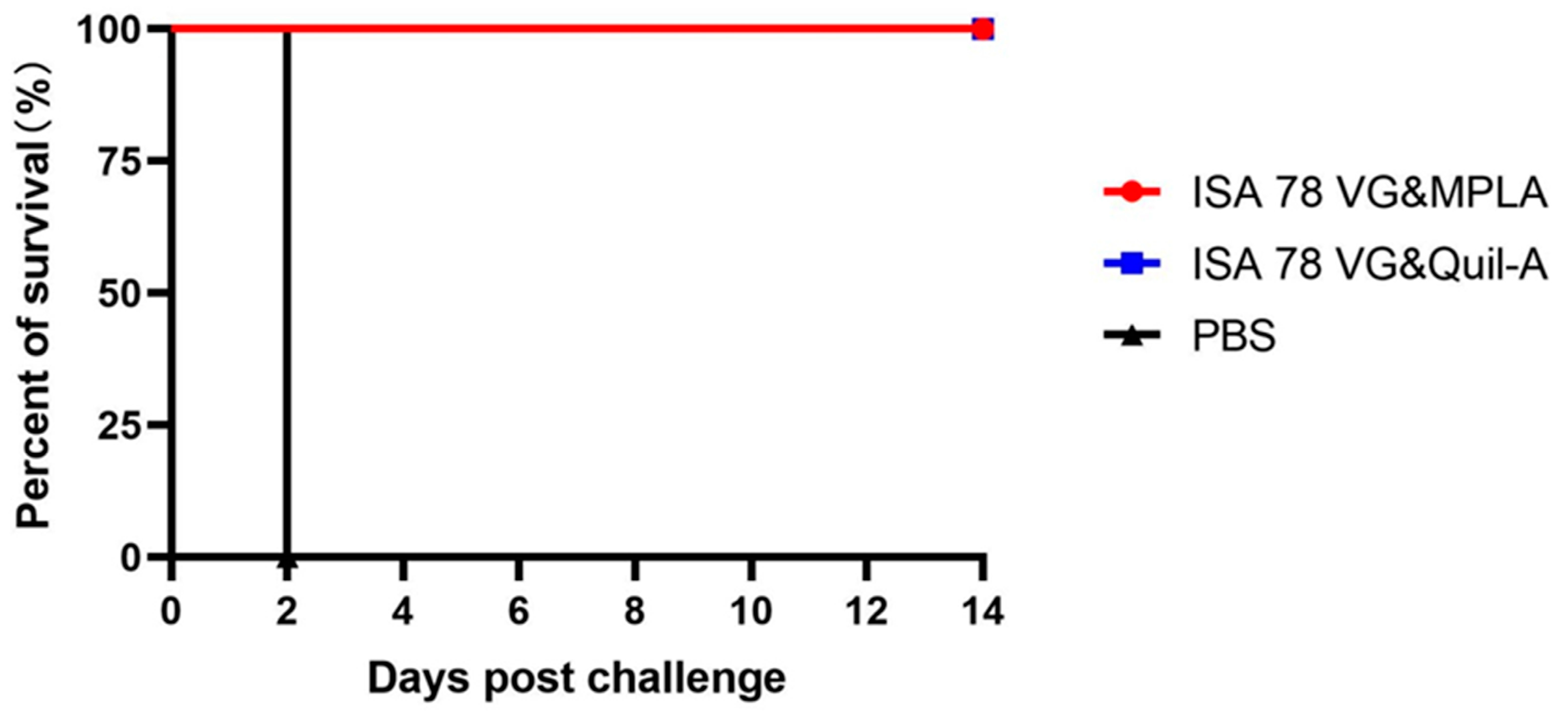
3.6. Combination Adjuvant Vaccines Protect Against Higher Doses of H5N6-20053 Virus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Zhu, W.; Li, X.; Bo, H.; Zhang, Y.; Zou, S.; Gao, R.; Dong, J.; Zhao, X.; Chen, W.; et al. Genesis and Dissemination of Highly Pathogenic H5N6 Avian Influenza Viruses. J. Virol. 2017, 91, e02199-16. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, Y.; Li, M.; Zhao, L.; Wang, D.; Tian, J.; Bai, X.; Ci, Y.; Wu, S.; Wang, F.; et al. Evolution and extensive reassortment of H5 influenza viruses isolated from wild birds in China over the past decade. Emerg. Microbes Infect. 2020, 9, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; Willgert, K.; et al. Avian influenza overview December 2023–March 2024. EFSA J. Eur. Food Saf. Auth. 2024, 22, e8754. [Google Scholar] [CrossRef]
- Swayne, D.E.; Kapczynski, D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008, 225, 314–331. [Google Scholar] [CrossRef]
- Swayne, D.E.; Pavade, G.; Hamilton, K.; Vallat, B.; Miyagishima, K. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev. Sci. Tech. (Int. Off. Epizoot.) 2011, 30, 839–870. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Wilson, I.A.; Cox, N.J. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 1990, 8, 737–771. [Google Scholar] [CrossRef]
- Han, T.; Marasco, W.A. Structural basis of influenza virus neutralization. Ann. New York Acad. Sci. 2011, 1217, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Landreth, S.; Liu, G.; Brownlie, R.; Gaba, A.; Littel-van den Hurk, S.V.D.; Gerdts, V.; Zhou, Y. Innate immunemodulator containing adjuvant formulated HA based vaccine protects mice from lethal infection of highly pathogenic avian influenza H5N1 virus. Vaccine 2020, 38, 2387–2395. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Orbegozo-Medina, R.A.; Martínez-Sernández, V.; González-Warleta, M.; Castro-Hermida, J.A.; Mezo, M.; Ubeira, F.M. Vaccination of sheep with Quil-A® adjuvant expands the antibody repertoire to the Fasciola MF6p/FhHDM-1 antigen and administered together impair the growth and antigen release of flukes. Vaccine 2018, 36, 1949–1957. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Chea, E.K.; George, C.; Pillarsetty, N.; Gardner, J.R.; Livingston, P.O.; Ragupathi, G.; Lewis, J.S.; Tan, D.S.; Gin, D.Y. Development of a minimal saponin vaccine adjuvant based on QS-21. Nat. Chem. 2014, 6, 635–643. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N, Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Vohra-Miller, S.; Schwartz, I.S. NVX-CoV2373, a protein-based vaccine against SARS-CoV-2 infection. Can. Med. Assoc. J. 2022, 194, E1214. [Google Scholar] [CrossRef]
- Nian, X.; Zhang, J.; Deng, T.; Liu, J.; Gong, Z.; Lv, C.; Yao, L.; Li, J.; Huang, S.; Yang, X. AddaVax Formulated with PolyI:C as a Potential Adjuvant of MDCK-based Influenza Vaccine Enhances Local, Cellular, and Antibody Protective Immune Response in Mice. AAPS PharmSciTech 2021, 22, 270. [Google Scholar] [CrossRef]
- Lee, C.W.; Bakre, A.; Olivier, T.L.; Alvarez-Narvaez, S.; Harrell, T.L.; Conrad, S.J. Toll-like Receptor Ligands Enhance Vaccine Efficacy against a Virulent Newcastle Disease Virus Challenge in Chickens. Pathogens 2023, 12, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Hill, E.; McNeel, D.G. Toll-like receptor agonists as cancer vaccine adjuvants. Hum. Vaccines Immunother. 2024, 20, 2297453. [Google Scholar] [CrossRef] [PubMed]
- Hyer, R.N.; Janssen, R.S. Immunogenicity and safety of a 2-dose hepatitis B vaccine, HBsAg/CpG 1018, in persons with diabetes mellitus aged 60–70 years. Vaccine 2019, 37, 5854–5861. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Nisbet, D.J.; Kogut, M.H. Profile of Toll-like receptor expressions and induction of nitric oxide synthesis by Toll-like receptor agonists in chicken monocytes. Mol. Immunol. 2006, 43, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Van Mechelen, M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 2011, 10, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Deifl, S.; Kitzmüller, C.; Steinberger, P.; Himly, M.; Jahn-Schmid, B.; Fischer, G.F.; Zlabinger, G.J.; Bohle, B. Differential activation of dendritic cells by toll-like receptors causes diverse differentiation of naïve CD4+ T cells from allergic patients. Allergy 2014, 69, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Deza, G.; Martin-Ezquerra, G.; Curto-Barredo, L.; Villar García, J.; Pujol, R.M. Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod. J. Dermatol. 2015, 42, 1176–1178. [Google Scholar] [CrossRef]
- Chen, T.; Kong, D.; Hu, X.; Gao, Y.; Lin, S.; Liao, M.; Fan, H. Influenza H7N9 Virus Hemagglutinin with T169A Mutation Possesses Enhanced Thermostability and Provides Effective Immune Protection against Lethal H7N9 Virus Challenge in Chickens. Vaccines 2023, 11, 1318. [Google Scholar] [CrossRef]
- Kong, D.; He, Y.; Wang, J.; Chi, L.; Ao, X.; Ye, H.; Qiu, W.; Zhu, X.; Liao, M.; Fan, H. A single immunization with H5N1 virus-like particle vaccine protects chickens against divergent H5N1 influenza viruses and vaccine efficacy is determined by adjuvant and dosage. Emerg. Microbes Infect. 2024, 13, 2287682. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Sitaras, I. Hemagglutination Inhibition Assay. Methods Mol. Biol. 2020, 2123, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.J.S.; Obadan, A.O.; Garcia, S.C.; Carnaccini, S.; Kapczynski, D.R.; Pantin-Jackwood, M.; Suarez, D.L.; Perez, D.R. Short- and long-term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime-boost vaccination in turkeys. Vaccine 2017, 35, 5637–5643. [Google Scholar] [CrossRef]
- Moingeon, P.; Haensler, J.; Lindberg, A. Towards the rational design of Th1 adjuvants. Vaccine 2001, 19, 4363–4372. [Google Scholar] [CrossRef] [PubMed]
- Mount, A.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E.; Morelli, A.B. Combination of adjuvants: The future of vaccine design. Expert Rev. Vaccines 2013, 12, 733–746. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Comin, A.; Toft, N.; Stegeman, A.; Klinkenberg, D.; Marangon, S. Serological diagnosis of avian influenza in poultry: Is the haemagglutination inhibition test really the ‘gold standard’? Influenza Other Respir. Viruses 2013, 7, 257–264. [Google Scholar] [CrossRef]
- Nazmi, A.; Mukherjee, S.; Kundu, K.; Dutta, K.; Mahadevan, A.; Shankar, S.K.; Basu, A. TLR7 is a key regulator of innate immunity against Japanese encephalitis virus infection. Neurobiol. Dis. 2014, 69, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xuan, D.; Wang, H.; Zhou, M.; Deng, B.; Ma, F.; Lu, Y.; Zhang, J. Biodegradable Imiquimod-Loaded Mesoporous Organosilica as a Nanocarrier and Adjuvant for Enhanced and Prolonged Immunity against Foot-and-Mouth Disease Virus in Mice. ACS Appl. Bio Mater. 2022, 5, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Peng, M.; Zhu, S.; Wu, Y.; Ji, R.; Shen, C. Screening of Efficient Adjuvants for the RBD-Based Subunit Vaccine of SARS-CoV-2. Vaccines 2023, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Davies, J.E.; Dollinger, E.P.; Pone, E.J.; Felgner, J.; Liang, L.; Strohmeier, S.; Jan, S.; Albin, T.J.; Jain, A.; Nakajima, R.; et al. Magnitude and breadth of antibody cross-reactivity induced by recombinant influenza hemagglutinin trimer vaccine is enhanced by combination adjuvants. Sci. Rep. 2022, 12, 9198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ding, X.; Zhao, R.; Liu, X.; Shen, H.; Cai, C.; Ferrari, M.; Wang, H.Y.; Wang, R.F. Co-delivery of tumor antigen and dual toll-like receptor ligands into dendritic cell by silicon microparticle enables efficient immunotherapy against melanoma. J. Control. Release Off. J. Control. Release Soc. 2018, 272, 72–82. [Google Scholar] [CrossRef]
- Ziegler, A.; Gerber, V.; Marti, E. In vitro effects of the toll-like receptor agonists monophosphoryl lipid A and CpG-rich oligonucleotides on cytokine production by equine cells. Vet. J. 2017, 219, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, K.A.; Wang, T.; Russell, K.S.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. Montanide™ ISA 763A VG and ISA 761 VG induce different immune pathway responses in rainbow trout (Oncorhynchus mykiss) when used as adjuvant for an Aeromonas salmonicida bacterin. Fish Shellfish Immunol. 2021, 114, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, J.; Lee, M.J.; Peng, C.; Luo, T.; Tillman, L.; Weichselbaum, R.R.; Lin, W. Nanoscale coordination polymer synergizes photodynamic therapy and toll-like receptor activation for enhanced antigen presentation and antitumor immunity. Biomaterials 2023, 302, 122334. [Google Scholar] [CrossRef]
- Tripp, R.A. Understanding immunity to influenza: Implications for future vaccine development. Expert Rev. Vaccines 2023, 22, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Çokçalışkan, C.; Türkoğlu, T.; Sareyyüpoğlu, B.; Uzunlu, E.; Babak, A.; Özbilge, B.B.; Gülyaz, V. QS-21 enhances the early antibody response to oil adjuvant foot-and-mouth disease vaccine in cattle. Clin. Exp. Vaccine Res. 2016, 5, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Gan, F.; Chen, X.; Deng, K.; Crowe, S.A.; Hudson, G.A.; Belcher, M.S.; Schmidt, M.; Astolfi, M.C.T.; et al. Complete biosynthesis of QS-21 in engineered yeast. Nature 2024, 629, 937–944. [Google Scholar] [CrossRef]


| Group | Antigen (μg) | Adjuvant (μg) | Emulsion (μL) | |||
|---|---|---|---|---|---|---|
| rHA | CpG | MPLA | Imiquimod | Quil-A | ISA78VG | |
| 1 | 50 | 150 | ||||
| 2 | 50 | 25 | 150 | |||
| 3 | 50 | 20 | 150 | |||
| 4 | 50 | 100 | 150 | |||
| 5 | 50 | 50 | 150 | |||
| 6 | 50 | 25 | 20 | 150 | ||
| 7 | 50 | 25 | 50 | 150 | ||
| 8 | - | - | - | - | - | - |
| Gene | Primer Sequence (5′-3′) | Product Size |
|---|---|---|
| IFN-γ | F: ACCTTCCTGATGGCGTGAAG | 102 bp |
| R: TGAAGAGTTCATTCGCGGCT | ||
| IL-4 | F: ATGACATCCAGGGAGAGGTTT | 235 bp |
| R: ATTGGAGTAGTGTTGCCTGCT | ||
| β-actin | F: TGGGTATGGAGTCCTGTGGT | 136 bp |
| R: CTGTCAGCAATGCCAGGGTA |
| Group | Oropharyngeal Swab (Virus Shedding Number/Total Number) | Cloacal Swab (Virus Shedding Number/Total Number) | Total Virus Shedding Number/Total Number | ||||
|---|---|---|---|---|---|---|---|
| 3 dpc | 5 dpc | 7 dpc | 3 dpc | 5 dpc | 7 dpc | ||
| ISA78VG | 2/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 2/7 |
| ISA78VG&CpG | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| ISA78VG&MPLA | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| ISA78VG&Imiquimod | 2/7 | 1/7 | 1/7 | 0/7 | 0/7 | 0/7 | 3 a/7 |
| ISA78VG&Quil-A | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| ISA78VG&CpG&MPLA | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| ISA78VG&CpG&Quil-A | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| PBS | - | - | - | - | - | - | - |
| Group | Positive Conversion Rate (%) | ||||
|---|---|---|---|---|---|
| 8 d | 10 d | 12 d | 14 d | 16 d | |
| ISA78VG&MPLA | 0 | 0 | 0 | 60 | 80 |
| ISA78VG&Quil-A | 0 | 20 | 80 | 100 | 100 |
| PBS | 0 | 0 | 0 | 0 | 0 |
| Group | HI Titer with Different Antigens (log2) | Virus Shedding from Chickens at Five Days Post-Challenge | Mortality | ||
|---|---|---|---|---|---|
| rHA | H5N6-20053 | Oropharyngeal | Cloacal | ||
| ISA78VG&MPLA | 7.8 | 6.8 | 0/5 | 0/5 | 0/5 |
| ISA78VG&Quil-A | 9.8 | 8.0 | 0/5 | 0/5 | 0/5 |
| PBS | 2 | 0 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, J.; Chi, L.; Dong, Y.; Chen, H.; Meng, X.; Liao, M.; Luo, Y.; Fan, H. Combination Adjuvants Enhance Recombinant H5 Hemagglutinin Vaccine Protection Against High-Dose Viral Challenge in Chickens. Vaccines 2024, 12, 1448. https://doi.org/10.3390/vaccines12121448
He Y, Wang J, Chi L, Dong Y, Chen H, Meng X, Liao M, Luo Y, Fan H. Combination Adjuvants Enhance Recombinant H5 Hemagglutinin Vaccine Protection Against High-Dose Viral Challenge in Chickens. Vaccines. 2024; 12(12):1448. https://doi.org/10.3390/vaccines12121448
Chicago/Turabian StyleHe, Yanjuan, Jiaxin Wang, Lanyan Chi, Yajing Dong, Huixin Chen, Xiaocui Meng, Ming Liao, Yongwen Luo, and Huiying Fan. 2024. "Combination Adjuvants Enhance Recombinant H5 Hemagglutinin Vaccine Protection Against High-Dose Viral Challenge in Chickens" Vaccines 12, no. 12: 1448. https://doi.org/10.3390/vaccines12121448
APA StyleHe, Y., Wang, J., Chi, L., Dong, Y., Chen, H., Meng, X., Liao, M., Luo, Y., & Fan, H. (2024). Combination Adjuvants Enhance Recombinant H5 Hemagglutinin Vaccine Protection Against High-Dose Viral Challenge in Chickens. Vaccines, 12(12), 1448. https://doi.org/10.3390/vaccines12121448






