Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations
Abstract
1. Introduction
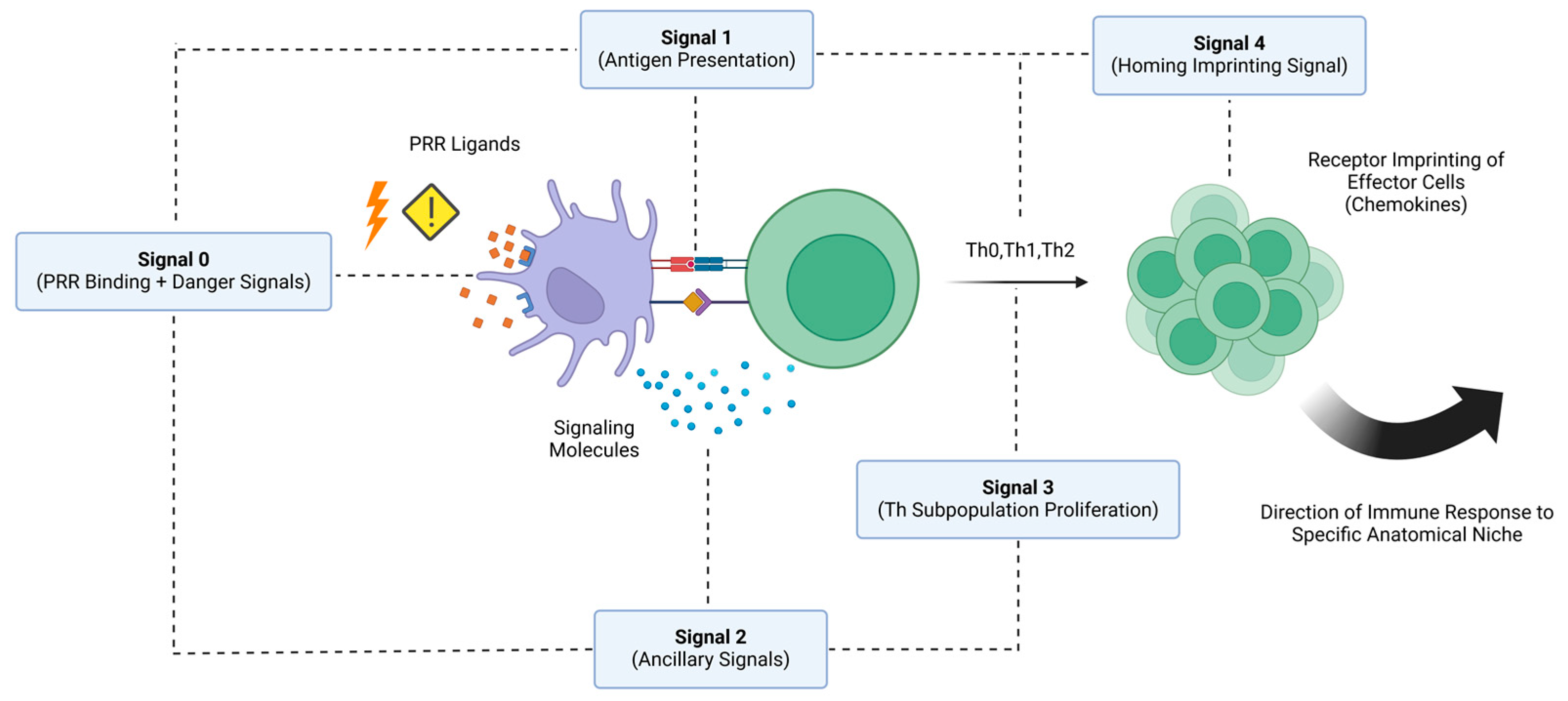
2. Mode of Action: The Two-Signal Model
2.1. Type 1 Signals
2.2. Type 2 Signals
2.3. Further Elaborations on the Two-Signal Model
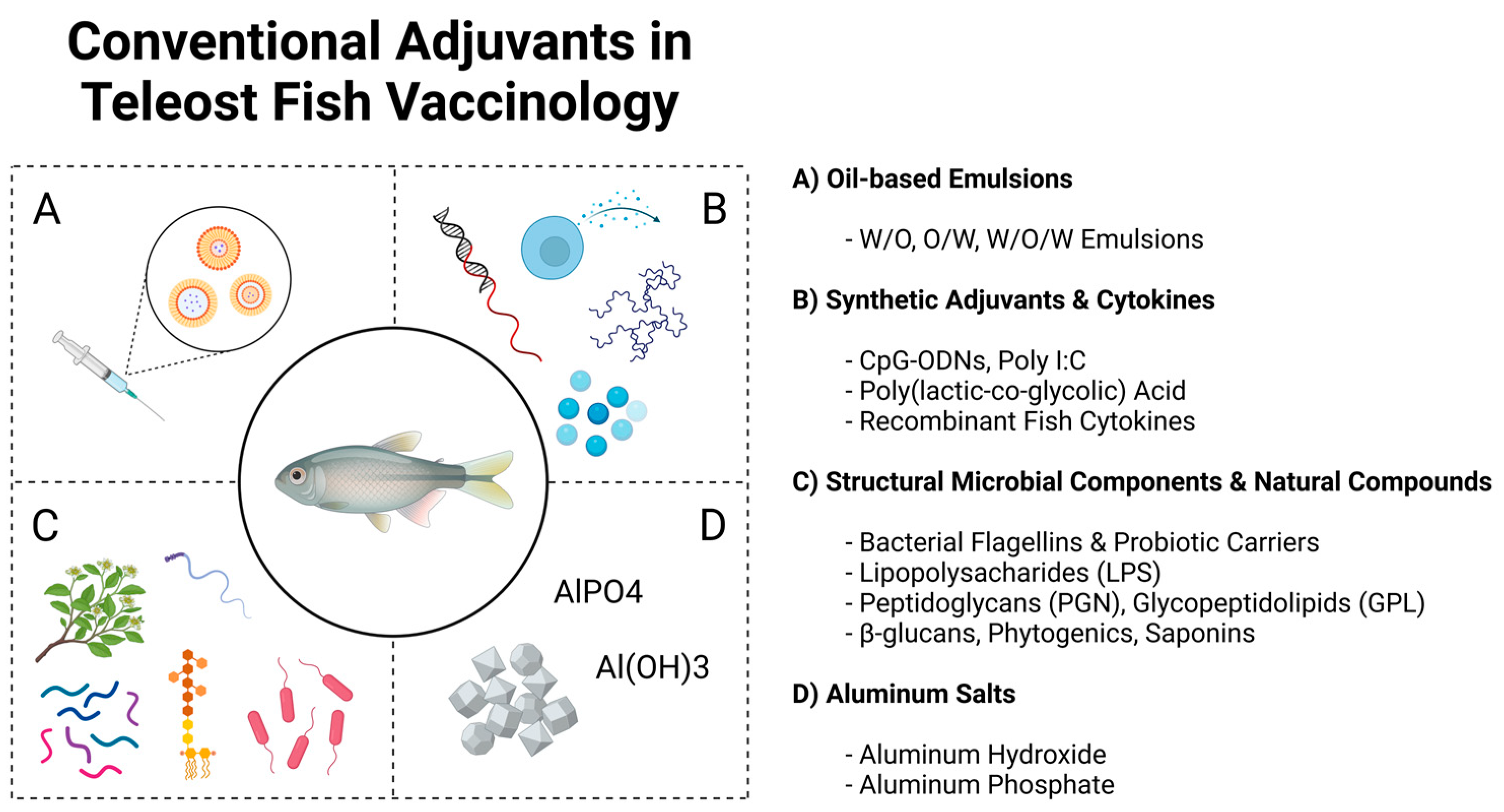
3. Traditional Adjuvants Used in Aquaculture Vaccinology
3.1. Oil-Based Emulsions
3.2. Aluminum-Based Compounds
3.3. Synthetic Adjuvants and Cytokines
3.4. Structural Microbial Components and Natural Compounds
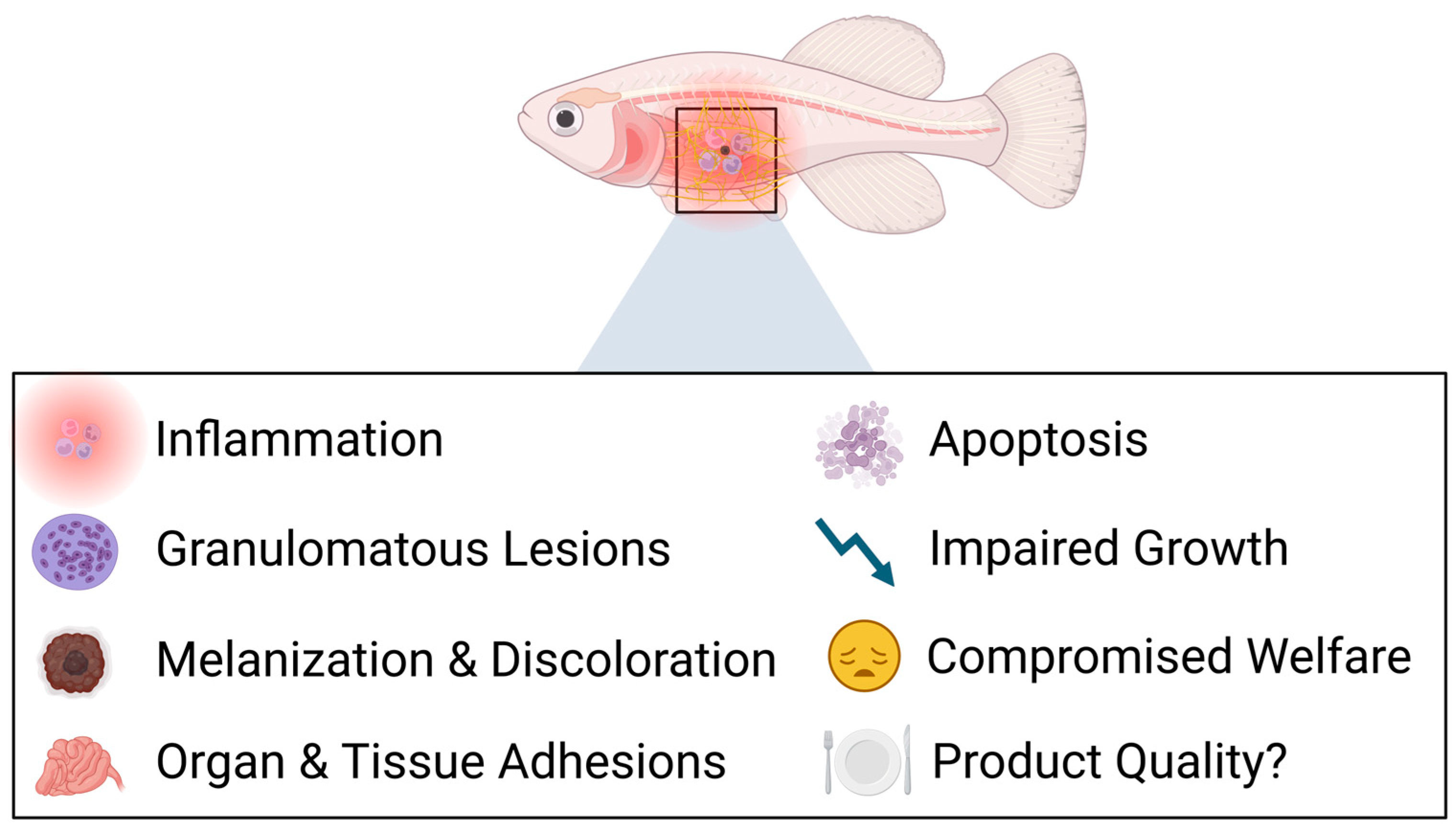
4. Assessing the Side Effects of Traditional Adjuvants
5. The Promise of Nanoparticle-Based Formulations for the Future
5.1. Polymeric Nanoparticle Formulations
5.2. Lipid-Based Nanoparticle Formulations
5.3. Carbon Nanotubes and Inorganic Nanoparticle Formulations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tammas, I.; Bitchava, K.; Gelasakis, A.I. Transforming Aquaculture through Vaccination: A Review on Recent Developments and Milestones. Vaccines 2024, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Vincent, A.T.; Gauthier, J.; Derome, N.; Charette, S.J. The Rise and Fall of Antibiotics in Aquaculture. In Microbial Communities in Aquaculture Ecosystems: Improving Productivity and Sustainability; Derome, N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–19. ISBN 978-3-030-16190-3. [Google Scholar]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Uglem, I.; Mladineo, I. Reared Fish, Farmed Escapees and Wild Fish Stocks—A Triangle of Pathogen Transmission of Concern to Mediterranean Aquaculture Management. Aquac. Environ. Interact. 2013, 3, 153–161. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Miller, K.M.; Bass, A.L.; Bateman, A.W.; Teffer, A.K.; Caleta, J.M.; Di Cicco, E.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; et al. Aquaculture Mediates Global Transmission of a Viral Pathogen to Wild Salmon. Sci. Adv. 2021, 7, eabe2592. [Google Scholar] [CrossRef]
- Johansen, L.-H.; Jensen, I.; Mikkelsen, H.; Bjørn, P.-A.; Jansen, P.A.; Bergh, Ø. Disease Interaction and Pathogens Exchange between Wild and Farmed Fish Populations with Special Reference to Norway. Aquaculture 2011, 315, 167–186. [Google Scholar] [CrossRef]
- Dalmo, R.; Bøgwald, J.; Tafalla, C. Adjuvants and Delivery Methods: Current and Novel. In Fish Vaccines; Adams, A., Ed.; Springer: Basel, Switzerland, 2016; pp. 75–103. ISBN 978-3-0348-0980-1. [Google Scholar]
- Mondal, H.; Thomas, J. A Review on the Recent Advances and Application of Vaccines against Fish Pathogens in Aquaculture. Aquacult Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Subramani, P.A.; Michael, R.D. Chapter 4—Prophylactic and Prevention Methods Against Diseases in Aquaculture. In Fish Diseases; Jeney, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 81–117. ISBN 978-0-12-804564-0. [Google Scholar]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; El-Sayed Ali, T. A Review Article on Nanotechnology in Aquaculture Sustainability as a Novel Tool in Fish Disease Control. Aquacult Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in Nanotechnology for Sustainable Aquaculture and Fisheries. Rev. Aquac. 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Sarkar, B.; Mahanty, A.; Gupta, S.K.; Choudhury, A.R.; Daware, A.; Bhattacharjee, S. Nanotechnology: A next-Generation Tool for Sustainable Aquaculture. Aquaculture 2022, 546, 737330. [Google Scholar] [CrossRef]
- Thompson, K.D.; Rodkhum, C.; Bunnoy, A.; Thangsunan, P.; Kitiyodom, S.; Sukkarun, P.; Yostawornkul, J.; Yata, T.; Pirarat, N. Addressing Nanovaccine Strategies for Tilapia. Vaccines 2023, 11, 1356. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Vaseeharan, B.; Ramasamy, P.; Jeyachandran, S. Oral Vaccination for Sustainable Disease Prevention in Aquaculture—An Encapsulation Approach. Aquacult. Int. 2023, 31, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Turley, J.L.; Lavelle, E.C. Resolving Adjuvant Mode of Action to Enhance Vaccine Efficacy. Curr. Opin. Immunol. 2022, 77, 102229. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.S.; Schijns, V.E.J.C. Immunology of Vaccine Adjuvants. In Vaccine Adjuvants: Methods and Protocols; Davies, G., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 1–14. ISBN 978-1-60761-585-9. [Google Scholar]
- Schijns, V.E. Immunological Concepts of Vaccine Adjuvant Activity: Commentary. Curr. Opin. Immunol. 2000, 12, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Belmonte, R. Overview of the Fish Adaptive Immune System. In Fish Vaccines; Adams, A., Ed.; Springer: Basel, Switzerland, 2016; pp. 35–52. ISBN 978-3-0348-0978-8. [Google Scholar]
- Kordon, A.O.; Pinchuk, L.; Karsi, A. Adaptive Immune System in Fish. Turk. J. Fish. Aquat. Sci. 2021, 22, TRJFAS20235. [Google Scholar] [CrossRef]
- Díaz-Rosales, P.; Muñoz-Atienza, E.; Tafalla, C. Role of Teleost B Cells in Viral Immunity. Fish Shellfish Immunol. 2019, 86, 135–142. [Google Scholar] [CrossRef]
- Johnstone, C.; Chaves-Pozo, E. Antigen Presentation and Autophagy in Teleost Adaptive Immunity. Int. J. Mol. Sci. 2022, 23, 4899. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Ehl, S.; Aichele, P.; Oehen, S.; Kündig, T.; Hengartner, H. Antigen Localisation Regulates Immune Responses in a Dose- and Time-Dependent Fashion: A Geographical View of Immune Reactivity. Immunol. Rev. 1997, 156, 199–209. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Kordon, A.O.; Karsi, A.; Pinchuk, L. Innate Immune Responses in Fish: Antigen Presenting Cells and Professional Phagocytes. Turk. J. Fish. Aquat. Sci. 2018, 18, 1123–1139. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of Fish Toll-like Receptors (TLR) and NOD-like Receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Su, J. Progresses on Three Pattern Recognition Receptor Families (TLRs, RLRs and NLRs) in Teleost. Dev. Comp. Immunol. 2021, 122, 104131. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like Receptor Recognition of Bacteria in Fish: Ligand Specificity and Signal Pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.A.; Peixoto, D.; Losada, A.P.; Quiroga, M.I.; Vale, A.D.; Costas, B. Early Innate Immune Responses in European Sea Bass (Dicentrarchus labrax L.) Following Tenacibaculum Maritimum Infection. Front. Immunol. 2023, 14, 1254677. [Google Scholar] [CrossRef]
- Leiva-Rebollo, R.; Gémez-Mata, J.; Castro, D.; Borrego, J.J.; Labella, A.M. Immune Response of DNA Vaccinated-Gilthead Seabream (Sparus aurata) against LCDV-Sa Infection: Relevance of the Inflammatory Process. Front. Immunol. 2023, 14, 1209926. [Google Scholar] [CrossRef]
- Franch, R.; Cardazzo, B.; Antonello, J.; Castagnaro, M.; Patarnello, T.; Bargelloni, L. Full-Length Sequence and Expression Analysis of Toll-like Receptor 9 in the Gilthead Seabream (Sparus aurata L.). Gene 2006, 378, 42–51. [Google Scholar] [CrossRef]
- Miccoli, A.; Buonocore, F.; Picchietti, S.; Scapigliati, G. The Sea Bass Dicentrarchus labrax as a Marine Model Species in Immunology: Insights from Basic and Applied Research. Aquac. Fish. 2024, 9, 136–143. [Google Scholar] [CrossRef]
- Muñoz, I.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Toll-like Receptor 22 of Gilthead Seabream, Sparus aurata: Molecular Cloning, Expression Profiles and Post-Transcriptional Regulation. Dev. Comp. Immunol. 2014, 44, 173–179. [Google Scholar] [CrossRef]
- Chen, Z.; Ceballos-Francisco, D.; Guardiola, F.A.; Huang, D.; Esteban, M.Á. The Alleviation of Skin Wound-Induced Intestinal Barrier Dysfunction via Modulation of TLR Signalling Using Arginine in Gilthead Seabream (Sparus aurata L). Fish Shellfish Immunol. 2020, 107, 519–528. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Zhao, J.; Cao, M.; Yu, Z.; Fu, Q.; Tan, F.; Yang, N.; Li, C. Characterization, Evolution and Expression Analysis of Toll-like Receptor 7 (TLR7) in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2022, 125, 9–16. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Liu, D.; Liu, Q.; Hu, G. Cloning and Expression Analysis of a Toll-like Receptor 21 (TLR21) Gene from Turbot, Scophthalmus maximus. Dev. Comp. Immunol. 2017, 73, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, G.; Liu, Q.; Zhang, S. Cloning and Expression Study of a Toll-like Receptor 2 (Tlr2) Gene from Turbot, Scophthalmus maximus. Fish Shellfish Immunol. 2016, 59, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Su, B.; Zhou, S.; Shang, M.; Yan, H.; Liu, F.; Gao, C.; Tan, F.; Li, C. Identification and Expression Analysis of Toll-like Receptor Genes (TLR8 and TLR9) in Mucosal Tissues of Turbot (Scophthalmus maximus L.) Following Bacterial Challenge. Fish Shellfish Immunol. 2016, 58, 309–317. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Yang, N.; Wang, B.; Su, B.; Fu, Q.; Zhang, M.; Tan, F.; Li, C. Characterization of Toll-like Receptor 1 (TLR1) in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2021, 115, 27–34. [Google Scholar] [CrossRef]
- Hu, G.-B.; Zhang, S.-F.; Yang, X.; Liu, D.-H.; Liu, Q.-M.; Zhang, S.-C. Cloning and Expression Analysis of a Toll-like Receptor 22 (Tlr22) Gene from Turbot, Scophthalmus maximus. Fish Shellfish Immunol. 2015, 44, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Su, B.; Fu, Q.; Shang, M.; Gao, C.; Tan, F.; Li, C. Identification, Characterization and Expression Analysis of TLR5 in the Mucosal Tissues of Turbot (Scophthalmus maximus L.) Following Bacterial Challenge. Fish Shellfish Immunol. 2017, 68, 272–279. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Tanekhy, M. The Role of Toll-like Receptors in Innate Immunity and Infectious Diseases of Teleost. Aquac. Res. 2016, 47, 1369–1391. [Google Scholar] [CrossRef]
- Savelkoul, H.F.J.; Ferro, V.A.; Strioga, M.M.; Schijns, V.E.J.C. Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines 2015, 3, 148–171. [Google Scholar] [CrossRef]
- Salinas, I.; Ding, Y.; Fernández-Montero, Á.; Sunyer, J.O. Mucosal Immunity in Fish. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 387–443. ISBN 978-3-030-85420-1. [Google Scholar]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The Mucosal Immune System of Fish: The Evolution of Tolerating Commensals While Fighting Pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Villumsen, K.R.; Koppang, E.O.; Christensen, D.; Bojesen, A.M. Alternatives to Mineral Oil Adjuvants in Vaccines against Aeromonas salmonicida Subsp. salmonicida in Rainbow Trout Offer Reductions in Adverse Effects. Sci. Rep. 2017, 7, 5930. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.C.; Sacco, N.; Bregni, C. The Studies on Hydrophilic-Lipophilic Balance (HLB): Sixty Years after William C. Griffin’s Pioneer Work (1949–2009). Lat. Am. J. Pharm 2009, 28, 313–317. [Google Scholar]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB Value on Oil-in-Water Emulsions: Droplet Size, Rheological Behavior, Zeta-Potential, and Creaming Index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Guldan, A.-C.; Paulus, N.; Runkel, F. Multiple W/O/W Emulsions—Using the Required HLB for Emulsifier Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Raman, R.P.; Kumar, S. Adjuvants for Fish Vaccines. In Fish Immune System and Vaccines; Makesh, M., Rajendran, K.V., Eds.; Springer Nature: Singapore, 2022; pp. 231–244. ISBN 978-981-19126-8-9. [Google Scholar]
- Tepparin, S.; Unajak, S.; Hirono, I.; Kondo, H.; Areechon, N. Efficacy of Adjuvanted Streptococcus agalactiae Vaccine by Montanide ISA 763 A VG in Nile Tilapia (Oreochromis niloticus Linn.). J. Fish. Environ. 2018, 42, 26–38. [Google Scholar]
- Soltani, M.; Mokhtari, A.; Mirzargar, S.S.; Taherimirghaed, A.; Zargar, A.; Shafiei, S.; Hosseini Shekarabi, S.P. Efficacy and Immune Response of Intraperitoneal Vaccination of Rainbow Trout (Oncorhynchus mykiss) with a Yersinia Ruckeri Bacterin Formulated with Montanide (TM) ISA 763 AVG Adjuvant. Bull. Eur. Assoc. Fish Pathol. 2016, 36, 225–236. [Google Scholar]
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of MontanideTM ISA 763 A VG as Aquatic Adjuvant Administrated with an Inactivated Vibrio harveyi Vaccine in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019, 84, 56–61. [Google Scholar] [CrossRef]
- Jaafar, R.M.; Chettri, J.K.; Dalsgaard, I.; Al-Jubury, A.; Kania, P.W.; Skov, J.; Buchmann, K. Effects of Adjuvant MontanideTM ISA 763 A VG in Rainbow Trout Injection Vaccinated against Yersinia Ruckeri. Fish Shellfish Immunol. 2015, 47, 797–806. [Google Scholar] [CrossRef]
- Wangkahart, E.; Thongsrisuk, A.; Vialle, R.; Pholchamat, S.; Sunthamala, P.; Phudkliang, J.; Srisapoome, P.; Wang, T.; Secombes, C.J. Comparative Study of the Effects of MontanideTM ISA 763A VG and ISA 763B VG Adjuvants on the Immune Response against Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 134, 108563. [Google Scholar] [CrossRef]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune Response and Protective Efficacy of Two New Adjuvants, MontanideTM ISA 763B VG and MontanideTM GEL02, Administered with a Streptococcus agalactiae Ghost Vaccine in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 116, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, K.A.; Wang, T.; Russell, K.S.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. MontanideTM ISA 763A VG and ISA 761 VG Induce Different Immune Pathway Responses in Rainbow Trout (Oncorhynchus mykiss) When Used as Adjuvant for an Aeromonas salmonicida Bacterin. Fish Shellfish Immunol. 2021, 114, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Thim, H.L.; Villoing, S.; McLoughlin, M.; Christie, K.E.; Grove, S.; Frost, P.; Jørgensen, J.B. Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines 2014, 2, 228–251. [Google Scholar] [CrossRef]
- Huang, M.; Wang, W. Factors Affecting Alum–Protein Interactions. Int. J. Pharm. 2014, 466, 139–146. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H. Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Front. Immunol. 2013, 3, 406. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in Aluminum Hydroxide-Based Adjuvant Research and Its Mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the Utilization of Aluminum Adjuvants in Vaccines: You Might Just Get What You Want. npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef]
- Angosto, D.; López-Muñoz, A.; García-Alcazar, A.; Meseguer, J.; Sepulcre, M.P.; Mulero, V. Aluminum Is a Powerful Adjuvant in Teleost Fish despite Failing to Induce Interleukin-1β Release. Dev. Comp. Immunol. 2018, 85, 18–24. [Google Scholar] [CrossRef]
- Compan, V.; Baroja-Mazo, A.; López-Castejón, G.; Gomez, A.I.; Martínez, C.M.; Angosto, D.; Montero, M.T.; Herranz, A.S.; Bazán, E.; Reimers, D.; et al. Cell Volume Regulation Modulates NLRP3 Inflammasome Activation. Immunity 2012, 37, 487–500. [Google Scholar] [CrossRef]
- Angosto, D.; López-Castejón, G.; López-Muñoz, A.; Sepulcre, M.P.; Arizcun, M.; Meseguer, J.; Mulero, V. Evolution of Inflammasome Functions in Vertebrates: Inflammasome and Caspase-1 Trigger Fish Macrophage Cell Death but Are Dispensable for the Processing of IL-1β. Innate Immun. 2012, 18, 815–824. [Google Scholar] [CrossRef]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of Interleukin-1β. Cytokine Growth Factor Rev. 2002, 13, 483–502. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Burchill, M.A.; Munks, M.W.; Jin, L.; Kappler, J.W.; Friedman, R.S.; Jacobelli, J.; Marrack, P. Host DNA Released in Response to Aluminum Adjuvant Enhances MHC Class II-Mediated Antigen Presentation and Prolongs CD4 T-Cell Interactions with Dendritic Cells. Proc. Natl. Acad. Sci. USA 2013, 110, E1122–E1131. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA Released from Dying Host Cells Mediates Aluminum Adjuvant Activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
- Kool, M.; Pétrilli, V.; De Smedt, T.; Rolaz, A.; Hammad, H.; van Nimwegen, M.; Bergen, I.M.; Castillo, R.; Lambrecht, B.N.; Tschopp, J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol. 2008, 181, 3755–3759. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Villegas, J.; García-Alcazar, A.; Meseguer, J.; Mulero, V. Aluminum Adjuvant Potentiates Gilthead Seabream Immune Responses but Induces Toxicity in Splenic Melanomacrophage Centers. Fish Shellfish Immunol. 2019, 85, 31–43. [Google Scholar] [CrossRef]
- Guo, M.; Li, C. An Overview of Cytokine Used as Adjuvants in Fish: Current State and Future Trends. Rev. Aquac. 2021, 13, 996–1014. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current Use and Development of Fish Vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, E.; Vimal, S.; Sathishkumar, R.; Ravi, M.; Karthick, V.; Ramya, S.; Thomas, J.; Kumar, V.; Kamaraj, C.; Citarasu, T. DNA Vaccine Incorporated Poly (Lactic-Co-Glycolic) Acid (PLGA) Microspheres Offer Enhanced Protection against Aeromonas hydrophila Infection. Int. J. Biol. Macromol. 2023, 253, 127182. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. PLGA Encapsulated Inactivated-Viral Vaccine: Formulation and Evaluation of Its Protective Efficacy against Viral Haemorrhagic septicaemia Virus (VHSV) Infection in Olive Flounder (Paralichthys olivaceus) Vaccinated by Mucosal Delivery Routes. Vaccine 2019, 37, 973–983. [Google Scholar] [CrossRef]
- Jiang, C.; Huo, X.; Tang, L.; Hu, M.; Yang, C.; Luo, D.; Su, J. Oral PLGA-Based DNA Vaccines Using Interferons as Adjuvants Remarkably Promote the Immune Protection of Grass Carp (Ctenopharyngodon idella) against GCRV Infection. Water Biol. Secur. 2023, 2, 100143. [Google Scholar] [CrossRef]
- Yogeshwari, G. Poly D, L-Lactide-Co-Glycolic Acid (PLGA)-Encapsulated CpG-Oligonucleotide (ODN) on Immune Response in Cyprinus carpio against Aeromonas hydrophila. J. Aquac. Res. Dev. 2015, 6, 327. [Google Scholar] [CrossRef]
- Diao, J.; Ye, H.; Yu, X.; Fan, Y.; Xu, L.; Li, T.; Wang, Y. Adjuvant and Immunostimulatory Effects of LPS and β-Glucan on Immune Response in Japanese Flounder, Paralichthys olivaceus. Vet. Immunol. Immunopathol. 2013, 156, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wangkahart, E.; Secombes, C.J.; Wang, T. Studies on the Use of Flagellin as an Immunostimulant and Vaccine Adjuvant in Fish Aquaculture. Front. Immunol. 2019, 9, 3054. [Google Scholar] [CrossRef]
- Sahoo, L.; Parhi, J.; Debnath, C.; Prasad, K.P. Effect of Feeding Lipopolysaccharide as an Immunostimulant on Immune Response and Immune Gene Expression of Labeo bata. Vet. Immunol. Immunopathol. 2017, 188, 48–58. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Salinas, I.; Tafalla, C.; Dalmo, R.A. Editorial: Vaccines and Immunostimulants for Finfish. Front. Immunol. 2020, 11, 573771. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The Response of Fish to Immunostimulant Diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Chi, C.; Jun, J.W.; Park, S.C. Use of Bacterial Subcellular Components as Immunostimulants in Fish Aquaculture. Rev. Aquac. 2018, 10, 474–492. [Google Scholar] [CrossRef]
- Hoel, K.; Lillehaug, A. Adjuvant Activity of Polar Glycopeptidolipids from Mycobacterium Chelonaein Experimental Vaccines against Aeromonas salmonicida in Salmonid Fish. Fish Shellfish Immunol. 1997, 7, 365–376. [Google Scholar] [CrossRef]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef]
- Vinay, T.-N.; Park, C.-S.; Kim, H.-Y.; Jung, S.-J. Toxicity and Dose Determination of Quillaja Saponin, Aluminum Hydroxide and Squalene in Olive Flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2014, 158, 73–85. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Huang, J.; Li, J. Adjuvant Effect of Quillaja Saponaria Saponin (QSS) on Protective Efficacy and IgM Generation in Turbot (Scophthalmus maximus) upon Immersion Vaccination. Int. J. Mol. Sci. 2016, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H.; Jung, S.-J.; Kim, T. Saponin and Chitosan-Based Oral Vaccine against Viral Haemorrhagic septicaemia Virus (VHSV) Provides Protective Immunity in Olive Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2022, 126, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-H.; Kole, S.; Jung, S.-J. Efficacy of Saponin-Based Inactivated Rock Bream Iridovirus (RBIV) Vaccine in Rock Bream (Oplegnathus fasciatus). Fish Shellfish Immunol. 2022, 121, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, J.; Zhang, Y.; Liu, Q.; Wang, Q.; Liu, X. A Compound Ginseng Stem Leaf Saponins and Aluminium Adjuvant Enhances the Potency of Inactivated Aeromonas salmonicida Vaccine in Turbot. Fish Shellfish Immunol. 2022, 128, 60–66. [Google Scholar] [CrossRef]
- Sun, F.; Wu, Y.; Zhang, Y.; Liu, Q.; Wang, Q.; Liu, X. An Aluminium Adjuvant Compound with Ginseng Stem Leaf Saponins Enhances the Potency of Inactivated Pseudomonas plecoglossicida Vaccine in Large Yellow Croaker (Larimichthys crocea). Fish Shellfish Immunol. 2024, 144, 109243. [Google Scholar] [CrossRef]
- Soltani, M.; Lymbery, A.; Song, S.K.; Hosseini Shekarabi, P. Adjuvant Effects of Medicinal Herbs and Probiotics for Fish Vaccines. Rev. Aquac. 2019, 11, 1325–1341. [Google Scholar] [CrossRef]
- Beck, B.R.; Lee, S.H.; Kim, D.; Park, J.H.; Lee, H.K.; Kwon, S.-S.; Lee, K.H.; Lee, J.I.; Song, S.K. A Lactococcus lactis BFE920 Feed Vaccine Expressing a Fusion Protein Composed of the OmpA and FlgD Antigens from Edwardsiella tarda Was Significantly Better at Protecting Olive Flounder (Paralichthys olivaceus) from Edwardsiellosis than Single Antigen Vaccines. Fish Shellfish Immunol. 2017, 68, 19–28. [Google Scholar] [CrossRef]
- Lee, S.H.; Beck, B.R.; Hwang, S.-H.; Song, S.K. Feeding Olive Flounder (Paralichthys olivaceus) with Lactococcus lactis BFE920 Expressing the Fusion Antigen of Vibrio OmpK and FlaB Provides Protection against Multiple Vibrio Pathogens: A Universal Vaccine Effect. Fish Shellfish Immunol. 2021, 114, 253–262. [Google Scholar] [CrossRef]
- Kim, D.; Beck, B.R.; Lee, S.M.; Jeon, J.; Lee, D.W.; Lee, J.I.; Song, S.K. Pellet Feed Adsorbed with the Recombinant Lactococcus lactis BFE920 Expressing SiMA Antigen Induced Strong Recall Vaccine Effects against Streptococcus iniae Infection in Olive Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2016, 55, 374–383. [Google Scholar] [CrossRef]
- Anuradha, K.; Foo, H.L.; Mariana, N.S.; Loh, T.C.; Yusoff, K.; Hassan, M.D.; Sasan, H.; Raha, A.R. Live Recombinant Lactococcus lactis Vaccine Expressing Aerolysin Genes D1 and D4 for Protection against Aeromonas hydrophila in Tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2010, 109, 1632–1642. [Google Scholar] [CrossRef]
- Naderi-Samani, M.; Soltani, M.; Dadar, M.; Taheri-Mirghaed, A.; Zargar, A.; Ahmadivand, S.; Hassanzadeh, R.; Goudarzi, L.M. Oral Immunization of Trout Fry with Recombinant Lactococcus lactis NZ3900 Expressing G Gene of Viral Hemorrhagic Septicaemia Virus (VHSV). Fish Shellfish Immunol. 2020, 105, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Docando, F.; Nuñez-Ortiz, N.; Gonçalves, G.; Serra, C.R.; Gomez-Casado, E.; Martín, D.; Abós, B.; Oliva-Teles, A.; Tafalla, C.; Díaz-Rosales, P. Bacillus subtilis Expressing the Infectious Pancreatic Necrosis Virus VP2 Protein Retains Its Immunostimulatory Properties and Induces a Specific Antibody Response. Front. Immunol. 2022, 13, 888311. [Google Scholar] [CrossRef]
- Chen, D.-D.; Yao, Y.-Y.; Cui, Z.-W.; Zhang, X.-Y.; Guo, X.; Zhou, Y.-Y.; Zhang, Y.-A. Comparative Study of the Immunoprotective Effect of Two Grass Carp-Sourced Bacillus subtilis Spore-Based Vaccines against Grass Carp Reovirus. Aquaculture 2019, 504, 88–95. [Google Scholar] [CrossRef]
- Gonçalves, G.; Santos, R.A.; Coutinho, F.; Pedrosa, N.; Curado, M.; Machado, M.; Costas, B.; Bonneville, L.; Serrano, M.; Carvalho, A.P.; et al. Oral Vaccination of Fish against Vibriosis Using Spore-Display Technology. Front. Immunol. 2022, 13, 1012301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, X.; Ning, Y.; Liu, S.; Liang, Z.; Zhang, Z.; Chen, Y.; Cao, J.; Wang, F.; Lan, L.; et al. Surface Display of Major Capsid Protein on Bacillus subtilis Spores against Largemouth Bass Virus (LMBV) for Oral Administration. Fish Shellfish Immunol. 2023, 135, 108627. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Wang, Y.; Niu, C.; Liu, Z.; Yao, X.; Jiang, X.; Pan, R.; Jia, S.; Li, D.; et al. Oral Probiotic Vaccine Expressing Koi Herpesvirus (KHV) ORF81 Protein Delivered by Chitosan-Alginate Capsules Is a Promising Strategy for Mass Oral Vaccination of Carps against KHV Infection. J. Virol. 2021, 95, e00415-21. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Ganesan, A.R.; Divya, D.; Johansen, J.; Zhang, S. Chitin, Chitosan and Chitooligosaccharides as Potential Growth Promoters and Immunostimulants in Aquaculture: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 251, 126285. [Google Scholar] [CrossRef]
- Collado-González, M.; Esteban, M.Á. Chitosan-Nanoparticles Effects on Mucosal Immunity: A Systematic Review. Fish Shellfish Immunol. 2022, 130, 1–8. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J.; Sun, X. Chitosan Microspheres as Candidate Plasmid Vaccine Carrier for Oral Immunisation of Japanese Flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2008, 126, 220–229. [Google Scholar] [CrossRef]
- León-Rodríguez, L.; Luzardo-Álvarez, A.; Blanco-Méndez, J.; Lamas, J.; Leiro, J. A Vaccine Based on Biodegradable Microspheres Induces Protective Immunity against Scuticociliatosis without Producing Side Effects in Turbot. Fish Shellfish Immunol. 2012, 33, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Sun, F.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. An Oral Vaccine Based on Chitosan/Aluminum Adjuvant Induces Both Local and Systemic Immune Responses in Turbot (Scophthalmus maximus). Vaccine 2021, 39, 7477–7484. [Google Scholar] [CrossRef]
- Xu, F.-F.; Jiang, F.-Y.; Zhou, G.-Q.; Xia, J.-Y.; Yang, F.; Zhu, B. The Recombinant Subunit Vaccine Encapsulated by Alginate-Chitosan Microsphere Enhances the Immune Effect against Micropterus salmoides Rhabdovirus. J. Fish Dis. 2022, 45, 1757–1765. [Google Scholar] [CrossRef]
- Wang, E.; Wang, X.; Wang, K.; He, J.; Zhu, L.; He, Y.; Chen, D.; Ouyang, P.; Geng, Y.; Huang, X.; et al. Preparation, Characterization and Evaluation of the Immune Effect of Alginate/Chitosan Composite Microspheres Encapsulating Recombinant Protein of Streptococcus iniae Designed for Fish Oral Vaccination. Fish Shellfish Immunol. 2018, 73, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Behera, T.; Swain, P. Antigen Encapsulated Alginate-Coated Chitosan Microspheres Stimulate Both Innate and Adaptive Immune Responses in Fish through Oral Immunization. Aquacult Int 2014, 22, 673–688. [Google Scholar] [CrossRef]
- Behera, T.; Swain, P. Alginate–Chitosan–PLGA Composite Microspheres Induce Both Innate and Adaptive Immune Response through Parenteral Immunization in Fish. Fish Shellfish Immunol. 2013, 35, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Dezfuly, Z.T.; Alishahi, M.; Ghorbanpoor, M.; Tabandeh, M.R.; Mesbah, M. Immunogenicity and Protective Efficacy of Yersinia Ruckeri Lipopolysaccharide (LPS), Encapsulated by Alginate-Chitosan Micro/Nanoparticles in Rainbow Trout (Oncorhyncus Mykiss). Fish Shellfish Immunol. 2020, 104, 25–35. [Google Scholar] [CrossRef] [PubMed]
- González-Chavarría, I.; Roa, F.J.; Sandoval, F.; Muñoz-Flores, C.; Kappes, T.; Acosta, J.; Bertinat, R.; Altamirano, C.; Valenzuela, A.; Sánchez, O.; et al. Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss. Int. J. Mol. Sci. 2023, 24, 14685. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and Future Perspectives of Vaccines for Industrialised Fin-Fish Farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef]
- Hoare, R.; Jung, S.-J.; Ngo, T.P.H.; Bartie, K.; Bailey, J.; Thompson, K.D.; Adams, A. Efficacy and Safety of a Non-Mineral Oil Adjuvanted Injectable Vaccine for the Protection of Atlantic Salmon (Salmo salar L.) against Flavobacterium psychrophilum. Fish Shellfish Immunol. 2019, 85, 44–51. [Google Scholar] [CrossRef]
- Poppe, T.T.; Koppang, E.O. Side-Effects of Vaccination. In Fish Vaccination; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 153–161. ISBN 978-1-118-80691-3. [Google Scholar]
- Mutoloki, S.; Cooper, G.A.; Marjara, I.S.; Koop, B.F.; Evensen, Ø. High Gene Expression of Inflammatory Markers and IL-17A Correlates with Severity of Injection Site Reactions of Atlantic Salmon Vaccinated with Oil-Adjuvanted Vaccines. BMC Genom. 2010, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Cheng, S.; Hu, Y.; Sun, L. Comparative Study of the Effects of Aluminum Adjuvants and Freund’s Incomplete Adjuvant on the Immune Response to an Edwardsiella tarda Major Antigen. Vaccine 2010, 28, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A Sequential Study of Incomplete Freund’s Adjuvant-Induced Peritonitis in Atlantic Cod. Fish Shellfish Immunol. 2012, 32, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Spinos, E.; Kokkoris, G.D.; Bakopoulos, V. Prevention of Sea Bass (Dicentrarchus labrax, L. 1758) Photobacteriosis and Vibriosis. Long Term Efficacy Study of Intraperitoneally Administered Bivalent Commercial Vaccines. Aquaculture 2017, 471, 172–184. [Google Scholar] [CrossRef]
- Veenstra, K.A.; Wang, T.; Alnabulsi, A.; Douglas, A.; Russell, K.S.; Tubbs, L.; Arous, J.B.; Secombes, C.J. Analysis of Adipose Tissue Immune Gene Expression after Vaccination of Rainbow Trout with Adjuvanted Bacterins Reveals an Association with Side Effects. Mol. Immunol. 2017, 88, 89–98. [Google Scholar] [CrossRef]
- Li, J.; Tang, L.; Li, S.; Li, G.; Mo, Z. The Efficacy and Side-Effects of Oil-Based Adjuvants Emulsified Vibrio anguillarum Bivalent Inactivated Vaccine in Turbot (Scophthalmus maximus) under Production Mode. Aquaculture 2020, 524, 735259. [Google Scholar] [CrossRef]
- Tziouvas, H.; Varvarigos, P. Intensity Scale of Side Effects in European Sea Bass (Dicentrarchus labrax) Post Intraperitoneal Injection with Commercial Oil-Adjuvanted Vaccines. Bull. EAFP 2021, 41, 103–110. [Google Scholar] [CrossRef]
- Midtlyng, P.J.; Reitan, L.J.; Speilberg, L. Experimental Studies on the Efficacy and Side-Effects of Intraperitoneal Vaccination of Atlantic Salmon (Salmo salar L.) against Furunculosis. Fish Shellfish Immunol. 1996, 6, 335–350. [Google Scholar] [CrossRef]
- Harshitha, M.; Nayak, A.; Disha, S.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; Karunasagar, I.; Maiti, B. Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines 2023, 11, 1555. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Grip, J. PLGA/PLA Micro- and Nanoparticle Formulations Serve as Antigen Depots and Induce Elevated Humoral Responses after Immunization of Atlantic Salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Sævareid, K.; McAuley, L.; Lane, M.E.; Bøgwald, J.; Dalmo, R.A. Early Immune Responses in Atlantic Salmon (Salmo salar L.) after Immunization with PLGA Nanoparticles Loaded with a Model Antigen and β-Glucan. Vaccine 2011, 29, 8338–8349. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.; St-Hilaire, S.; Zheng, Y.; Eley, J.; Marcum, R.D.; Sealey, W.; Donahower, B.C.; LaPatra, S.; Sheridan, P.P. Oral DNA Vaccination of Rainbow Trout, Oncorhynchus mykiss (Walbaum), against Infectious Haematopoietic Necrosis Virus Using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] Nanoparticles. J. Fish Dis. 2012, 35, 203–214. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J. Poly(Lactic-Co-Glycolic Acid) Nanoparticles as Candidate DNA Vaccine Carrier for Oral Immunization of Japanese Flounder (Paralichthys olivaceus) against Lymphocystis Disease Virus. Fish Shellfish Immunol. 2011, 30, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Paul, J.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; et al. Aeromonas hydrophila OmpW PLGA Nanoparticle Oral Vaccine Shows a Dose-Dependent Protective Immunity in Rohu (Labeo rohita). Vaccines 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Harshitha, M.; D’souza, R.; Akshay, S.D.; Nayak, A.; Disha, S.; Aditya, V.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; et al. Oral Administration of Recombinant Outer Membrane Protein A-Based Nanovaccine Affords Protection against Aeromonas hydrophila in Zebrafish. World J. Microbiol. Biotechnol. 2024, 40, 250. [Google Scholar] [CrossRef]
- Alishahi, M.; Vaseghi, M.; Tabandeh, M.R.; Khosravi, M. Immunogenic and Protective Effects of an Oral Polylactic-Co-Glycolic Acid Nano Encapsulated DNA Vaccine Encoding aopB Gene of Aeromonas hydrophila in Common Carp. Aquac. Int. 2024, 32, 1169–1190. [Google Scholar] [CrossRef]
- Alishahi, M.; Lababian, H.; Heidari, H.; Tabandeh, M.R.; Khosravi, M. Development of an Injectable DNA Vaccine Against Aeromonas hydrophila Infection Nanoencapsulated With Poly(Lactic-Co-Glycolic) Acid (PLGA) in Common Carp. Aquac. Res. 2024, 2024, 7270489. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, P.-Q.; Guo, S.; Zhao, Z.; Wang, G.-X.; Zhu, B. Dual-Targeting Polymer Nanoparticles Efficiently Deliver DNA Vaccine and Induce Robust Prophylactic Immunity against Spring Viremia of Carp Virus Infection. Microbiol. Spectr. 2022, 10, e03085-22. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent Progress in Biomedical Applications of Chitosan and Its Nanocomposites in Aquaculture: A Review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef]
- El-Naggar, M.; Medhat, F.; Taha, A. Applications of Chitosan and Chitosan Nanoparticles in Fish Aquaculture. Egypt. J. Aquat. Biol. Fish. 2022, 26, 23–43. [Google Scholar] [CrossRef]
- Kole, S.; Dar, S.A.; Shin, S.-M.; Jeong, H.-J.; Jung, S.-J. Potential Efficacy of Chitosan-Poly (Lactide-Co-Glycolide)-Encapsulated Trivalent Immersion Vaccine in Olive Flounder (Paralichthys olivaceus) Against Viral Hemorrhagic Septicemia Virus, Streptococcus Parauberis Serotype I, and Miamiensis avidus (Scuticociliate). Front. Immunol. 2021, 12, 761130. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Hassanzadeh, R.; Soltani, E. Oral DNA Vaccines Based on CS-TPP Nanoparticles and Alginate Microparticles Confer High Protection against Infectious Pancreatic Necrosis Virus (IPNV) Infection in Trout. Dev. Comp. Immunol. 2017, 74, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.M.; Kole, S.; Gireesh-Babu, P.; Sharma, R.; Tripathi, G.; Bedekar, M.K. Evaluation of Persistence, Bio-Distribution and Environmental Transmission of Chitosan/PLGA/pDNA Vaccine Complex against Edwardsiella tarda in Labeo rohita. Aquaculture 2019, 500, 385–392. [Google Scholar] [CrossRef]
- Halimi, M.; Alishahi, M.; Abbaspour, M.R.; Ghorbanpoor, M.; Tabandeh, M.R. Valuable Method for Production of Oral Vaccine by Using Alginate and Chitosan against Lactococcus garvieae/Streptococcus iniae in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 90, 431–439. [Google Scholar] [CrossRef]
- Huo, X.; Tang, L.; Liu, Q.; Zhu, W.; Zhang, J.; Hu, M.; Zhao, F.; Wang, P.; Yuan, G.; Yang, C.; et al. Oral pcDNA3.1-VP4/VP56-FlaC DNA Vaccine Encapsulated by Chitosan/Sodium Alginate Nanoparticles Confers Remarkable Protection against GCRV Infection in Grass Carp. Aquaculture 2023, 577, 739996. [Google Scholar] [CrossRef]
- Leya, T.; Ahmad, I.; Sharma, R.; Tripathi, G.; Kurcheti, P.P.; Rajendran, K.V.; Bedekar, M.K. Bicistronic DNA Vaccine Macromolecule Complexed with Poly Lactic-Co-Glycolic Acid-Chitosan Nanoparticles Enhanced the Mucosal Immunity of Labeo rohita against Edwardsiella tarda Infection. Int. J. Biol. Macromol. 2020, 156, 928–937. [Google Scholar] [CrossRef]
- Li, L.; Lin, S.-L.; Deng, L.; Liu, Z.-G. Potential Use of Chitosan Nanoparticles for Oral Delivery of DNA Vaccine in Black Seabream Acanthopagrus schlegelii Bleeker to Protect from Vibrio parahaemolyticus. J. Fish Dis. 2013, 36, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Kumar, S.; Ishaq Ahmed, V.P.; Parameswaran, V.; Sudhakaran, R.; Sarath Babu, V.; Sahul Hameed, A.S. Potential Use of Chitosan Nanoparticles for Oral Delivery of DNA Vaccine in Asian Sea Bass (Lates calcarifer) to Protect from Vibrio (Listonella) Anguillarum. Fish Shellfish Immunol. 2008, 25, 47–56. [Google Scholar] [CrossRef]
- Sukkarun, P.; Kitiyodom, S.; Yostawornkul, J.; Chaiin, P.; Yata, T.; Rodkhum, C.; Boonrungsiman, S.; Pirarat, N. Chitosan-Polymer Based Nanovaccine as Promising Immersion Vaccine against Aeromonas veronii Challenge in Red Tilapia (Oreochromis sp.). Fish Shellfish Immunol. 2022, 129, 30–35. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Yata, T.; Yostawornkul, J.; Kaewmalun, S.; Nittayasut, N.; Suktham, K.; Surassmo, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Enhanced Efficacy of Immersion Vaccination in Tilapia against Columnaris Disease by Chitosan-Coated “Pathogen-like” Mucoadhesive Nanovaccines. Fish Shellfish Immunol. 2019, 95, 213–219. [Google Scholar] [CrossRef]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; Karunasagar, I.; et al. Edwardsiella tarda OmpA Encapsulated in Chitosan Nanoparticles Shows Superior Protection over Inactivated Whole Cell Vaccine in Orally Vaccinated Fringed-Lipped Peninsula Carp (Labeo fimbriatus). Vaccines 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, E.; Lelin, C.; Sathishkumar, R.; Vimal, S.; Anand, S.B.; Babu, M.M.; Citarasu, T. Oral Delivery of pVAX-OMP and pVAX-Hly DNA Vaccine Using Chitosan-Tripolyphosphate (Cs-TPP) Nanoparticles in Rohu, (Labeo rohita) for Protection against Aeromonas hydrophila Infection. Fish Shellfish Immunol. 2021, 115, 189–197. [Google Scholar] [CrossRef]
- Kole, S.; Kumari, R.; Anand, D.; Kumar, S.; Sharma, R.; Tripathi, G.; Makesh, M.; Rajendran, K.V.; Bedekar, M.K. Nanoconjugation of Bicistronic DNA Vaccine against Edwardsiella tarda Using Chitosan Nanoparticles: Evaluation of Its Protective Efficacy and Immune Modulatory Effects in Labeo rohita Vaccinated by Different Delivery Routes. Vaccine 2018, 36, 2155–2165. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.; Wang, G.; Ling, F. Mannosylated Chitosan Nanoparticles Loaded with ABP Antigen Server as a Novel Nucleic Acid Vaccine against Nocardia Seriolae Infection in Micropterus salmoides. Aquaculture 2023, 574, 739635. [Google Scholar] [CrossRef]
- Ponce, M.; Zuasti, E.; Anguís, V.; Fernández-Díaz, C. Anti-Bacterial and Immunostimulatory Properties of Ulvan-Loaded Chitosan Nanoparticles for Use in Aquaculture. Mar. Biotechnol. 2024, 26, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sukkarun, P.; Kitiyodom, S.; Kamble, M.T.; Bunnoy, A.; Boonanuntanasarn, S.; Yata, T.; Boonrungsiman, S.; Thompson, K.D.; Rodkhum, C.; Pirarat, N. Systemic and Mucosal Immune Responses in Red Tilapia (Oreochromis sp.) Following Immersion Vaccination with a Chitosan Polymer-Based Nanovaccine against Aeromonas veronii. Fish Shellfish Immunol. 2024, 146, 109383. [Google Scholar] [CrossRef]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the Mucosal Immune Response of Red Tilapia (Oreochromis sp.) against Columnaris Disease Using a Biomimetic-Mucoadhesive Nanovaccine. Fish Shellfish Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kitiyodom, S.; Yata, T.; Thompson, K.D.; Costa, J.; Elumalai, P.; Katagiri, T.; Temisak, S.; Namdee, K.; Rodkhum, C.; Pirarat, N. Immersion Vaccination by a Biomimetic-Mucoadhesive Nanovaccine Induces Humoral Immune Response of Red Tilapia (Oreochromis sp.) against Flavobacterium Columnare Challenge. Vaccines 2021, 9, 1253. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Z.; Zhou, J.; Wang, W.; Su, J.; Yuan, G. Carboxymethyl Chitosan Nanoparticles Loaded with Ctenopharyngodon idella Interferon-Γ2 (CiIFN-Γ2) Enhance Protective Efficacy against Bacterial Infection in Grass Carp. Aquaculture 2023, 572, 739554. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Kitiyodom, S.; Yata, T.; Jantharadej, K.; Adamek, M.; Surachetpong, W. Chitosan Nanoparticle Immersion Vaccine Offers Protection against Tilapia Lake Virus in Laboratory and Field Studies. Fish Shellfish Immunol. 2022, 131, 972–979. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, H.; Sun, X.; Zhang, Y.; Zhang, B.; Teng, Z.; Hou, Y.; Wang, B. Development of Oral DNA Vaccine Based on Chitosan Nanoparticles for the Immunization against Reddish Body Iridovirus in Turbots (Scophthalmus maximus). Aquaculture 2016, 452, 263–271. [Google Scholar] [CrossRef]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. Nanoencapsulation of Inactivated-Viral Vaccine Using Chitosan Nanoparticles: Evaluation of Its Protective Efficacy and Immune Modulatory Effects in Olive Flounder (Paralichthys olivaceus) against Viral Haemorrhagic septicaemia Virus (VHSV) Infection. Fish Shellfish Immunol. 2019, 91, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.; Abdul Majeed, S.; Nambi, K.S.N.; Madan, N.; Farook, M.A.; Venkatesan, C.; Taju, G.; Venu, S.; Subburaj, R.; Thirunavukkarasu, A.R.; et al. Delivery of DNA Vaccine Using Chitosan–Tripolyphosphate (CS/TPP) Nanoparticles in Asian Sea Bass, Lates calcarifer (Bloch, 1790) for Protection against Nodavirus Infection. Aquaculture 2014, 420–421, 240–246. [Google Scholar] [CrossRef]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.Á.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An Oral Chitosan DNA Vaccine against Nodavirus Improves Transcription of Cell-Mediated Cytotoxicity and Interferon Genes in the European Sea Bass Juveniles Gut and Survival upon Infection. Dev. Comp. Immunol. 2016, 65, 64–72. [Google Scholar] [CrossRef]
- Thu Lan, N.G.; Dong, H.T.; Vinh, N.T.; Salin, K.R.; Senapin, S.; Pimsannil, K.; St-Hilaire, S.; Shinn, A.P.; Rodkhum, C. A Novel Vaccination Strategy against Vibrio harveyi Infection in Asian Seabass (Lates calcarifer) with the Aid of Oxygen Nanobubbles and Chitosan. Fish Shellfish Immunol. 2024, 149, 109557. [Google Scholar] [CrossRef]
- Andresen, A.M.S.; Gjøen, T. Chitosan Nanoparticle Formulation Attenuates Poly (I:C) Induced Innate Immune Responses against Inactivated Virus Vaccine in Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100915. [Google Scholar] [CrossRef]
- Ji, J.; Merino, S.; Tomás, J.M.; Roher, N. Nanoliposomes Encapsulating Immunostimulants Modulate the Innate Immune System and Elicit Protection in Zebrafish Larvae. Fish Shellfish Immunol. 2019, 92, 421–429. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Kitiyodom, S.; Srisapoome, P.; Rodkhum, C. Mucoadhesive Cationic Lipid-Based Flavobacterium oreochromis Nanoencapsulation Enhanced the Efficacy of Mucoadhesive Immersion Vaccination against Columnaris Disease and Strengthened Immunity in Asian Sea Bass (Lates calcarifer). Fish Shellfish Immunol. 2022, 127, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Thangsunan, P.; Kitiyodom, S.; Srisapoome, P.; Pirarat, N.; Yata, T.; Thangsunan, P.; Boonrungsiman, S.; Bunnoy, A.; Rodkhum, C. Novel Development of Cationic Surfactant-Based Mucoadhesive Nanovaccine for Direct Immersion Vaccination against Francisella Noatunensis Subsp. Orientalis in Red Tilapia (Oreochromis sp.). Fish Shellfish Immunol. 2022, 127, 1051–1060. [Google Scholar] [CrossRef]
- Ruyra, A.; Cano-Sarabia, M.; MacKenzie, S.A.; Maspoch, D.; Roher, N. A Novel Liposome-Based Nanocarrier Loaded with an LPS-dsRNA Cocktail for Fish Innate Immune System Stimulation. PLoS ONE 2013, 8, e76338. [Google Scholar] [CrossRef]
- Ruyra, A.; Cano-Sarabia, M.; García-Valtanen, P.; Yero, D.; Gibert, I.; Mackenzie, S.A.; Estepa, A.; Maspoch, D.; Roher, N. Targeting and Stimulation of the Zebrafish (Danio rerio) Innate Immune System with LPS/dsRNA-Loaded Nanoliposomes. Vaccine 2014, 32, 3955–3962. [Google Scholar] [CrossRef] [PubMed]
- Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA–Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines 2024, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Kwon, J.; Lee, S.B.; Jung, W.J.; Park, S.C. Applications of Carbon Nanotubes and Polymeric Micro-/Nanoparticles in Fish Vaccine Delivery: Progress and Future Perspectives. Rev. Aquac. 2021, 13, 1844–1863. [Google Scholar] [CrossRef]
- Giri, S.S.; Park, S.C. Application of Carbon Nanotubes in the Advancement of Fish Vaccine. In Biotechnological Advances in Aquaculture Health Management; Gupta, S.K., Giri, S.S., Eds.; Springer Nature: Singapore, 2021; pp. 61–78. ISBN 9789811651953. [Google Scholar]
- Cimbaluk, G.V.; Ramsdorf, W.A.; Perussolo, M.C.; Santos, H.K.F.; Da Silva De Assis, H.C.; Schnitzler, M.C.; Schnitzler, D.C.; Carneiro, P.G.; Cestari, M.M. Evaluation of Multiwalled Carbon Nanotubes Toxicity in Two Fish Species. Ecotoxicol. Environ. Saf. 2018, 150, 215–223. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of Functionalized Multiwalled Carbon Nanotubes and Comparison of Their Cellular Toxicity between HEK 293 Cells and Zebra Fish in Vivo. Heliyon 2019, 5, e02605. [Google Scholar] [CrossRef]
- Su, Y.; Yan, X.; Pu, Y.; Xiao, F.; Wang, D.; Yang, M. Risks of Single-Walled Carbon Nanotubes Acting as Contaminants-Carriers: Potential Release of Phenanthrene in Japanese Medaka (Oryzias latipes). Environ. Sci. Technol. 2013, 47, 4704–4710. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, X.; Gao, S.; Huang, Y.; Xiong, J.; Ren, H. Toxicity of Amine-Functionalized Single-Carbon Nanotube (NH2 f-SWCNT) to Channel Catfish (Ietalurus punetaus): Organ Pathologies, Oxidative Stress, Inflammation, and Apoptosis. Chemosphere 2021, 282, 131133. [Google Scholar] [CrossRef]
- Smith, C.J.; Shaw, B.J.; Handy, R.D. Toxicity of Single Walled Carbon Nanotubes to Rainbow Trout, (Oncorhynchus mykiss): Respiratory Toxicity, Organ Pathologies, and Other Physiological Effects. Aquat. Toxicol. 2007, 82, 94–109. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Ling, F.; Song, L.-S.; Wang, G.-X. Development Toxicity of Functionalized Single-Walled Carbon Nanotubes on Rare Minnow Embryos and Larvae. Nanotoxicology 2015, 9, 579–590. [Google Scholar] [CrossRef]
- Li, Y.; Men, B.; He, Y.; Xu, H.; Liu, M.; Wang, D. Effect of Single-Wall Carbon Nanotubes on Bioconcentration and Toxicity of Perfluorooctane Sulfonate in Zebrafish (Danio rerio). Sci. Total Environ. 2017, 607–608, 509–518. [Google Scholar] [CrossRef]
- Bisesi, J.H.; Ngo, T.; Ponnavolu, S.; Liu, K.; Lavelle, C.M.; Afrooz, A.R.M.N.; Saleh, N.B.; Ferguson, P.L.; Denslow, N.D.; Sabo-Attwood, T. Examination of Single-Walled Carbon Nanotubes Uptake and Toxicity from Dietary Exposure: Tracking Movement and Impacts in the Gastrointestinal System. Nanomaterials 2015, 5, 1066–1086. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.K.; Chung, Y.S.; Johari, S.A.; Kim, T.G.; Kim, J.K.; Lee, J.H.; Lee, Y.H.; Kang, S.W.; Yu, I.J. Acute Toxicity Comparison of Single-Walled Carbon Nanotubes in Various Freshwater Organisms. BioMed Res. Int. 2015, 2015, 323090. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Gou, N.; Lin, Y.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Toxicity of Single-Walled Carbon Nanotubes (SWCNTs): Effect of Lengths, Functional Groups and Electronic Structures Revealed by a Quantitative Toxicogenomics Assay. Environ. Sci. Nano 2020, 7, 1348–1364. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, K.S.; Bhat, I.A.; Chanu, T.I.; Kumar, P.; Pathakota, G.-B.; Nayak, S.K.; Walke, P.; Sharma, R. Chitosan Grafting onto Single-Walled Carbon Nanotubes Increased Their Stability and Reduced the Toxicity in Vivo (Catfish) Model. Int. J. Biol. Macromol. 2020, 155, 697–707. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; González-Ortega, O. Carbon Nanotubes-Based Mucosal Vaccines. In Nanovaccines: An Innovative Technology to Fight Human and Animal Diseases; Rosales-Mendoza, S., González-Ortega, O., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 159–179. ISBN 978-3-030-31668-6. [Google Scholar]
- Gong, Y.-X.; Zhu, B.; Liu, G.-L.; Liu, L.; Ling, F.; Wang, G.-X.; Xu, X.-G. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effects of a Recombinant Vaccine against Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 42, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gong, Y.-X.; Liu, G.-L.; Zhu, B.; Wang, G.-X. Protective Immunity of Grass Carp Immunized with DNA Vaccine against Aeromonas hydrophila by Using Carbon Nanotubes as a Carrier Molecule. Fish Shellfish Immunol. 2016, 55, 516–522. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Ma, R.; Qi, X.; Wang, G.; Zhu, B.; Ling, F. The Immunoprotective Effect of Whole-Cell Lysed Inactivated Vaccine with SWCNT as a Carrier against Aeromonas hydrophila Infection in Grass Carp. Fish Shellfish Immunol. 2020, 97, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, J.; Liu, G.; Du, H.; Liu, T.; Liu, T.; Li, P.; Yu, Q.; Wang, G.; Wang, E. A Nanocarrier Immersion Vaccine Encoding Surface Immunogenic Protein Confers Cross-Immunoprotection against Streptococcus agalactiae and Streptococcus iniae Infection in Tilapia. Fish Shellfish Immunol. 2024, 144, 109267. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, C.; Zhao, Z.; Wang, G.-X. Targeted Delivery of Mannosylated Nanoparticles Improve Prophylactic Efficacy of Immersion Vaccine against Fish Viral Disease. Vaccines 2020, 8, 87. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Gong, Y.-X.; Ling, F.; Song, L.-S.; Wang, G.-X. Single-Walled Carbon Nanotubes as Candidate Recombinant Subunit Vaccine Carrier for Immunization of Grass Carp against Grass Carp Reovirus. Fish Shellfish Immunol. 2014, 41, 279–293. [Google Scholar] [CrossRef]
- Qiu, D.-K.; Jia, Y.-J.; Gong, Y.-M.; Zheng, Y.-Y.; Wang, G.-X.; Zhu, B. Optimizing the Immunization Procedure of Single-Walled Carbon Nanotubes Based Vaccine against Grass Carp Reovirus for Grass Carp. Aquaculture 2021, 533, 736152. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.-L.; Li, D.-L.; Ling, F.; Zhu, B.; Wang, G.-X. The Protective Immunity against Grass Carp Reovirus in Grass Carp Induced by a DNA Vaccination Using Single-Walled Carbon Nanotubes as Delivery Vehicles. Fish Shellfish Immunol. 2015, 47, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, G.-L.; Gong, Y.-X.; Ling, F.; Wang, G.-X. Protective Immunity of Grass Carp Immunized with DNA Vaccine Encoding the Vp7 Gene of Grass Carp Reovirus Using Carbon Nanotubes as a Carrier Molecule. Fish Shellfish Immunol. 2015, 42, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Y.; Qiu, D.-K.; Guo, Z.-R.; Gong, Y.-M.; Wang, G.-X.; Zhu, B. Evaluation of SWCNTs-Loaded DNA Vaccine Encoding Predominant Antigen Epitope VP4-3 against Type II GCRV. Aquaculture 2021, 534, 736197. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Liu, G.-Y.; Li, J.; Wang, G.-X.; Zhu, B. Immune Response and Protective Effect against Spring Viremia of Carp Virus Induced by Intramuscular Vaccination with a SWCNTs-DNA Vaccine Encoding Matrix Protein. Fish Shellfish Immunol. 2018, 79, 256–264. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.-H.; Wang, J.; Zhao, Z.; Li, J.; Tu, X.; Huang, A.-G.; Wang, G.-X.; Zhu, B. Enhanced Protective Immunity against Spring Viremia of Carp Virus Infection Can Be Induced by Recombinant Subunit Vaccine Conjugated to Single-Walled Carbon Nanotubes. Vaccine 2018, 36, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Zha, J.-W.; Wang, G.-X.; Zhu, B. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effect of a DNA Vaccine against Spring Viremia of Carp Virus in Common Carp. Fish Shellfish Immunol. 2017, 71, 191–201. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.-Y.; Gong, Y.-M.; Zhao, Z.; Guo, Z.-R.; Jia, Y.-J.; Wang, G.-X.; Zhu, B. Evaluation of Immune Response and Protection against Spring Viremia of Carp Virus Induced by a Single-Walled Carbon Nanotubes-Based Immersion DNA Vaccine. Virology 2019, 537, 216–225. [Google Scholar] [CrossRef]
- Gong, Y.-M.; Zhang, C.; Li, Y.; Chen, G.; Wang, G.-X.; Zhu, B. Optimization of Immunization Procedure for SWCNTs-Based Subunit Vaccine with Mannose Modification against Spring Viraemia of Carp Virus in Common Carp. J. Fish Dis. 2021, 44, 1925–1936. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Lin, Q.; Li, N.-Q.; Huang, Z.-B.; Fu, X.-Z.; Wang, G.-X.; Zhu, B. Immersion Vaccination of Mandarin Fish Siniperca chuatsi against Infectious Spleen and Kidney Necrosis Virus with a SWCNTs-Based Subunit Vaccine. Fish Shellfish Immunol. 2019, 92, 133–140. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiong, Y.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Wang, G.-X.; Zhu, B. Optimization of the Efficacy of a SWCNTs-Based Subunit Vaccine against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish. Fish Shellfish Immunol. 2020, 106, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, C.; Lin, Q.; Li, N.; Huang, Z.-B.; Zhao, M.; Fu, X.-Z.; Wang, G.-X.; Zhu, B. Single-Walled Carbon Nanotubes as Delivery Vehicles Enhance the Immunoprotective Effect of an Immersion DNA Vaccine against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish. Fish Shellfish Immunol. 2020, 97, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Chen, G.; Zhang, C.; Wang, G.-X.; Zhu, B. Protective Immunity against Infectious Spleen and Kidney Necrosis Virus Induced by Mannose Modified Subunit Vaccine with Carbon Nanotubes in Mandarin Fish. Aquac. Res. 2022, 53, 2175–2184. [Google Scholar] [CrossRef]
- Zhao, Z.; Meng, Q.; Sun, T.-Z.; Zhu, B. Mannose Modified Targeted Immersion Vaccine Delivery System Improves Protective Immunity against Infectious Spleen and Kidney Necrosis Virus in Mandarin Fish (Siniperca chuatsi). Vaccine 2024, 42, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Wang, E.-L.; Qu, X.-Y.; Yang, K.-C.; Zhang, Z.-Y.; Liu, J.-Y.; Zhang, C.; Zhu, B.; Wang, G.-X. Single-Walled Carbon Nanotubes Enhance the Immune Protective Effect of a Bath Subunit Vaccine for Pearl Gentian Grouper against Iridovirus of Taiwan. Fish Shellfish Immunol. 2020, 106, 510–517. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Liu, J.; Zhu, B.; Wang, G.; Ling, F. Epitope Screening of the Major Capsid Protein within Grouper Iridovirus of Taiwan and the Immunoprotective Effect with SWCNTs as the Vaccine Carrier. Fish Shellfish Immunol. 2021, 117, 17–23. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Wang, G.; Zhu, B.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Carbon Nanotube-Based DNA Vaccine against Koi Herpesvirus given by Intramuscular Injection. Fish Shellfish Immunol. 2020, 98, 810–818. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wang, Q.; Zhu, B.; Wu, S.; Wang, Y.; Zeng, W.; Yin, J.; Liu, C.; Bergmann, S.M.; et al. Immersion Immunization of Koi (Cyprinus carpio) against Cyprinid Herpesvirus 3 (CyHV-3) with Carbon Nanotube-Loaded DNA Vaccine. Aquaculture 2021, 539, 736644. [Google Scholar] [CrossRef]
- Guo, Z.-R.; Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Zhu, B.; Wang, G.-X. Carbon Nanotubes-Loaded Subunit Vaccine Can Increase Protective Immunity against Rhabdovirus Infections of Largemouth Bass (Micropterus salmoides). Fish Shellfish Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Li, J.; Zhang, Z.-Y.; Liu, J.-Y.; Zhang, C.; Zhu, B.; Wang, G.-X. An Immersion Subunit Vaccine Loaded by Single-Walled Carbon Nanotube Protects Pearl Gentian Grouper from Viral Nervous Necrosis Virus. Aquaculture 2021, 541, 736813. [Google Scholar] [CrossRef]
- Jia, Y.-J.; Guo, Z.-R.; Ma, R.; Qiu, D.-K.; Zhao, Z.; Wang, G.-X.; Zhu, B. Immune Efficacy of Carbon Nanotubes Recombinant Subunit Vaccine against Largemouth Bass Ulcerative Syndrome Virus. Fish Shellfish Immunol. 2020, 100, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Tsigkou, K.; Zuorro, A.; Sun, Y.-Z.; Rabetafika, H.; Razafindralambo, H. Inorganic Nanoparticles for Use in Aquaculture. Rev. Aquac. 2023, 15, 1600–1617. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered Composite Based on Halloysite and Natural Polymers: A Carrier for the pH Controlled Release of Drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Pumchan, A.; Sae-Ueng, U.; Prasittichai, C.; Sirisuay, S.; Areechon, N.; Unajak, S. A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.). Vaccines 2022, 10, 1180. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Xiao, F.; Liu, X.; Xie, A.; Chen, F.; Dong, P.; Lin, P.; Zheng, C.; Zhang, H.; et al. pH-Controlled Release of Antigens Using Mesoporous Silica Nanoparticles Delivery System for Developing a Fish Oral Vaccine. Front. Immunol. 2021, 12, 644396. [Google Scholar] [CrossRef]
| Toll-like Receptors (TLRs) | ||
|---|---|---|
| Receptor | Associated Ligands | Indicative Teleost Fish Species |
| TLR1 | Lipopeptides | Rainbow Trout, Large Yellow Croaker, Carp, Pufferfish, Orange-Spotted Grouper, European Sea Bass, Turbot |
| TLR2 | Lipopeptides, PGN, LTA, Pam3CSK4 | Carp, Catfish, Orange-Spotted Grouper, European Sea Bass, Turbot, Gilthead Seabream |
| TLR3 | dsRNA, poly(I:C) | Carp, Pufferfish, Zebrafish |
| TLR4 | Unknown | Carp, Catfish, Rare Minnow, Zebrafish |
| TLR5 | Flagellin | Atlantic Salmon, Japanese Flounder, Channel Catfish, Gilthead Seabream, Rainbow Trout, Pufferfish, Zebrafish, Turbot |
| TLR7 | ssRNA | Channel Catfish, Grass Carp, Pufferfish, Rainbow Trout, Zebrafish, Turbot |
| TLR8 | ssRNA | Atlantic Salmon, Channel Catfish, Pufferfish, Rainbow Trout, Turbot |
| TLR9 | CpG motifs | Atlantic Salmon, Cobia, Japanese Flounder, Rainbow Trout, Zebrafish, European Seabass, Gilthead Seabream, Turbot |
| TLR13 | rRNA | Atlantic Salmon, Channel Catfish, Orange-Spotted Grouper |
| TLR14 | Unknown | Japanese Flounder, Orange-Spotted Grouper, Pufferfish |
| TLR18 | Unknown | Channel Catfish, Grass Carp, Zebrafish |
| TLR19 | dsRNA | Channel Catfish, Grass Carp, Zebrafish |
| TLR20 | Unknown | Carp, Channel Catfish, Zebrafish |
| TLR21 | CpG motifs | Channel Catfish, Grass Carp, Orange-Spotted Grouper, Zebrafish, Turbot |
| TLR22 | dsRNA, poly(I:C) | Atlantic Cod, Channel Catfish, Grass Carp, Pufferfish, Zebrafish, European Seabass, Turbot, Gilthead Seabream |
| TLR23 | Unknown | Atlantic Cod, Pufferfish |
| TLR25 | Unknown | Channel Catfish, Fathead Minnow, Nile Tilapia |
| TLR26 | LPS, poly(I:C) | Channel Catfish, Yellow Catfish |
| TLR28 | LPS, poly(I:C) | Brown Croaker |
| NOD-like Receptor (NLR) Superfamilies | ||
| NLR-A | LPS, PGN (iE-DAP, MDP), Poly (I:C) | Grass Carp, Nile Tilapia, Rainbow Trout, Channel Catfish, Zebrafish |
| NLR-B | Unknown | Grass Carp |
| NLR-C | Unknown | Grass Carp, Brown Croaker |
| RIG-1-like Receptors (RLRs) | ||
| RIG-1 | dsRNA | Zebrafish |
| MDA5 | dsRNA | Grass Carp |
| LGP2 | dsRNA | Grass Carp |
| Currently Available MontanideTM Adjuvants for Use in Aquaculture Vaccinology | ||
|---|---|---|
| Series Name | Technology | Route |
| MontanideTM ISA 61 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 50 V2 | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 70 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 71 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 71 R VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 761 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 78 VG | Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 763B VG | Non-Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM ISA 660 VG | Non-Mineral Oil base for W/O Emulsions | Injection |
| MontanideTM GR | Oil base for W/O Emulsions containing a Protective Matrix | Oral |
| MontanideTM IMS 1312 VG | Combination of Micro-Emulsions with an Immunostimulating Compound | Immersion |
| Score (0–6) | Spielberg Scale [126] (Atlantic Salmon) | Tziouvas and Varvarigos Scale [125] (European Sea Bass) |
|---|---|---|
| 0 |
|
|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
| Vaccine Technology | Delivery Route | Pathogen (Antigen) | Teleost Species | Efficacy | Source |
|---|---|---|---|---|---|
| Encapsulating Nanoliposomes | Immersion | Aeromonas hydrophila (E. coli LPS + Poly I:C) | Zebrafish (Danio rerio) | Increased survival rate post challenge and upregulation of immune-related genes | [165] |
| CTAB-Cationic Lipid Nanoemulsion | Immersion | Flavobacterium oreochromis (Formalin-Inactivated) | Asian Seabass (Lates calcalifer) | Increased survival rate post challenge and upregulation of immune-related genes | [166] |
| CTAB-Cationic Lipid Nanoemulsion | Immersion | Francisella noatunensis subsp. Orientalis (Formalin Inactivated) | Red Tilapia (Oreochromis sp.) | Reduced bacterial load in tissues, enhanced antibody titers, and upregulation of immune-related genes | [167] |
| Encapsulating Nanoliposomes | Injection and Immersion | Pseudomonas aeruginosa and SVCV (E. coli LPS + Poly I:C) | Zebrafish (Danio rerio) | Increased survival rates against both bacterial and viral challenges | [169] |
| CNT Type | Vaccine Technology | Delivery Route | Pathogen (Antigen) | Teleost Species | Source |
|---|---|---|---|---|---|
| Single-Walled | Recombinant Subunit | Intramuscular Injection and Bath Immersion | Aeromonas hydrophila (aerA) | Grass Carp | [185] |
| Single-Walled | DNA Vaccine | Intramuscular Injection | Aeromonas hydrophila (aerA) | Grass Carp | [186] |
| Single-Walled | Whole-cell Inactivated Vaccine | Intraperitoneal Injection and Bath Immersion | Aeromonas hydrophila (Bacterial Lysate) | Grass Carp | [187] |
| Single-Walled | Recombinant Subunit | Bath Immersion | Streptococcus sp. (rSip) | Tilapia | [188] |
| Single-Walled (Mannose Modified) | Recombinant Subunit | Bath Immersion | GCRV (VP7) | Grass Carp | [189] |
| Single-Walled | Recombinant Subunit | Bath Immersion | GCRV (VP7) | Grass Carp | [190] |
| Single-Walled | Recombinant Subunit | Bath Immersion | GCRV (VP4-3) | Grass Carp | [191] |
| Single-Walled | DNA Vaccine | Intramuscular Injection and Bath Immersion | GCRV (VP4-3, VP5, VP7) | Grass Carp | [192,193,194] |
| Single-Walled | DNA Vaccine | Intramuscular Injection | SVCV (M) | Common Carp | [195] |
| Single-Walled | Recombinant Subunit | Intramuscular Injection and Bath Immersion | SVCV (G) | Common Carp | [196] |
| Single-Walled | DNA Vaccine | Intramuscular Injection and Bath Immersion | SVCV (G) | Common Carp | [197] |
| Single-Walled | DNA Vaccine | Bath Immersion | SVCV (M) | Common Carp | [198] |
| Single-Walled (Mannose Modified) | Recombinant Subunit | Bath Immersion | SVCV (G) | Common Carp | [199] |
| Single-Walled | Recombinant Subunit | Bath Immersion | ISKNV (MCP) | Mandarin Fish | [200,201] |
| Single-Walled | DNA Vaccine | Bath Immersion | ISKNV (MCP) | Mandarin Fish | [202] |
| Single-Walled (Mannose Modified) | Recombinant Subunit | Intramuscular Injection and Bath Immersion | ISKNV (MCP) | Mandarin Fish | [203,204] |
| Single-Walled | Recombinant Subunit | Bath Immersion | TGIV (MCP, P2) | Pearl Gentian Grouper | [205,206] |
| Single-Walled | DNA Vaccine | Intramuscular Injection and Bath Immersion | KHV (ORF 149) | Koi Fish | [207,208] |
| Single-Walled | Recombinant Subunit | Bath Immersion | MSRV (G) | Largemouth Bass | [209] |
| Single-Walled | Recombinant Subunit | Bath Immersion | NNV (MCP) | Pearl Gentian Grouper | [210] |
| Single-Walled | Recombinant Subunit | Bath Immersion | LBUSV (MCP) | Largemouth Bass | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tammas, I.; Bitchava, K.; Gelasakis, A.I. Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations. Vaccines 2024, 12, 1347. https://doi.org/10.3390/vaccines12121347
Tammas I, Bitchava K, Gelasakis AI. Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations. Vaccines. 2024; 12(12):1347. https://doi.org/10.3390/vaccines12121347
Chicago/Turabian StyleTammas, Iosif, Konstantina Bitchava, and Athanasios I. Gelasakis. 2024. "Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations" Vaccines 12, no. 12: 1347. https://doi.org/10.3390/vaccines12121347
APA StyleTammas, I., Bitchava, K., & Gelasakis, A. I. (2024). Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations. Vaccines, 12(12), 1347. https://doi.org/10.3390/vaccines12121347







