Abstract
Multidrug-resistant tuberculosis (MDR-TB) poses a significant global health threat, especially when it involves the central nervous system (CNS). Tuberculous meningitis (TBM), a severe manifestation of TB, is linked to high mortality rates and long-term neurological complications, further exacerbated by drug resistance and immune evasion mechanisms employed by Mycobacterium tuberculosis (Mtb). Although pulmonary TB remains the primary focus of research, MDR-TBM introduces unique challenges in diagnosis, treatment, and patient outcomes. The effectiveness of current treatments is frequently compromised by poor CNS penetration of anti-TB drugs and the necessity for prolonged therapy, which often involves considerable toxicity. This review explores the potential of cytokine-based adjunct immunotherapies for MDR-TBM, addressing the challenges of balancing pro-inflammatory and anti-inflammatory signals within the CNS. A central focus is the prospective role of glutathione, not only in reducing oxidative stress but also in enhancing host immune defenses against Mtb’s immune evasion strategies. Furthermore, the development of vaccines aimed at upregulating glutathione synthesis in macrophages represents a promising strategy to bolster the immune response and improve treatment outcomes. By integrating glutathione and innovative vaccine approaches into MDR-TBM management, this review proposes a comprehensive strategy that targets Mtb directly while supporting immune modulation, with the potential to enhance patient outcomes and reduce treatment related adverse effects. We underscore the urgent need for further research into adjunctive therapies and immunomodulatory strategies to more effectively combat MDR-TBM.
1. Introduction
Multidrug-resistant tuberculosis (MDR-TB) remains a major global health concern, particularly when it affects extrapulmonary sites such as the central nervous system (CNS) [,,]. Tuberculous meningitis (TBM), a severe manifestation of TB, is associated with high mortality and long-term neurological complications, especially when associated with drug resistance [,]. TBM accounts for 5–10% of extrapulmonary TB cases and around 1% of all TB cases; however, these are likely underestimates due to limited microbiological confirmation and a lack of robust epidemiological studies []. While pulmonary TB continues to dominate research and treatment efforts, the emergence of MDR-TBM has introduced new complexities in diagnosis, treatment, and outcomes [,,]. Studies indicate a significantly higher mortality rate in MDR-TBM compared to drug-susceptible TBM, with mortality rates ranging from 7.8% to 67%, depending on setting and drug resistance status, emphasizing the urgent need for more effective treatments for this severe form of TB [,,].
In response to the MDR-TB crisis, the World Health Organization (WHO) released updated treatment guidelines, incorporating novel drugs bedaquiline (a diarylquinoline) and delamanid (a nitroimidazole), alongside shorter, more effective regimens for pulmonary MDR-TB []. These advancements have significantly improved the prognosis for pulmonary MDR-TB, offering hope for more effective management [,,]. However, the management of TBM remains particularly challenging due to the limited penetration of many anti-TB drugs into the cerebrospinal fluid (CSF), leading to reduced efficacy in treating infections within the CNS []. MDR-TBM is further complicated by factors such as delayed diagnosis, the absence of optimized CNS-specific drug regimens, and prolonged treatment courses that often carry toxic side effects [,,].
The unique pathogenesis of Mycobacterium tuberculosis (Mtb) within the CNS presents a significant challenge in managing MDR-TBM. Mtb in the CNS utilizes immune evasion mechanisms, such as resistance to oxidative stress, which allow it to survive within host cells and evade immune clearance. Recent research suggests that enhancing the host’s oxidative defenses through adjunctive glutathione therapy could provide a novel approach to countering Mtb’s survival strategies []. Glutathione, a critical antioxidant, not only bolsters immune responses but may also mitigate oxidative stress, potentially counteracting Mtb’s immune evasion mechanisms and improving patient outcomes.
This review examines current therapeutic approaches for MDR-TBM, evaluates the efficacy of newly recommended CNS-specific regimens, and explores promising adjunctive therapies such as GSH. By addressing both antimicrobial and immune modulation strategies, we aim to provide a comprehensive overview for improving MDR-TBM management and patient prognosis, aligning with recent insights into Mtb’s immune evasion tactics and the role of host oxidative defenses.
2. Current Treatment Options for MDR-TBM
MDR-TBM treatment is challenging due to the need for drugs that effectively cross the blood–brain barrier (BBB) while targeting resistant TB strains. Unlike pulmonary MDR-TB, which has standardized treatments, MDR-TBM lacks a consensus on optimal therapy []. This is due to the complex nature of TB meningitis and limited CNS penetration of many MDR-TB drugs, which reduces their efficacy against brain infections. Current approaches include modified regimens and research-driven strategies under evaluation for improved CNS effectiveness (Table 1).

Table 1.
Experimental Treatment Options for MDR-TBM.
2.1. Pretomanid-Based Combinations
Research has highlighted pretomanid’s promising penetration into the CNS, positioning it as a potential core drug in MDR-TBM regimens []. Studies demonstrated that pretomanid achieves higher concentrations in brain tissue compared to other MDR-TB drugs like bedaquiline and linezolid. Experimental regimens such as Pa_100SMx (pretomanid, sutezolid, moxifloxacin) have shown significant effectiveness in reducing bacterial load in the brain in animal models, which could make it an option for MDR-TBM in the future [].
2.2. Modified WHO Regimens
The WHO’s guidelines recommend regimens for MDR-TB, and some studies have explored potential modifications to adapt these regimens for MDR-TBM. However, such adaptations remain experimental, and further research is necessary to confirm their efficacy and safety specifically for TB meningitis [,,].
2.3. Ongoing Research
The WHO’s current guidelines and MDR-TB-focused trials, such as end TB and TB-PRACTECAL, emphasize shorter and less toxic treatments for pulmonary MDR-TB [,]. However, they do not directly address MDR-TBM, underscoring the need for further research and clinical trials to determine effective CNS-specific regimens for TB meningitis.
3. Challenges of Treatment Regimens in MDR-TBM
The evidence supporting MDR-TBM treatment is limited and largely extrapolated from pulmonary TB experience, presenting specific challenges when using MDR-TB regimens for TBM. A primary challenge is the limited drug penetration into the CNS.
Many drugs in MDR-TB regimens, such as bedaquiline and clofazimine, exhibit restricted CNS penetration, potentially reducing efficacy against MDR-TBM (Table 2) [,]. Bedaquiline reaches CSF, but at lower concentrations than in plasma, and data on its role in TBM treatment are scarce [,]. Similarly, pretomanid’s CNS penetration in the MDR-TB regimen is not well-documented, raising concerns about its impact on MDR-TBM [,]. Moxifloxacin achieves therapeutic levels in CSF at doses of 400 mg to 800 mg daily, but monitoring may be needed for MDR-TBM due to higher concentration requirements [,,].

Table 2.
Challenges of Anti-TB Drugs in Treating MDR-TBM.
Linezolid shows better CNS penetration and is being evaluated with high-dose rifampicin, but it carries risks of severe side effects [,,,].
The prolonged treatment duration for MDR-TBM complicates standard regimens, as effective CNS clearance often requires extended therapy []. Key challenges include achieving adequate drug levels in the CSF, managing side effects, and adjusting treatment duration.
4. Adjunct Immunotherapies for MDR-TBM
The persistent challenges of MDR-TBM, such as poor treatment outcomes and limited CNS penetration of drugs, have spurred increased interest in adjunct immunotherapy options []. This strategy leverages the host immune system as a vital component for both containing and curing TB (Figure 1). However, while most studies focus on TB and MDR-TB, data specific to MDR-TBM remain limited, highlighting the need for further research in this area.
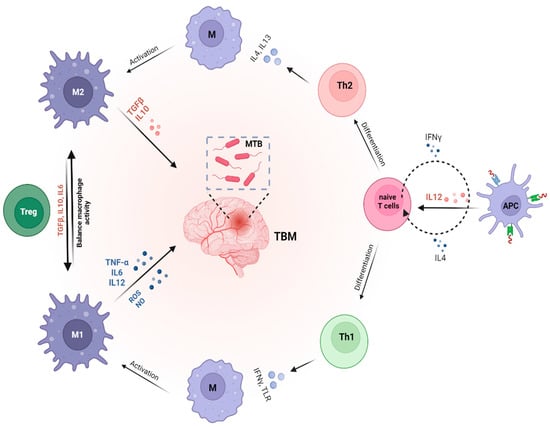
Figure 1.
The role of cytokines in modulating immune response and Mtb susceptibility. Macrophages, which can differentiate into pro-inflammatory (M1) or anti-inflammatory (M2) subtypes, play a critical role in TBM by interacting with T cell subsets, including Th1, Th2, and regulatory T (Treg) cells. M1 macrophages are key for eliminating Mtb in the central nervous system, while M2 macrophages support tissue repair and regulate inflammation. Th1 cells, through cytokines such as IFN-γ, activate M1 macrophages to enhance the clearance of Mtb. In contrast, Th2 cells secrete cytokines like IL-4 and IL-13, promoting M2 macrophage polarization to facilitate tissue repair. Treg cells, by producing cytokines like IL-10 and TGF-β, help control excessive inflammation and maintain immune homeostasis.
Using immunotherapy with cytokines alongside anti-TB treatment offers multiple potential benefits: it may improve treatment success rates, reduce treatment duration, and enhance the immune system’s ability to eliminate Mtb (Table 3). Cytokine immunotherapy can be divided into three categories based on their mechanisms of action: effector cytokine therapies, strategies for promoting a favorable immune response, and immunosuppressive strategies [].

Table 3.
Mechanisms and Advantages of Immunotherapies in MDR-TBM.
4.1. Effector Cytokine Therapies
These immunotherapies, which may be used for MDR-TBM, include recombinant IL-2, IL-7, IL-12, IL-15, IL-24, and IFN-γ, may enhance the immune response, improving microbicidal effects and overall management of TB and TBM.
Randomized controlled trials indicated that recombinant human IL-2, significantly improved sputum culture conversion and smear conversion in patients receiving at least three months of anti-TB therapy, including those with MDR-TB [,].
Similarly, trials on adjunctive therapy with IFN-γ, which could also be used for MDR-TBM, have demonstrated that both aerosolized and intramuscular administration can significantly improve sputum negative conversion rates and chest radiograph outcomes in patients with pulmonary MDR-TB [,].
In preclinical studies, recombinant adenoviruses IL-12 effectively reduced Mtb loads in infected mice [], suggesting potential applicability for MDR-TBM. Additionally, in research on macaques infected with MDR-TB, IL-2 enhanced T-effector cell responses, leading to lower Mtb burdens and milder lung pathology [].
Other studies found that mice supplemented with IL-7 and IL-15 exhibited a statistically significant reduction in mycobacterial burden in the lungs []. Moreover, both IL-7 and IL-15 were shown to enhance the survival of Mtb-infected mice [], which could offer insights into new strategies for tackling MDR-TBM.
In an animal study using a mouse model infected with Mtb, exogenous IL-24 demonstrated protective effects by activating CD8+ T cells to produce IFN-γ through IL-24 receptors. Suppression of IL-24 may increase susceptibility to TB and contribute to chronic infection []. This highlights IL-24′s potential role in enhancing immune defenses, which may have implications for addressing MDR-TBM.
4.2. Promoting a Favorable Immune Response
Strategies such as using Toll-Like Receptor (TLR) agonists, like imiquimod, can effectively influence immune responses in TB. A recent study indicated that imiquimod significantly suppressed intracellular Mtb growth in macrophages through autophagy induction linked to increased nitric oxide production []. Moreover, imiquimod activate innate immune responses, stimulating macrophages and dendritic cells to release pro-inflammatory cytokines such as IL-12, which promotes Th1 differentiation and enhances Interferon-gamma (IFN-γ) production, further bolstering the body’s ability to combat Mtb.
A preclinical study also showed that anti-IL-4 neutralizing antibodies significantly reduced the Mtb burden in a murine model, indicating a shift toward a more effective Th1 immune response []. This suggests that targeting and suppressing the Th2 cytokine component in TB patients could enhance treatment outcomes. This suggests that targeting and suppressing Th2 cytokines in TB patients may shift the immune response toward a Th1 profile, enhancing IFN-γ production and macrophage activation. This shift could improve treatment outcomes, offering a promising strategy for managing MDR-TBM.
4.3. Immunosuppressive Strategies
Managing excessive inflammation in TBM is crucial. Corticosteroids, which lower Tumor Necrosis Factor-alpha (TNF-α) levels, are effective options for controlling inflammation in TBM. Multiple clinical studies indicated that corticosteroids, particularly dexamethasone, effectively reduce mortality in TBM. In a randomized, double-blind, placebo-controlled trial, patients receiving tapering doses of dexamethasone over eight weeks experienced a significant decrease in death risk []. Furthermore, long-term follow-up showed that adjunctive dexamethasone improved survival rates among TBM patients []. This suggests that adjunctive dexamethasone could similarly improve survival outcomes in MDR-TBM by addressing inflammation.
4.4. Balancing Pro- and Anti-Inflammatory Responses
Cytokine therapy for MDR-TBM must strike a delicate balance between enhancing the immune system’s ability to combat Mtb and controlling excessive inflammation that could damage the CNS. Clinical and preclinical studies provide valuable insights into how specific cytokines can be used to achieve this balance.
IFN-γ: In clinical trials for pulmonary MDR-TB, recombinant IFN-γ has been shown to activate macrophages, enhancing their ability to phagocytose and kill Mtb, thereby improving sputum conversion rates and reducing lung pathology. However, the strong pro-inflammatory response induced by IFN-γ necessitates careful management in CNS infections like MDR-TBM, where excessive inflammation can be harmful. In TBM, the co-administration of corticosteroids such as dexamethasone with IFN-γ can help manage this balance. Corticosteroids effectively lower TNF-α levels, reducing inflammation without compromising IFN-γ’s macrophage-activating properties. This combination has been shown to enhance clinical outcomes by promoting bacterial clearance while preventing inflammatory damage in TBM patients.
IL-10: As an anti-inflammatory cytokine, IL-10 has been explored in various clinical contexts, including autoimmune diseases such as Crohn’s disease and rheumatoid arthritis. IL-10 suppresses excessive immune activation by inhibiting the production of pro-inflammatory cytokines, offering a protective effect against tissue damage. In MDR-TBM, the controlled use of IL-10 could help mitigate the potentially damaging effects of pro-inflammatory cytokines like IFN-γ and TNF-α. Administering low doses of IL-10 in combination with Th1 cytokines may prevent neuroinflammation while preserving the immune system’s ability to control Mtb, providing a balanced therapeutic approach.
IL-12: Preclinical studies have demonstrated that IL-12 drives a robust Th1 immune response, reducing Mtb loads in animal models. However, clinical application of IL-12 must be carefully monitored due to its potent immune-activating effects, which can lead to excessive inflammation. In cancer immunotherapy, where IL-12 is used to stimulate anti-tumor immunity, it is often combined with immune checkpoint inhibitors to regulate immune activation. A similar approach could be applied in MDR-TBM, where IL-12 is paired with immunosuppressive agents to maintain an effective immune response against Mtb while avoiding overactivation that could damage CNS tissue.
TLR Agonists: TLR agonists such as imiquimod have been explored in clinical trials for infectious diseases and cancer. These agents stimulate innate immune responses by activating macrophages and dendritic cells, enhancing the production of pro-inflammatory cytokines like IL-12 and promoting a Th1 response. In MDR-TBM, TLR agonists could be used to trigger an initial immune response against Mtb. However, to prevent excessive inflammation in the CNS, TLR agonists could be combined with adjunctive anti-inflammatory treatments, ensuring that the immune response is potent enough to control the infection without causing harmful inflammation.
These clinical and preclinical examples highlight the importance of carefully regulating cytokine therapy to maintain a balance between pro-inflammatory and anti-inflammatory responses in MDR-TBM.
5. Challenges of Immunotherapies for MDR-TBM
While immunotherapies and cytokine therapies could aid MDR-TBM treatment, their application presents challenges that must be managed carefully. The complex immune environment, particularly in the CNS, requires precise control to prevent unintended effects. Below, we outline key obstacles and considerations necessary to advance cytokine use in MDR-TBM.
5.1. Balancing Challenges in Cytokine Therapy for MDR-TB
Cytokine therapy for MDR-TBM must be carefully balanced to manage the immune response effectively. The immune response against Mtb relies on a delicate equilibrium between pro-inflammatory and anti-inflammatory cytokines. Imbalances in this response could lead to adverse effects, making it essential that cytokine therapy is implemented cautiously to maintain this balance and prevent complications.
5.2. Limited Research in TBM-Specific Contexts
Most immunotherapeutic research has focused on pulmonary TB, leaving a significant gap in understanding TBM-specific responses. The interactions between anti-TB drugs and the immune system in MDR-TBM are not well understood, especially concerning how these drugs might affect the cytokine balance within the CNS. Anti-TB drugs may disrupt this balance, potentially exacerbating inflammation and worsening TBM outcomes. Further research is necessary to clarify how anti-TB therapies interact with immune responses in MDR-TBM [,,].
5.3. Variability in Patient Response to Cytokine Therapy
There is considerable variability in individual responses to cytokine therapy due to factors such as genetics, pre-existing immune conditions (e.g., HIV, autoimmune diseases), and TB infection characteristics (e.g., drug susceptibility, drug resistance). Patients with drug-resistant TB often display different immune responses compared to those with drug-susceptible TB, underscoring the need for tailored immunotherapy approaches. To enable more targeted cytokine therapies, cytokine-release assays and immunomonitoring could be beneficial for assessing immune responses to Mtb antigens [,,,,,]. However, implementing these assays in TB management, particularly in MDR-TBM, is costly and challenging, as TB predominantly affects low-resource populations.
5.4. The Impact of Cytokines on the CNS Immune Environment
Using cytokines in MDR-TBM therapy presents unique challenges due to their pronounced effects on the CNS immune environment. Cytokine therapy in the CNS can influence bacterial metabolism and survival strategies. In MDR-TBM, these alterations may inadvertently support bacterial survival mechanisms, including the upregulation of efflux pumps and modifications to the cell wall structure. Both adaptations are associated with enhanced persistence of drug-resistant Mtb strains, complicating treatment and potentially promoting bacterial resilience [,,].
6. Glutathione and MDR-TBM Management
As researchers continue to explore adjunct therapies for TBM, glutathione (GSH) has emerged as a potential candidate due to its critical role in modulating immune responses and protecting against oxidative stress in the CNS (Figure 2) (Table 4) [].
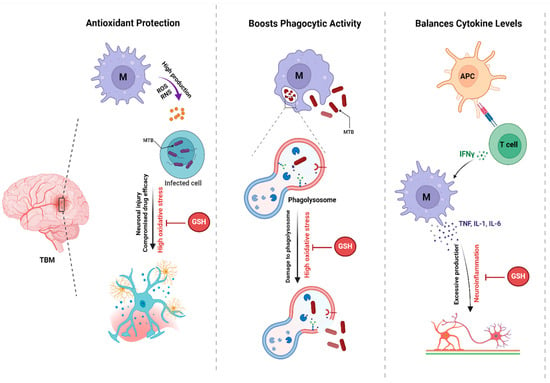
Figure 2.
The Protective Role of Glutathione in TBM. This figure illustrates the mechanisms by which antioxidants contribute to host defense against Mtb infection. Antioxidants protect against oxidative stress, enhance phagocytic activity, and balance cytokine levels, all of which are crucial for effective immune responses.
GSH, which is composed of glutamine, cysteine, and glycine, is a powerful antioxidant that helps maintain cellular redox balance, particularly critical in TBM, where inflammation can lead to neuronal damage []. It supports lymphocyte and macrophage functions, enhancing immune responses against Mtb and potentially benefiting MDR-TBM management [,,,,].

Table 4.
Clinical Benefit of Glutathione in Managing MDR-TBM.
Table 4.
Clinical Benefit of Glutathione in Managing MDR-TBM.
| Benefit | Description | Mechanism | Clinical Relevance to MDR-TBM |
|---|---|---|---|
| Antioxidant Properties [] | Glutathione acts as a key antioxidant, protecting cells from oxidative stress. | Maintains redox balance and reduces oxidative damage during inflammation, critical in TBM. | Protects neuronal integrity and function, which is vital in preventing neurological damage in TBM. |
| Immune Modulation [,,,,] | Enhances immune response by improving lymphocyte and macrophage function. | Increases levels of Th1 cytokines (IFN-γ, TNF-α, IL-2) and reduces anti-inflammatory cytokines (IL-6, IL-10). | Strengthens host defenses specifically against MDR strains, improving treatment efficacy in MDR-TBM. |
| Synergistic Effect with NAC [,] | Combining glutathione with N-acetylcysteine (NAC) boosts intracellular levels. | NAC replenishes glutathione, enhancing its antioxidant effects and cellular protection. | May enhance the therapeutic response to standard TB drugs, addressing drug resistance in MDR-TBM. |
| Enhanced Bioavailability [,,,] | Liposomal formulations improve stability and delivery. | Encapsulates glutathione to protect against degradation, ensuring effective transport across the BBB. | Ensures effective delivery of antioxidants to the CNS, critical for managing TBM complications. |
| Prevention of Drug Resistance [,,,] | Cysteine (a glutathione derivative) prevents emergence of drug-resistant strains. | Inhibits cells from entering a persister state and induces an oxidative burst, enhancing drug activity. | Helps mitigate the development of drug resistance, a major concern in treating MDR-TBM. |
| Hepatoprotection [,,,] | NAC protects against hepatotoxicity associated with anti-TB drugs. | Reduces liver enzyme levels and mitigates drug-induced liver damage. | Essential for maintaining liver health, thus ensuring continuity of MDR-TBM treatment. |
| Cost-Effectiveness [] | Could alleviate the financial burden on MDR-TB patients. | Enhances treatment efficacy and may reduce length of hospital stays. | Addresses the significant economic strain on patients, making treatment more accessible and sustainable. |
MDR: Multidrug-Resistant, TB: Tuberculosis, CNS: Central Nervous System, NAC: N-acetylcysteine, BBB: Blood–Brain Barrier.
Advances in delivery systems, such as liposomal GSH, improve its bioavailability and CNS delivery, potentially enhancing its therapeutic impact on MDR-TBM cases where effective CNS penetration is critical [,,,,]. Beyond liposomal formulations, other advanced delivery strategies like nanoformulations, polymeric nanoparticles, and conjugated drug systems are being explored for their ability to enhance the CNS targeting of therapies. These systems can be designed to improve cellular uptake, stability, and the controlled release of glutathione, ensuring that it reaches therapeutic levels in the brain without causing systemic toxicity. Moreover, the use of cell-penetrating peptides or receptor-mediated transport systems may further enhance the selective targeting of liposomal GSH to CNS tissues, improving its efficacy in treating MDR-TBM. These advancements in delivery technologies are expected to significantly improve the clinical management of MDR-TBM, where high CNS concentrations of glutathione are necessary to combat inflammation, oxidative damage, and bacterial persistence in the brain.
The combination of GSH with NAC amplifies immune function by increasing Th1 cytokine production (IFN-γ, TNF-α) and decreasing IL-6 and IL-10 levels, which could be particularly beneficial in strengthening the immune response against MDR-TBM [,].
When used alongside standard anti-TB treatments, GSH and NAC enhance drug efficacy, reduce hepatotoxicity, and prevent drug resistance, positioning them as powerful adjuncts in MDR-TB and potentially in MDR-TBM management [,,,]. Additionally, GSH’s role in reducing treatment duration and hospital stays may alleviate financial burdens for MDR-TB patients, as 81% face catastrophic treatment costs []. These economic benefits could also extend to MDR-TBM patients, where prolonged and complex treatment further increases financial strain.
7. Glutathione and Oxidative Stress in MDR-TBM
To effectively manage MDR-TBM, understanding how GSH influences the host’s relationship with Mtb is essential. This involves examining oxidative stress mechanisms that allow Mtb to survive and evade immune responses, highlighting the importance of GSH in developing effective treatments for MDR-TBM.
One strategy Mtb employs to evade immune defenses is the synthesis of mycothiol, a GSH analog that protects the bacterium against oxidative stress [,,]. This mycothiol-based defense is essential for Mtb’s persistence within infected cells. Mycothiol, a key thiol in Mtb, consists of glucosamine, myo-inositol, and cysteine, playing a vital role in detoxifying harmful substances and maintaining redox balance within the bacterial cell.
The synthesis of mycothiol begins with the enzyme mycothiol synthetase, which catalyzes its formation from N-acetylcysteine and glucosamine-1-phosphate [,,]. Acting as an antioxidant like GSH in human cells, mycothiol neutralizes harmful compounds, enabling Mtb to evade immune defenses and survive within host cells, particularly in macrophages where oxidative stress poses a significant threat.
The interplay between host GSH and Mtb’s mycothiol is critical in TB therapy for MDR-TBM. Increased GSH levels in host cells can challenge Mtb’s defenses, as elevated GSH in macrophages may overwhelm mycothiol’s detoxification role, making Mtb more vulnerable to immune clearance. Additionally, higher levels of GSH can outcompete mycothiol in neutralizing harmful compounds, weakening Mtb’s ability to evade immune attacks and increasing its susceptibility to oxidative damage.
Glutathione also directly interferes with several key strategies Mtb employs to evade the host immune system. One major evasion tactic used by Mtb is the alteration of the host’s redox environment to resist oxidative damage. By increasing glutathione levels, the host can enhance the production of reactive oxygen species (ROS) and reactive nitrogen species, which create a hostile intracellular environment. Glutathione also influences macrophage function by promoting autophagy, a process where Mtb-containing phagosomes are engulfed and degraded. Elevated glutathione enhances the formation of autophagosomes, facilitating better bacterial clearance. Additionally, glutathione regulates key immune signaling pathways, including NF-kB and MAPK pathways, which control the release of pro-inflammatory cytokines like TNF-α and IL-12. These cytokines not only recruit immune cells to the site of infection but also activate macrophages and other immune cells, leading to an increased immune response. By modulating both oxidative stress and immune signaling, glutathione enhances the host’s ability to recognize and destroy Mtb, counteracting its immune evasion strategies. Moreover, glutathione’s role in maintaining redox homeostasis helps to prevent immune dysfunction, such as excessive inflammation, which could otherwise allow Mtb to survive and replicate within the host.
8. Implications of Glutathione for Vaccine Development
Insights into GSH’s potential to counteract Mtb immune evasion open new avenues in vaccine development for MDR-TBM. A targeted vaccine could aim to upregulate GSH synthesis within macrophages, where Mtb primarily resides, thereby creating an oxidative environment hostile to Mtb. This section explores specific strategies for developing such a vaccine (Table 5).

Table 5.
Strategies to Enhance Glutathione for MDR-TBM Vaccines.
8.1. Upregulating Glutathione Synthesis via the Nrf2 Pathway
One promising approach is to stimulate the Nuclear factor erythroid 2–related factor 2 (Nrf2) pathway, a master regulator of antioxidant production, including GSH [,,]. By including adjuvants or immunostimulatory molecules that activate Nrf2, a vaccine could promote the expression of genes responsible for GSH synthesis, such as glutamate-cysteine ligase, which catalyzes the rate-limiting step in GSH production. Enhancing Nrf2 activation would drive macrophages to produce higher GSH levels, increasing ROS and creating conditions that challenge Mtb’s ability to evade immune responses.
8.2. Increasing Availability of Glutathione Precursors
Another approach is to boost intracellular cysteine levels, a crucial precursor for glutathione production. A vaccine could include components that upregulate transporters like SLC7A11, enhancing cystine import into macrophages []. This steady supply of cysteine would sustain GSH synthesis within infected macrophages, supporting prolonged oxidative stress against Mtb and undermining its immune evasion mechanisms.
8.3. Enhancing NADPH Production for Sustained Glutathione Activity
To maintain GSH in its active, reduced form, an adequate NADPH supply is essential []. A vaccine could promote pathways that increase NADPH production by activating the pentose phosphate pathway or enhancing the expression of enzymes such as glucose-6-phosphate dehydrogenase. This steady NADPH supply would allow macrophages to maintain high levels of active GSH, strengthening their oxidative response and reducing Mtb’s capacity to survive under oxidative stress.
Moreover, these GSH-centric strategies could be integrated with other vaccine approaches targeting Mtb antigens or enhancing T-cell responses [,,]. This multifaceted strategy could provide a more robust defense against MDR-TBM by not only enhancing the oxidative capacity of macrophages but also improving overall immune recognition and response to the pathogen.
9. Feasibility and Challenges of Glutathione-Targeted Vaccines for MDR-TBM
While the idea of leveraging glutathione synthesis in macrophages to combat MDR-TBM is promising, there are several practical considerations and challenges that must be addressed to translate this concept into viable vaccine strategies.
9.1. Practical Feasibility of Glutathione-Targeted Vaccines
Developing vaccines to enhance glutathione synthesis in macrophages requires identifying effective adjuvants or immunostimulatory molecules, such as Nrf2 activators. Key challenges include precise macrophage targeting to avoid systemic side effects and optimizing the bioavailability of adjuvants to activate Nrf2 without causing overactivation and excessive inflammation. Additionally, balancing glutathione precursor supplementation, particularly cysteine, is critical, as overstimulation could disrupt redox homeostasis, leading to potential immune cell damage. Careful dose optimization is essential to enhance oxidative stress against Mtb while minimizing immune pathology.
9.2. Potential Limitations and Side Effects
Upregulating macrophage glutathione raises concerns about chronic immune activation and its systemic effects. Excessive glutathione levels could lead to oxidative stress, potentially damaging host tissues, especially in individuals with compromised immune systems, such as those with autoimmune diseases or HIV. Additionally, tissue-specific variations in macrophage responses to glutathione modulation may affect vaccine efficacy. Targeting Nrf2 activation also carries risks, as excessive activation could disrupt immune balance, leading to autoimmune reactions or chronic inflammation. Therefore, controlling the timing and intensity of Nrf2 activation is crucial to minimize these risks.
9.3. Current Progress in Glutathione-Based Vaccine Research
While no vaccines targeting glutathione upregulation in macrophages have reached clinical trials, preclinical research is progressing. Nrf2 activators, being tested for diseases like cancer and neurodegenerative disorders, show promise in enhancing antioxidant defenses and may offer insights for TB immune modulation. In MDR-TBM, preclinical studies have shown that boosting glutathione synthesis enhances macrophage activity and pathogen clearance in TB mouse models. However, more research is needed to assess safety, efficacy, and side effects. Combining glutathione-based strategies with conventional TB vaccines may provide a more effective defense against MDR-TBM.
10. Future Research Directions
Future research on MDR-TBM should prioritize enhancing CNS drug delivery to improve the bioavailability of anti-TB medications, exploring innovative formulations like nanoparticles or liposomal carriers. Additionally, studies should focus on effectively boosting GSH levels in the CNS macrophages through Nrf2 pathway modulation or cysteine supplementation, while investigating the role of elevated GSH in immune responses. Cytokine-based immunotherapy also needs further exploration to identify optimal combinations that balance inflammatory responses and interact with oxidative stress modulators. Moreover, vaccine development should aim to enhance GSH synthesis and immune responses against Mtb’s redox balance, utilizing suitable adjuvants. Finally, clinical trials assessing new treatment combinations, drug formulations, and patient-specific factors are crucial for developing personalized approaches to improve outcomes in MDR-TBM. Understanding the variability in patient responses, along with optimizing GSH dose, administration, and its combination with other drugs and vaccines, will be key to tailoring therapeutic regimens effectively. Careful consideration of therapeutic regimens that incorporate GSH supplementation, its precise dosing, and how it synergizes with anti-TB drugs and vaccines will enhance treatment efficacy and minimize side effects. Tailoring these strategies based on individual patient profiles will be essential for achieving better treatment outcomes in this challenging disease.
11. Conclusions
MDR-TBM remains a challenging and life-threatening condition, worsened by limited CNS penetration of conventional anti-TB drugs and Mtb’s immune evasion mechanisms. This review discusses strategies to overcome these barriers, such as enhancing GSH synthesis and developing cytokine and vaccine-based immunotherapies to bolster host immune responses.
Innovations in GSH-centered therapies, CNS-targeted drug formulations, and vaccines that enhance GSH production in macrophages may significantly improve patient outcomes. However, further research is needed to validate these approaches. By integrating antimicrobial and immune-based strategies, including novel vaccines, future treatments may provide a comprehensive solution to combat MDR-TBM and improve the quality of life for affected patients.
Author Contributions
Conceptualization, M.J.N. and V.V.; methodology, M.J.N. and V.V.; software, V.V.; validation, M.J.N., V.V. and K.L.; formal analysis, M.J.N.; investigation, M.J.N.; resources, V.V.; data curation, M.J.N.; writing—original draft preparation, M.J.N.; writing—review and editing, V.V.; and K.L.; visualization, M.J.N.; supervision, V.V.; project administration, V.V.; funding acquisition, V.V. All authors have read and agreed to the published version of the manuscript.
Funding
We appreciate the funding support from the NIH-NHLBI (2R15HL143545-02).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Figures were created with BioRender.com (accessed on 25 October 2024). We thank Frederick Guilford, Your Energy Systems, LLC, Palo Alto, California 94301.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization (WHO). Global Tuberculosis Report; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Dheda, K.; Mirzayev, F.; Cirillo, D.M.; Udwadia, Z.; Dooley, K.E.; Chang, K.-C.; Omar, S.V.; Reuter, A.; Perumal, T.; Horsburgh, C.R., Jr. Multidrug-resistant tuberculosis. Nat. Rev. Dis. Primers 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Biswas, P.; Ansar, W.; Mukherjee, P.; Jawed, J.J. Tuberculosis of the central nervous system: Pathogenicity and molecular mechanism. In A Review on Diverse Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2024; pp. 93–102. [Google Scholar]
- Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; Van Crevel, R.; Mai, N.T.H.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R. Tuberculous meningitis. Nat. Rev. Neurol. 2017, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Donovan, J.; Phu, N.H.; Nghia, H.D.T.; Thuong, N.T.T.; Thwaites, G.E. Tuberculous meningitis: Progress and remaining questions. Lancet Neurol. 2022, 21, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Boubour, A.; Paradkar, M.; Thakur, K.T. Global and regional burden of tuberculosis and tuberculous meningitis. In Tuberculous Meningitis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Byrd, T.F.; Davis, L.E. Multidrug-resistant tuberculous meningitis. Curr. Neurol. Neurosci. Rep. 2007, 7, 470–475. [Google Scholar] [CrossRef]
- Garg, R.K.; Rizvi, I.; Malhotra, H.S.; Uniyal, R.; Kumar, N. Management of complex tuberculosis cases: A focus on drug-resistant tuberculous meningitis. Expert Rev. Anti-Infect. Ther. 2018, 16, 813–831. [Google Scholar] [CrossRef]
- Inbaraj, L.R.; Daniel, B.D.; Padmapriyadarsini, C. Drug resistant tuberculous meningitis: A neglected foe in ending tuberculosis epidemic. Indian J. Tuberc. 2024, in press. [Google Scholar]
- Smith, A.; Gujabidze, M.; Avaliani, T.; Blumberg, H.; Collins, J.; Sabanadze, S.; Bakuradze, T.; Avaliani, Z.; Kempker, R.; Kipiani, M. Clinical outcomes among patients with tuberculous meningitis receiving intensified treatment regimens. Int. J. Tuberc. Lung Dis. 2021, 25, 632–639. [Google Scholar] [CrossRef]
- Evans, E.E.; Avaliani, T.; Gujabidze, M.; Bakuradze, T.; Kipiani, M.; Sabanadze, S.; Smith, A.G.; Avaliani, Z.; Collins, J.M.; Kempker, R.R. Long term outcomes of patients with tuberculous meningitis: The impact of drug resistance. PLoS ONE 2022, 17, e0270201. [Google Scholar] [CrossRef]
- Thee, S.; Basu Roy, R.; Blázquez-Gamero, D.; Falcón-Neyra, L.; Neth, O.; Noguera-Julian, A.; Lillo, C.; Galli, L.; Venturini, E.; Buonsenso, D. Treatment and outcome in children with tuberculous meningitis: A multicenter pediatric tuberculosis network European trials group study. Clin. Infect. Dis. 2022, 75, 372–381. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Migliori, G.B.; Sotgiu, G.; Richardson, M.A.; Centis, R.; Facchini, A.; Guenther, G.; Spanevello, A.; Lange, C. MDR-TB and XDR-TB: Drug resistance and treatment outcomes. Eur. Respir. J. 2009, 34, 778–779. [Google Scholar] [CrossRef]
- Hatami, H.; Sotgiu, G.; Bostanghadiri, N.; Abadi, S.S.D.; Mesgarpour, B.; Goudarzi, H.; Migliori, G.B.; Nasiri, M.J. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. J. Bras. Pneumol. 2022, 48, e20210384. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Zangiabadian, M.; Arabpour, E.; Amini, S.; Khalili, F.; Centis, R.; D’Ambrosio, L.; Denholm, J.T.; Schaaf, H.S.; van den Boom, M. Delamanid-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 124, S90–S103. [Google Scholar] [CrossRef] [PubMed]
- Méchaï, F.; Bouchaud, O. Tuberculous meningitis: Challenges in diagnosis and management. Rev. Neurol. 2019, 175, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, F.V.; Te Brake, L.; Atherton, R.; Ruslami, R.; Dooley, K.E.; Aarnoutse, R.; Van Crevel, R. Intensified antibiotic treatment of tuberculosis meningitis. Expert Rev. Clin. Pharmacol. 2019, 12, 267–288. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Musubire, A.K.; Århem, K.M.J. Treatment guidelines for tuberculosis and tuberculous meningitis. In Tuberculous Meningitis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–101. [Google Scholar]
- Huynh, J.; Abo, Y.-N.; du Preez, K.; Solomons, R.; Dooley, K.E.; Seddon, J.A. Tuberculous meningitis in children: Reducing the burden of death and disability. Pathogens 2021, 11, 38. [Google Scholar] [CrossRef]
- Venketaraman, V.; Millman, A.; Salman, M.; Swaminathan, S.; Goetz, M.; Lardizabal, A.; Hom, D.; Connell, N.D. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 2008, 44, 255–261. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Key Updates to the Treatment of Drug-Resistant Tuberculosis: Rapid Communication, June 2024. Available online: https://www.who.int/publications/i/item/B09123 (accessed on 1 June 2024).
- Chen, X.; Arun, B.; Nino-Meza, O.J.; Sarhan, M.O.; Singh, M.; Jeon, B.; Mane, K.; Shah, M.; Tucker, E.W.; Carroll, L.S. Dynamic PET reveals compartmentalized brain and lung tissue antibiotic exposures of tuberculosis drugs. Nat. Commun. 2024, 15, 6657. [Google Scholar] [CrossRef]
- Akkerman, O.W.; Odish, O.F.; Bolhuis, M.S.; de Lange, W.C.; Kremer, H.P.; Luijckx, G.-J.R.; van der Werf, T.S.; Alffenaar, J.-W. Pharmacokinetics of bedaquiline in cerebrospinal fluid and serum in multidrug-resistant tuberculous meningitis. Clin. Infect. Dis. 2016, 62, 523–524. [Google Scholar] [CrossRef]
- Fang, M.-T.; Su, Y.-F.; An, H.-R.; Zhang, P.-Z.; Deng, G.-F.; Liu, H.-M.; Mao, Z.; Zeng, J.-F.; Li, G.; Yang, Q.-T. Decreased mortality seen in rifampicin/multidrug-resistant tuberculous meningitis treated with linezolid in Shenzhen, China. BMC Infect. Dis. 2021, 21, 1015. [Google Scholar] [CrossRef]
- Nyang’wa, B.-T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Solodovnikova, V.; Liverko, I.; Moodliar, R.; Dodd, M. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 2022, 387, 2331–2343. [Google Scholar] [CrossRef]
- Cholo, M.C.; Mothiba, M.T.; Fourie, B.; Anderson, R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J. Antimicrob. Chemother. 2016, 72, dkw426. [Google Scholar] [CrossRef] [PubMed]
- Upton, C.M.; Steele, C.I.; Maartens, G.; Diacon, A.H.; Wiesner, L.; Dooley, K.E. Pharmacokinetics of bedaquiline in cerebrospinal fluid (CSF) in patients with pulmonary tuberculosis (TB). J. Antimicrob. Chemother. 2022, 77, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Upton, C.M.; Wiesner, L.; Dooley, K.E.; Maartens, G. Cerebrospinal fluid and tuberculous meningitis. Clin. Infect. Dis. 2023, 77, 158. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Tobin, D.M.; Tucker, E.W.; Venketaraman, V.; Ordonez, A.A.; Jayashankar, L.; Siddiqi, O.K.; Hammoud, D.A.; Prasadarao, N.V.; Sandor, M. Tuberculous meningitis: A roadmap for advancing basic and translational research. Nat. Immunol. 2018, 19, 521–525. [Google Scholar] [CrossRef]
- Mota, F.; Ruiz-Bedoya, C.A.; Tucker, E.W.; Holt, D.P.; De Jesus, P.; Lodge, M.A.; Erice, C.; Chen, X.; Bahr, M.; Flavahan, K. Dynamic 18F-Pretomanid PET imaging in animal models of TB meningitis and human studies. Nat. Commun. 2022, 13, 7974. [Google Scholar] [CrossRef]
- Alffenaar, J.; Van Altena, R.; Bökkerink, H.; Luijckx, G.; Van Soolingen, D.; Aarnoutse, R.; Van Der Werf, T. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin. Infect. Dis. 2009, 49, 1080–1082. [Google Scholar] [CrossRef]
- Ruslami, R.; Ganiem, A.R.; Dian, S.; Apriani, L.; Achmad, T.H.; van der Ven, A.J.; Borm, G.; Aarnoutse, R.E.; van Crevel, R. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: An open-label, randomised controlled phase 2 trial. Lancet Infect. Dis. 2013, 13, 27–35. [Google Scholar] [CrossRef]
- Pranger, A.; Van Der Werf, T.; Kosterink, J.; Alffenaar, J. The role of fluoroquinolones in the treatment of tuberculosis in 2019. Drugs 2019, 79, 161–171. [Google Scholar] [CrossRef]
- Tucker, E.W.; Pieterse, L.; Zimmerman, M.D.; Udwadia, Z.F.; Peloquin, C.A.; Gler, M.T.; Ganatra, S.; Tornheim, J.A.; Chawla, P.; Caoili, J.C. Delamanid central nervous system pharmacokinetics in tuberculous meningitis in rabbits and humans. Antimicrob. Agents Chemother. 2019, 63, e00913-19. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Liu, J.; Zhao, Y.; Ni, X.; Zhao, S. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr. Infect. Dis. J. 2016, 35, 607–610. [Google Scholar] [CrossRef]
- Li, C.-p.; Li, X.; Huang, S.-m.; Wang, X.-x.; Li, X.-q.; Hu, H.-x.; Fang, Y.-m. Efficacy and safety of linezolid in the treatment of children with severe tuberculous meningitis. China Trop. Med. 2021, 21, 1062–1065. [Google Scholar]
- Kempker, R.R.; Smith, A.G.; Avaliani, T.; Gujabidze, M.; Bakuradze, T.; Sabanadze, S.; Avaliani, Z.; Collins, J.M.; Blumberg, H.M.; Alshaer, M.H. Cycloserine and linezolid for tuberculosis meningitis: Pharmacokinetic evidence of potential usefulness. Clin. Infect. Dis. 2022, 75, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, N.; Wasserman, S.; Abdelwahab, M.T.; Davis, A.; Stek, C.; Wiesner, L.; Black, J.; Meintjes, G.; Wilkinson, R.J.; Denti, P. Linezolid population pharmacokinetic model in plasma and cerebrospinal fluid among patients with tuberculosis meningitis. J. Infect. Dis. 2024, 229, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.A.; Maartens, G.; Meintjes, G.; Wasserman, S. Clofazimine for the treatment of tuberculosis. Front. Pharmacol. 2023, 14, 1100488. [Google Scholar] [CrossRef]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef]
- Garg, R. Tuberculous meningitis. Acta Neurol. Scand. 2010, 122, 75–90. [Google Scholar] [CrossRef]
- Uhlin, M.; Andersson, J.; Zumla, A.; Maeurer, M. Adjunct immunotherapies for tuberculosis. J. Infect. Dis. 2012, 205 (Suppl. S2), S325–S334. [Google Scholar] [CrossRef]
- Johnson, J.L.; Ssekasanvu, E.; Okwera, A.; Mayanja, H.; Hirsch, C.S.; Nakibali, J.G.; Jankus, D.D.; Eisenach, K.D.; Boom, W.H.; Ellner, J.J. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 168, 185–191. [Google Scholar] [CrossRef]
- Tan, Q.; Min, R.; Dai, G.-q.; Wang, Y.-l.; Nan, L.; Yang, Z.; Xia, J.; Pan, S.-y.; Mao, H.; Xie, W.-p. Clinical and immunological effects of rhIL-2 therapy in eastern Chinese patients with multidrug-resistant tuberculosis. Sci. Rep. 2017, 7, 17854. [Google Scholar] [CrossRef]
- Maeurer, M.J.; Trinder, P.; Hommel, G.; Walter, W.; Freitag, K.; Atkins, D.; Störkel, S. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 2000, 68, 2962–2970. [Google Scholar] [CrossRef]
- Singh, V.; Gowthaman, U.; Jain, S.; Parihar, P.; Banskar, S.; Gupta, P.; Gupta, U.D.; Agrewala, J.N. Coadministration of interleukins 7 and 15 with bacille Calmette-Guerin mounts enduring T cell memory response against Mycobacterium tuberculosis. J. Infect. Dis. 2010, 202, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Mata-Espinosa, D.A.; Francisco-Cruz, A.; Marquina-Castillo, B.; Barrios-Payan, J.; Ramos-Espinosa, O.; Bini, E.I.; Xing, Z.; Hernández-Pando, R. Immunotherapeutic effects of recombinant adenovirus encoding interleukin 12 in experimental pulmonary tuberculosis. Scand. J. Immunol. 2019, 89, e12743. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, E.; Guo, M.; Yang, R.; Huang, G.; Peng, Y.; Sha, W.; Wang, F.; Shen, L. Adjunctive Zoledronate+ IL-2 administrations enhance anti-tuberculosis Vγ2Vδ2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis1. Emerg. Microbes Infect. 2022, 11, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, H.-D.; Wang, Y.; Wang, Q.; Li, Y.; Zhao, Y.; Zhang, X.-l. Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes Infect. 2011, 13, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Condos, R.; Tse, D.; Huie, M.L.; Ress, S.; Tseng, C.-H.; Brauns, C.; Weiden, M.; Hoshino, Y.; Bateman, E. Immunomodulation with recombinant interferon-γ1b in pulmonary tuberculosis. PLoS ONE 2009, 4, e6984. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Guo, L.; Lin, M.; Chen, X. A short-term efficacy on aerosolizing inhalation recombinant human gamma interferon in multidrug-resistant pulmonary tuberculosis. Chin. J. Antituberc. 2009, 31, 660. [Google Scholar]
- Lee, H.-J.; Kang, S.-J.; Woo, Y.; Hahn, T.-W.; Ko, H.-J.; Jung, Y.-J. TLR7 stimulation with imiquimod induces selective autophagy and controls Mycobacterium tuberculosis growth in mouse macrophages. Front. Microbiol. 2020, 11, 1684. [Google Scholar] [CrossRef]
- Roy, E.; Brennan, J.; Jolles, S.; Lowrie, D.B. Beneficial effect of anti-interleukin-4 antibody when administered in a murine model of tuberculosis infection. Tuberculosis 2008, 88, 197–202. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Bang, N.D.; Dung, N.H.; Quy, H.T.; Oanh, D.T.T.; Thoa, N.T.C.; Hien, N.Q.; Thuc, N.T.; Hai, N.N.; Lan, N.T.N. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 2004, 351, 1741–1751. [Google Scholar] [CrossRef]
- Török, M.E.; Bang, N.D.; Chau, T.T.H.; Yen, N.T.B.; Thwaites, G.E.; Thi Quy, H.; Dung, N.H.; Hien, T.T.; Chinh, N.T.; Thi Thanh Hoang, H. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS ONE 2011, 6, e27821. [Google Scholar] [CrossRef]
- Lahat, G.; Halperin, D.; Barazovsky, E.; Shalit, I.; Rabau, M.; Klausner, J.; Fabian, I. Immunomodulatory effects of ciprofloxacin in TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2007, 13, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Beigelman, A.; Mikols, C.L.; Gunsten, S.P.; Cannon, C.L.; Brody, S.L.; Walter, M.J. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir. Res. 2010, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Geffner, L.; Yokobori, N.; Basile, J.; Schierloh, P.; Balboa, L.; Romero, M.M.; Ritacco, V.; Vescovo, M.; González Montaner, P.; Lopez, B.; et al. Patients with multidrug-resistant tuberculosis display impaired Th1 responses and enhanced regulatory T-cell levels in response to an outbreak of multidrug-resistant Mycobacterium tuberculosis M and Ra strains. Infect. Immun. 2009, 77, 5025–5034. [Google Scholar] [CrossRef]
- Basile, J.I.; Geffner, L.J.; Romero, M.M.; Balboa, L.; Sabio, Y.G.C.; Ritacco, V.; García, A.; Cuffré, M.; Abbate, E.; López, B.; et al. Outbreaks of mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J. Infect. Dis. 2011, 204, 1054–1064. [Google Scholar] [CrossRef]
- Moideen, K.; Kumar, N.P.; Bethunaickan, R.; Banurekha, V.V.; Nair, D.; Babu, S. Heightened systemic levels of anti-inflammatory cytokines in pulmonary tuberculosis and alterations following anti-tuberculosis treatment. Cytokine 2020, 127, 154929. [Google Scholar] [CrossRef]
- Riou, C.; Du Bruyn, E.; Ruzive, S.; Goliath, R.T.; Lindestam Arlehamn, C.S.; Sette, A.; Sher, A.; Barber, D.L.; Wilkinson, R.J. Disease extent and anti-tubercular treatment response correlates with Mycobacterium tuberculosis-specific CD4 T-cell phenotype regardless of HIV-1 status. Clin. Transl. Immunol. 2020, 9, e1176. [Google Scholar] [CrossRef]
- Russomando, G.; Sanabria, D.; Díaz Acosta, C.C.; Rojas, L.; Franco, L.; Arenas, R.; Delogu, G.; Ndiaye, M.D.B.; Bayaa, R.; Rakotosamimanana, N. C1q and HBHA-specific IL-13 levels as surrogate plasma biomarkers for monitoring tuberculosis treatment efficacy: A cross-sectional cohort study in Paraguay. Front. Immunol. 2024, 15, 1308015. [Google Scholar] [CrossRef]
- Sun, M.; Phan, J.M.; Kieswetter, N.S.; Huang, H.; Yu, K.K.; Smith, M.T.; Liu, Y.E.; Wang, C.; Gupta, S.; Obermoser, G. Specific CD4+ T cell phenotypes associate with bacterial control in people who ‘resist’infection with Mycobacterium tuberculosis. Nat. Immunol. 2024, 25, 1411–1421. [Google Scholar] [CrossRef]
- Nguyen, L. Antibiotic resistance mechanisms in M. tuberculosis: An update. Arch. Toxicol. 2016, 90, 1585–1604. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Haeili, M.; Ghazi, M.; Goudarzi, H.; Pormohammad, A.; Imani Fooladi, A.A.; Feizabadi, M.M. New insights in to the intrinsic and acquired drug resistance mechanisms in mycobacteria. Front. Microbiol. 2017, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Bekale, R.B.; Du Plessis, S.-M.; Hsu, N.-J.; Sharma, J.R.; Sampson, S.L.; Jacobs, M.; Meyer, M.; Morse, G.D.; Dube, A. Mycobacterium tuberculosis and interactions with the host immune system: Opportunities for nanoparticle based immunotherapeutics and vaccines. Pharm. Res. 2019, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Morris, D.; Sipin, A.; Kung, S.; Franklin, M.; Gray, D.; Tanzil, M.; Guilford, F.; Khasawneh, F.T.; Venketaraman, V. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PloS ONE 2011, 6, e28378. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Bailey, C.; Cahatol, I.; Dodge, L.; Yim, J.; Kassissa, C.; Luong, J.; Kasko, S.; Pandya, S.; Venketaraman, V. Mechanisms of control of Mycobacterium tuberculosis by NK cells: Role of glutathione. Front. Immunol. 2015, 6, 508. [Google Scholar] [CrossRef]
- Cao, R.; Teskey, G.; Islamoglu, H.; Abrahem, R.; Munjal, S.; Gyurjian, K.; Zhong, L.; Venketaraman, V. Characterizing the effects of glutathione as an immunoadjuvant in the treatment of tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e01132-18. [Google Scholar] [CrossRef]
- Singh, M.; Vaughn, C.; Sasaninia, K.; Yeh, C.; Mehta, D.; Khieran, I.; Venketaraman, V. Understanding the relationship between glutathione, TGF-β, and vitamin D in combating Mycobacterium tuberculosis infections. J. Clin. Med. 2020, 9, 2757. [Google Scholar] [CrossRef]
- Abnousian, A.; Vasquez, J.; Sasaninia, K.; Kelley, M.; Venketaraman, V. Glutathione modulates efficacious changes in the immune response against tuberculosis. Biomedicines 2023, 11, 1340. [Google Scholar] [CrossRef]
- Vilchèze, C.; Jacobs, W.R., Jr. The promises and limitations of N-acetylcysteine as a potentiator of first-line and second-line tuberculosis drugs. Antimicrob. Agents Chemother. 2021, 65, e01703-20. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Appeldoorn, C.C.; Rip, J.; Dorland, R.; van der Pol, S.M.; Kooij, G.; de Vries, H.E.; Reijerkerk, A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J. Control. Release 2012, 164, 364–369. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Nguyen, T.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Medina, A. Effects of oral liposomal glutathione in altering the immune responses against mycobacterium tuberculosis and the mycobacterium bovis bcg strain in individuals with type 2 diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 657775. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Sathananthan, A.; Venketaraman, V. The Effects of Oral Liposomal Glutathione and In Vitro Everolimus in Altering the Immune Responses against Mycobacterium bovis BCG Strain in Individuals with Type 2 Diabetes. Biomol. Concepts 2021, 12, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kachour, N.; Beever, A.; Owens, J.; Cao, R.; Kolloli, A.; Kumar, R.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E. Liposomal glutathione helps to mitigate mycobacterium tuberculosis infection in the lungs. Antioxidants 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jain, P.; Weisbrod, T.R.; Leung, L.W.; Freundlich, J.S.; Jacobs, W.R., Jr. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.S.; Maartens, G.; Gunter, H.; Allie, S.; Chughlay, M.F.; Setshedi, M.; Wasserman, S.; Stead, D.F.; Hickman, N.; Stewart, A. A Randomized controlled trial of intravenous N-acetylcysteine in the management of anti-tuberculosis drug–induced liver injury. Clin. Infect. Dis. 2021, 73, e3377–e3383. [Google Scholar] [CrossRef]
- Sukumaran, D.; Usharani, P.; Paramjyothi, G.; Subbalaxmi, M.; Sireesha, K.; Ali, M.A. A study to evaluate the hepatoprotective effect of N-acetylcysteine on anti tuberculosis drug induced hepatotoxicity and quality of life. Indian J. Tuberc. 2023, 70, 303–310. [Google Scholar] [CrossRef]
- Akalu, T.Y.; Clements, A.C.; Wolde, H.F.; Alene, K.A. Economic burden of multidrug-resistant tuberculosis on patients and households: A global systematic review and meta-analysis. Sci. Rep. 2023, 13, 22361. [Google Scholar] [CrossRef]
- Ung, K.S.; Av-Gay, Y. Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett. 2006, 580, 2712–2716. [Google Scholar] [CrossRef]
- Sao Emani, C.; Williams, M.; Wiid, I.; Baker, B. The functional interplay of low molecular weight thiols in Mycobacterium tuberculosis. J. Biomed. Sci. 2018, 25, 55. [Google Scholar] [CrossRef]
- Sao Emani, C.; Gallant, J.; Wiid, I.; Baker, B. The role of low molecular weight thiols in Mycobacterium tuberculosis. Tuberculosis 2019, 116, 44–55. [Google Scholar] [CrossRef]
- Fontán, P.; Aris, V.; Ghanny, S.; Soteropoulos, P.; Smith, I. Global transcriptional profile of Mycobacterium tuberculosis during THP-1 human macrophage infection. Infect. Immun. 2008, 76, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.M.; Pedre, B.; De Armas, M.I.; Tossounian, M.-A.; Radi, R.; Messens, J.; Trujillo, M. Chemistry and redox biology of mycothiol. Antioxid. Redox Signal. 2018, 28, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The immune escape mechanisms of Mycobacterium tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, G.S.; Kirk, N.M.; Ackart, D.F.; Shanley, C.A.; Orme, I.M.; Basaraba, R.J. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS ONE 2011, 6, e26254. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Matsuyama, M.; Nonaka, M.; Nakajima, M.; Morishima, Y.; Ishii, Y.; Hizawa, N. The role of Nrf2 in mycobacterial infection. Antioxidants 2021, 10, 1861. [Google Scholar] [CrossRef]
- Jiang, Q.; Shi, L. Coordination of the uptake and metabolism of amino acids in Mycobacterium tuberculosis-infected macrophages. Front. Immunol. 2021, 12, 711462. [Google Scholar] [CrossRef]
- Connell, N.D.; Venketaraman, V. Control of Mycobacterium tuberculosis infection by glutathione. Recent Pat. Anti-Infect. Drug Discov. 2009, 4, 214–226. [Google Scholar] [CrossRef]
- Whitlow, E.; Mustafa, A.; Hanif, S. An overview of the development of new vaccines for tuberculosis. Vaccines 2020, 8, 586. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Fang, R.; Li, X.; Xing, J.; Li, Z.; Song, N. Enhancing TB Vaccine Efficacy: Current Progress on Vaccines, Adjuvants and Immunization Strategies. Vaccines 2023, 12, 38. [Google Scholar] [CrossRef]
- Zhuang, L.; Ye, Z.; Li, L.; Yang, L.; Gong, W. Next-generation TB vaccines: Progress, challenges, and prospects. Vaccines 2023, 11, 1304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).