Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Condition or Study Domain
2.3. Participants
2.4. Intervention
2.5. Comparator
2.6. Types of Studies
2.7. Inclusion and Exclusion Criteria
2.8. Measures of Effect
2.9. Data Extraction (Selection and Coding)
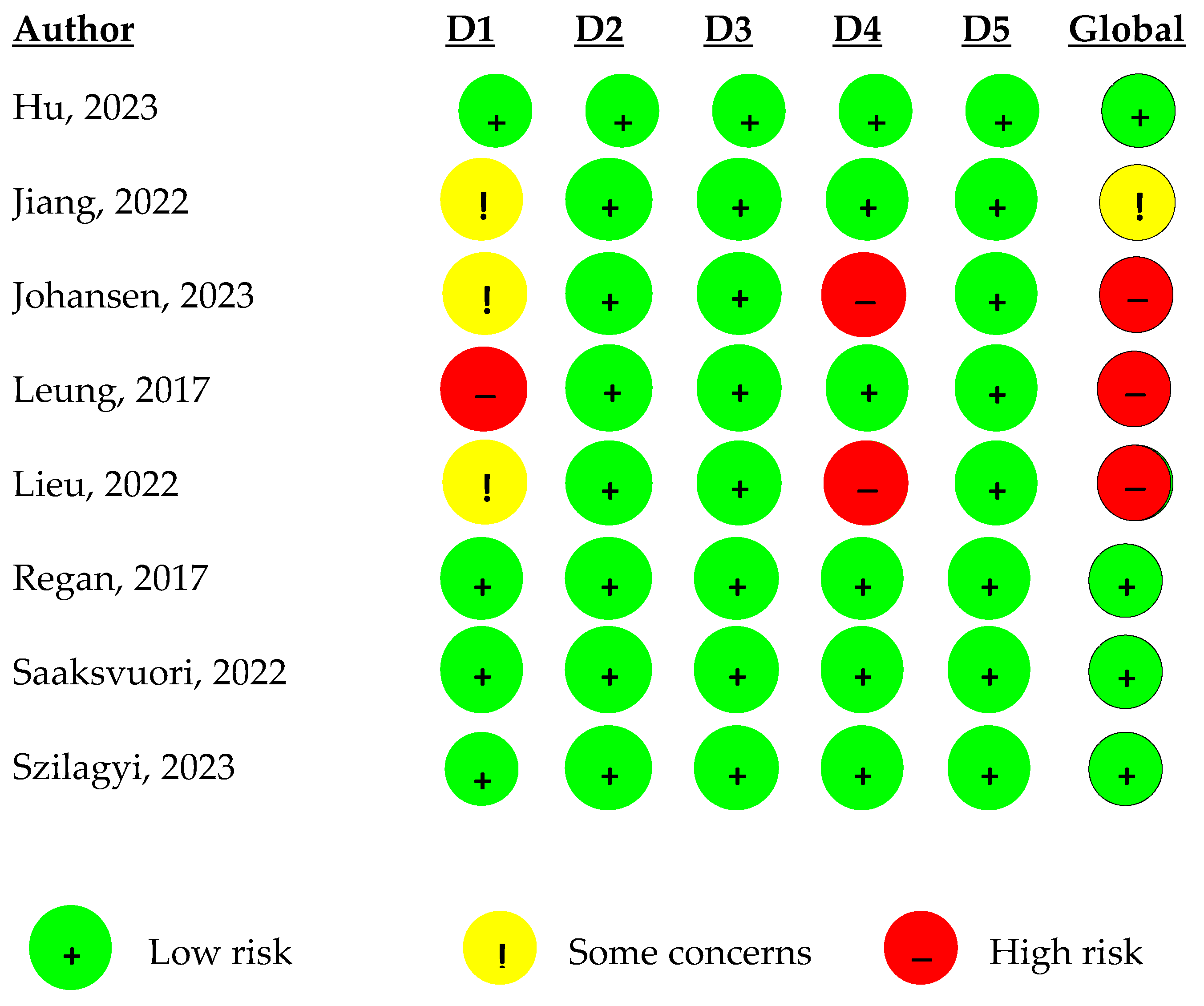
2.10. Risk of Bias Assessment
2.11. Data Synthesis Strategy
2.12. Subgroup or Subset Analysis
3. Results
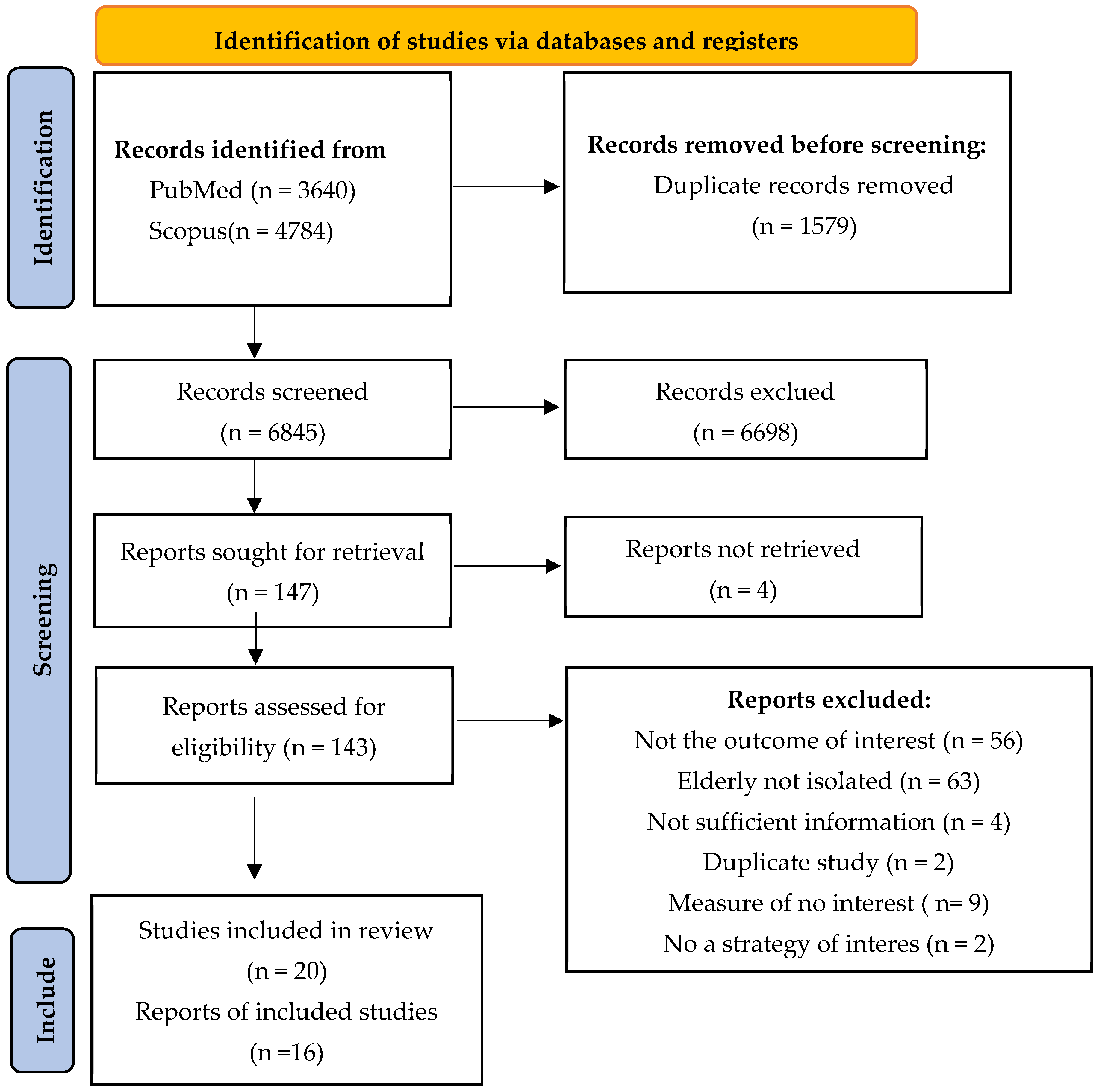
3.1. Selection of the Studies
3.2. Quality of the Studies Included
3.3. Meta-Analysis
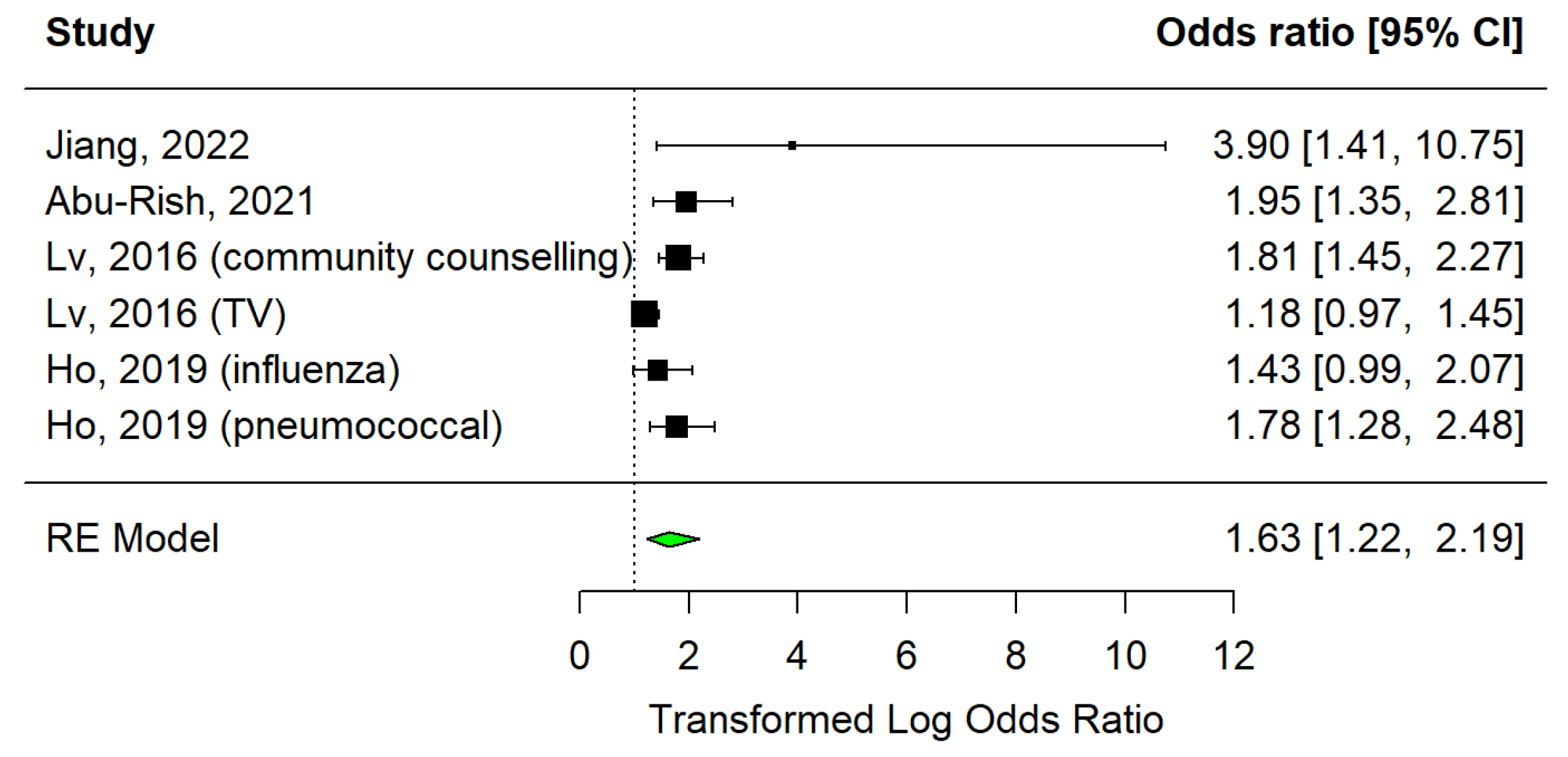
3.3.1. Educational Sessions
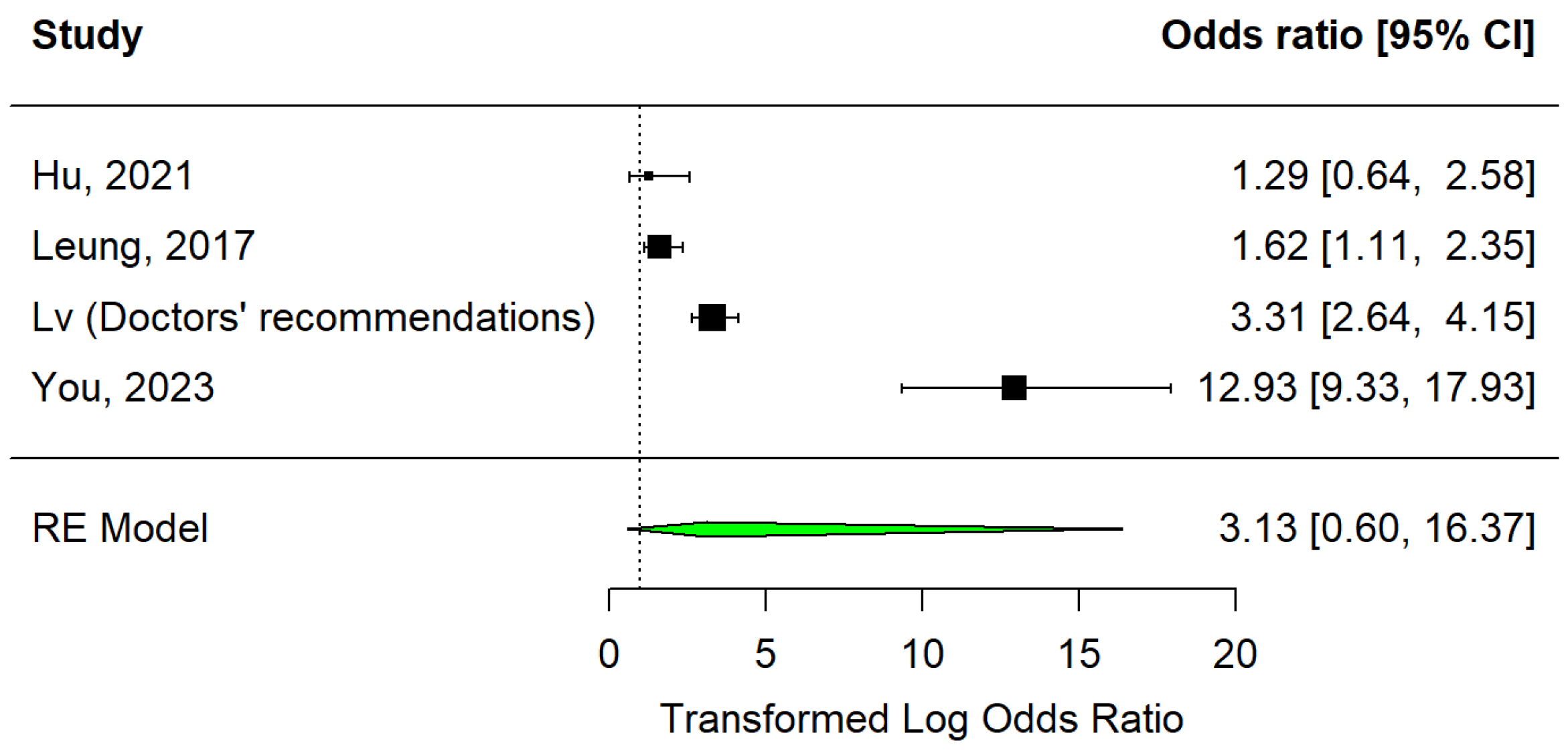
3.3.2. Medical Advice
3.3.3. Free and Subsidised Vaccination
3.3.4. Writing Strategy
3.3.5. Comparison of Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado-Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef]
- Kayser, V.; Ramzan, I. Vaccines and vaccination: History and emerging issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Australian Government, Department of Health and Aged Care. Immunisation Protects You, Your Family, and Others in the Community from Serious Diseases. Learn About the Benefits of Immunisation and How Vaccines Work. June 2022. Available online: https://www.health.gov.au/topics/immunisation/about-immunisation (accessed on 1 December 2024).
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza Vaccination in the Elderly. Hum. Vaccines Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef]
- de Oliveira Gomes, J.; Gagliardi, A.M.; Andriolo, B.N.; Torloni, M.R.; Andriolo, R.B.; Puga, M.E.D.S.; Canteiro Cruz, E. Vaccines for Preventing Herpes Zoster in Older Adults. Cochrane Database Syst. Rev. 2023, 10, CD008858. [Google Scholar] [CrossRef]
- Rizzo, C.; Rezza, G.; Ricciardi, W. Strategies in Recommending Influenza Vaccination in Europe and the US. Hum. Vaccines Immunother. 2018, 14, 693–698. [Google Scholar] [CrossRef]
- Wei, L.; Zeng, W.; Huang, Y.; Ye, G.; Chen, Y.; Yang, L.; Cai, Y. COVID-19 Vaccination Coverage and Its Cognitive Determinants among Older Adults in Shanghai, China, during the COVID-19 Epidemic. Front. Public Health 2023, 11, 1163616. [Google Scholar] [CrossRef]
- Gül, A.; Oncel, S.S. The Importance of Vaccines in a Sustainable Healthy Society. In A Sustainable Green Future; Oncel, S.S., Ed.; Springer: Cham, Switzerland, 2023; pp. 183–212. [Google Scholar] [CrossRef]
- Schuchat, A. Human Vaccines and Their Importance to Public Health. Procedia Vaccinol. 2011, 5, 120–126. [Google Scholar] [CrossRef]
- Lisenby, K.M.; Patel, K.N.; Uichanco, M.T. The role of pharmacists in addressing vaccine hesitancy and the measles outbreak. J. Pharm. Pract. 2021, 34, 127–132. [Google Scholar] [CrossRef]
- Bardosh, K.; de Figueiredo, A.; Gur-Arie, R.; Jamrozik, E.; Doidge, J.; Lemmens, T.; Keshavjee, S.; Graham, J.E.; Baral, S. The Unintended Consequences of COVID-19 Vaccine Policy: Why Mandates, Passports, and Restrictions May Cause More Harm than Good. BMJ Glob. Health 2022, 7, e008684. [Google Scholar] [CrossRef]
- Velicia Peñas, C.; Del Campo Pérez, V.M.; Rivero Calle, I.; Armenteros Del Olmo, L.; Pérez Rodríguez, M.T.; Gestal Otero, J.J. Expert opinion on strategies to improve vaccination coverage against seasonal influenza. Rev. Española Quimioter. 2022, 35, 435–443. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lv, M.; Fang, R.; Wu, J.; Pang, X.; Deng, Y.; Lei, T.; Xie, Z. The free vaccination policy of influenza in Beijing, China: The vaccine coverage and its associated factors. Vaccine 2016, 34, 2135–2140. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, M.; Losos, M.; Tugwell, P.; Ga, S.W.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://api.semanticscholar.org/CorpusID:79550924 (accessed on 1 December 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Martins, J.P.; Santos, M.; Martins, A.; Felgueiras, M.; Santos, R. Seasonal influenza vaccine effectiveness in persons aged 15–64 years: A systematic review and meta-analysis. Vaccines 2023, 11, 1322. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G.; Sinha, B. Statistical Meta-Analysis with Applications; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- UNDP (United Nations Development Programme). Human Development Report 2023–24. UNDP (United Nations Development Programme). 2024. Available online: http://report.hdr.undp.org (accessed on 4 December 2024).
- Du, Y.; Jin, C.; Jit, M.; Chantler, T.; Lin, L.; Larson, H.J.; Li, J.; Gong, W.; Yang, F.; Ren, N.; et al. Influenza vaccine uptake among children and older adults in China: A secondary analysis of a quasi-experimental study. BMC Infect. Dis. 2023, 23, 225. [Google Scholar] [CrossRef]
- Jiang, X.; Shang, X.; Lin, J.; Zhao, Y.; Wang, W.; Qiu, Y. Impacts of free vaccination policy and associated factors on influenza vaccination behavior of the elderly in China: A quasi-experimental study. Vaccine 2021, 39, 846–852. [Google Scholar] [CrossRef]
- Wu, D.; Jin, C.; Bessame, K.; Tang, F.F.Y.; Ong, J.J.; Wang, Z.; Xie, Y.; Jit, M.; Larson, H.J.; Chantler, T.; et al. Effectiveness of a pay-it-forward intervention compared with user-paid vaccination to improve influenza vaccine uptake and community engagement among children and older adults in China: A quasi-experimental pragmatic trial. Lancet Infect. Dis. 2022, 22, 1484–1492. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Domínguez-Berjón, M.F.; García-Riolobos, C.; Zoni, A.C.; Aréjula Torres, J.L.; Sánchez-Perruca, L.; Astray-Mochales, J. Effect of mobile phone text messaging for improving the uptake of influenza vaccination in patients with rare diseases. Vaccine 2019, 37, 5257–5264. [Google Scholar] [CrossRef]
- Regan, A.K.; Bloomfield, L.; Peters, I.; Effler, P.V. Randomized controlled trial of text message reminders for increasing influenza vaccination. Ann. Fam. Med. 2017, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, P.G.; Casillas, A.; Duru, O.K.; Ong, M.K.; Vangala, S.; Tseng, C.H.; Albertin, C.; Humiston, S.G.; Ross, M.K.; Friedman, S.R.; et al. Evaluation of behavioral economic strategies to raise influenza vaccination rates across a health system: Results from a randomized clinical trial. Prev. Med. 2023, 170, 107474. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rish, E.Y.; Barakat, N.A. The impact of pharmacist-led educational intervention on pneumococcal vaccine awareness and acceptance among the elderly in Jordan. Hum. Vaccines Immunother. 2021, 17, 1181–1189. [Google Scholar] [CrossRef]
- Leung, K.C.; Mui, C.; Chiu, W.Y.; Ng, Y.Y.; Chen, M.H.Y.; Ho, P.H.; Kwok, C.P.; Lam, S.S.M.; Wong, C.Y.; Wong, K.Y.; et al. Impact of patient education on influenza vaccine uptake among community-dwelling elderly: A randomized controlled trial. Health Educ. Res. 2017, 32, 455–464. [Google Scholar] [CrossRef]
- Sääksvuori, L.; Betsch, C.; Nohynek, H.; Salo, H.; Sivelä, J.; Böhm, R. Information nudges for influenza vaccination: Evidence from a large-scale cluster-randomized controlled trial in Finland. PLoS Med. 2022, 19, e1003919. [Google Scholar] [CrossRef]
- Johansen, N.D.; Vaduganathan, M.; Bhatt, A.S.; Lee, S.G.; Modin, D.; Claggett, B.L.; Dueger, E.L.; Samson, S.; Loiacono, M.M.; Harris, R.C.; et al. Electronic nudges to increase influenza vaccination uptake among patients with heart failure: A pre-specified analysis of the NUDGE-FLU trial. Eur. J. Heart Fail. 2023, 25, 1450–1458. [Google Scholar] [CrossRef]
- You, Y.; Li, X.; Jiang, S.; Liang, J.; Xie, P.; Zou, X.; Liu, G.; Han, X. Can primary care physician recommendation improve influenza vaccine uptake among older adults? A community health centre-based experimental study in China. BMC Prim. Care 2023, 24, 16. [Google Scholar] [CrossRef]
- Hu, P.L.; Koh, E.Y.L.; Tay, J.S.H.; Chan, V.X.B.; Goh, S.S.M.; Wang, S.Z. Assessing the impact of educational methods on influenza vaccine uptake and patient knowledge and attitudes: A randomized controlled trial. Singap. Med. J. 2023, 64, 98–104. [Google Scholar] [CrossRef]
- Lee, S.K.; Sun, J.; Jang, S.; Connelly, S. Misinformation of COVID-19 Vaccines and Vaccine Hesitancy. Sci. Rep. 2022, 12, 13681. [Google Scholar] [CrossRef]
- Ho, H.J.; Tan, Y.R.; Cook, A.R.; Koh, G.; Tham, T.Y.; Anwar, E.; Hui Chiang, G.S.; Lwin, M.O.; Chen, M.I. Increasing influenza and pneumococcal vaccination uptake in seniors using point-of-care informational interventions in primary care in Singapore: A pragmatic, cluster-randomized crossover trial. Am. J. Public Health 2019, 109, 1776–1783. [Google Scholar] [CrossRef]
- Jiang, M.; Yao, X.; Li, P.; Fang, Y.; Feng, L.; Hayat, K.; Shi, X.; Gong, Y.; Peng, J.; Atif, N. Impact of video-led educational intervention on uptake of influenza vaccine among the elderly in Western China: A community-based randomized controlled trial. BMC Public Health 2022, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Lieu, T.A.; Elkin, E.P.; Escobar, P.R.; Finn, L.; Klein, N.P.; Durojaiye, C.; Prausnitz, S.; Quesenberry, C.P.; Sawyer, D.; Teran, S.; et al. Effect of electronic and mail outreach by primary care physicians for COVID-19 vaccination of Black and Latinoolder adults. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2793497 (accessed on 1 December 2024).
- Whitehead, H.S.; French, C.E.; Caldwell, D.M.; Letley, L.; Mounier-Jack, S. A systematic review of communication interventions for countering vaccine misinformation. Vaccine 2023, 41, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- Ganczak, M.; Gil, K.; Korzeń, M.; Bażydło, M. Coverage and Influencing Determinants of Influenza Vaccination in Elderly Patients in a Country with a Poor Vaccination Implementation. Int. J. Environ. Res. Public Health 2017, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Naeim, A.; Guerin, R.J.; Baxter-King, R.; Okun, A.H.; Wenger, N.; Sepucha, K.; Stanton, A.L.; Rudkin, A.; Holliday, D.; Rossell Hayes, A.; et al. Strategies to increase the intention to get vaccinated against COVID-19: Findings from a nationally representative survey of US adults, October 2020 to October 2021. Vaccine 2022, 40, 7571–7578. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, S.T.; Kaushal, A.; Grimani, A.; Von Wagner, C.; Sniehotta, F.F.; Vlaev, I. Effect of communicating community immunity on COVID-19 vaccine-hesitant people from ethnically diverse backgrounds: An experimental vignette study in the UK. BMJ Open 2022, 12, e065804. [Google Scholar] [CrossRef]
- Sudharsanan, N.; Favaretti, C.; Hachaturyan, V.; Bärnighausen, T.; Vandormael, A. Effects of side-effect risk framing strategies on COVID-19 vaccine intentions: A randomized controlled trial. eLife 2022, 11, e78765. [Google Scholar] [CrossRef]
- Okuhara, T.; Ishikawa, H.; Ueno, H.; Okada, H.; Kato, M.; Kiuchi, T. Readability Assessment of Vaccine Information: A Systematic Review for Addressing Vaccine Hesitancy. Patient Educ. Couns. 2022, 105, 331–338. [Google Scholar] [CrossRef]
- Singh, P.; Dhalaria, P.; Kashyap, S.; Soni, G.K.; Nandi, P.; Ghosh, S.; Mohapatra, M.K.; Rastogi, A.; Prakash, D. Strategies to Overcome Vaccine Hesitancy: A Systematic Review. Syst. Rev. 2022, 11, 78. [Google Scholar] [CrossRef]
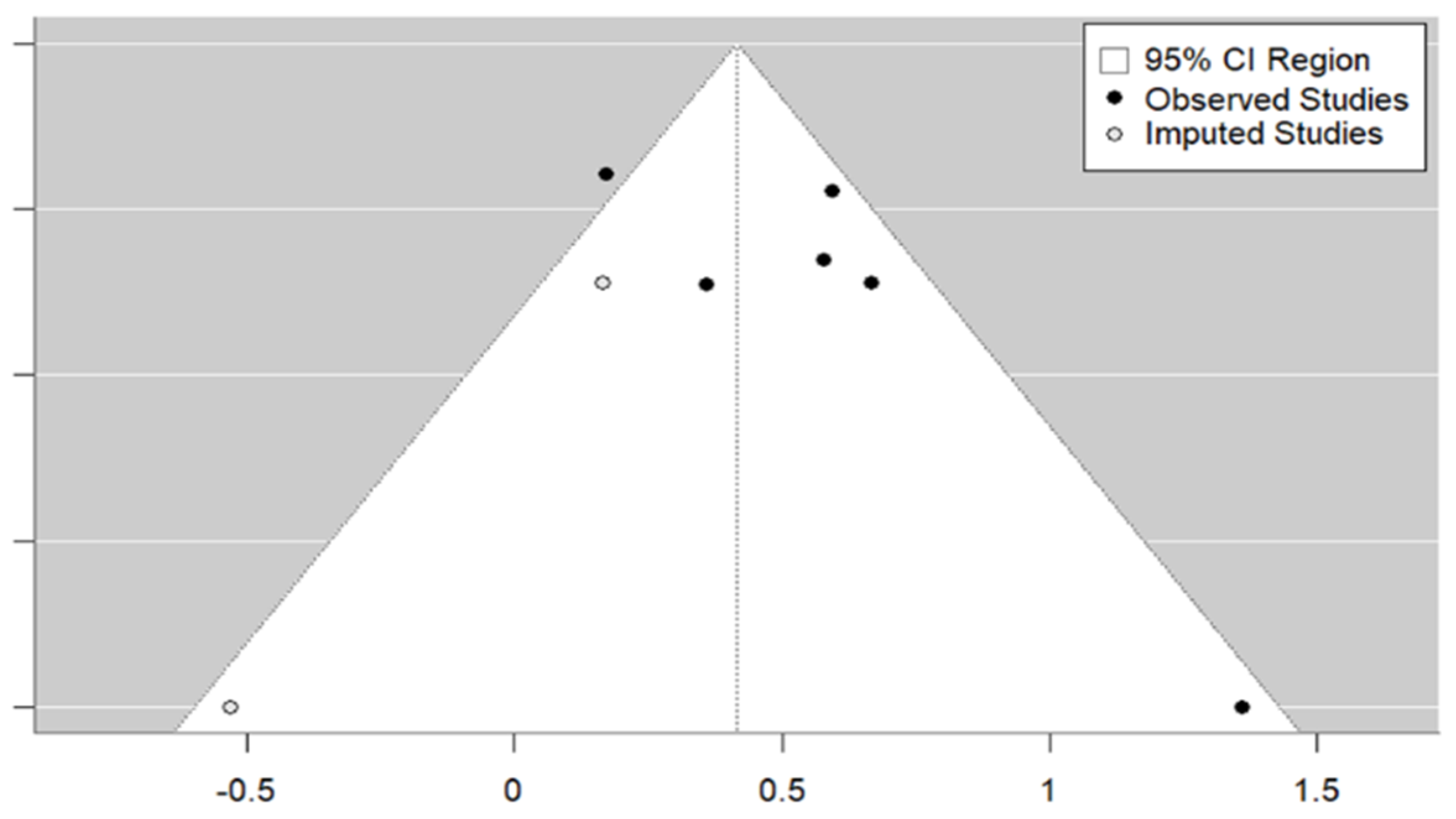
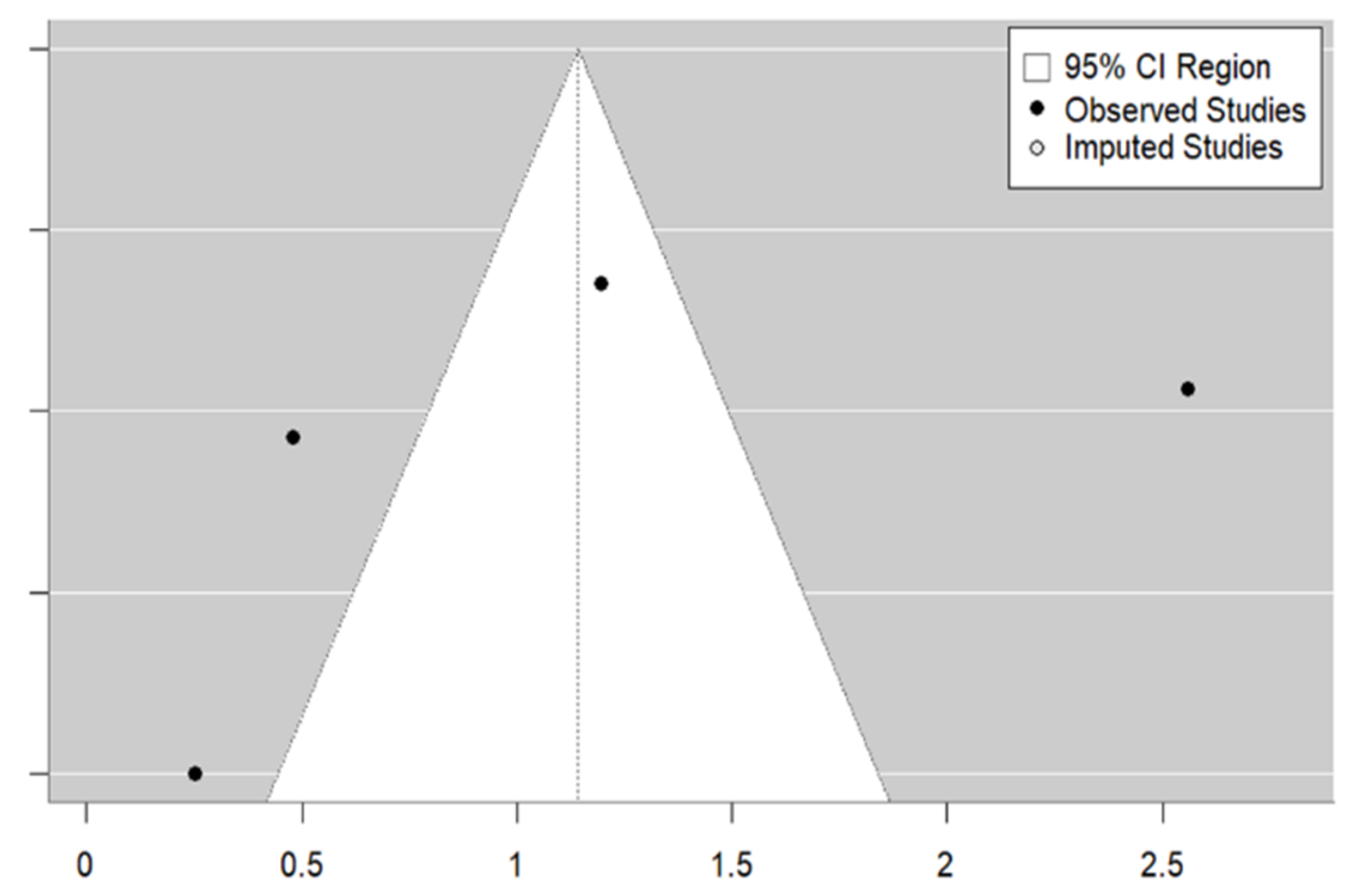
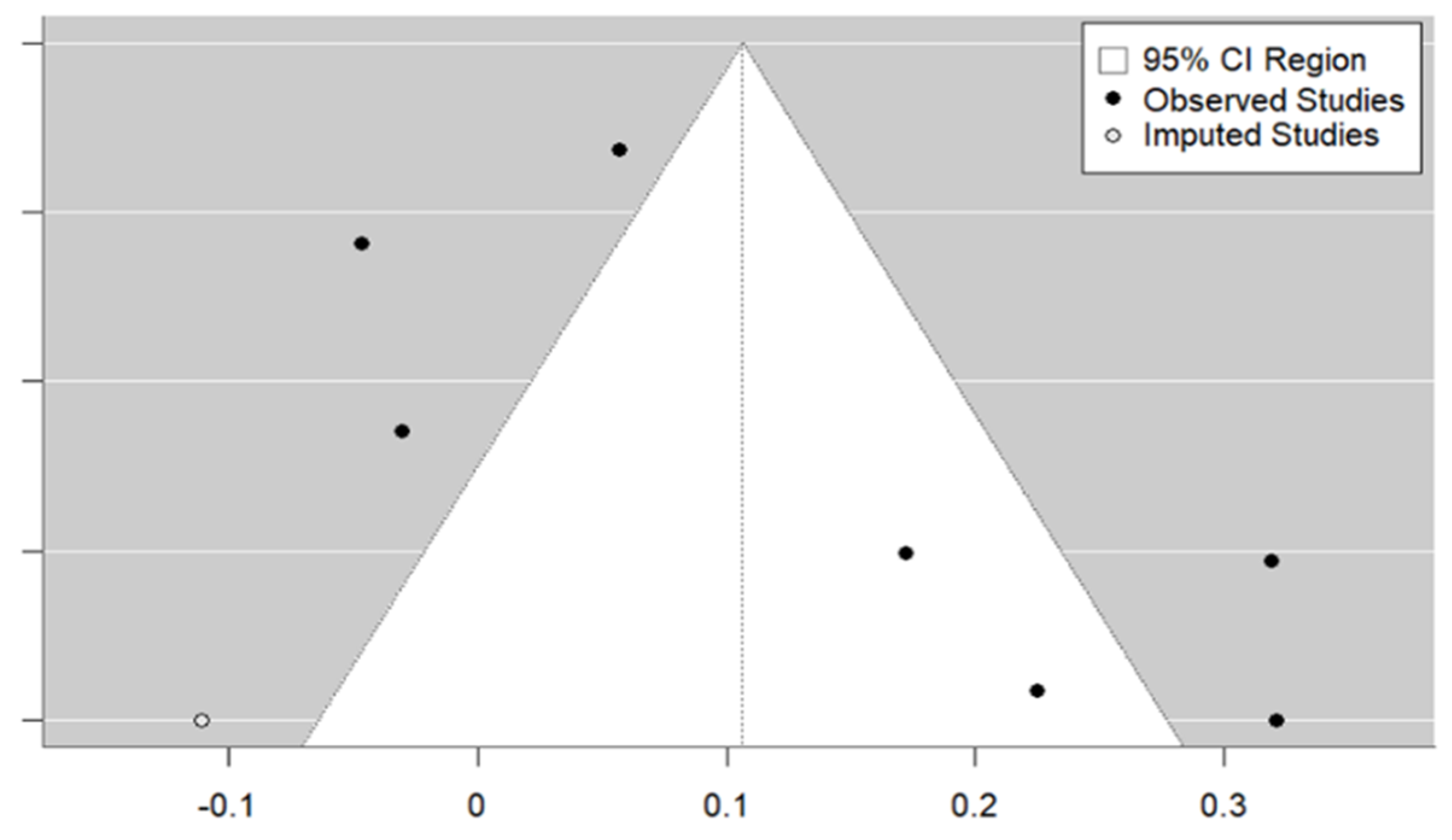
| Study | Country | HDI | Rural/ Urban | Age Mean | Female (%) | Type of Study | Vaccine | Population | Sample Size | Type of Strategy | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abu-rish & Barakat, 2021 [28] | Jordan | High (0.736) | Urban | 70.0 | 78.8 | Intervention study | Pneumococcal | Unvaccinated ≥ 65 years old | 700 | Educational charter | * 1.95 (1.35–2.81) |
| Du et al., 2023 [22] | China | High (0.788) | Rural and urban | NA | NA | Quasi-experimental trial | Influenza | Unvaccinated ≥ 60 years old | 225 | Free and subsidised vaccination | 6.91 (3.59–13.32) |
| Esteban-Vasallo et al., 2019 [25] | Spain | Very high (0.911) | Mostly urban | NA | NA | Quasi-experimental study, pre/post intervention | Influenza | Unvaccinated > 64 years old with concomitant comorbidities and rare disease | 17695 | SMS | 1.38 (1.21–1.57) |
| Ho et al., 2019 [35] | Singapore | Very high (0.949) | Urban | NA | 55.6 | Randomised, crossover study | Influenza | Unvaccinated people aged ≥ 65 years, with or without illness and who have visited the clinic | 4378 | Information brochures | * 1.43 (0.99-2.07) |
| Pneumococcal | * 1.78 (1.28-2.48) | ||||||||||
| Hu et al., 2023 [33] | Singapore | Very high (0.949) | Urban | 71.0 | 49.1 | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old | 320 | Individualised counselling | * 1.29 (0.64–2.58) |
| Jiang et al., 2021 [23] | China | High (0.788) | NA | 69.5 | 47.2 | Quasi-experimental trial | Influenza | Unvaccinated people aged ≥ 60 from local health centres | 1210 | Free vaccination | * 30.46 (20.86–44.49) |
| Jiang et al., 2022 [36] | China | High (0.788) | Urban | NA | 54.9 | Randomised clinical trial | Influenza | Unvaccinated ≥ 60 years old | 350 | Educational video | * 3.90 (1.41–10.75) |
| Johansen et al., 2023 [31] | Denmark | Very high (0.952) | NA | NA | NA | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old with heart failure, excludes residents of nursing homes | 384913 | Electronic letters | 1.06 (1.03–1.09) |
| Leung et al., 2017 [29] | Hong Kong | Very high (0.956) | Urban | 74.6 | 52.5 | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old | 529 | Individualised verbal education | 1.62 (1.11–2.35) |
| Lieu et al., 2022 [37] | United States | Very high (0.927) | NA | 72.6 | 56.3 | Randomised clinical trial | COVID-19 | Unvaccinated ≥ 65 years old | 3858 | Electronic messages | 1.25 (1.06–1.47) |
| Letters | 1.19 (1.04–1.35) | ||||||||||
| Lv et al., 2016 [15] | China | High (0.788) | Mostly urban (83.2%) | NA | 57.2 | Retrospective cross-sectional study | Influenza | Unvaccinated people who have lived in Beijing for at least one year and are ≥ 60 years old | 1673 | Community counselling | * 1.812 (1.446–2.27) |
| Television | * 1.18 (0.97–1.45) | ||||||||||
| Doctors’ recommendations | * 3.31 (2.64–4.15) | ||||||||||
| Regan et al., 2017 [26] | Australia | Very high (0.946) | Mostly urban | NA | NA | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old | 3613 | SMS reminders | * 1.38 (1.16–1.63) |
| Sääksvuori et al., 2022 [30] | Finland | Very high (0.942) | Rural | 75.5 | NA | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old with no vaccination data from the previous year | 7324 | Letter reminders | 0.97 (0.88–1.07) |
| Szilagyi et al., 2023 [27] | United States | Very high (0.927) | Mostly urban | NA | NA | Randomised clinical trial | Influenza | Unvaccinated ≥ 65 years old | 39235 | SMS reminders | 0.95 (0.91–1.00) |
| Wu et al., 2022 [24] | China | High (0.788) | Rural (33%) and urban (67%) | 68.0 | 68.7 | Quasi-experimental trial | Influenza | Unvaccinated ≥ 60 years old | 150 | Subsidised vaccine | * 5 (2.3–10.08) |
| You et al., 2023 [32] | China | High (0.788) | Urban | NA | NA | Experimental study | Influenza | Unvaccinated ≥ 60 years old | 2158 | Doctors’ recommendations | 12.93 (9.33–17.93) |
| Study ID | Items | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | Score | ||||||
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | ||
| Abu-rish & Barakat, 2021 [28] | * | * | - | * | ** | - | * | * | 7/9 |
| Du et al., 2023 [22] | * | * | * | - | ** | - | * | - | 6/9 |
| Esteban-Vasallo et al., 2019 [25] | * | * | * | * | ** | * | * | * | 9/9 |
| Ho et al., 2019 [35] | * | * | - | * | ** | * | * | * | 8/9 |
| Jiang et al., 2021 [23] | * | * | * | - | ** | - | * | * | 7/9 |
| Lv et al., 2016 [15] | * | * | * | - | ** | - | * | * | 7/9 |
| Wu et al., 2022 [24] | * | * | * | * | ** | * | * | * | 9/9 |
| You et al., 2023 [32] | * | * | - | - | ** | * | * | * | 8/9 |
| Subgroup | N.s (N.i) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Type of intervention | 0.019 | |||
| Educational | 6 (4378) | 1.63 (1.22–2.19) | 59.4 | |
| Medical advice | 4 (2158) | 3.13 (0.60–16.37) | 97.0 | |
| Writing | 7 (384,913) | 1.14 (0.99–1.32) | 93.3 | |
| HDI | <0.001 | |||
| High | 9 (2158) | 4.43 (2.06–9.53) | 97.3 | |
| Very high | 11 (384,913) | 1.22 (1.07–1.39) | 92.3 | |
| Age (mean) | 0.099 | |||
| ≤71 | 4 (1210) | 4.43 (0.54–36.6) | 96.6 | |
| >71 | 4 (7324) | 1.18 (0.88–1.58) | 79.3 | |
| Age (minimum) | <0.001 | |||
| 60 | 8 (2158) | 4.92 (2.11–11.50) | 97.5 | |
| 65 | 12 (39,235) | 1.26 (1.10–1.45) | 93.4 | |
| Female (%) | 0.935 | |||
| ≤57 | 8 (3858) | 2.27 (0.95–5.41) | 98.2 | |
| >57 | 5 (1673) | 2.29 (1.10–4.78) | 93.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Pinho, C.; Oliveira, A.; Santos, R.; Felgueiras, M.; Martins, J.P. Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis. Vaccines 2024, 12, 1395. https://doi.org/10.3390/vaccines12121395
Pereira A, Pinho C, Oliveira A, Santos R, Felgueiras M, Martins JP. Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis. Vaccines. 2024; 12(12):1395. https://doi.org/10.3390/vaccines12121395
Chicago/Turabian StylePereira, Ana, Cláudia Pinho, Adriana Oliveira, Rui Santos, Miguel Felgueiras, and João P. Martins. 2024. "Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis" Vaccines 12, no. 12: 1395. https://doi.org/10.3390/vaccines12121395
APA StylePereira, A., Pinho, C., Oliveira, A., Santos, R., Felgueiras, M., & Martins, J. P. (2024). Vaccination Promotion Strategies in the Elderly: Systematic Review and Meta-Analysis. Vaccines, 12(12), 1395. https://doi.org/10.3390/vaccines12121395