An Inactivated West Nile Virus Vaccine Candidate Based on the Lineage 2 Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Animals
2.4. Titration of WNV
2.5. Production of Hyperimmune Ascites Fluids
2.6. Preparation of WNV-Containing Fluid
- The cultivation of monolayer culture was conducted in a 2 L roller bottle (TufRol, Thermo Fisher Scientific, Waltham, MA, USA) in cell production roller apparatus (BELLCO Biotechnology, Vineland, NJ, USA) in Vero cells at 37 °C;
- Pseudo-suspension cultivation on Cytodex-3 type microcarrier beads (Cytiva, Marlborough, MA, USA) in 1.5 L spinner flasks at 37 °C. The seed concentration of Vero cells was 600–900 thousand cells/mL, and the concentration of Cytodex-3 microcarrier was 5–6 g/L.
2.7. Inactivation of Virus-Containing Fluid
2.8. Preparation of Purified Inactivated WNV
2.9. Determination of Total Protein
2.10. Determination of Total DNA
2.11. Preparation of the Standard iWNV Sample
2.12. Electrophoresis and Western Blotting
2.13. Enzyme-Linked Immunosorbent Assay
2.14. Mouse Immune Serum After iWNV Immunization
2.15. Neutralization Assay
2.16. Control of Specific Safety of the Vaccine Candidate In Vivo
2.17. Titration of WNV in Mice
2.18. Evaluation of the Protectivity of the Vaccine Preparation
2.19. Reverse Transcription and Real-Time PCR
2.20. Genome Sequencing of WNV Strains
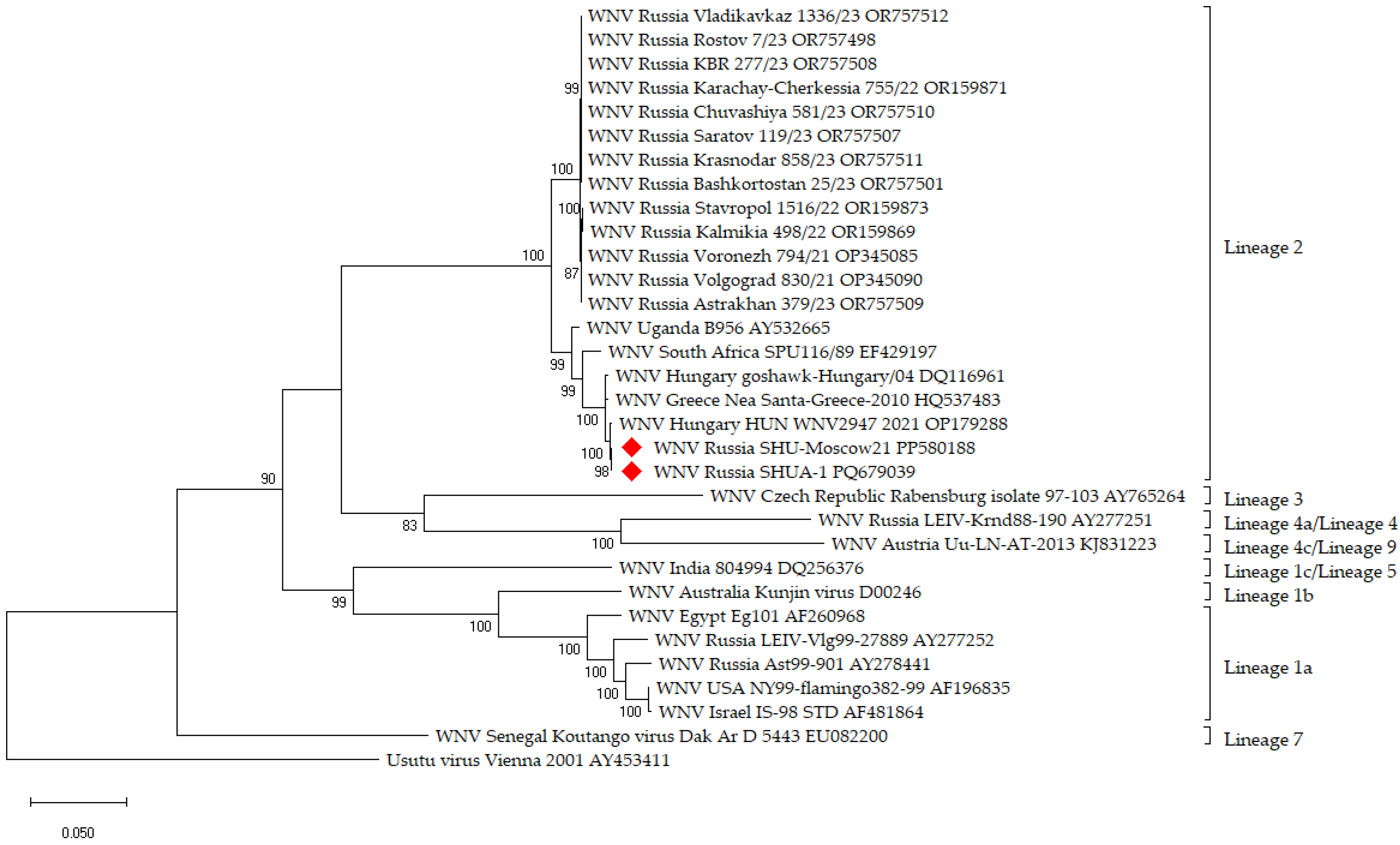
2.21. Phylogenetic Analysis
2.22. Statistical Analysis
3. Results
3.1. Vaccine Strain Selection
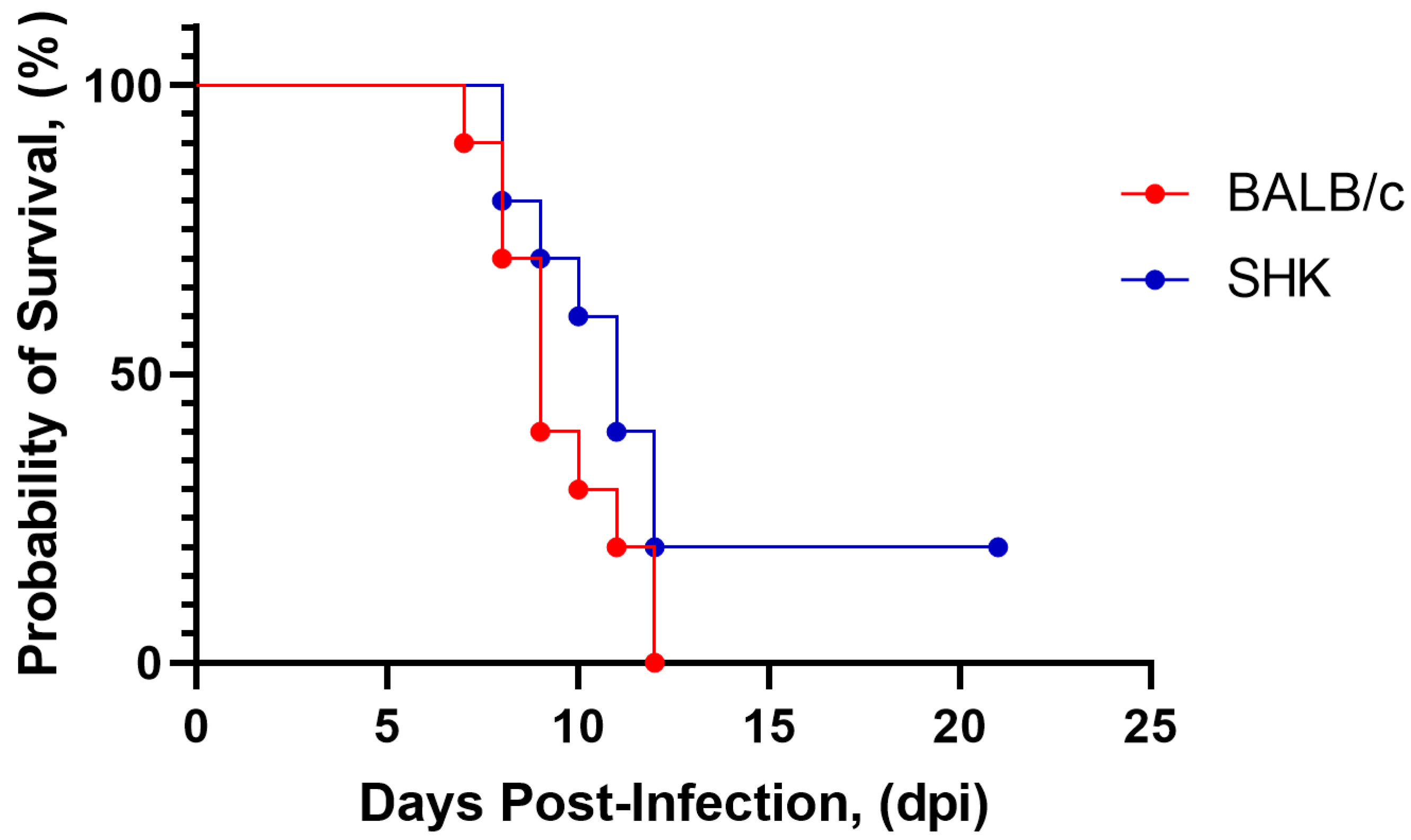
3.2. Selection of Animal Models
3.3. Inactivation of Viral Suspension
3.4. Preparation of a Purified, Inactivated West Nile Virus (iWNV) Vaccine Candidate
3.5. Safety of Purified iWNV Antigen In Vivo
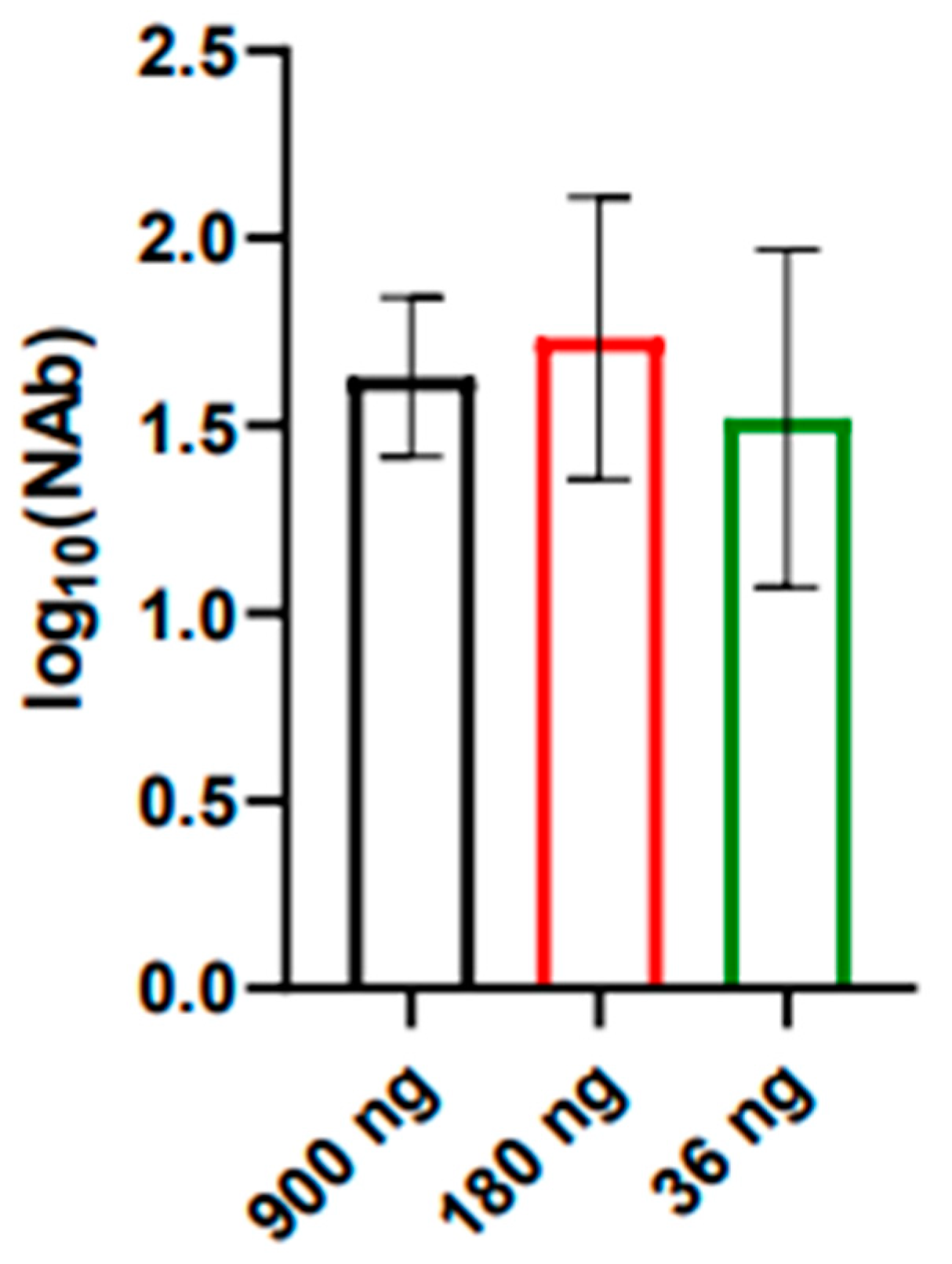
3.6. Immunogenicity and Protectivity of iWNV Antigen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeller, H.G.; Schuffenecker, I. West Nile Virus: An Overview of Its Spread in Europe and the Mediterranean Basin in Contrast to Its Spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Mertens, E.; Desprès, P. West Nile Virus and Its Emergence in the United States of America. Vet. Res. 2010, 41, 67. [Google Scholar] [CrossRef]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A Systematic Review of Environmental Factors Related to WNV Circulation in European and Mediterranean Countries. One Health 2023, 16, 100478. [Google Scholar] [CrossRef]
- Fonzo, M.; Bertoncello, C.; Tudor, L.; Miccolis, L.; Serpentino, M.; Petta, D.; Amoruso, I.; Baldovin, T.; Trevisan, A. Do We Protect Ourselves against West Nile Virus? A Systematic Review on Knowledge, Attitudes, and Practices and Their Determinants. J. Infect. Public Health 2024, 17, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308. [Google Scholar] [CrossRef]
- Van Der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile Virus in the Vertebrate World. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Frasca, F.; Sorrentino, L.; Fracella, M.; D’Auria, A.; Coratti, E.; Maddaloni, L.; Bugani, G.; Gentile, M.; Pierangeli, A.; d’Ettorre, G.; et al. An Update on the Entomology, Virology, Pathogenesis, and Epidemiology Status of West Nile and Dengue Viruses in Europe (2018–2023). Trop. Med. Infect. Dis. 2024, 9, 166. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Stuchin, M.; Machalaba, C.C.; Karesh, W.B. Vector-Borne Diseases: Animals Snd Patterns. In Global Health Impacts of Vector-Borne Diseases: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Dey, D.; Poudyal, S.; Rehman, A.; Hasan, S.S. Structural and Biochemical Insights into Flavivirus Proteins. Virus Res. 2021, 296, 198343. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Koch, R.T.; Erazo, D.; Folly, A.J.; Johnson, N.; Dellicour, S.; Grubaugh, N.D.; Vogels, C.B.F. Genomic Epidemiology of West Nile Virus in Europe. One Health 2024, 18, 100664. [Google Scholar] [CrossRef] [PubMed]
- Rajaiah, P.; Mayilsamy, M.; Kumar, A. West Nile Virus in India: An Update on Its Genetic Lineages. J. Vector Borne Dis. 2023, 60, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Neglected Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef]
- Toporkov, A.V.; Putintseva, E.V.; Udovichenko, S.K.; Boroday, N.V.; Molchanova, E.V.; Bondareva, O.S.; Antonov, A.S. Study of the circulation and properties of the West Nile virus in Russia in 2022. J. Microbiol. Epidemiol. Immunobiol. 2024, 101, 114–126. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Williams, D.T. The Zoonotic Flaviviruses of Southern, South-Eastern and Eastern Asia, and Australasia: The Potential for Emergent Viruses. Zoonoses Public Health 2009, 56, 338–356. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive Spread of a Neuroinvasive Lineage 2 West Nile Virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef]
- Pietsch, C.; Michalski, D.; Münch, J.; Petros, S.; Bergs, S.; Trawinski, H.; Lübbert, C.; Liebert, U.G. Autochthonous West Nile Virus Infection Outbreak in Humans, Leipzig, Germany, August to September 2020. Eurosurveillance 2020, 25, 2001786. [Google Scholar] [CrossRef]
- Baturin, A.A.; Tkachenko, G.A.; Ledeneva, M.L.; Lemasova, L.V.; Bondareva, O.S.; Kaysarov, I.D.; Shpak, I.M.; Boroday, N.V.; Teteryatnikova, N.N. Teteryatnikova Molecular Genetic Analysis of West Nile Virus Variants Circulating in Russia between 2010 and 2019. J. Microbiol. Epidemiol. Immunobiol. 2021, 98, 308–318. [Google Scholar] [CrossRef]
- Barzon, L.; Montarsi, F.; Quaranta, E.; Monne, I.; Pacenti, M.; Michelutti, A.; Toniolo, F.; Danesi, P.; Marchetti, G.; Gobbo, F.; et al. Early Start of Seasonal Transmission and Co-Circulation of West Nile Virus Lineage 2 and a Newly Introduced Lineage 1 Strain, Northern Italy, June 2022. Eurosurveillance 2022, 27, 2200548. [Google Scholar] [CrossRef]
- Saiz, J.-C.; Martín-Acebes, M.A.; Blázquez, A.B.; Escribano-Romero, E.; Poderoso, T.; Jiménez De Oya, N. Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation. Virulence 2021, 12, 1145–1173. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Jiménez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The Challenge of West Nile Virus in Europe: Knowledge Gaps and Research Priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, F.; Oude Munnink, B.B.; Munger, E.; Sikkema, R.S.; Pappa, S.; Tsioka, K.; Sinigaglia, A.; Dal Molin, E.; Shih, B.B.; et al. West Nile Virus Spread in Europe: Phylogeographic Pattern Analysis and Key Drivers. PLoS Pathog. 2024, 20, e1011880. [Google Scholar] [CrossRef] [PubMed]
- Toporkov, A.V.; Putintseva, E.V.; Udovichenko, S.K. West Nile fever as a relevant health hazard: The history of studying and measures of its prevention in Russia. Health Risk Anal. 2023, 3, 138–149. [Google Scholar] [CrossRef]
- Naveed, A.; Eertink, L.G.; Wang, D.; Li, F. Lessons Learned from West Nile Virus Infection:Vaccinations in Equines and Their Implications for One Health Approaches. Viruses 2024, 16, 781. [Google Scholar] [CrossRef] [PubMed]
- Fagre, A.C.; Lyons, S.; Staples, J.E.; Lindsey, N. West Nile Virus and Other Nationally Notifiable Arboviral Diseases. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 901–906. [Google Scholar] [CrossRef]
- Putintseva, E.V.; Alekseychik, I.O.; Chesnokova, S.N.; Udovichenko, S.K.; Boroday, N.V.; Nikitin, D.N.; Agarkova, E.A.; Baturin, A.A.; Shpak, I.M.; Fomina, V.K.; et al. Results of the West Nile Fever Agent Monitoring in the Russian Federation in 2019 and the Forecast of Epidemic Situation Development in 2020. Probl. Osob. Opasnykh Infektsii 2020, 1, 51–60. [Google Scholar] [CrossRef][Green Version]
- Klimova, E.A.; Karetkina, G.N.; Shakaryan, A.K.; Sayfullin, M.A.; Karan, L.S.; Larichev, V.F.; Grigoreva, Y.E.; Morozkin, E.S.; Lyapeikova, E.A.; Abramova, E.N. West Nile fever on the territory of the Moscow agglomeration. Infect. Dis. News Opin. Train. 2021, 10, 13–21. [Google Scholar] [CrossRef]
- Filette, M.D.; Ulbert, S.; Diamond, M.S.; Sanders, N.N. Recent Progress in West Nile Virus Diagnosis and Vaccination. Vet. Res. 2012, 43, 16. [Google Scholar] [CrossRef]
- Kaiser, J.A.; Barrett, A.D.T. Twenty Years of Progress Toward West Nile Virus Vaccine Development. Viruses 2019, 11, 823. [Google Scholar] [CrossRef]
- Ulbert, S. West Nile Virus Vaccines–Current Situation and Future Directions. Human. Vaccines Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef]
- Venter, M.; van Vuren, P.J.; Mentoor, J.; Paweska, J.; Williams, J. Inactivated West Nile Virus (WNV) Vaccine, Duvaxyn WNV, Protects against a Highly Neuroinvasive Lineage 2 WNV Strain in Mice. Vaccine 2013, 31, 3856–3862. [Google Scholar] [CrossRef]
- Minke, J.M.; Siger, L.; Cupillard, L.; Powers, B.; Bakonyi, T.; Boyum, S.; Nowotny, N.; Bowen, R. Protection Provided by a Recombinant ALVAC®-WNV Vaccine Expressing the prM/E Genes of a Lineage 1 Strain of WNV against a Virulent Challenge with a Lineage 2 Strain. Vaccine 2011, 29, 4608–4612. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.; Pugachev, K.; Bevilacqua, J.; Lang, J.; Monath, T. Preclinical and Clinical Development of a YFV 17 D-Based Chimeric Vaccine against West Nile Virus. Viruses 2013, 5, 3048–3070. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.M.; Nerurkar, V.R.; Luo, H.; Cropp, B.; Carrion, R.; De La Garza, M.; Coller, B.-A.; Clements, D.; Ogata, S.; Wong, T.; et al. Immunogenicity and Protective Efficacy of a Recombinant Subunit West Nile Virus Vaccine in Rhesus Monkeys. Clin. Vaccine Immunol. 2009, 16, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.K.; Richner, J.M.; Poore, E.A.; Patil, P.P.; Amanna, I.J.; Slifka, M.K.; Diamond, M.S. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J. Virol. 2013, 87, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Vorovitch, M.F.; Grishina, K.G.; Volok, V.P.; Chernokhaeva, L.L.; Grishin, K.V.; Karganova, G.G.; Ishmukhametov, A.A. Evervac: Phase I/II Study of Immunogenicity and Safety of a New Adjuvant-Free TBE Vaccine Cultivated in Vero Cell Culture. Hum. Vaccines Immunother. 2020, 16, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Romanova, L.I.; Gmyl, A.P.; Dzhivanian, T.I.; Bakhmutov, D.V.; Lukashev, A.N.; Gmyl, L.V.; Rumyantsev, A.A.; Burenkova, L.A.; Lashkevich, V.A.; Karganova, G.G. Microevolution of Tick-Borne Encephalitis Virus in Course of Host Alternation. Virology 2007, 362, 75–84. [Google Scholar] [CrossRef]
- Baryshnikova, V.; Turchenko, Y.; Tuchynskaya, K.; Belyaletdinova, I.; Butenko, A.; Dereventsova, A.; Ignatiev, G.; Kholodilov, I.; Larichev, V.; Lyapeykova, E.; et al. Recombinant TBEV Protein E of the Siberian Subtype Is a Candidate Antigen in the ELISA Test System for Differential Diagnosis. Diagnostics 2023, 13, 3277. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. ISBN 978-1-59745-198-7. [Google Scholar]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Lorenz, R.J.; Bögel, K. Laboratory Techniques in Rabies: Methods of Calculation; Monograph Series; World Health Organization: Geneva, Switzerland, 1973; pp. 321–335. [Google Scholar]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real Time PCR Assay for Detection of All Known Lineages of West Nile Virus. J. Virol. Methods 2016, 236, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz , C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated Virus Vaccines from Chemistry to Prophylaxis: Merits, Risks and Challenges. Expert. Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.N.; Terpening, S.J.; Snow, D.; Cobb, R.R.; Kistner, O. Vero Cell Technology for Rapid Development of Inactivated Whole Virus Vaccines for Emerging Viral Diseases. Expert. Rev. Vaccines 2017, 16, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Flaviviruses and Flavivirus Vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Yang, X.; Wei, Y.; Chen, J.-T.; Wang, X.-J.; Peng, H.-J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Htay-Htay, H. Safety and Immunogenicity of a Purified Inactivated Zika Virus Vaccine Candidate in Healthy Adults: An Observer-Blind, Randomised, Phase 1 Trial-The Lancet Infectious Diseases. Lancet Infect. Dis. 2021, 21, 1282–1292. [Google Scholar]
- Fehér, O.; Bakonyi, T.; Barna, M.; Nagy, A.; Takács, M.; Szenci, O.; Joó, K.; Sárdi, S.; Korbacska-Kutasi, O. Serum Neutralising Antibody Titres against a Lineage 2 Neuroinvasive West Nile Virus Strain in Response to Vaccination with an Inactivated Lineage 1 Vaccine in a European Endemic Area. Vet. Immunol. Immunopathol. 2020, 227, 110087. [Google Scholar] [CrossRef]
- Amanna, I.J.; Thomas, A.; Engelmann, F.; Hammarlund, E.; Raué, H.-P.; Bailey, A.L.; Poore, E.A.; Quintel, B.K.; Lewis, A.D.; Axthelm, M.K.; et al. Development of a Hydrogen Peroxide-Inactivated Vaccine That Protects against Viscerotropic Yellow Fever in a Non-Human Primate Model. CR Med. 2024, 5, 101655. [Google Scholar] [CrossRef]
- Charlier, N.; Leyssen, P.; Clercq, E.D.; Neyts, J. Rodent Models for the Study of Therapy against Flavivirus Infections. Antivir. Res. 2004, 63, 67–77. [Google Scholar] [CrossRef]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a Chimeric Zika Vaccine Using a Licensed Live-Attenuated Flavivirus Vaccine as Backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S.; Hart, C.E.; Hermance, M.E.; Brining, D.L.; Thangamani, S. An Overview of Animal Models for Arthropod-Borne Viruses. Comp. Med. 2017, 67, 232–241. [Google Scholar] [PubMed]
- Chernokhaeva, L.L.; Rogova, Y.V.; Kozlovskaya, L.I.; Romanova, L.I.; Osolodkin, D.I.; Vorovitch, M.F.; Karganova, G.G. Experimental Evaluation of the Protective Efficacy of Tick-Borne Encephalitis (TBE) Vaccines Based on European and Far-Eastern TBEV Strains in Mice and in Vitro. Front. Microbiol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Perelygin, A.A.; Scherbik, S.V.; Zhulin, I.B.; Stockman, B.M.; Li, Y.; Brinton, M.A. Positional Cloning of the Murine Flavivirus Resistance Gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9322–9327. [Google Scholar] [CrossRef] [PubMed]
- Brinton, M.A.; Perelygin, A.A. Genetic Resistance to Flaviviruses. Adv. Virus Res. 2003, 60, 43–85. [Google Scholar] [CrossRef]
- Moiseenko, A.; Zhang, Y.; Vorovitch, M.F.; Ivanova, A.L.; Liu, Z.; Osolodkin, D.I.; Egorov, A.M.; Ishmukhametov, A.A.; Sokolova, O.S. Structural Diversity of Tick-Borne Encephalitis Virus Particles in the Inactivated Vaccine Based on Strain Sofjin. Emerg. Microbes Infect. 2024, 13, 2290833. [Google Scholar] [CrossRef]
- Pichkur, E.B.; Vorovitch, M.F.; Ivanova, A.L.; Protopopova, E.V.; Loktev, V.B.; Osolodkin, D.I.; Ishmukhametov, A.A.; Samygina, V.R. The Structure of Inactivated Mature Tick-Borne Encephalitis Virus at 3.0 Å Resolution. Emerg. Microbes Infect. 2024, 13, 2313849. [Google Scholar] [CrossRef]
- Elbert, L.B.; Terletskay, E.N.; Timofeev, A.V.; Mironova, L.L.; Khapchaev, U.K.; Amosenko, F.A.; Svitkin, Y.V.; Alpatova, G.A.; Krutyanskaya, G.L. Inactivated Vaccine against Tick-Borne Encephalitis (TBE) Derived from Heteroploid Continuous Monkey Cell Line. Vaccine 1989, 7, 477. [Google Scholar] [CrossRef]
- Karpovich, L.V.; Kalashnikova, T.V.; Gorbunov, M.A. Comparative Study of Immunogenicity of Inactivated Vaccine against Hepatitis A ‘HEP-A-in-VAC’ According to Experimental and Clinical Studies. Vopr. Virusol. 1995, 6, 268–270. [Google Scholar]
- Ivanov, A.P.; Klebleeva, T.D.; Ivanova, O.E.; Ipatova, E.G.; Gmyl, L.V.; Ishmuhametov, A.A. Experimental Approaches to the Development of Inactivated Poliovirus Vaccine Based on Sabin Strains. Epidemiol. Vaccinal Prev. 2016, 15, 59–64. [Google Scholar] [CrossRef][Green Version]
- Piniaeva, A.; Ignatyev, G.; Kozlovskaya, L.; Ivin, Y.; Kovpak, A.; Ivanov, A.; Shishova, A.; Antonova, L.; Khapchaev, Y.; Feldblium, I.; et al. Immunogenicity and Safety of Inactivated Sabin-Strain Polio Vaccine “PoliovacSin”: Clinical Trials Phase I and II. Vaccines 2021, 9, 565. [Google Scholar] [CrossRef]
- Ivanov, A.P.; Klebleeva, T.D.; Rogova, Y.V.; Ivanova, O.G. Development of inactivated cultural yellow fever vaccine. Probl. Virol. 2020, 65, 212–217. [Google Scholar] [CrossRef]
- Gordeychuk, I.V.; Kozlovskaya, L.I.; Siniugina, A.A.; Yagovkina, N.V.; Kuzubov, V.I.; Zakharov, K.A.; Volok, V.P.; Dodina, M.S.; Gmyl, L.V.; Korotina, N.A.; et al. Safety and Immunogenicity of Inactivated Whole Virion COVID-19 Vaccine CoviVac in Clinical Trials in 18–60 and 60+ Age Cohorts. Viruses 2023, 15, 1828. [Google Scholar] [CrossRef]
- Dzagurova, T.K.; Siniugina, A.A.; Ishmukhametov, A.A.; Egorova, M.S.; Kurashova, S.S.; Balovneva, M.V.; Deviatkin, A.A.; Tkachenko, P.E.; Leonovich, O.A.; Tkachenko, E.A. Pre-Clinical Studies of Inactivated Polyvalent HFRS Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 545372. [Google Scholar] [CrossRef]
- Muraki, Y.; Fujita, T.; Matsuura, M.; Fuke, I.; Manabe, S.; Ishikawa, T.; Okuno, Y.; Morita, K. The Efficacy of Inactivated West Nile Vaccine (WN-VAX) in Mice and Monkeys. Virol. J. 2015, 12, 54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quintel, B.K.; Thomas, A.; Poer DeRaad, D.E.; Slifka, M.K.; Amanna, I.J. Advanced Oxidation Technology for the Development of a Next-Generation Inactivated West Nile Virus Vaccine. Vaccine 2019, 37, 4214–4221. [Google Scholar] [CrossRef] [PubMed]

| Indicators | Inactivation Temperature, °C | Initial VCF | Inactivated VCF | ||
|---|---|---|---|---|---|
| Time of Inactivation, hours | |||||
| 24 | 48 | 72 | |||
| CPE | 24 ± 1 | + | - | - | - |
| 32 ± 1 | + | - | - | - | |
| E, ng/mL | 24 ± 1 | 125 ± 12 | 80 ± 10 | 68 ± 4 | 63 ± 5 |
| 32 ± 1 | 125 ± 8 | 70 ± 6 | 50 ± 4 | 53 ± 2 | |
| Inoculum | Survival of SHK Mice, 7–8 g, Simultaneous i/p and i/c Inoculation, N = 10, % | Survival of SHK Suckling Mice, i/c Inoculation, N = 7, % |
|---|---|---|
| WNV | 30 | 0 |
| iVCF | 100 | 100 * |
| E Protein Dose (ng per Mouse) | Route of iWNV Antigen Administration | Survival, N = 10, % |
|---|---|---|
| 900 | intraperitoneally (0.5 mL) and intracerebrally (0.03 mL) | 100% |
| 180 | 100% | |
| 36 | 100% |
| Route of Inoculation | Number of the Positive Samples (PCR) in the Mouse Brain/Number of Examined Mice | |
|---|---|---|
| Dpi, hours | i/c | i/p |
| 1 h | 3/3 | 0/3 |
| 10 | 0/5 | 0/5 |
| 25 | 0/5 | 0/5 |
| Antigen Dose, ng E Protein per Mouse | Mice, (Survival/Total) | Survival, % | Median Survival, Days | Mice Without Clinical Symptoms (Healthy/Total) | Mice Without WNV Viral RNA in the Brains, 25 Days After Infection (RNA Negative/Total) * |
|---|---|---|---|---|---|
| 180 | 10/10 ** | 100 | - | 10/10 | 10/10 |
| 36 | 9/10 ** | 90 | 18 | 9/10 | 9/10 |
| Control group | 2/10 | 20 | 13 [11,12,13,14,15] | 2/10 | 1/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorovitch, M.F.; Tuchynskaya, K.K.; Kruglov, Y.A.; Peunkov, N.S.; Mostipanova, G.F.; Kholodilov, I.S.; Ivanova, A.L.; Fedina, M.P.; Gmyl, L.V.; Morozkin, E.S.; et al. An Inactivated West Nile Virus Vaccine Candidate Based on the Lineage 2 Strain. Vaccines 2024, 12, 1398. https://doi.org/10.3390/vaccines12121398
Vorovitch MF, Tuchynskaya KK, Kruglov YA, Peunkov NS, Mostipanova GF, Kholodilov IS, Ivanova AL, Fedina MP, Gmyl LV, Morozkin ES, et al. An Inactivated West Nile Virus Vaccine Candidate Based on the Lineage 2 Strain. Vaccines. 2024; 12(12):1398. https://doi.org/10.3390/vaccines12121398
Chicago/Turabian StyleVorovitch, Mikhail F., Ksenia K. Tuchynskaya, Yuriy A. Kruglov, Nikita S. Peunkov, Guzal F. Mostipanova, Ivan S. Kholodilov, Alla L. Ivanova, Maria P. Fedina, Larissa V. Gmyl, Evgeny S. Morozkin, and et al. 2024. "An Inactivated West Nile Virus Vaccine Candidate Based on the Lineage 2 Strain" Vaccines 12, no. 12: 1398. https://doi.org/10.3390/vaccines12121398
APA StyleVorovitch, M. F., Tuchynskaya, K. K., Kruglov, Y. A., Peunkov, N. S., Mostipanova, G. F., Kholodilov, I. S., Ivanova, A. L., Fedina, M. P., Gmyl, L. V., Morozkin, E. S., Roev, G. V., Karan, L. S., & Karganova, G. G. (2024). An Inactivated West Nile Virus Vaccine Candidate Based on the Lineage 2 Strain. Vaccines, 12(12), 1398. https://doi.org/10.3390/vaccines12121398





