Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
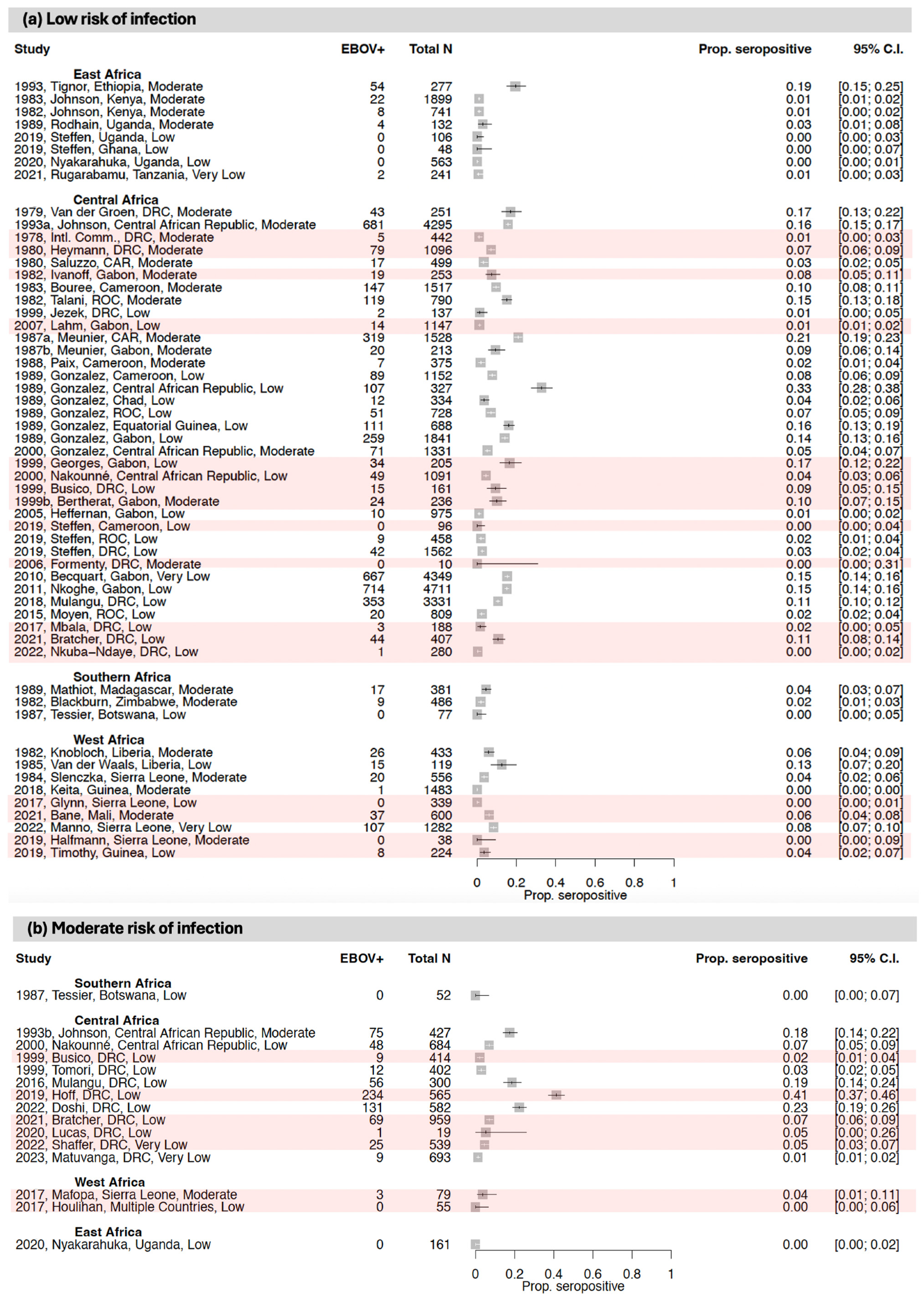
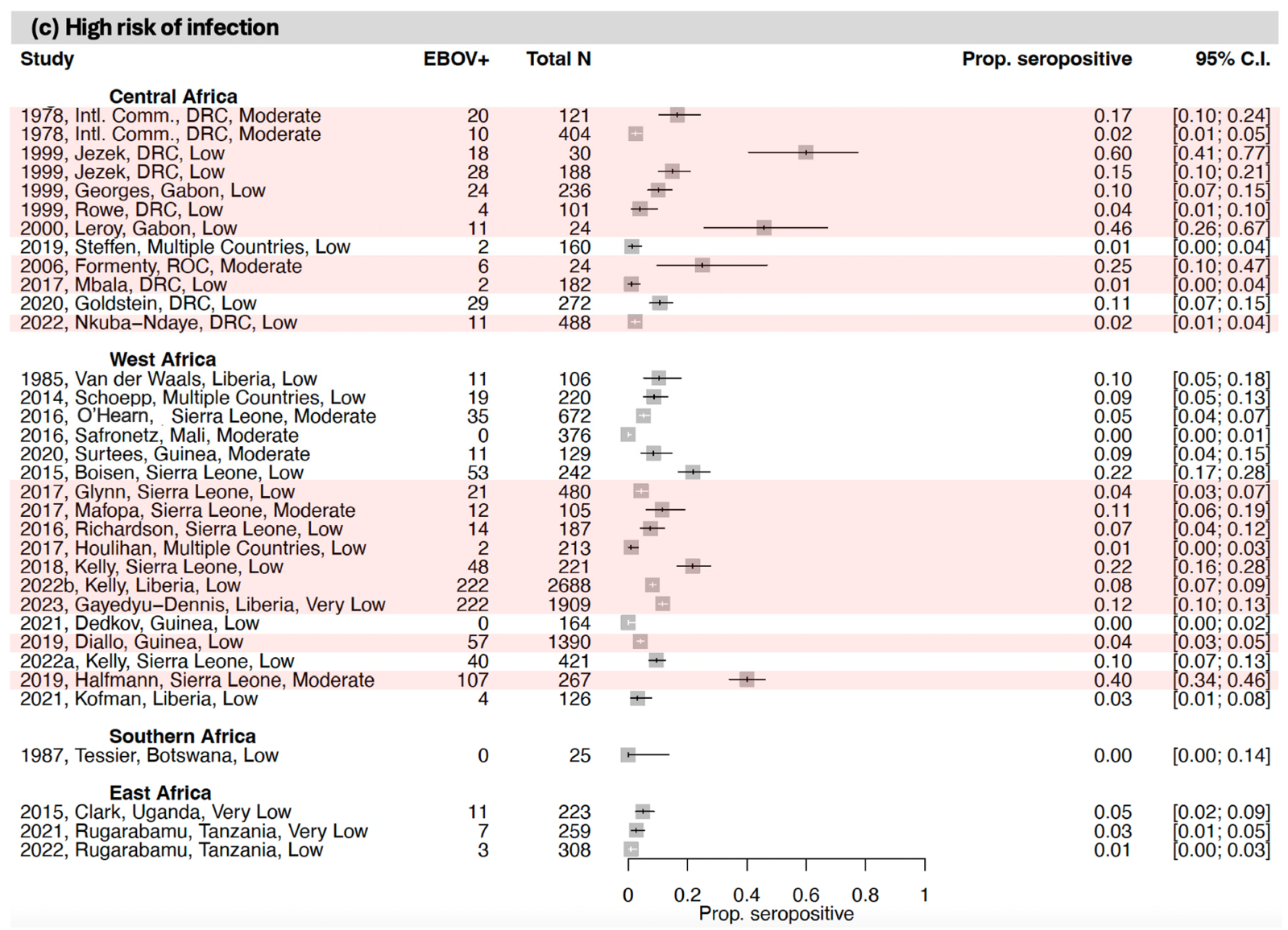
2.4. Assessment of Quality and Strength of Evidence
2.5. Data Synthesis and Analysis
3. Results
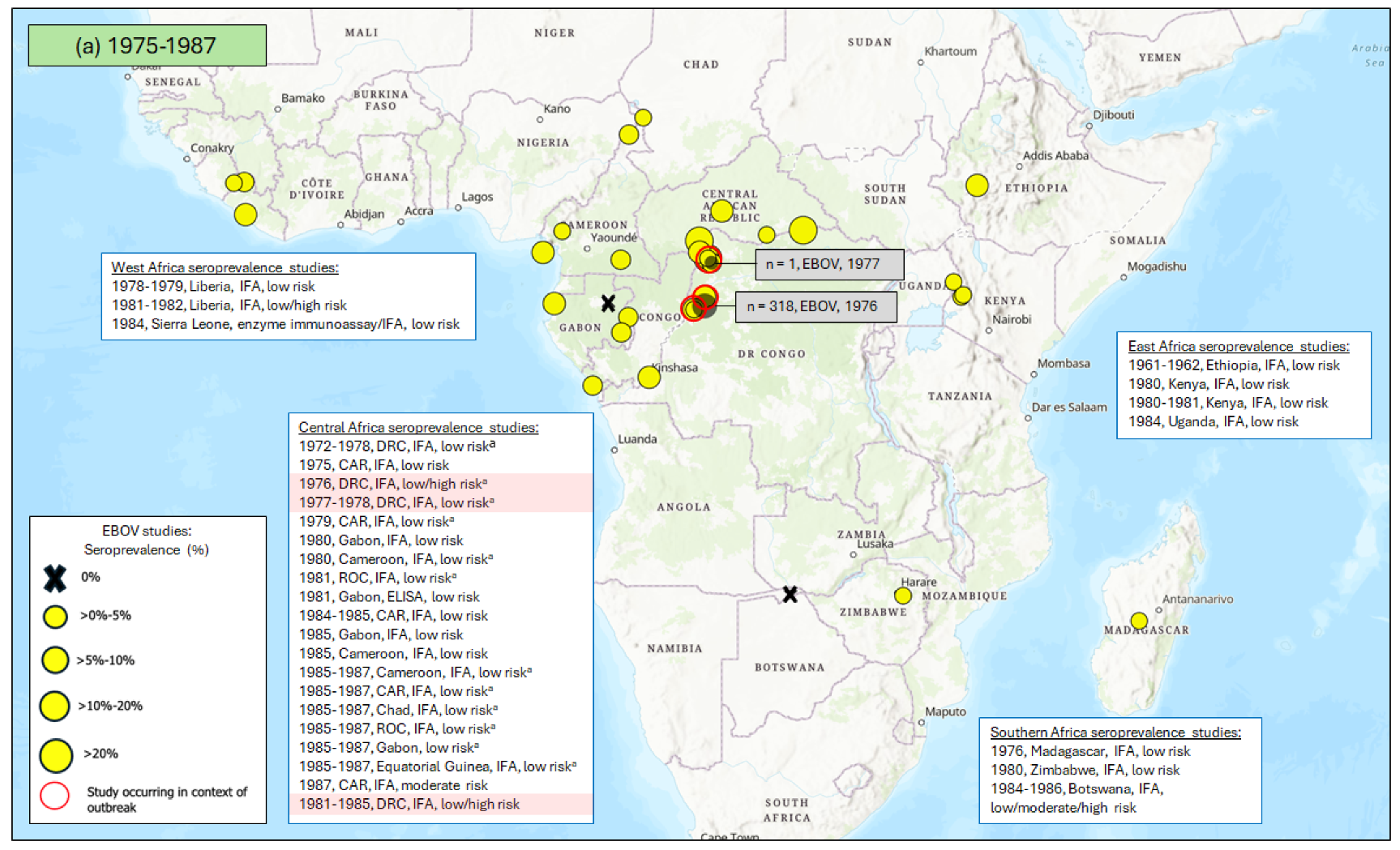
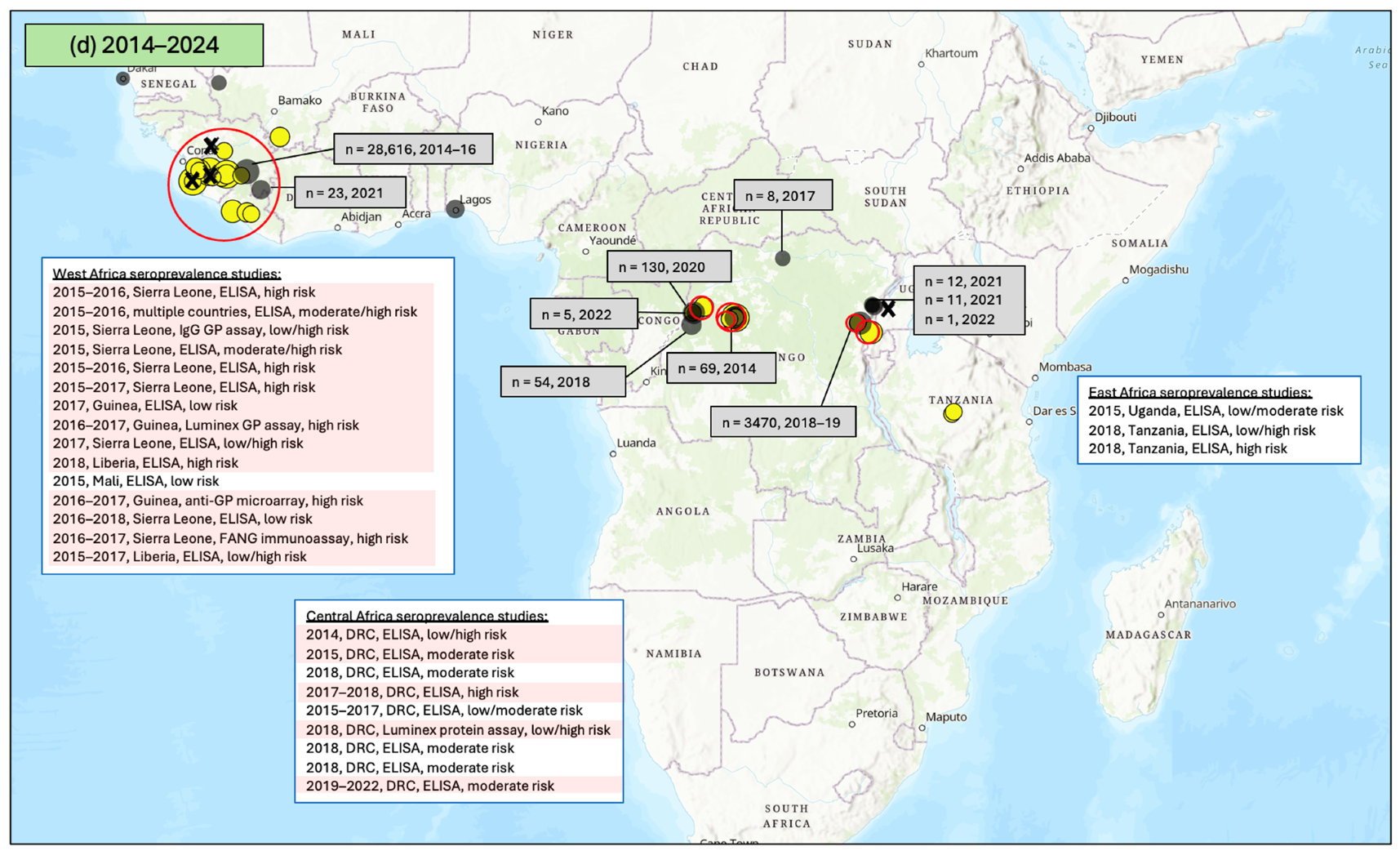
3.1. Seroprevalence of Antibodies to Ebola Virus (EBOV)
3.1.1. East Africa
3.1.2. Central Africa
3.1.3. West Africa
3.1.4. Southern Africa
3.2. Seroprevalence of Antibodies to Other Orthoebolaviruses
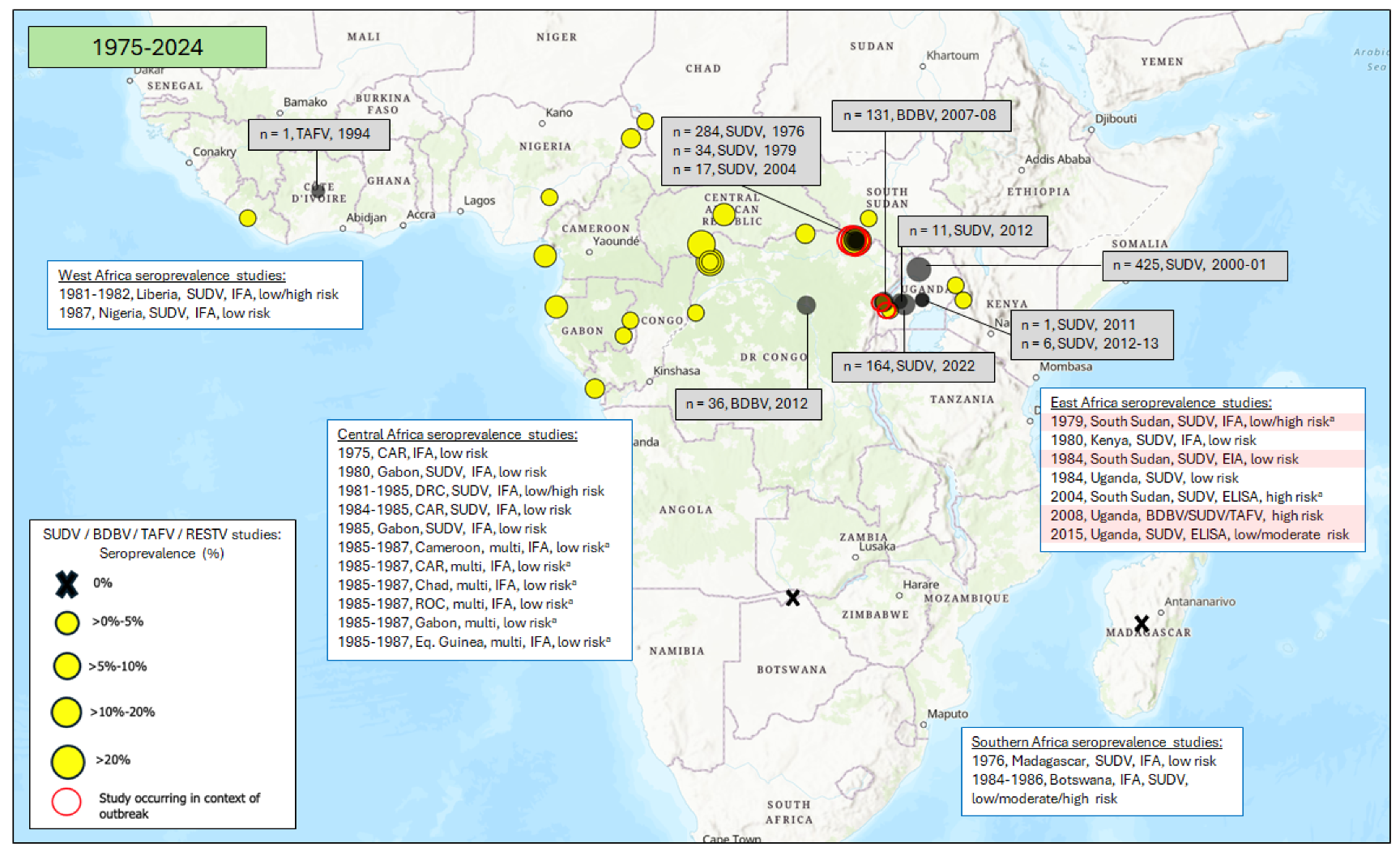
3.2.1. East Africa
3.2.2. Central Africa
3.2.3. West Africa
3.2.4. Southern Africa
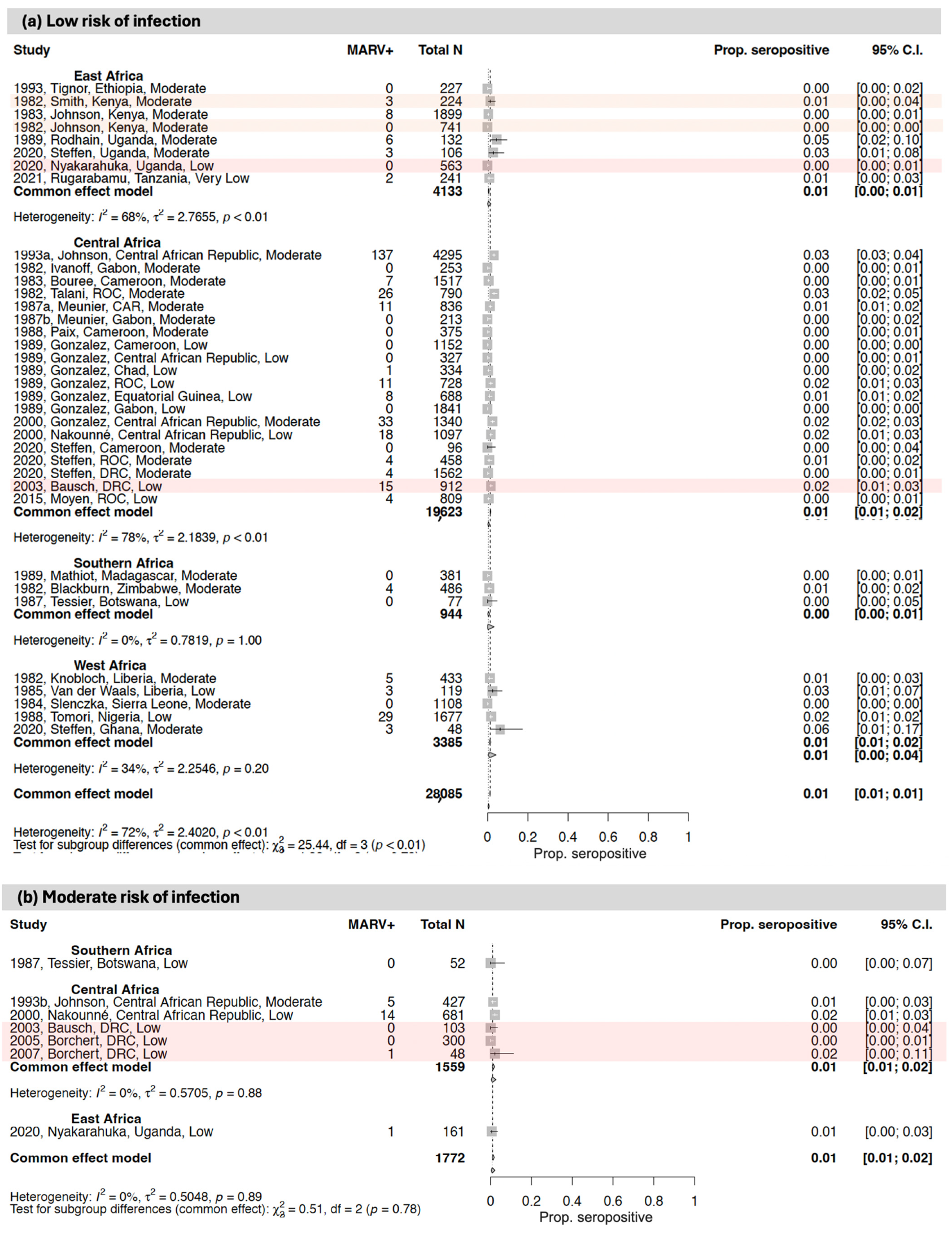
3.3. Seroprevalence of Antibodies to Orthomarburgviruses
3.3.1. East Africa
3.3.2. Central Africa
3.3.3. West Africa
3.3.4. Southern Africa
3.4. Assays Used for Detection of Antibodies to Orthoebolaviruses and Orthomarburgviruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldmann, H.; Klank, H.-D. Filoviruses. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Volume 4. [Google Scholar]
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Di Paola, N.; Formenty, P.B.H.; Griffiths, A.; Hume, A.J.; Muhlberger, E.; Netesov, S.V.; Palacios, G.; et al. Renaming of genera Ebolavirus and Marburgvirus to Orthoebolavirus and Orthomarburgvirus, respectively, and introduction of binomial species names within family Filoviridae. Arch. Virol. 2023, 168, 220. [Google Scholar] [CrossRef] [PubMed]
- Ebola Virus Disease. Available online: https://www.paho.org/en/topics/ebola-virus-disease#:~:text=The%20average%20EVD%20case%20fatality,to%2090%25%20in%20past%20outbreaks (accessed on 10 October 2024).
- Marburg Virus Disease. Available online: https://www.who.int/health-topics/marburg-virus-disease#tab=tab_1 (accessed on 10 October 2024).
- Marburg Virus Disease—Ghana. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON409 (accessed on 10 October 2024).
- Languon, S.; Quaye, O. Filovirus Disease Outbreaks: A Chronological Overview. Virology 2019, 10, 1178122X19849927. [Google Scholar] [CrossRef] [PubMed]
- History of Ebola Disease Outbreaks. Available online: https://www.cdc.gov/ebola/outbreaks/?CDC_AAref_Val=https://www.cdc.gov/vhf/ebola/history/chronology.html (accessed on 10 October 2024).
- History of Marburg Disease Outbreaks. Available online: https://www.cdc.gov/marburg/outbreaks/?CDC_AAref_Val=https://www.cdc.gov/vhf/marburg/outbreaks/chronology.html (accessed on 10 October 2024).
- Kuhn, J.H. Filoviruses. A compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch. Virol. Suppl. 2008, 20, 13–360. [Google Scholar] [PubMed]
- Marburg Virus Disease (Marburg) Situation Summary. Available online: https://www.cdc.gov/marburg/situation-summary/index.html#:~:text=Approximately%2075%20percent%20of%20patients,PCR%20separated%20by%2048%20hours (accessed on 10 October 2024).
- Keita, A.K.; Koundouno, F.R.; Faye, M.; Dux, A.; Hinzmann, J.; Diallo, H.; Ayouba, A.; Le Marcis, F.; Soropogui, B.; Ifono, K.; et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature 2021, 597, 539–543. [Google Scholar] [CrossRef]
- Meakin, S.; Nsio, J.; Camacho, A.; Kitenge, R.; Coulborn, R.M.; Gignoux, E.; Johnson, J.; Sterk, E.; Musenga, E.M.; Mustafa, S.H.B.; et al. Effectiveness of rVSV-ZEBOV vaccination during the 2018–20 Ebola virus disease epidemic in the Democratic Republic of the Congo: A retrospective test-negative study. Lancet Infect. Dis. 2024, 24, 1357–1365. [Google Scholar] [CrossRef]
- Woolsey, C.; Geisbert, T.W. Current state of Ebola virus vaccines: A snapshot. PLoS Pathog. 2021, 17, e1010078. [Google Scholar] [CrossRef]
- WHO Technical Advisory Group—Candidate Vaccine Prioritization. Summary of the Evaluations and Recommendations on the Four Marburg Vaccines. Available online: https://www.who.int/publications/m/item/who-technical-advisory-group---candidate-vaccine-prioritization.--summary-of-the-evaluations-and-recommendations-on-the-four-marburg-vaccines (accessed on 10 October 2024).
- Sudan Ebolavirus Candidate Vaccines—What Additional Research Should Be Conducted to Advance the Evaluation of These Candidate Vaccines? Available online: https://www.who.int/publications/m/item/sudan-ebolavirus-candidate-vaccines-what-additional-research-should-be-conducted-to-advance-the-evaluation-of-these-candidate-vaccines (accessed on 10 October 2024).
- Cross, R.W.; Longini, I.M.; Becker, S.; Bok, K.; Boucher, D.; Carroll, M.W.; Diaz, J.V.; Dowling, W.E.; Draghia-Akli, R.; Duworko, J.T.; et al. An introduction to the Marburg virus vaccine consortium, MARVAC. PLoS Pathog. 2022, 18, e1010805. [Google Scholar] [CrossRef]
- Cooper, C.L.; Morrow, G.; Yuan, M.; Coleman, J.W.; Hou, F.; Reiserova, L.; Li, S.L.; Wagner, D.; Carpov, A.; Wallace-Selman, O.; et al. Nonhuman Primates Are Protected against Marburg Virus Disease by Vaccination with a Vesicular Stomatitis Virus Vector-Based Vaccine Prepared under Conditions to Allow Advancement to Human Clinical Trials. Vaccines 2022, 10, 1582. [Google Scholar] [CrossRef]
- Sabin Vaccine Institute. Vaccine Development Using the ChAd3 Platform. Available online: https://www.sabin.org/our-impact/programs/vaccine-research-and-development/ (accessed on 10 October 2024).
- Oxford Vaccine Group. Oxford Scientists Launch First-in-Human Vaccine Trial for Deadly Marburg Virus. Available online: https://www.ovg.ox.ac.uk/news/oxford-scientists-launch-first-in-human-vaccine-trial-for-deadly-marburg-virus (accessed on 10 October 2024).
- Marzi, A.; Feldmann, H. Filovirus vaccines as a response paradigm for emerging infectious diseases. npj Vaccines 2024, 9, 186. [Google Scholar] [CrossRef]
- Falzarano, D.; Geisbert, T.W.; Feldmann, H. Progress in filovirus vaccine development: Evaluating the potential for clinical use. Expert. Rev. Vaccines 2011, 10, 63–77. [Google Scholar] [CrossRef]
- Markham, A. REGN-EB3: First Approval. Drugs 2021, 81, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Bienes, K.M.; Xiii, A.; Fausther-Bovendo, H.; Kobinger, G.P. Ebola-specific therapeutic antibodies from lab to clinic: The example of ZMapp. Antivir. Res. 2024, 226, 105873. [Google Scholar] [CrossRef] [PubMed]
- Wirchnianski, A.S.; Nyakatura, E.K.; Herbert, A.S.; Kuehne, A.I.; Abbasi, S.A.; Florez, C.; Storm, N.; McKay, L.G.A.; Dailey, L.; Kuang, E.; et al. Design and characterization of protective pan-ebolavirus and pan-filovirus bispecific antibodies. PLoS Pathog. 2024, 20, e1012134. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating next-generation vaccine development for global disease prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef]
- Bower, H.; Glynn, J.R. A systematic review and meta-analysis of seroprevalence surveys of ebolavirus infection. Sci. Data 2017, 4, 160133. [Google Scholar] [CrossRef][Green Version]
- Nyakarahuka, L.; Kankya, C.; Krontveit, R.; Mayer, B.; Mwiine, F.N.; Lutwama, J.; Skjerve, E. How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence. BMC Infect. Dis. 2016, 16, 708. [Google Scholar] [CrossRef]
- Semancik, C.S.; Cooper, C.L.; Postler, T.S.; Price, M.; Yun, H.; Zaric, M.; Kuteesa, M.; Malkevich, N.; Kilianski, A.; Gupta, S.B.; et al. Prevalence of human filovirus infections in sub-Saharan Africa: A systematic review and meta-analysis protocol. Syst. Rev. 2024, 13, 218. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. Establishing a minimum dataset for prospective registration of systematic reviews: An international consultation. PLoS ONE 2011, 6, e27319. [Google Scholar] [CrossRef] [PubMed]
- Checklist for Prevalence Studies. Available online: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf (accessed on 10 October 2024).
- What is GRADE? Available online: https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/ (accessed on 10 October 2024).
- African Development Bank. African Countries. Available online: https://www.afdb.org/en/countries (accessed on 10 October 2024).
- Johnson, B.K.; Ocheng, D.; Gitau, L.G. Viral haemorrhagic fever surveillance in Kenya, 1980–1981. Trop. Geogr. Med. 1983, 35, 43–47. [Google Scholar] [PubMed]
- Nyakarahuka, L.; Schafer, I.J.; Balin, S.; Mulei, S.; Tumusiime, A.; Kyondo, J.; Knust, B.; Lutwama, J.; Rollin, P.; Nichol, S.; et al. A retrospective cohort investigation of seroprevalence of Marburg virus and ebolaviruses in two different ecological zones in Uganda. BMC Infect. Dis. 2020, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Rugarabamu, S.; Rumisha, S.F.; Mwanyika, G.O.; Sindato, C.; Lim, H.Y.; Misinzo, G.; Mboera, L.E.G. Viral haemorrhagic fevers and malaria co-infections among febrile patients seeking health care in Tanzania. Infect. Dis. Poverty 2022, 11, 33. [Google Scholar] [CrossRef]
- Rugarabamu, S.; Mwanyika, G.O.; Rumisha, S.F.; Sindato, C.; Lim, H.Y.; Misinzo, G.; Mboera, L.E.G. Seroprevalence and associated risk factors of selected zoonotic viral hemorrhagic fevers in Tanzania. Int. J. Infect. Dis. 2021, 109, 174–181. [Google Scholar] [CrossRef]
- Steffen, I.; Lu, K.; Yamamoto, L.K.; Hoff, N.A.; Mulembakani, P.; Wemakoy, E.O.; Muyembe-Tamfum, J.J.; Ndembi, N.; Brennan, C.A.; Hackett, J.; et al. Serologic Prevalence of Ebola Virus in Equatorial Africa. Emerg. Infect. Dis. 2019, 25, 911–918. [Google Scholar] [CrossRef]
- Rodhain, F.; Gonzalez, J.P.; Mercier, E.; Helynck, B.; Larouze, B.; Hannoun, C. Arbovirus infections and viral haemorrhagic fevers in Uganda: A serological survey in Karamoja district, 1984. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 851–854. [Google Scholar] [CrossRef]
- Johnson, B.K.; Ocheng, D.; Gichogo, A.; Okiro, M.; Libondo, D.; Tukei, P.M.; Ho, M.; Mugambi, M.; Timms, G.L.; French, M. Antibodies against haemorrhagic fever viruses in Kenya populations. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 731–733. [Google Scholar] [CrossRef]
- Clark, D.V.; Kibuuka, H.; Millard, M.; Wakabi, S.; Lukwago, L.; Taylor, A.; Eller, M.A.; Eller, L.A.; Michael, N.L.; Honko, A.N.; et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 905–912. [Google Scholar] [CrossRef]
- Tignor, G.H.; Casals, J.; Shope, R.E. The yellow fever epidemic in Ethiopia, 1961–1962: Retrospective serological evidence for concomitant Ebola or Ebola-like virus infection. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 162. [Google Scholar] [CrossRef]
- Jezek, Z.; Szczeniowski, M.Y.; Muyembe-Tamfum, J.J.; McCormick, J.B.; Heymann, D.L. Ebola between outbreaks: Intensified Ebola hemorrhagic fever surveillance in the Democratic Republic of the Congo, 1981–1985. J. Infect. Dis. 1999, 179 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Formenty, P.; Leroy, E.M.; Epelboin, A.; Libama, F.; Lenzi, M.; Sudeck, H.; Yaba, P.; Allarangar, Y.; Boumandouki, P.; Nkounkou, V.B.; et al. Detection of Ebola virus in oral fluid specimens during outbreaks of Ebola virus hemorrhagic fever in the Republic of Congo. Clin. Infect. Dis. 2006, 42, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Nkuba-Ndaye, A.; Mukadi-Bamuleka, D.; Bulabula-Penge, J.; Thaurignac, G.; Edidi-Atani, F.; Mambu-Mbika, F.; Danga-Yema, B.; Matondo-Kuamfumu, M.; Kinganda-Lusamaki, E.; Bisento, N.; et al. Added Value of an Anti-Ebola Serology for the Management of Clinically Suspected Ebola Virus Disease Patients Discharged as Negative in an Epidemic Context. J. Infect. Dis. 2022, 226, 352–356. [Google Scholar] [CrossRef]
- Report of an International Commission. Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 1978, 56, 271–293. [Google Scholar]
- Mbala, P.; Baguelin, M.; Ngay, I.; Rosello, A.; Mulembakani, P.; Demiris, N.; Edmunds, W.J.; Muyembe, J.J. Evaluating the frequency of asymptomatic Ebola virus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160303. [Google Scholar] [CrossRef]
- Rowe, A.K.; Bertolli, J.; Khan, A.S.; Mukunu, R.; Muyembe-Tamfum, J.J.; Bressler, D.; Williams, A.J.; Peters, C.J.; Rodriguez, L.; Feldmann, H.; et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidemies a Kikwit. J. Infect. Dis. 1999, 179 (Suppl. S1), S28–S35. [Google Scholar] [CrossRef]
- Hoff, N.A.; Mukadi, P.; Doshi, R.H.; Bramble, M.S.; Lu, K.; Gadoth, A.; Sinai, C.; Spencer, D.; Nicholson, B.P.; Williams, R.; et al. Serologic Markers for Ebolavirus Among Healthcare Workers in the Democratic Republic of the Congo. J. Infect. Dis. 2019, 219, 517–525. [Google Scholar] [CrossRef]
- Bratcher, A.; Hoff, N.A.; Doshi, R.H.; Gadoth, A.; Halbrook, M.; Mukadi, P.; Musene, K.; Ilunga-Kebela, B.; Spencer, D.; Bramble, M.S.; et al. Zoonotic risk factors associated with seroprevalence of Ebola virus GP antibodies in the absence of diagnosed Ebola virus disease in the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2021, 15, e0009566. [Google Scholar] [CrossRef]
- Shaffer, K.C.L.; Hui, S.; Bratcher, A.; King, L.B.; Mutombe, R.; Kavira, N.; Kompany, J.P.; Tambu, M.; Musene, K.; Mukadi, P.; et al. Pan-ebolavirus serology study of healthcare workers in the Mbandaka Health Region, Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2022, 16, e0010167. [Google Scholar] [CrossRef]
- Doshi, R.H.; Hoff, N.A.; Bratcher, A.; Mukadi, P.; Gadoth, A.; Nicholson, B.P.; Williams, R.; Mukadi, D.; Mossoko, M.; Wasiswa, J.; et al. Risk Factors for Ebola Exposure in Health Care Workers in Boende, Tshuapa Province, Democratic Republic of the Congo. J. Infect. Dis. 2022, 226, 608–615. [Google Scholar] [CrossRef]
- Tomori, O.; Bertolli, J.; Rollin, P.E.; Fleerackers, Y.; Guimard, Y.; De Roo, A.; Feldmann, H.; Burt, F.; Swanepoel, R.; Killian, S.; et al. Serologic survey among hospital and health center workers during the Ebola hemorrhagic fever outbreak in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999, 179 (Suppl. S1), S98–S101. [Google Scholar] [CrossRef] [PubMed]
- Zola Matuvanga, T.; Marien, J.; Lariviere, Y.; Osang’ir, B.I.; Milolo, S.; Meta, R.; Esanga, E.; Maketa, V.; Matangila, J.; Mitashi, P.; et al. Low seroprevalence of Ebola virus in health care providers in an endemic region (Tshuapa province) of the Democratic Republic of the Congo. PLoS ONE 2023, 18, e0286479. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Baize, S.; Volchkov, V.E.; Fisher-Hoch, S.P.; Georges-Courbot, M.C.; Lansoud-Soukate, J.; Capron, M.; Debre, P.; McCormick, J.B.; Georges, A.J. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000, 355, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.J.; Leroy, E.M.; Renaut, A.A.; Benissan, C.T.; Nabias, R.J.; Ngoc, M.T.; Obiang, P.I.; Lepage, J.P.; Bertherat, E.J.; Benoni, D.D.; et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: Epidemiologic and health control issues. J. Infect. Dis. 1999, 179 (Suppl. S1), S65–S75. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.; Belaganahalli, M.N.; Syaluha, E.K.; Lukusa, J.K.; Greig, D.J.; Anthony, S.J.; Tremeau-Bravard, A.; Thakkar, R.; Caciula, A.; Mishra, N.; et al. Spillover of ebolaviruses into people in eastern Democratic Republic of Congo prior to the 2018 Ebola virus disease outbreak. One Health Outlook 2020, 2, 21. [Google Scholar] [CrossRef]
- Meunier, D.M.Y.; Dupont, A.; Madelon, M.C. Serological study of haemorrhagic fevers in the province of Haut-Ogooue, Gabon. Ann. De L’institut Pasteur Virol. 1987, 138, 229–235. [Google Scholar] [CrossRef]
- Moyen, N.; Thirion, L.; Emmerich, P.; Dzia-Lepfoundzou, A.; Richet, H.; Boehmann, Y.; Dimi, Y.; Gallian, P.; Gould, E.A.; Günther, S.; et al. Risk Factors Associated with Ebola and Marburg Viruses Seroprevalence in Blood Donors in the Republic of Congo. PLoS Negl. Trop. Dis. 2015, 9, e0003833. [Google Scholar] [CrossRef]
- Mulangu, S.; Borchert, M.; Paweska, J.; Tshomba, A.; Afounde, A.; Kulidri, A.; Swanepoel, R.; Muyembe-Tamfum, J.J.; Van der Stuyft, P. High prevalence of IgG antibodies to Ebola virus in the Efe pygmy population in the Watsa region, Democratic Republic of the Congo. BMC Infect. Dis. 2016, 16, 263. [Google Scholar] [CrossRef]
- Nakounné, E.; Selekon, B.; Morvan, J. Microbiological surveillance: Viral hemorrhagic fever in Central African Republic: Current serological data in man. Bull. De La Société De Pathol. Exot. (1990) 2000, 93, 340–347. [Google Scholar]
- Johnson, E.D.; Gonzalez, J.P.; Georges, A. Haemorrhagic fever virus activity in equatorial Africa: Distribution and prevalence of filovirus reactive antibody in the Central African Republic. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 530–535. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Josse, R.; Johnson, E.D.; Merlin, M.; Georges, A.J.; Abandja, J.; Danyod, M.; Delaporte, E.; Dupont, A.; Ghogomu, A.; et al. Antibody prevalence against haemorrhagic fever viruses in randomized representative Central African populations. Res. Virol. 1989, 140, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Bertherat, E.; Renaut, A.; Nabias, R.; Dubreuil, G.; Georges-Courbot, M.C. Leptospirosis and Ebola virus infection in five gold-panning villages in northeastern Gabon. Am. J. Trop. Med. Hyg. 1999, 60, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Busico, K.M.; Marshall, K.L.; Ksiazek, T.G.; Roels, T.H.; Fleerackers, Y.; Feldmann, H.; Khan, A.S.; Peters, C.J. Prevalence of IgG antibodies to Ebola virus in individuals during an Ebola outbreak, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999, 179, S102–S107. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, R.T.; Pambo, B.; Hatchett, R.J.; Leman, P.A.; Swanepoel, R.; Ryder, R.W. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooué-Ivindo region, Northeastern Gabon, 1997. J. Infect. Dis. 2005, 191, 964–968. [Google Scholar] [CrossRef]
- Becquart, P.; Wauquier, N.; Mahlakoiv, T.; Nkoghe, D.; Padilla, C.; Souris, M.; Ollomo, B.; Gonzalez, J.P.; De Lamballerie, X.; Kazanji, M.; et al. High prevalence of both humoral and cellular immunity to Zaire ebolavirus among rural populations in Gabon. PLoS ONE 2010, 5, e9126. [Google Scholar] [CrossRef]
- Nkoghe, D.; Padilla, C.; Becquart, P.; Wauquier, N.; Moussavou, G.; Akué, J.P.; Ollomo, B.; Pourrut, X.; Souris, M.; Kazanji, M.; et al. Risk factors for zaire ebolavirus-specific IgG in rural gabonese populations. J. Infect. Dis. 2011, 204, S768–S775. [Google Scholar] [CrossRef]
- Lucas, A.; Kumakamba, C.; Lange, C.E.; Obel, E.; Miningue, G.; Likofata, J.; Gillis, A.; LeBreton, M.; McIver, D.J.; Euren, J.; et al. Serology and Behavioral Perspectives on Ebola Virus Disease Among Bushmeat Vendors in Equateur, Democratic Republic of the Congo, After the 2018 Outbreak. Open Forum Infect. Dis. 2020, 7, ofaa295. [Google Scholar] [CrossRef]
- Ivanoff, B.; Duquesnoy, P.; Languillat, G.; Saluzzo, J.F.; Georges, A.; Gonzalez, J.P.; McCormick, J. Haemorrhagic fever in Gabon. I. Incidence of Lassa, Ebola and Marburg viruses in Haut-Ogooue. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 719–720. [Google Scholar] [CrossRef]
- Heymann, D.L.; Weisfeld, J.S.; Webb, P.A.; Johnson, K.M.; Cairns, T.; Berquist, H. Ebola hemorrhagic fever: Tandala, Zaire, 1977–1978. J. Infect. Dis. 1980, 142, 372–376. [Google Scholar] [CrossRef]
- Saluzzo, J.F.; Gonzalez, J.P.; Herve, J.P.; Georges, A.J.; Johnson, K.M. [Preliminary note on the presence of antibodies to Ebola virus in the human population in the eastern part of the Central African Republic]. Bull. Soc. Pathol. Exot. Fil. 1980, 73, 238–241. [Google Scholar]
- Talani, P.; AI Gromyko, J.D.K. Fièvre Hémorragique: Prévalence des Anticorps Anti-Fièvres Hémorragiques D’origine Virale dans la Région du Pool (Congo-Brazzaville). Available online: https://www.santetropicale.com/biblio.asp?id=94&action=lire (accessed on 10 October 2024).
- Bouree, P.; Bergmann, J.F. Ebola virus infection in man: A serological and epidemiological survey in the Cameroons. Am. J. Trop. Med. Hyg. 1983, 32, 1465–1466. [Google Scholar] [CrossRef] [PubMed]
- Meunier, D.M.; Johnson, E.D.; Gonzalez, J.P.; Georges-Courbot, M.C.; Madelon, M.C.; Georges, A.J. [Current serologic data on viral hemorrhagic fevers in the Central African Republic]. Bull. Soc. Pathol. Exot. Fil. 1987, 80, 51–61. [Google Scholar]
- Lahm, S.A.; Kombila, M.; Swanepoel, R.; Barnes, R.F. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994–2003. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Van der Groen, G.; Pattyn, S.R. Measurement of antibodies to Ebola virus in human sera from N. W.-Zaire. Ann. Soc. Belg. Med. Trop. 1979, 59, 87–92. [Google Scholar]
- Mulangu, S.; Alfonso, V.H.; Hoff, N.A.; Doshi, R.H.; Mulembakani, P.; Kisalu, N.K.; Okitolonda-Wemakoy, E.; Kebela, B.I.; Marcus, H.; Shiloach, J.; et al. Serologic Evidence of Ebolavirus Infection in a Population With No History of Outbreaks in the Democratic Republic of the Congo. J. Infect. Dis. 2018, 217, 529–537. [Google Scholar] [CrossRef]
- Paix, M.A.; Poveda, J.D.; Malvy, D.; Bailly, C.; Merlin, M.; Fleury, H.J.A. Sero-epidemiological study of Hemorrhagic Fever Viruses in an urban population of Cameroon. Bull. De La Soc. De Pathol. Exot. Et De Ses Fil. 1988, 81, 679–682. [Google Scholar]
- Johnson, E.D.; Gonzalez, J.P.; Georges, A. Filovirus activity among selected ethnic groups inhabiting the tropical forest of equatorial Africa. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 536–538. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Nakoune, E.; Slenczka, W.; Vidal, P.; Morvan, J.M. Ebola and Marburg virus antibody prevalence in selected populations of the Central African Republic. Microbes Infect. 2000, 2, 39–44. [Google Scholar] [CrossRef]
- Dedkov, V.G.; Magassouba, N.; Stukolova, O.A.; Savina, V.A.; Camara, J.; Soropogui, B.; Safonova, M.V.; Semizhon, P.; Platonov, A.E. Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers. Int. J. Environ. Res. Public Health 2021, 18, 6022. [Google Scholar] [CrossRef]
- Richardson, E.T.; Kelly, J.D.; Barrie, M.B.; Mesman, A.W.; Karku, S.; Quiwa, K.; Marsh, R.H.; Koedoyoma, S.; Daboh, F.; Barron, K.P.; et al. Minimally Symptomatic Infection in an Ebola ‘Hotspot’: A Cross-Sectional Serosurvey. PLoS Negl. Trop. Dis. 2016, 10, e0005087. [Google Scholar] [CrossRef]
- Glynn, J.R.; Bower, H.; Johnson, S.; Houlihan, C.F.; Montesano, C.; Scott, J.T.; Semple, M.G.; Bangura, M.S.; Kamara, A.J.; Kamara, O.; et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: A cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect. Dis. 2017, 17, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Barrie, M.B.; Mesman, A.W.; Karku, S.; Quiwa, K.; Drasher, M.; Schlough, G.W.; Dierberg, K.; Koedoyoma, S.; Lindan, C.P.; et al. Anatomy of a Hotspot: Chain and Seroepidemiology of Ebola Virus Transmission, Sukudu, Sierra Leone, 2015–2016. J. Infect. Dis. 2018, 217, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.S.K.; Rabilloud, M.; Ayouba, A.; Touré, A.; Thaurignac, G.; Keita, A.K.; Butel, C.; Kpamou, C.; Barry, T.A.; Sall, M.D.; et al. Prevalence of infection among asymptomatic and paucisymptomatic contact persons exposed to Ebola virus in Guinea: A retrospective, cross-sectional observational study. Lancet Infect. Dis. 2019, 19, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Eisfeld, A.J.; Watanabe, T.; Maemura, T.; Yamashita, M.; Fukuyama, S.; Armbrust, T.; Rozich, I.; N’Jai, A.; Neumann, G.; et al. Serological analysis of Ebola virus survivors and close contacts in Sierra Leone: A cross-sectional study. PLoS Negl. Trop. Dis. 2019, 13, e0007654. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Frankfurter, R.G.; Tavs, J.M.; Barrie, M.B.; McGinnis, T.; Kamara, M.; Freeman, A.; Quiwah, K.; Davidson, M.C.; Dighero-Kemp, B.; et al. Association of Lower Exposure Risk With Paucisymptomatic/Asymptomatic Infection, Less Severe Disease, and Unrecognized Ebola Virus Disease: A Seroepidemiological Study. Open Forum Infect. Dis. 2022, 9, ofac052. [Google Scholar] [CrossRef]
- Kelly, J.D.; Van Ryn, C.; Badio, M.; Fayiah, T.; Johnson, K.; Gayedyu-Dennis, D.; Weiser, S.D.; Porco, T.C.; Martin, J.N.; Sneller, M.C.; et al. Clinical sequelae among individuals with pauci-symptomatic or asymptomatic Ebola virus infection and unrecognised Ebola virus disease in Liberia: A longitudinal cohort study. Lancet Infect. Dis. 2022, 22, 1163–1171. [Google Scholar] [CrossRef]
- Gayedyu-Dennis, D.; Fallah, M.P.; Drew, C.; Badio, M.; Moses, J.S.; Fayiah, T.; Johnson, K.; Richardson, E.T.; Weiser, S.D.; Porco, T.C.; et al. Identifying Paucisymptomatic or Asymptomatic and Unrecognized Ebola Virus Disease Among Close Contacts Based on Exposure Risk Assessments and Screening Algorithms. J. Infect. Dis. 2023, 227, 878–887. [Google Scholar] [CrossRef]
- Houlihan, C.F.; McGowan, C.R.; Dicks, S.; Baguelin, M.; Moore, D.A.J.; Mabey, D.; Roberts, C.H.; Kumar, A.; Samuel, D.; Tedder, R.; et al. Ebola exposure, illness experience, and Ebola antibody prevalence in international responders to the West African Ebola epidemic 2014-2016: A cross-sectional study. PLoS Med. 2017, 14, e1002300. [Google Scholar] [CrossRef]
- Timothy, J.; Hall, Y.; Akoi-Bore, J.; Diallo, B.; Tipton, T.; Bower, H.; Glynn, J.; Carroll, M. Seroprevalence Study of Anti-Ebolavirus (Ebov) Immunoglobulin G (IGG) at the Index Site of 2013–2016 West African Ebov Outbreak: Insight into Early Transmission and Case Fatality Rate. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, S48. [Google Scholar]
- Bane, S.; Rosenke, K.; Maiga, O.; Feldmann, F.; Meade-White, K.; Callison, J.; Safronetz, D.; Sogoba, N.; Feldmann, H. Ebola Virus IgG Seroprevalence in Southern Mali. Emerg. Infect. Dis. 2021, 27, 1681–1684. [Google Scholar] [CrossRef]
- Manno, D.; Ayieko, P.; Ishola, D.; Afolabi, M.O.; Rogers, B.; Baiden, F.; Serry-Bangura, A.; Bah, O.M.; Kohn, B.; Swaray, I.; et al. Ebola Virus Glycoprotein IgG Seroprevalence in Community Previously Affected by Ebola, Sierra Leone. Emerg. Infect. Dis. 2022, 28, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Boisen, M.L.; Schieffelin, J.S.; Goba, A.; Oottamasathien, D.; Jones, A.B.; Shaffer, J.G.; Hastie, K.M.; Hartnett, J.N.; Momoh, M.; Fullah, M.; et al. Multiple circulating infections can mimic the early stages of viral hemorrhagic fevers and possible human exposure to filoviruses in Sierra Leone prior to the 2014 outbreak. Viral Immunol. 2015, 28, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Van der Waals, F.W.; Pomeroy, K.L.; Goudsmit, J.; Asher, D.M.; Gajdusek, D.C. Hemorrhagic fever virus infections in an isolated rainforest area of central Liberia. Limitations of the indirect immunofluorescence slide test for antibody screening in Africa. Trop. Geogr. Med. 1986, 38, 209–214. [Google Scholar] [PubMed]
- Safronetz, D.; Sacko, M.; Sogoba, N.; Rosenke, K.; Martellaro, C.; Traoré, S.; Cissé, I.; Maiga, O.; Boisen, M.; Nelson, D.; et al. Vectorborne infections, Mali. Emerg. Infect. Dis. 2016, 22, 340–342. [Google Scholar] [CrossRef]
- Keita, A.K.; Butel, C.; Thaurignac, G.; Diallo, A.; Nioke, T.; Traoré, F.; Koivogui, L.; Peeters, M.; Delaporte, E.; Ayouba, A. Serological evidence of ebola virus infection in rural Guinea before the 2014 West African epidemic outbreak. Am. J. Trop. Med. Hyg. 2018, 99, 425–427. [Google Scholar] [CrossRef]
- Knobloch, J.; Albiez, E.J.; Schmitz, H. A Serological Survey on Viral Haemorrhagic Fevers in Liberia. Ann. de l’Institut Pasteur/ Virol. 1982, 133, pp. 125–128. Available online: https://www.sciencedirect.com/science/article/pii/S0769261782800282 (accessed on 10 October 2024).
- Slenczka, W.; Rietschel, M.; Hoffmann, C.; Sixl, W. Seroepidemiologische Untersuchungen über das Vorkommen von Antikörpern gegen Marburg- und Ebola-Virus in Afrika. Available online: https://www.zobodat.at/pdf/MOGTP_6_0053-0060.pdf (accessed on 10 October 2024).
- Tomori, O.; Fabiyi, A.; Sorungbe, A.; Smith, A.; McCormick, J.B. Viral hemorrhagic fever antibodies in Nigerian populations. Am. J. Trop. Med. Hyg. 1988, 38, 407–410. [Google Scholar] [CrossRef]
- Schoepp, R.J.; Rossi, C.A.; Khan, S.H.; Goba, A.; Fair, J.N. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg. Infect. Dis. 2014, 20, 1176–1182. [Google Scholar] [CrossRef]
- Surtees, R.; Stern, D.; Ahrens, K.; Kromarek, N.; Lander, A.; Kreher, P.; Weiss, S.; Hewson, R.; Punch, E.K.; Barr, J.N.; et al. Development of a multiplex microsphere immunoassay for the detection of antibodies against highly pathogenic viruses in human and animal serum samples. PLoS Negl. Trop. Dis. 2020, 14, e0008699. [Google Scholar] [CrossRef]
- Mathiot, C.C.; Fontenille, D.; Georges, A.J.; Coulanges, P. Antibodies to haemorrhagic fever viruses in Madagascar populations. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 407–409. [Google Scholar] [CrossRef]
- Blackburn, N.K.; Searle, L.; Taylor, P. Viral haemorrhagic fever antibodies in Zimbabwe schoolchildren. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 803–805. [Google Scholar] [CrossRef]
- Tessier, S.F.; Rollin, P.E.; Sureau, P. Viral haemorrhagic fever survey in Chobe (Botswana). Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.C.; McCormick, J.B.; Zubeir, O.A. Ebola virus disease in southern Sudan: Hospital dissemination and intrafamilial spread. Bull. World Health Organ. 1983, 61, 997–1003. [Google Scholar] [PubMed]
- Onyango, C.O.; Opoka, M.L.; Ksiazek, T.G.; Formenty, P.; Ahmed, A.; Tukei, P.M.; Sang, R.C.; Ofula, V.O.; Konongoi, S.L.; Coldren, R.L.; et al. Laboratory diagnosis of Ebola hemorrhagic fever during an outbreak in Yambio, Sudan, 2004. J. Infect. Dis. 2007, 196 (Suppl. S2), S193–S198. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Farnon, E.C.; Tschioko, F.; Wamala, J.F.; Byaruhanga, E.; Bwire, G.S.; Kansiime, E.; Kagirita, A.; Ahimbisibwe, S.; Katunguka, F.; et al. Outbreak of Marburg hemorrhagic fever among miners in kamwenge and ibanda Districts, Uganda, 2007. J. Infect. Dis. 2011, 204, S796–S799. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; Shoemaker, T.R.; Balin, S.; Chemos, G.; Kwesiga, B.; Mulei, S.; Kyondo, J.; Tumusiime, A.; Kofman, A.; Masiira, B.; et al. Marburg virus disease outbreak in Kween District Uganda, 2017: Epidemiological and laboratory findings. PLoS Negl. Trop. Dis. 2018, 13, e0007257. [Google Scholar] [CrossRef]
- Knust, B.; Schafer, I.J.; Wamala, J.; Nyakarahuka, L.; Okot, C.; Shoemaker, T.; Dodd, K.; Gibbons, A.; Balinandi, S.; Tumusiime, A.; et al. Multidistrict Outbreak of Marburg Virus Disease-Uganda, 2012. J. Infect. Dis. 2015, 212 (Suppl. S2), S119–S128. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, B.K.; Isaacson, M.; Swanapoel, R.; Johnson, K.M.; Killey, M.; Bagshawe, A.; Siongok, T.; Keruga, W.K. Marburg-virus disease in Kenya. Lancet 1982, 1, 816–820. [Google Scholar] [CrossRef]
- Steffen, I.; Lu, K.; Hoff, N.A.; Mulembakani, P.; Okitolonda Wemakoy, E.; Muyembe-Tamfum, J.J.; Ndembi, N.; Brennan, C.A.; Hackett, J., Jr.; Switzer, W.M.; et al. Seroreactivity against Marburg or related filoviruses in West and Central Africa. Emerg. Microbes Infect. 2020, 9, 124–128. [Google Scholar] [CrossRef]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.J.; Tugume, B.; et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003, 9, 1531–1537. [Google Scholar] [CrossRef]
- Borchert, M.; Mulangu, S.; Swanepoel, R.; Libande, M.L.; Tshomba, A.; Kulidri, A.; Muyembe-Tamfum, J.J.; Van Der Stuyft, P. Serosurvey on household contacts of marburg hemorrhagic fever patients. Emerg. Infect. Dis. 2006, 12, 433–439. [Google Scholar] [CrossRef]
- Borchert, M.; Mulangu, S.; Lefèvre, P.; Tshomba, A.; Libande, M.L.; Kulidri, A.; Muyembe-Tamfum, J.J.; Van Der Stuyft, P. Use of protective gear and the occurrence of occupational Marburg hemorrhagic fever in health workers from Watsa Health Zone, Democratic Republic of the Congo. J. Infect. Dis. 2007, 196, S168–S175. [Google Scholar] [CrossRef] [PubMed]
- Borchert, M.; Mulangu, S.; Swanepoel, R.; Tshomba, A.; Afounde, A.; Kulidri, A.; Muyembe-Tamfum, J.J.; Van Der Stuyft, P. Pygmy populations seronegative for Marburg virus. Emerg. Infect. Dis. 2005, 11, 174–177. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, A.E.; Voorhees, M.A.; Fetterer, D.P.; Wauquier, N.; Coomber, M.R.; Bangura, J.; Fair, J.N.; Gonzalez, J.P.; Schoepp, R.J. Serosurveillance of viral pathogens circulating in West Africa. Virol. J. 2016, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.L.; Isaacson, M.; Smith, E.B.; Wulff, H.; Crees, M.; Geldenhuys, P.; Johnston, J. Epidemiologic investigation of Marburg virus disease, Southern Africa, 1975. Am. J. Trop. Med. Hyg. 1978, 27, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.J.; Amman, B.R.; Sealy, T.S.; Flietstra, T.D.; Guito, J.C.; Nichol, S.T.; Towner, J.S. Comparative analysis of serologic cross-reactivity using convalescent sera from filovirus-experimentally infected fruit bats. Sci. Rep. 2019, 9, 6707. [Google Scholar] [CrossRef]
- Nakayama, E.; Yokoyama, A.; Miyamoto, H.; Igarashi, M.; Kishida, N.; Matsuno, K.; Marzi, A.; Feldmann, H.; Ito, K.; Saijo, M.; et al. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 2010, 17, 1723–1728. [Google Scholar] [CrossRef]
- Dean, N.E.; Halloran, M.E.; Yang, Y.; Longini, I.M. Transmissibility and Pathogenicity of Ebola Virus: A Systematic Review and Meta-analysis of Household Secondary Attack Rate and Asymptomatic Infection. Clin. Infect. Dis. 2016, 62, 1277–1286. [Google Scholar] [CrossRef]
- Cuomo-Dannenburg, G.; McCain, K.; McCabe, R.; Unwin, H.J.T.; Doohan, P.; Nash, R.K.; Hicks, J.T.; Charniga, K.; Geismar, C.; Lambert, B.; et al. Marburg virus disease outbreaks, mathematical models, and disease parameters: A systematic review. Lancet Infect. Dis. 2024, 24, e307–e317. [Google Scholar] [CrossRef]
- Cantoni, D.; Hamlet, A.; Michaelis, M.; Wass, M.N.; Rossman, J.S. Risks Posed by Reston, the Forgotten Ebolavirus. mSphere 2016, 1, e00322-16. [Google Scholar] [CrossRef]
- Prehaud, C.; Hellebrand, E.; Coudrier, D.; Volchkov, V.E.; Volchkova, V.A.; Feldmann, H.; Le Guenno, B.; Bouloy, M. Recombinant Ebola virus nucleoprotein and glycoprotein (Gabon 94 strain) provide new tools for the detection of human infections. J. Gen. Virol. 1998, 79 Pt 11, 2565–2572. [Google Scholar] [CrossRef]
- Niikura, M.; Ikegami, T.; Saijo, M.; Kurata, T.; Kurane, I.; Morikawa, S. Analysis of linear B-cell epitopes of the nucleoprotein of ebola virus that distinguish ebola virus subtypes. Clin. Diagn. Lab. Immunol. 2003, 10, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Trappier, S.G.; Stroher, U.; Nichol, S.T.; Bowen, M.D.; Feldmann, H. Variation in the glycoprotein and VP35 genes of Marburg virus strains. Virology 1998, 240, 138–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guven, E.; Duus, K.; Lydolph, M.C.; Jorgensen, C.S.; Laursen, I.; Houen, G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J. Immunol. Methods 2014, 403, 26–36. [Google Scholar] [CrossRef] [PubMed]





| Population Group | Variable | Studies Conducted | |||||
|---|---|---|---|---|---|---|---|
| EBOV | SUDV | BDBV | RESTV | TAFV | MARV | ||
| Asymptomatic, healthy individuals (low risk) | Total N a | 11 | 6 | 0 | 0 | 0 | 5 |
| African region b | - | - | - | ||||
| East | 1 | 2 | 1 | ||||
| Central | 6 | 1 | 1 | ||||
| West | 3 | 2 | 2 | ||||
| Southern | 1 | 1 | 1 | ||||
| Outbreak area c | - | - | - | ||||
| Yes | 6 | 2 | 0 | ||||
| No | 5 | 4 | 5 | ||||
| Seroprevalence (%) d | 0.0–15.3 | 0.0–17.8 | - | - | - | 0.0–4.5 | |
| General population (low risk) | Total N a | 33 | 8 | 1 | 0 | 0 | 20 |
| African region b | - | - | |||||
| East | 3 | 2 | 1 | 7 | |||
| Central | 21 | 5 | 0 | 10 | |||
| West | 6 | 0 | 0 | 1 | |||
| Southern | 2 | 1 | 0 | 2 | |||
| Multi-region | 1 | 0 | 0 | 0 | |||
| Outbreak area c | - | - | |||||
| Yes | 15 | 2 | 0 | 3 | |||
| No | 18 | 6 | 1 | 17 | |||
| Seroprevalence (%) d | 0.0–20.9 | 0.0–19.9 | 0.2 | - | - | 0.0–3.2 | |
| Healthcare workers e (moderate risk) | Total N a | 8 | 1 | 1 | 0 | 0 | 2 |
| African region b | - | - | |||||
| East | 0 | 0 | 0 | 0 | |||
| Central | 6 | 1 | 1 | 2 | |||
| West | 2 | 0 | 0 | 0 | |||
| Outbreak area c | - | - | |||||
| Yes | 8 | 1 | 1 | 2 | |||
| No | 0 | 0 | 0 | 0 | |||
| Seroprevalence (%) d | 0.0–41.4 | 2.2 | 2.4 | - | - | 0.0–2.1 | |
| People exposed to wildlife f (moderate risk) | Total N a | 7 | 4 | 2 | 0 | 1 | 6 |
| African region b | - | ||||||
| East | 1 | 1 | 1 | ||||
| Central | 5 | 2 | 1 | 0 | 4 | ||
| West | 0 | 0 | 1 | 1 | 0 | ||
| Southern | 1 | 1 | 0 | 0 | 1 | ||
| Outbreak area c | - | ||||||
| Yes | 3 | 2 | 2 | 1 | 3 | ||
| No | 4 | 2 | 0 | 0 | 3 | ||
| Seroprevalence (%) d | 0.0–18.7 | 0.0–10.5 | 0.0–5.2 | - | 5.2 | 0.0–5.2 | |
| Close contacts of confirmed cases (high risk) | Total N a | 18 | 3 | 1 | 0 | 1 | 8 |
| African region b | - | ||||||
| East | 2 | 2 | 1 | 1 | 6 | ||
| Central | 6 | 1 | 0 | 0 | 1 | ||
| West | 10 | 0 | 0 | 0 | 0 | ||
| Southern | 0 | 0 | 0 | 0 | 1 | ||
| Outbreak area c | - | ||||||
| Yes | 17 | 3 | 1 | 0 | 5 | ||
| No | 1 | 0 | 0 | 1 | 3 | ||
| Seroprevalence (%) d | 0.9–45.8 | 1.3–32.0 | 3.6 | - | 0.4 | 0.0–3.5 | |
| Symptomatic/Suspected Cases g (high risk) | Total N a | 20 | 6 | 1 | 1 | 0 | 10 |
| African region b | - | ||||||
| East | 3 | 1 | 0 | 0 | 2 | ||
| Central | 5 | 2 | 1 | 1 | 1 | ||
| West | 10 | 1 | 0 | 0 | 5 | ||
| Southern | 1 | 1 | 1 | ||||
| Multi-region | 1 | 0 | 0 | 0 | 1 | ||
| Outbreak area c | - | ||||||
| Yes | 7 | 2 | 0 | 0 | 0 | ||
| No | 13 | 4 | 1 | 1 | 10 | ||
| Seroprevalence (%) d | 0.0–70.6 | 0.0–70.6 | 0.4 | 0.0 | - | 0.0–18.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semancik, C.S.; Whitworth, H.S.; Price, M.A.; Yun, H.; Postler, T.S.; Zaric, M.; Kilianski, A.; Cooper, C.L.; Kuteesa, M.; Talasila, S.; et al. Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment. Vaccines 2024, 12, 1394. https://doi.org/10.3390/vaccines12121394
Semancik CS, Whitworth HS, Price MA, Yun H, Postler TS, Zaric M, Kilianski A, Cooper CL, Kuteesa M, Talasila S, et al. Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment. Vaccines. 2024; 12(12):1394. https://doi.org/10.3390/vaccines12121394
Chicago/Turabian StyleSemancik, Christopher S., Hilary S. Whitworth, Matt A. Price, Heejin Yun, Thomas S. Postler, Marija Zaric, Andrew Kilianski, Christopher L. Cooper, Monica Kuteesa, Sandhya Talasila, and et al. 2024. "Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment" Vaccines 12, no. 12: 1394. https://doi.org/10.3390/vaccines12121394
APA StyleSemancik, C. S., Whitworth, H. S., Price, M. A., Yun, H., Postler, T. S., Zaric, M., Kilianski, A., Cooper, C. L., Kuteesa, M., Talasila, S., Malkevich, N., Gupta, S. B., & Francis, S. C. (2024). Seroprevalence of Antibodies to Filoviruses with Outbreak Potential in Sub-Saharan Africa: A Systematic Review to Inform Vaccine Development and Deployment. Vaccines, 12(12), 1394. https://doi.org/10.3390/vaccines12121394






