Polio Epidemiology: Strategies and Challenges for Polio Eradication Post the COVID-19 Pandemic
Abstract
1. Introduction
2. Polio Epidemiology After the COVID-19 Pandemic
3. Strategies Recommended by the WHO for Polio Eradication
4. Discussion
4.1. Vaccine Coverage Rates
4.2. Polio Surveillance
4.3. Benefits and Risks Associated with Live and Inactivated Vaccines Recommended by the WHO
4.4. Plannings for the End of the Game and Post-Eradication Era
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms and Abbreviations
| aVDPV | ambiguous vaccine derived poliovirus |
| AFP | acute flaccid paralysis |
| bOPV | bivalent OPV |
| cVDPV | circulating virus derived of polio vaccine |
| DRC | Democratic Republic of the Congo |
| DTP | diphtheria tetanus and poliomyelitis vaccine |
| DTPa | DPT based acellular pertussis vaccine |
| DTPw | DPT based whole-cell vaccine |
| ES | environmental surveillance |
| fIPV | fractional IPV |
| GMT | geometric medium titers of antibody |
| GMC | geometric medium concentration of antibody titers |
| GPEI | Global Polio Eradication Initiative |
| HIC | high-income countries |
| IMI | intestinal mucosal immunity |
| LIC | low-income countries |
| MIC | medium-income countries |
| mOPV | monovalent OPV |
| nOPV | novel OPV |
| IPV | inactivated polio vaccine |
| iVDPV | vaccine derived poliovirus in immunocompromised individuals |
| NIP | national immunization program |
| OPV | oral polio vaccine |
| PHEIC | Public Health Emergency of International Concern |
| RI | routine immunization |
| sIPV | IPV based on Sabin strains |
| tOPV | trivalent oral polio vaccine |
| USA | United States of America |
| UK | United Kingdom |
| VAPP | vaccine associated paralytic poliomyelitis |
| VCR | vaccine coverage rate |
| VLPs | virus-like particles |
| WPV | wild poliovirus |
| WGS | whole genome sequencing |
| WHO | World Health Organization |
References
- Poliomyelitis. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 25 July 2024).
- AFP/Polio Data. Available online: https://extranet.who.int/polis/public/CaseCount.aspx (accessed on 24 October 2024).
- Cavestany, R.L.; Eisenhawer, M.; Diop, O.M.; Verma, H.; Quddus, A.; Mach, O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens 2024, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Polioeradication. Global wild AFP and Environmental Samples, 2018–2024. p. 1. Available online: https://polioeradication.org/wild-poliovirus-count/ (accessed on 24 October 2024).
- Polioeradication. Global Circulation Vaccine Derived (cVDPV) AFP and Environmental Samples, 2021–2024. p. 2. Available online: https://polioeradication.org/wild-poliovirus-count/ (accessed on 21 October 2024).
- Vaccination and Immunization Statistics—UNICEF DATA. Available online: https://data.unicef.org/resources/immunization-coverage-estimates-data-visualization/ (accessed on 4 September 2024).
- Polio (Pol3) Immunization Coverage Among 1-Year-Olds (%). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-) (accessed on 24 July 2024).
- Sutter, R.W.; Kew, O.M.; Cochi, S.L.; Aylward, R.B. Poliovirus vaccine live. In Plotkin’s Vaccines, 7th ed.; Plotkin, S., Orenstein, W.A., Offit, P., Eds.; Elsevier: Philadelhia, PA, USA, 2018; pp. 866–917. [Google Scholar]
- Vidor, E. Poliovirus vaccine inactivated. In Plotkin’s Vaccines, 7th ed.; Plotkin, S., Orenstein, W.A., Offit, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 841–865. [Google Scholar]
- Muslin, C.; Mac Kain, A.; Bessaud, M.; Blondel, B.; Delpeyroux, F. Recombination in Enteroviruses, a Multi-Step Modular Evolutionary Process. Viruses 2019, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Vaccination Schedule for Poliomyelitis. Available online: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-poliomyelitis (accessed on 29 July 2024).
- Update on Wild Poliovirus Type 1 Outbreak—Southeastern Africa, 2021–2022 MMWR. Available online: https://www.cdc.gov/mmwr/volumes/72/wr/mm7215a3.htm (accessed on 26 July 2024).
- Geiger, K.; Stehling-Ariza, T.; Bigouette, J.P.; Bennett, S.D.; Burns, C.C.; Quddus, A.; Wassilak, S.G.; Bolu, O. Progress Toward Poliomyelitis Eradication—Worldwide, January 2022–December 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Statement Following the Thirty-Eighth Meeting of the IHR Emergency Committee for Polio. 8 April 2024. Available online: https://www.who.int/news/item/08-04-2024-statement-following-the-thirty-eighth-meeting-of-the-ihr-emergency-committee-for-polio (accessed on 24 July 2024).
- GPEI Press Release on nOPV2 Prequalification—GPEI. Available online: https://polioeradication.org/news/gpei-press-release-on-nopv2-prequalification/ (accessed on 25 July 2024).
- GPEI Statement on cVDPV2 detections in Burundi and Democratic Republic of the Congo. Available online: https://polioeradication.org/news-post/gpei-statement-on-cvdpv2-detections-in-burundi-and-democratic-republic-of-the-congo (accessed on 27 July 2024).
- Namageyo-Funa, A.; Greene, S.A.; Henderson, E.; Traoré, M.A.; Shaukat, S.; Bigouette, J.P.; Jorba, J.; Wiesen, E.; Bolu, O.; Diop, O.M.; et al. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2023–June 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Variant Type 2 Poliovirus Isolated from Sewage Samples in Gaza—GPEI. Available online: https://polioeradication.org/news/variant-type-2-poliovirus-isolated-from-sewage-samples-in-gaza/ (accessed on 24 July 2024).
- Statement of the Thirty-Ninth Meeting of the Polio IHR Emergency Committee. 13 August 2024. Available online: https://www.who.int/news/item/13-08-2024-statement-of-the-thirty-ninth-meeting-of-the-polio-ihr-emergency-committee (accessed on 11 September 2024).
- Falleiros-Arlant, L.H.; Ayala, S.E.G.; Domingues, C.; Brea, J.; De Colsa-Ranero, A. Estado actual de la poliomielitis en Latinoamérica. Rev. Chil. Infectologia 2020, 37, 701–709, (In English, Spanish). [Google Scholar] [CrossRef]
- Alfaro-Murillo, J.A.; Ávila-Agüero, M.L.; Fitzpatrick, M.C.; Crystal, C.J.; Falleiros-Arlant, L.-H.; Galvani, A.P. The case for replacing live oral polio vaccine with inactivated vaccine in the Americas. Lancet 2020, 395, 1163–1166. [Google Scholar] [CrossRef]
- Macklin, G.; Diop, O.M.; Humayun, A.; Shahmahmoodi, S.; El-Sayed, Z.A.; Triki, H.; Rey, G.; Avagyan, T.; Grabovac, V.; Jorba, J.; et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses—Worldwide, July 2018–December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 913–917. [Google Scholar] [CrossRef]
- Pan American Health Organization; World Health Organization. Epidemiological Update: Poliomyelitis in the Region of the Americas; PAHO/WHO: Washington, DC, USA, 2023. Available online: https://www.paho.org/en/documents/epidemiological-update-poliomyelitis-region-americas-23-march-2023 (accessed on 26 September 2024).
- Rodríguez, R.; Juárez, E.; Estívariz, C.F.; Cajas, C.; Rey-Benito, G.; Amézquita, M.O.B.; Miles, S.J.; Orantes, O.; Freire, M.C.; Chévez, A.-E.; et al. Response to Vaccine-Derived Polioviruses Detected through Environmental Surveillance, Guatemala, 2019. Emerg. Infect. Dis. 2023, 29, 1524–1530. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhang, W.; Huang, S.; Zhu, S.; Li, C.; Guo, X.; Zeng, H.; Fang, L.; Ke, B.; et al. Dynamic changes in polioviruses identified by environmental surveillance in Guangzhou, 2009–2021. J. Med Virol. 2023, 95, e28668. [Google Scholar] [CrossRef]
- Al-Qassimi, M.A.; Al Amad, M.; Al-Dar, A.; Al Sakaf, E.; Al Hadad, A.; Raja’a, Y.A. Circulating vaccine derived polio virus type 2 outbreak and response in Yemen, 2021–2022, a retrospective descriptive analysis. BMC Infect. Dis. 2024, 24, 321. [Google Scholar] [CrossRef]
- Cooper, L.V.; Bandyopadhyay, A.S.; Gumede, N.; Mach, O.; Mkanda, P.; Ndoutabé, M.; Okiror, S.O.; Ramirez-Gonzalez, A.; Touray, K.; Wanyoike, S.; et al. Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: A retrospective analysis of surveillance data for 51 countries in Africa. Lancet Infect. Dis. 2022, 22, 284–294, Erratum in Lancet Infect. Dis. 2022, 22, e41. [Google Scholar] [CrossRef] [PubMed]
- Suares, J.E.; Khan, S.; Aadrika, A.; Poojari, P.G.; Rashid, M.; Thunga, G. Vaccine-associated paralytic poliomyelitis in oral polio vaccine recipients: Disproportionality analysis using VAERS and systematic review. Expert Opin. Drug Saf. 2024, 23, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.C.; Govindaraj, G.; Ahmad, M.; Varose, S.Y.; Tatkare, M.; Shete, A.; Yadav, S.; Joshi, Y.; Yadav, P.; Sharma, D.; et al. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Excretion in an Infant with Severe Combined Immune Deficiency with Spillover to a Parent. Vaccines 2024, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhu, S.; Wang, D.; Li, X.; Zhu, H.; Song, Y.; Liu, X.; Xiao, F.; Zhao, H.; Lu, H.; et al. Genetic characterization and molecular evolution of type 3 vaccine-derived polioviruses from an immunodeficient patient in China. Virus Res. 2023, 334, 199177. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.F.; Eisenberg, J.N.S.; Pomeroy, C.D.; Shulman, L.M.; Hindiyeh, M.; Manor, Y.; Grotto, I.; Koopman, J.S.; Eisenberg, M.C. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. USA 2018, 115, E10625–E10633. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Wassilak, S.G.; Pallansch, M.A.; Burns, C.C.; Wiesen, E.; Durry, E.; Badizadegan, K.; Thompson, K.M. Outbreak response strategies with type 2-containing oral poliovirus vaccines. Vaccine 2023, 41, A142–A152. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Badizadegan, K.; Thompson, K.M. Outbreak management strategies for cocirculation of multiple poliovirus types. Vaccine 2023, 41, 3718–3727. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Wassilak, S.G.F.; Wiesen, E.; Burns, C.C.; Pallansch, M.A.; Badizadegan, K.; Thompson, K.M. Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences. Risk Anal. 2024, 44, 366–378. [Google Scholar] [CrossRef]
- Connor, R.I.; Brickley, E.B.; Wieland-Alter, W.F.; Ackerman, M.E.; Weiner, J.A.; Modlin, J.F.; Bandyopadhyay, A.S.; Wright, P.F. Mucosal immunity to poliovirus. Mucosal Immunol. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Saleem, A.F.; Kazi, Z.U.; Zehra, S.M.; Parkar, S.; Macklin, G.; Sifontes, G.; A Mainou, B.; Alam, M.; Cavestany, R.L.; Mach, O. Mucosal Immunity to Poliovirus in Children 0–15 Years of Age: A Community-Based Study in Karachi, Pakistan in 2019. J. Infect. Dis. 2024, 230, 736–740. [Google Scholar] [CrossRef]
- Habib, M.A.; Soofi, S.B.; Hussain, I.; Ahmed, I.; Hussain, Z.; Tahir, R.; Anwar, S.; Cousens, S.; Bhutta, Z.A. Does IPV Boost Intestinal Immunity among Children under Five Years of Age? An Experience from Pakistan. Vaccines 2023, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Cavestany, R.L.; Blake, I.M.; Macklin, G.; Cooper, L.; Grassly, N.; Nery, A.L.M.d.S.; Mach, O. Use of inactivated poliovirus vaccine for poliovirus outbreak response. Lancet Infect. Dis. 2023, 24, e328–e342, Erratum in Lancet Infect. Dis. 2024, 24, e83. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Pontarotti, P.; Levasseur, A.; Colson, P.; Raoult, D. Is it time to switch to a formulation other than the live attenuated poliovirus vaccine to prevent poliomyelitis? Front. Public Health 2024, 11, 1284337. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.B.; Macklin, G.R.; Ross, G.M.; Cavestany, R.L.; A Moukom, R.; Jones, K.A.V.; A Mainou, B.; Massaquoi, M.B.F.; Kieh, M.W.S.; Mach, O. Poliovirus antibodies following two rounds of campaigns with a type 2 novel oral poliovirus vaccine in Liberia: A clustered, population-based seroprevalence survey. Lancet Glob. Health 2023, 11, e917–e923. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Erbeto, T.B.; A Danzomo, A.; Abdullahi, H.W.; Boateng, K.; Adamu, U.S.; Shuaib, F.; Modjirom, N.; Gray, E.J.; Bandyopadhyay, A.S.; et al. Effectiveness of poliovirus vaccines against circulating vaccine-derived type 2 poliomyelitis in Nigeria between 2017 and 2022: A case-control study. Lancet Infect. Dis. 2024, 24, 427–436. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Eremeeva, T.P.; Baykova, O.Y.; Krasota, A.Y.; Yakovchuk, E.V.; Shustova, E.Y.; Malyshkina, L.P.; Mustafina, A.N.-I.; Mikhailova, Y.M.; Chirova, A.V.; et al. Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak. Vaccines 2024, 12, 718. [Google Scholar] [CrossRef]
- Gao, H.; Lau, E.H.Y.; Cowling, B.J. Waning Immunity After Receipt of Pertussis, Diphtheria, Tetanus, and Polio-Related Vaccines: A Systematic Review and Meta-analysis. J. Infect. Dis. 2021, 225, 557–566. [Google Scholar] [CrossRef]
- Larocca, A.M.V.; Bianchi, F.P.; Bozzi, A.; Tafuri, S.; Stefanizzi, P.; Germinario, C.A. Long-Term Immunogenicity of Inactivated and Oral Polio Vaccines: An Italian Retrospective Cohort Study. Vaccines 2022, 10, 1329. [Google Scholar] [CrossRef]
- Tan, Q.; Zhu, Q.; Zheng, H.; Zhang, B.; Wu, C.; Guo, X.; Li, H.; Liu, L.; Liu, Y.; Rutherford, S.; et al. Epidemiological serosurvey of poliovirus in Guangdong, China: A cross-sectional study. Hum. Vaccines Immunother. 2018, 14, 2644–2648. [Google Scholar] [CrossRef]
- Xu, J.; Kuang, S.; Rong, R.; Zhang, Y.; Tang, W.; Wang, Q. Sero-survey of polio antibodies and quality of acute flaccid paralysis surveillance in Chongqing, China: A cross-sectional study. Medicine 2020, 99, e21298. [Google Scholar] [CrossRef]
- Alfonso, V.H.; Voorman, A.; Hoff, N.A.; Weldon, W.C.; Gerber, S.; Gadoth, A.; Halbrook, M.; Goldsmith, A.; Mukadi, P.; Doshi, R.H.; et al. Poliovirus immunity among adults in the Democratic Republic of the Congo: A cross-sectional serosurvey. BMC Infect. Dis. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- John, T.J.; Dharmapalan, D.; Steinglass, R.; Hirschhorn, N. Novel OPV is Still not the Right Tool for Polio Eradication. Indian Pediatr. 2024, 61, 387. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.M. Population Immunity and Polio Eradication. Pathogens 2024, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Eisenhawer, M.; Molodecky, N.A.; Verma, H.; Okayasu, H. Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance. Pathogens 2024, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- John, T.J.; Dharmapalan, D.; Steinglass, R.; Hirschhorn, N. The respiratory route of transmission of virulent polioviruses. Infect. Dis. 2024, 56, 918–924. [Google Scholar] [CrossRef]
- Wahid, B.; Kumari, B.; Saifullah, K.M.; Idrees, M. The History and Current Killings of Polio Vaccinators in Pakistan: A Need for Targeted Surveillance Strategy. Asia Pac. J. Public Health 2023, 35, 183–188. [Google Scholar] [CrossRef]
- Abbasi, F.H.; Shaikh, A.A.; Mehraj, J.; Raza, S.M.; Rasool, S.; Bullo, U.F.; Mehraj, S.; Phul, Z.A.; Sahitia, S.; Zardari, A.A.; et al. Vaccine Hesitancy and Perceptions of the Community about Polio in High-Risk Areas of Karachi, Sindh, Pakistan. Vaccines 2022, 11, 70. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Shiferie, F.; Tsegaye, D.A.; Alemayehu, W.A.; Wondie, T.; Donofrio, J.; DelPizzo, F.; Belete, K.; Biks, G.A. Oral and Inactivated Polio Vaccine Coverage and Determinants of Coverage Inequality Among the Most At-Risk Populations in Ethiopia. Am. J. Trop. Med. Hyg. 2023, 109, 1148–1156. [Google Scholar] [CrossRef]
- Jacobson, A.; Spitzer, S.; Gorelik, Y.; Edelstein, M. Barriers and enablers to vaccination in the ultra-orthodox Jewish population: A systematic review. Front. Public Health 2023, 11, 1244368. [Google Scholar] [CrossRef]
- Kidd, S.; Clark, T.; Routh, J.; Cineas, S.; Bahta, L.; Brooks, O. Use of Inactivated Polio Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1327–1330. [Google Scholar] [CrossRef]
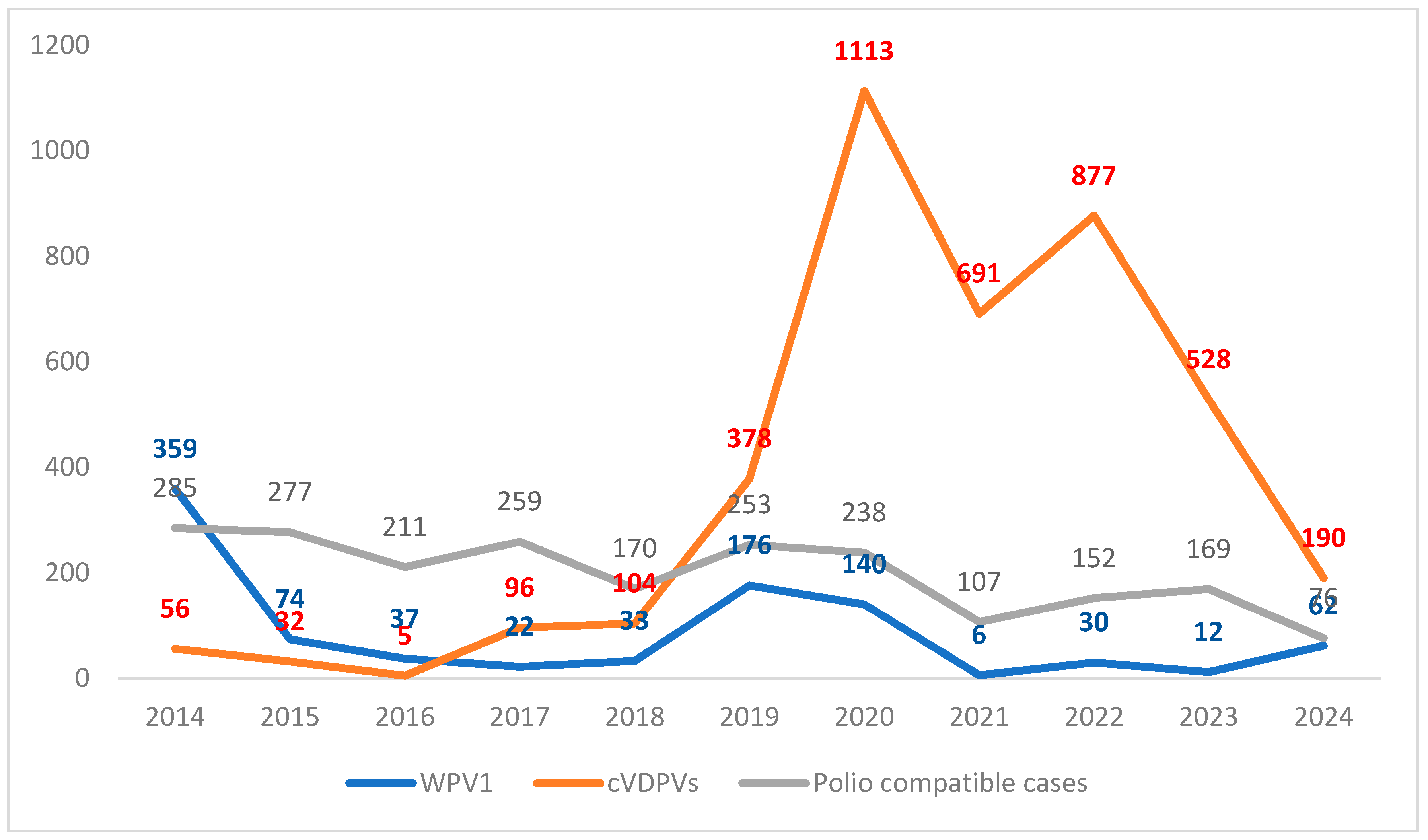

| AFP | 2020 Cases (No. Countries) | 2021 Cases (No. Countries) | 2022 Cases (No. Countries) | 2023 Cases (No. Countries) | 2024 Cases (No. Countries) * |
|---|---|---|---|---|---|
| WPV1 | 140 (2) | 6 (3) | 30 (3) | 12 (2) | 62 (2) |
| cVDPV1 | 35 (4) | 14 (2) | 192 (5) | 134 (3) | 8 (2) |
| cVDPV2 | 1082 (24) | 629 (22) | 367 (20) | 383 (23) | 182 (16) |
| cVDPV3 | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bricks, L.F.; Macina, D.; Vargas-Zambrano, J.C. Polio Epidemiology: Strategies and Challenges for Polio Eradication Post the COVID-19 Pandemic. Vaccines 2024, 12, 1323. https://doi.org/10.3390/vaccines12121323
Bricks LF, Macina D, Vargas-Zambrano JC. Polio Epidemiology: Strategies and Challenges for Polio Eradication Post the COVID-19 Pandemic. Vaccines. 2024; 12(12):1323. https://doi.org/10.3390/vaccines12121323
Chicago/Turabian StyleBricks, Lucia F., Denis Macina, and Juan C. Vargas-Zambrano. 2024. "Polio Epidemiology: Strategies and Challenges for Polio Eradication Post the COVID-19 Pandemic" Vaccines 12, no. 12: 1323. https://doi.org/10.3390/vaccines12121323
APA StyleBricks, L. F., Macina, D., & Vargas-Zambrano, J. C. (2024). Polio Epidemiology: Strategies and Challenges for Polio Eradication Post the COVID-19 Pandemic. Vaccines, 12(12), 1323. https://doi.org/10.3390/vaccines12121323